Abstract
Viral infections are among the major causes of acute liver failure (ALF) worldwide. While the role of agents such as hepatitis A, B, C, D and E viruses in precipitating ALF are well known, improvements in serological assays have led to the detection of viral agents such as Epstein Barr virus, cytomegalovirus etc. as atypical causes of ALF. Despite the plethora of literature available on viral hepatitis and ALF, there is very limited large-scale epidemiologic data on the prevalence, risk factors of progression and outcomes in ALF of viral causes. This is important as viral infections remain the leading cause of ALF in the East and in developing countries, while the impact of viral ALF in the West has largely been ameliorated by effective vaccination and sanitization programs. This review focuses specifically on the available prognostic scores that aid in the management of ALF of viral etiologies while also briefly reviewing the current literature on newer viral agents known to cause ALF, risk factors of progression, outcomes and how management algorithms can be developed by incorporation of prognostic scoring systems for referral and transplant listing.
1. Introduction
Acute liver failure (ALF) is clinically defined as coagulopathy (internationalized normalized ratio (INR) > 1.5) and encephalopathy following an acute hepatic insult in a patient without pre-existing liver disease [1]. The time interval between the onset of jaundice and encephalopathy varies from 4 weeks in India to 26 weeks in the United States [2]. It is considered a medical emergency and is associated with high mortality rates of 50–75% [3]. The etiology of ALF is myriad, varies globally and includes drugs, infectious etiologies, such as viruses, and rare metabolic diseases such as Wilson’s disease. In the West, most ALF cases are due to drugs and toxins with acetaminophen being the most prevalent. By contrast, in the East and the developing world, ALF is mainly due to viral infections. The common etiologies of ALF are provided in Table 1.

Table 1.
Etiologies of acute liver failure [1,3].
ALF is considered a success story in gastroenterology as the introduction of liver transplantation (LT) in its management has proven to be a definitive therapy for afflicted patients, and LT has decreased mortality rates to as low as 20% [4,5]. ALF patients are currently being given the highest priority for LT (“Status 1A”) by the United Network for Organ Sharing (UNOS), with median wait times as low as 48 h [6]. The introduction and widespread usage of live donor liver transplants has further reduced the waitlist times while also providing a potential solution to the ever-increasing disparity between organ donors and recipients [7]. In parallel with the developments in LT, there have been better understanding of pathophysiology and improvements in critical care medicine, which have led to improved transplant-free survival (TFS) rates [8].
Thus, it is increasingly being recognized that not all ALF patients require LT and there is a need for objective measures to identify those who do need it [9]. This subset of patients would benefit from early referral to specialized liver units (SLU) and transplant listing. Several prognostic scores exist which serve as prognostic models to identify patients with poor outcomes who would require LT [10]. The current article reviews the various prognostic models developed for patients with ALF due to a viral etiology, along with their performance characteristics, applicability and limitations.
2. Viral Etiologies of ALF
Viruses are one of the most common causes of ALF worldwide. Several viral agents are known to cause ALF, which may be classified as follows (Table 2):

Table 2.
Known viral agents which may cause acute liver failure [1,3].
3. Epidemiology
Despite the knowledge that several viruses may cause ALF, there is limited global epidemiological data on viral-ALF [11]. Some of the reasons behind this are: (i) recent identification of certain viruses as causative agents of liver diseases, (ii) development of better diagnostic assays which allowed for diagnosis of atypical viral agents causing ALF (which may have been classified as idiopathic ALF), (iii) the inconsistent implementation of vaccination programs for preventable viral diseases across countries. As a result of this, one finds that the burden of hepatitis A and B-related ALF is very high in Southeast Asia due to poor penetration of vaccination programs as compared to the West, where common etiologies of ALF are drugs, toxins and metabolic diseases. Patterson et al. reported that cases of hepatitis B-related ALF (HBV-ALF) in Europe have decreased to 19% following routine immunization, while similar changes in hepatitis A related ALF (HAV-ALF) have been seen in Argentina, with a decrease in incidence to 25% [12]. Similarly, hepatitis E virus-related ALF (HEV-ALF) was reported to have a point prevalence of 32% (range: 3–70%) in this review. The hepatitis E vaccine (Hecolin) was introduced in China in the year 2012 but is licensed by the WHO for use only in outbreaks. Despite being introduced over a decade, this vaccine has not been incorporated in national programs, but may potentially decrease the prevalence of HEV infections in endemic areas if approved [13]. The importance of knowing the epidemiology of viral ALF extends beyond the design of national programs and preventive healthcare. Multiple groups have reported that ALF most commonly presents in the fourth decade of life and is more prevalent in women [14,15,16]. Thus, most affected patients are relatively young and at a risk for significant morbidity and mortality. However, mortality rates vary in different countries owing to the availability of donor livers, the prevalent etiology of ALF and referral practices. The survival rates in ALF with expectant management range between 50 and 60%, which may improve to 80% after liver transplant (LT) [1]. Thus, 20% of patients with ALF will succumb to the illness despite receiving definitive care with LT. There are multiple reasons why an LT does not ensure survival in all ALF patients, which include: (i) incorrect timing of liver transplant (pre-transplant waiting >5 days), (ii) post-transplant infections, (iii) recipients’ characteristics (such as higher age, higher BMI, concomitant renal impairment, vasopressor requirement), (iv) deficiencies in referral systems with lack of SLU and (v) procedural morbidity and mortality associated with the transplant procedure.
The timing of the LT is a very pertinent but unanswered question in the management of ALF, despite the immense research and literature available on the subject. Current guidelines advocate that all patients should be considered for LT if they deteriorate clinically and should be referred to an SLU for specialized care while awaiting a transplant [17,18]. The impact of referral to an SLU in improving outcomes in these patients has also been noted in recent studies [19,20]. Several procedures that allow us to sustain a patient clinically have also become available, such as plasma exchange (PE) and an extracorporeal liver support (ECLS) device, which has shown some benefit in improving biochemical and clinical parameters (ECLS) and in survival rates (PE) in randomized trials [21,22]. Thus, when faced with a patient presenting with ALF, the clinician should be able to answer the following questions: (i) whether the patient require a liver transplant or expectant management; (ii) how to objectively identify parameters which would enable to identify those in need of LT; and (iii) how frequently these objective parameters should be reassessed in a patient. Prognostic scoring systems can help answer these in ALF patients. Several such prognostic systems are available, each with its benefits and shortcomings, which are discussed in this review article with respect to their use in viral-ALF.
4. Importance of Prognostic Systems
Prognostic systems can help objectively identify patients requiring an LT. To most efficiently manage such patients, the prognostic system should have the following properties [23]:
- (i)
- Dynamicity
ALF is a dynamic condition which requires close monitoring for early identification of poor prognostic parameters. A delay in identification may lead to complications such as infections, cerebral edema etc., significantly decreasing survival in these patients. Thus, the prognostic score should also reflect the changing prognosis of patients with changing clinical and biochemical parameters [24].
- (ii)
- Applicability
We have highlighted that a multitude of factors can cause ALF. An ideal prognostic system should be able to predict outcomes across etiologies of ALF. For example, high bilirubin values in anti-tubercular drug therapy-induced ALF are an independent risk factor determining poor outcomes, while it is not very relevant in patients with acetaminophen-induced ALF (APAP-ALF) [25]. An ideal score should thus be universally applicable and provide accurate results irrespective of etiology.
- (iii)
- Accuracy
The accuracy of a prognostic score in ALF would be its ability to identify those who require an LT. Thus, the test would be required to have a high sensitivity and specificity in order to increase true positives and eliminate false positives). It would also need to have a high positive predictive value (PPV) so that those who require a transplant are identified by the test, as well as a high negative predictive value (NPV) to ensure those who would survive without a transplant do not receive an organ. Unfortunately, no single prognostic score meets all the criteria mentioned above [9,26].
- (iv)
- Ease of use
The most commonly used clinical prognostic scores are those which are the least cumbersome with the highest yields. This is one of the major drawbacks of scores with multiple parameters (such as the acute physiology and chronic health evaluation (APACHE) score), which have multiple components and are thus cumbersome. Similarly, the components of the test should be commonly available. Several newer tests use serological markers that are not available at most centers, such as M30 and M65 (circulating apoptotic markers), limiting their use to a research setting or academic centers [27,28].
5. Clinical Presentation of Viral ALF
Clinically, the natural history of hepatitis caused by the hepatotropic viruses (HAV, HBV, HCV, HDV and HEV) is well known [29]. The mode of infection, incubation period and outcomes of acute viral hepatitis are provided in Table 3. There are limited data available on factors which predict the progression from viral hepatitis to ALF. In general, ALF is classified based on the time interval between the appearance of jaundice and the onset of encephalopathy [30]. This also has prognostic significance as a more rapid onset of encephalopathy (hyperacute or acute presentation) has better outcomes than a more subacute or delayed presentation. However, there is substantial variability in defining ALF based on icterus-encephalopathy interval. The proposed different classification systems used for ALF are provided in Table 4.

Table 3.
Clinical outcomes of acute viral hepatitis-related acute liver failure.

Table 4.
Classification of ALF based on the interval between onset of jaundice and encephalopathy.
6. Approach to Management
Ideally, all patients with ALF should receive care in an intensive care unit [43]. ALF is a dynamic condition where stringent monitoring is required to prevent complications which would adversely affect the outcome [42]. One of the most feared outcomes is cerebral edema which may lead to a progressive and irreversible decline in neurological functions and render a patient unfit for liver transplantation [44]. Thus, the primary target of management in ALF is good triaging (to identify patients with poor prognosis at baseline) and early referral to higher centers with SLU to be considered for early transplant listing or other bridging methods such as plasmapheresis or artificial liver support systems (ALSS), as may be relevant [45]. In this regard the clinical judgment of the physician is aided by several objective prognostic scores which have been detailed below. Some of these prognostic scores are applicable to ALF of any etiology (e.g., Clichy score, King’s college criteria (KCC) for non-acetaminophen ALF (non-APAP ALF) or model for end-stage liver disease (MELD) score) while some scoring systems are specific to the precipitating etiology (e.g., hepatitis A related-ALF (ALFA) score). These scoring systems have predominantly been derived from a retrospective analysis of ALF cohorts and prospectively validated. Each scoring system has its own benefits and drawbacks, but most have not been compared with each other in randomized trials; hence, there is no single best scoring system which is universally accepted. Similarly, when patients are considered for LT in cases of ALF, no single scoring system is relied upon and it is a composite decision based on clinical and objective parameters [4].
7. Prognostic Scoring Systems in Viral ALF
There are several prognostic systems available for use in ALF. As viral ALF is the leading cause of ALF in the East, most of these systems have been used for prognostication in viral ALF along with other etiologies. For this review, we have limited the discussion to scoring systems which have either been used in viral ALF or have been developed for specific viral etiologies. As noted from the data provided in Table 5, very few scoring systems have been developed exclusively for the prognosis of viral hepatitis. Further, the number of patients of viral ALF included in the derivation cohort of these scoring systems was limited. The etiology-specific scoring systems are largely limited to hepatitis A and E. The details of the various cut-off values of prognostic scoring systems in predicting outcomes are shown in Table 6. The scoring systems for hepatitis B virus largely relate to acute-on-chronic liver failure, while scoring systems for hepatitis C, hepatitis D and the other less commonly encountered viruses are not available [46,47].

Table 5.
Currently available scoring systems with individual components and limitations.

Table 6.
Utility of prognostic scores in predicting mortality/outcome in patients with viral-ALF.
8. Management of Viral ALF
Viral ALF can be precipitated by multiple agents, yet only a few have specific antiviral agents which may be used—hepatitis B (entecavir, tenofovir), HSV (acyclovir) and CMV (ganciclovir, valganciclovir) [74,75,76]. Ongoing research has highlighted the unique role of host factors such as very low density lipoproteins, low density lipoproteins, high density lipoproteins and apolipoproteins in mediating viral entry of HCV through suppression of the transforming growth factor beta pathway. Identification of such newer pathways not only provides us clearer understanding of pathways of viral replication and infection, but also provides newer targets for drug therapy. Better understanding of these interactions may also lead to identification of newer risk factors which predict progression of disease from hepatitis to liver failure [77,78]. Novel drug targets are being identified to combat entry of hepatotropic viruses into cells and replication such as nicotinamide in hepatitis A [79], recombinant HEV virion [80] and the newly introduced bepirovirsen in hepatitis B [81], although these are yet to be tested in acute liver failure. Emerging data from animal studies are also available on immunotherapy with agents such as thymosin alpha 1 [82], mesenchymal stem cells and their exosomes in ALF [83] as immunomodulators to prevent immune dysfunction in ALF. If successful, they would help decrease the burden on the organ pool and contribute to improving transplant free survival rates in ALF further. As the atypical viral infections that precipitate ALF most commonly occur in the immunocompromised, decreasing the doses of immunosuppressive drugs may prevent progression from acute viral hepatitis to ALF and thus drug therapy could be reserved for advanced or severe cases [84].
Once a patient progresses to ALF, the management protocol of the patient becomes standardized, as shown in Figure 1. Ideally, all ALF patients should receive care in an intensive care unit (ICU) or SLU which has been shown to improve survival in ALF patients in multiple studies [20,85]. The key decisions in management are to triage patients who need a transplant and to time the transplant correctly so as to maximize the chances of recovery and survival [4]. While clinical assessment is subjective and may show interobserver variations, objective prognostic scores form the backbone of decision-making in ALF. Ideally the prognostic scores are calculated at admission to identify patients with poor prognosis on entry to the system. Patients are resuscitated and started on supportive care. These scores are then repeated at various intervals to assess the progression or resolution of the underlying condition. Thus, we can objectively assess those who will recover with supportive care only (lower priority for transplant listing or delisting) and those who are clinically worsening and thus need to be listed for an LT [86].
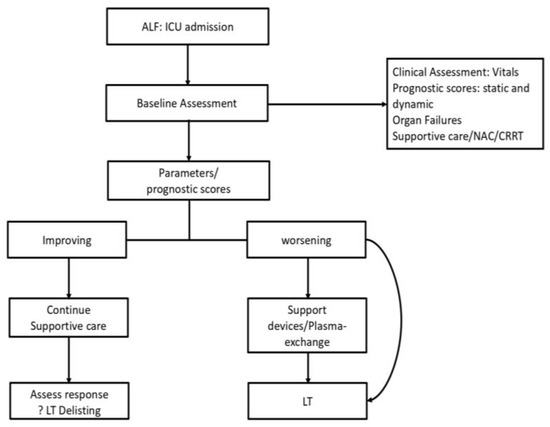
Figure 1.
Suggested management algorithm for ALF.
Current issues in the clinical management deal with the choice of score to be used, as there is no single “best scoring system” available, and the frequency at which these should be repeated. These practices are largely adapted to the centers at which the patient is admitted. For example, a high-volume transplant center would prefer more frequent reassessments (6–12 hourly) to identify early features of deterioration and proceed for an LT. At present, there is no consensus on the frequency of repeat scoring and assessment. If the patient is found to deteriorate clinically or have poor prognosis as per the scoring systems, they should be listed for transplantation and referred to an SLU (if not already admitted to one). Over here the decision may be to proceed to a transplant based on the availability of a donor organ or utilize a bridge to transplant such as plasmapheresis or artificial liver support systems (ALSS) which would sustain the patient clinically to allow more time for obtaining the donor organ [22,45,87,88]. In this respect, plasmapheresis has been shown to improve both clinical parameters as well as short-term survival, whereas several ALSS have been shown to improve clinical and biochemical parameters (bilirubin, hepatic encephalopathy) without impacting survival [22,89].
Advances in liver transplant such as availability of live donor liver transplants (LDLT), expanded criteria/marginal liver and hepatocyte transplants have changed the landscape of ALF management [90,91]. The availability of LDLT significantly reduces the waiting time for a donor organ for a recipient. LDLT is also associated with less cold ischemia time (CIT), although this does not translate to a clinically lower incidence of biliary strictures or lower rejection rates [92]. LDLT is thus a popular option in countries such as India where organ donation after death is not a popular option and thus a small donor pool is available for dead donor liver transplant (DDLT) [93]. Post-transplant survival rates in ALF are provided in Table 3 and vary as per the etiology. Overall, the TFS is best for hepatitis E while the 1- and 5-year post transplant survival is excellent for HBV-ALF.
9. Conclusions
A large number of viral agents, both typical and atypical, can cause acute liver failure. Over the past three decades, improvements in critical care management and a better knowledge of pathophysiology have led to a dramatic rise in the transplant free survival rate for viral-induced acute liver failure patients. However, a significant number of patients still need a liver transplant and the post-transplant survival rate has improved to about 80%. Several prognostic scoring systems exist for prognostication and the decision to proceed with liver transplantation in such patients. However, the lack of reliable prognostic models frequently makes it difficult to identify transplant candidates early and appropriately, necessitating further research to produce a more reliable and widely used prognostic model for viral-induced acute liver failure.
Author Contributions
S.B.: draft writing and critical review; R.K.: draft writing and critical review; S.K.A.: draft writing and critical review; S.: draft writing and critical review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Acharya, S.K. Acute Liver Failure: Indian Perspective. Clin. Liver Dis. 2021, 18, 143–149. [Google Scholar] [CrossRef]
- Shalimar; Acharya, S.K.; Lee, W.M. Worldwide Differences in Acute Liver Failure. In Critical Care in Acute Liver Failure; Future Medicine Ltd.: London, UK, 2013; pp. 32–46. [Google Scholar]
- Anand, A.C.; Nandi, B.; Acharya, S.K.; Arora, A.; Babu, S.; Batra, Y.; Chawla, Y.K.; Chowdhury, A.; Chaoudhuri, A.; Eapen, E.C.; et al. Indian National Association for the Study of the Liver Consensus Statement on Acute Liver Failure (Part 1): Epidemiology, Pathogenesis, Presentation and Prognosis. J. Clin. Exp. Hepatol. 2020, 10, 339–376. [Google Scholar] [CrossRef]
- Kumar, R.; Anand, U.; Priyadarshi, R.N. Liver Transplantation in Acute Liver Failure: Dilemmas and Challenges. World J. Transplant. 2021, 11, 187–202. [Google Scholar] [CrossRef]
- Bretherick, A.D.; Craig, D.G.N.; Masterton, G.; Bates, C.; Davidson, J.; Martin, K.; Iredale, J.P.; Simpson, K.J. Acute Liver Failure in Scotland between 1992 and 2009; Incidence, Aetiology and Outcome. QJM Int. J. Med. 2011, 104, 945–956. [Google Scholar] [CrossRef]
- Nephew, L.D.; Zia, Z.; Ghabril, M.; Orman, E.; Lammert, C.; Kubal, C.; Chalasani, N. Sex Disparities in Waitlisting and Liver Transplant for Acute Liver Failure. JHEP Rep. 2020, 3, 100200. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mehta, N.; Rao, P.S.; Lalwani, S.; Mangla, V.; Nundy, S. Live Donor Liver Transplantation for Acute Liver Failure: A Single Center Experience. Indian J. Gastroenterol. 2018, 37, 25–30. [Google Scholar] [CrossRef]
- Koch, D.G.; Tillman, H.; Durkalski, V.; Lee, W.M.; Reuben, A. Development of a Model to Predict Transplant-Free Survival of Patients with Acute Liver Failure. Clin. Gastroenterol. Hepatol. 2016, 14, 1199–1206.e2. [Google Scholar] [CrossRef]
- Shalimar; Sonika, U.; Kedia, S.; Mahapatra, S.J.; Nayak, B.; Yadav, D.P.; Gunjan, D.; Thakur, B.; Kaur, H.; Acharya, S.K. Comparison of Dynamic Changes among Various Prognostic Scores in Viral Hepatitis-Related Acute Liver Failure. Ann. Hepatol. 2018, 17, 403–412. [Google Scholar] [CrossRef]
- Bernal, W.; Lee, W.M.; Wendon, J.; Larsen, F.S.; Williams, R. Acute Liver Failure: A Curable Disease by 2024? J. Hepatol. 2015, 62, S112–S120. [Google Scholar] [CrossRef]
- Patterson, J.; Hussey, H.S.; Abdullahi, L.H.; Silal, S.; Goddard, L.; Setshedi, M.; Spearman, W.; Hussey, G.D.; Kagina, B.; Muloiwa, R. The Global Epidemiology of Viral-Induced Acute Liver Failure: A Systematic Review Protocol. BMJ Open 2019, 9, e029819. [Google Scholar] [CrossRef]
- Patterson, J.; Hussey, H.S.; Silal, S.; Goddard, L.; Setshedi, M.; Spearman, W.; Hussey, G.D.; Kagina, B.M.; Muloiwa, R. Systematic Review of the Global Epidemiology of Viral-Induced Acute Liver Failure. BMJ Open 2020, 10, e037473. [Google Scholar] [CrossRef]
- Ciglenecki, I.; Rumunu, J.; Wamala, J.F.; Nkemenang, P.; Duncker, J.; Nesbitt, R.; Gignoux, E.; Newport, T.; Heile, M.; Jamet, C.; et al. The First Reactive Vaccination Campaign against Hepatitis E. Lancet Infect. Dis. 2022, 22, 1110–1111. [Google Scholar] [CrossRef]
- Shalimar; Acharya, S.K.; Kumar, R.; Bharath, G.; Rout, G.; Gunjan, D.; Nayak, B. Acute Liver Failure of Non–A-E Viral Hepatitis Etiology—Profile, Prognosis, and Predictors of Outcome. J. Clin. Exp. Hepatol. 2020, 10, 453–461. [Google Scholar] [CrossRef]
- Thanapirom, K.; Treeprasertsuk, S.; Soonthornworasiri, N.; Poovorawan, K.; Chaiteerakij, R.; Komolmit, P.; Phaosawasdi, K.; Pinzani, M. The Incidence, Etiologies, Outcomes, and Predictors of Mortality of Acute Liver Failure in Thailand: A Population-Base Study. BMC Gastroenterol. 2019, 19, 18. [Google Scholar] [CrossRef]
- Simões, C.; Santos, S.; Vicente, M.; Sousa Cardoso, F. Epidemiology of Acute Liver Failure from a Regional Liver Transplant Center in Portugal. GE-Port. J. Gastroenterol. 2019, 26, 33–39. [Google Scholar] [CrossRef]
- Polson, J.; Lee, W.M. AASLD Position Paper: The Management of Acute Liver Failure. Hepatology 2005, 41, 1179–1197. [Google Scholar] [CrossRef]
- Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Nevens, F.; Samuel, D.; Simpson, K.J.; Yaron, I.; Bernardi, M. EASL Clinical Practical Guidelines on the Management of Acute (Fulminant) Liver Failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef]
- Donnelly, M.C.; Davidson, J.S.; Martin, K.; Baird, A.; Hayes, P.C.; Simpson, K.J. Acute Liver Failure in Scotland: Changes in Aetiology and Outcomes over Time (the Scottish Look-Back Study). Aliment. Pharmacol. Ther. 2017, 45, 833–843. [Google Scholar] [CrossRef]
- Ordys, B.B.; Robinson, O. Acute Liver Failure. Anaesth. Intensive Care Med. 2021, 22, 113–120. [Google Scholar] [CrossRef]
- Matar, A.J.; Subramanian, R. Extracorporeal Liver Support: A Bridge to Somewhere. Clin. Liver Dis. 2021, 18, 274–279. [Google Scholar] [CrossRef]
- Larsen, F.S.; Schmidt, L.E.; Bernsmeier, C.; Rasmussen, A.; Isoniemi, H.; Patel, V.C.; Triantafyllou, E.; Bernal, W.; Auzinger, G.; Shawcross, D.; et al. High-Volume Plasma Exchange in Patients with Acute Liver Failure: An Open Randomised Controlled Trial. J. Hepatol. 2016, 64, 69–78. [Google Scholar] [CrossRef]
- Jain, V.; Dhawan, A. Prognostic Modeling in Pediatric Acute Liver Failure. Liver Transpl. 2016, 22, 1418–1430. [Google Scholar] [CrossRef]
- Kumar, R.; Shalimar; Sharma, H.; Goyal, R.; Kumar, A.; Khanal, S.; Prakash, S.; Gupta, S.D.; Panda, S.K.; Acharya, S.K. Prospective Derivation and Validation of Early Dynamic Model for Predicting Outcome in Patients with Acute Liver Failure. Gut 2012, 61, 1068–1075. [Google Scholar] [CrossRef]
- Kumar, R.; Shalimar; Bhatia, V.; Khanal, S.; Sreenivas, V.; Gupta, S.D.; Panda, S.K.; Acharya, S.K. Antituberculosis Therapy–Induced Acute Liver Failure: Magnitude, Profile, Prognosis, and Predictors of Outcome. Hepatology 2010, 51, 1665–1674. [Google Scholar] [CrossRef]
- Sanders, S.; Flaws, D.; Than, M.; Pickering, J.W.; Doust, J.; Glasziou, P. Simplification of a Scoring System Maintained Overall Accuracy but Decreased the Proportion Classified as Low Risk. J. Clin. Epidemiol. 2016, 69, 32–39. [Google Scholar] [CrossRef]
- Gomez, D.; Cameron, I.C. Prognostic Scores for Colorectal Liver Metastasis: Clinically Important or an Academic Exercise? HPB 2010, 12, 227–238. [Google Scholar] [CrossRef]
- Cao, Z.; Li, F.; Xiang, X.; Liu, K.; Liu, Y.; Tang, W.; Lin, L.; Guo, Q.; Bao, S.; Xie, Q.; et al. Circulating Cell Death Biomarker: Good Candidates of Prognostic Indicator for Patients with Hepatitis B Virus Related Acute-on-Chronic Liver Failure. Sci. Rep. 2015, 5, 14240. [Google Scholar] [CrossRef]
- Castaneda, D.; Gonzalez, A.J.; Alomari, M.; Tandon, K.; Zervos, X.B. From Hepatitis A to E: A Critical Review of Viral Hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715. [Google Scholar] [CrossRef]
- Blackmore, L.; Bernal, W. Acute Liver Failure. Clin. Med. 2015, 15, 468–472. [Google Scholar] [CrossRef]
- Jung, D.-H.; Hwang, S.; Lim, Y.-S.; Kim, K.-H.; Ahn, C.-S.; Moon, D.-B.; Ha, T.-Y.; Song, G.-W.; Park, G.-C.; Lee, S.-G. Outcome Comparison of Liver Transplantation for Hepatitis A-Related versus Hepatitis B-Related Acute Liver Failure in Adult Recipients. Clin. Transplant. 2018, 32, e13140. [Google Scholar] [CrossRef]
- Taylor, R.M.; Davern, T.; Munoz, S.; Han, S.-H.; McGuire, B.; Larson, A.M.; Hynan, L.; Lee, W.M.; Fontana, R.J.; US Acute Liver Failure Study Group. Fulminant Hepatitis A Virus Infection in the United States: Incidence, Prognosis, and Outcomes. Hepatology 2006, 44, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.-F.; Xiong, T.; Huang, P.; Zhong, Y.-D.; Wang, H.-L.; Yang, Y.-F. Early Predictors of Acute Hepatitis B Progression to Liver Failure. PLoS ONE 2018, 13, e0201049. [Google Scholar] [CrossRef] [PubMed]
- Ichai, P.; Samuel, D. Management of Fulminant Hepatitis B. Curr. Infect. Dis. Rep. 2019, 21, 25. [Google Scholar] [CrossRef]
- Urban, S.; Neumann-Haefelin, C.; Lampertico, P. Hepatitis D Virus in 2021: Virology, Immunology and New Treatment Approaches for a Difficult-to-Treat Disease. Gut 2021, 70, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Karna, R. A Review of the Diagnosis and Management of Hepatitis E. Curr. Treat. Options Infect. Dis. 2020, 12, 310–320. [Google Scholar] [CrossRef]
- Shalimar; Kedia, S.; Gunjan, D.; Sonika, U.; Mahapatra, S.J.; Nayak, B.; Kaur, H.; Acharya, S.K. Acute Liver Failure Due to Hepatitis E Virus Infection Is Associated with Better Survival than Other Etiologies in Indian Patients. Dig. Dis. Sci. 2017, 62, 1058–1066. [Google Scholar] [CrossRef]
- Jensen, K.O.; Angst, E.; Hetzer, F.H.; Gingert, C. Acute Cytomegalovirus Hepatitis in an Immunocompetent Host as a Reason for Upper Right Abdominal Pain. Case Rep. Gastroenterol. 2016, 10, 36–43. [Google Scholar] [CrossRef]
- Chávez, S.M.; Poniachik, J.M.; Urzua, Á.M.; Roblero, J.P.; Cattaneo, M.J.; Jimenez, A.P.; Carreño, L.E.; Cornejo, R.A. Acute Liver Failure Due to Herpes Simplex Virus: Diagnostic Clues and Potential Role of Plasmapheresis. Medicine 2021, 100, e27139. [Google Scholar] [CrossRef]
- Mellinger, J.L.; Rossaro, L.; Naugler, W.E.; Nadig, S.N.; Appelman, H.; Lee, W.M.; Fontana, R.J. Epstein–Barr Virus (EBV) Related Acute Liver Failure: A Case Series from the US Acute Liver Failure Study Group. Dig. Dis. Sci. 2014, 59, 1630–1637. [Google Scholar] [CrossRef]
- Kye Mon, K.; Nontprasert, A.; Kittitrakul, C.; Tangkijvanich, P.; Leowattana, W.; Poovorawan, K. Incidence and Clinical Outcome of Acute Liver Failure Caused by Dengue in a Hospital for Tropical Diseases, Thailand. Am. J. Trop. Med. Hyg. 2016, 95, 1338–1344. [Google Scholar] [CrossRef]
- Rovegno, M.; Vera, M.; Ruiz, A.; Benítez, C. Current Concepts in Acute Liver Failure. Ann. Hepatol. 2019, 18, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Price, J.; Agarwal, B. Management of Acute Liver Failure in Intensive Care. BJA Educ. 2021, 21, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.R.; Kronsten, V.T.; Hughes, R.D.; Shawcross, D.L. Pathophysiology of Cerebral Oedema in Acute Liver Failure. World J. Gastroenterol. WJG 2013, 19, 9240–9255. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Froghi, S. Artificial Liver Support Systems. J. Gastroenterol. Hepatol. 2021, 36, 1164–1179. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; You, S.; Feng, T.; Zhou, X.; Zhu, B.; Luo, J.; Xin, J.; Jiang, J.; Shi, D.; et al. Development and Validation of a New Prognostic Score for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. J. Hepatol. 2021, 75, 1104–1115. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Shao, L.; Xin, J.; Jiang, L.; Zhou, Q.; Shi, D.; Jiang, J.; Sun, S.; Jin, L.; et al. Development of Diagnostic Criteria and a Prognostic Score for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Gut 2018, 67, 2181–2191. [Google Scholar] [CrossRef]
- Yantorno, S.E.; Kremers, W.K.; Ruf, A.E.; Trentadue, J.J.; Podestá, L.G.; Villamil, F.G. MELD Is Superior to King’s College and Clichy’s Criteria to Assess Prognosis in Fulminant Hepatic Failure. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2007, 13, 822–828. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Jain, S.; Maheshwari, U.; Bhalla, A.; Sharma, N.; Ahluwalia, J.; Duseja, A.; Chawla, Y. Early Indicators of Prognosis in Fulminant Hepatic Failure: An Assessment of the Model for End-Stage Liver Disease (MELD) and King’s College Hospital Criteria. Liver Transpl. 2007, 13, 814–821. [Google Scholar] [CrossRef]
- Katoonizadeh, A.; Decaestecker, J.; Wilmer, A.; Aerts, R.; Verslype, C.; Vansteenbergen, W.; Yap, P.; Fevery, J.; Roskams, T.; Pirenne, J.; et al. MELD Score to Predict Outcome in Adult Patients with Non-Acetaminophen-Induced Acute Liver Failure. Liver Int. Off. J. Int. Assoc. Study Liver 2007, 27, 329–334. [Google Scholar] [CrossRef]
- Bismuth, H.; Samuel, D.; Castaing, D.; Adam, R.; Saliba, F.; Johann, M.; Azoulay, D.; Ducot, B.; Chiche, L. Orthotopic Liver Transplantation in Fulminant and Subfulminant Hepatitis The Paul Brousse Experience. Ann. Surg. 1995, 222, 109. [Google Scholar] [CrossRef]
- Ichai, P.; Legeai, C.; Francoz, C.; Boudjema, K.; Boillot, O.; Ducerf, C.; Mathurin, P.; Pruvot, F.-R.; Suc, B.; Wolf, P.; et al. Patients with Acute Liver Failure Listed for Superurgent Liver Transplantation in France: Reevaluation of the Clichy-Villejuif Criteria. Liver Transpl. 2015, 21, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Hadem, J.; Stiefel, P.; Bahr, M.J.; Tillmann, H.L.; Rifai, K.; Klempnauer, J.; Wedemeyer, H.; Manns, M.P.; Schneider, A.S. Prognostic Implications of Lactate, Bilirubin, and Etiology in German Patients With Acute Liver Failure. Clin. Gastroenterol. Hepatol. 2008, 6, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, A.; King, L.Y.; Hynan, L.S.; Vedvyas, C.; Lin, W.; Lee, W.M.; Chung, R.T. Development of an Accurate Index for Predicting Outcomes of Patients with Acute Liver Failure. Gastroenterology 2012, 143, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Figorilli, F.; Putignano, A.; Roux, O.; Houssel-Debry, P.; Francoz, C.; Paugam-Burtz, C.; Soubrane, O.; Agarwal, B.; Durand, F.; Jalan, R. Development of an Organ Failure Score in Acute Liver Failure for Transplant Selection and Identification of Patients at High Risk of Futility. PLoS ONE 2017, 12, e0188151. [Google Scholar] [CrossRef] [PubMed]
- McPhail, M.J.W.; Wendon, J.A.; Bernal, W. Meta-Analysis of Performance of Kings’s College Hospital Criteria in Prediction of Outcome in Non-Paracetamol-Induced Acute Liver Failure. J. Hepatol. 2010, 53, 492–499. [Google Scholar] [CrossRef]
- Kim, J.D.; Cho, E.J.; Ahn, C.; Park, S.K.; Choi, J.Y.; Lee, H.C.; Kim, D.Y.; Choi, M.S.; Wang, H.J.; Kim, I.H.; et al. A Model to Predict 1-Month Risk of Transplant or Death in Hepatitis A-Related Acute Liver Failure: Hepatology. Hepatology 2019, 70, 621–629. [Google Scholar] [CrossRef]
- Wu, J.; Shi, C.; Sheng, X.; Xu, Y.; Zhang, J.; Zhao, X.; Yu, J.; Shi, X.; Li, G.; Cao, H.; et al. Prognostic Nomogram for Patients with Hepatitis E Virus-Related Acute Liver Failure: A Multicenter Study in China. J. Clin. Transl. Hepatol. 2021, 9, 828–837. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, P.-L.; Ding, H.-G. Comparison Scoring Model of Severe Viral Hepatitis and Model of End Stage Liver Disease for the Prognosis of Patients with Liver Failure in China. World J. Gastroenterol. WJG 2007, 13, 2999–3002. [Google Scholar] [CrossRef]
- Craig, D.G.N.; Reid, T.W.D.J.; Wright, E.C.; Martin, K.G.; Davidson, J.S.; Hayes, P.C.; Simpson, K.J. The Sequential Organ Failure Assessment (SOFA) Score Is Prognostically Superior to the Model for End-Stage Liver Disease (MELD) and MELD Variants Following Paracetamol (Acetaminophen) Overdose. Aliment. Pharmacol. Ther. 2012, 35, 705–713. [Google Scholar] [CrossRef]
- Cholongitas, E.; Theocharidou, E.; Vasianopoulou, P.; Betrosian, A.; Shaw, S.; Patch, D.; O’Beirne, J.; Agarwal, B.; Burroughs, A.K. Comparison of the Sequential Organ Failure Assessment Score with the King’s College Hospital Criteria and the Model for End-Stage Liver Disease Score for the Prognosis of Acetaminophen-Induced Acute Liver Failure. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2012, 18, 405–412. [Google Scholar] [CrossRef]
- Mitchell, I.; Bihari, D.; Chang, R.; Wendon, J.; Williams, R. Earlier Identification of Patients at Risk from Acetaminophen-Induced Acute Liver Failure. Crit. Care Med. 1998, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Singh, R.; Acharya, S.K. Predictive Value of Arterial Ammonia for Complications and Outcome in Acute Liver Failure. Gut 2006, 55, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shalimar; Sharma, H.; Prakash, S.; Panda, S.K.; Khanal, S.; Acharya, S.K. Persistent Hyperammonemia Is Associated With Complications and Poor Outcomes in Patients With Acute Liver Failure. Clin. Gastroenterol. Hepatol. 2012, 10, 925–931. [Google Scholar] [CrossRef]
- Bernal, W.; Wendon, J. More on Serum Phosphate and Prognosis of Acute Liver Failure. Hepatology 2003, 38, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Sitrin, M.D.; Te, H.S. Serum Phosphorus Levels Predict Clinical Outcome in Fulminant Hepatic Failure. Liver Transpl. 2003, 9, 248–253. [Google Scholar] [CrossRef]
- Bernal, W. Lactate Is Important in Determining Prognosis in Acute Liver Failure. J. Hepatol. 2010, 53, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Taurá, P.; Martinez-Palli, G.; Martinez-Ocon, J.; Beltran, J.; Sanchez-Etayo, G.; Balust, J.; Anglada, T.; Mas, A.; Garcia-Valdecasas, J.-C. Hyperlactatemia in Patients with Non-Acetaminophen-Related Acute Liver Failure. World J. Gastroenterol. WJG 2006, 12, 1949–1953. [Google Scholar] [CrossRef]
- Schiødt, F.V.; Ostapowicz, G.; Murray, N.; Satyanarana, R.; Zaman, A.; Munoz, S.; Lee, W.M. Alpha-Fetoprotein and Prognosis in Acute Liver Failure. Liver Transpl. 2006, 12, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.G.; Berry, P.A.; Davies, E.T.; Hussain, M.; Bernal, W.; Vergani, D.; Wendon, J. Reduced Monocyte HLA-DR Expression: A Novel Biomarker of Disease Severity and Outcome in Acetaminophen-Induced Acute Liver Failure. Hepatology 2006, 44, 34–43. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Triantafyllou, E.; Brenig, R.; Lebosse, F.J.; Singanayagam, A.; Patel, V.C.; Pop, O.T.; Khamri, W.; Nathwani, R.; Tidswell, R.; et al. CD14+ CD15− HLA-DR− Myeloid-Derived Suppressor Cells Impair Antimicrobial Responses in Patients with Acute-on-Chronic Liver Failure. Gut 2018, 67, 1155–1167. [Google Scholar] [CrossRef]
- Grama, A.; Burac, L.; Aldea, C.O.; Bulata, B.; Delean, D.; Samasca, G.; Abrudan, C.; Sirbe, C.; Pop, T.L. Vitamin D-Binding Protein (Gc-Globulin) in Acute Liver Failure in Children. Diagnostics 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Schiødt, F.V.; Rossaro, L.; Stravitz, R.T.; Shakil, A.O.; Chung, R.T.; Lee, W.M.; Group, A.L.F.S. Gc-Globulin and Prognosis in Acute Liver Failure. Liver Transpl. 2005, 11, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Oketani, M.; Uto, H.; Ido, A.; Tsubouchi, H. Management of Hepatitis B Virus-Related Acute Liver Failure. Clin. J. Gastroenterol. 2014, 7, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Norvell, J.P.; Blei, A.T.; Jovanovic, B.D.; Levitsky, J. Herpes Simplex Virus Hepatitis: An Analysis of the Published Literature and Institutional Cases. Liver Transpl. 2007, 13, 1428–1434. [Google Scholar] [CrossRef]
- Nakase, H.; Herfarth, H. Cytomegalovirus Colitis, Cytomegalovirus Hepatitis and Systemic Cytomegalovirus Infection: Common Features and Differences. Inflamm. Intest. Dis. 2016, 1, 15–23. [Google Scholar] [CrossRef]
- Zhang, F.; Sodroski, C.; Cha, H.; Li, Q.; Liang, T.J. Infection of Hepatocytes with HCV Increases Cell Surface Levels of Heparan Sulfate Proteoglycans, Uptake of Cholesterol and Lipoprotein, and Virus Entry by Up-Regulating SMAD6 and SMAD7. Gastroenterology 2017, 152, 257–270.e7. [Google Scholar] [CrossRef]
- Cosset, F.-L.; Mialon, C.; Boson, B.; Granier, C.; Denolly, S. HCV Interplay with Lipoproteins: Inside or Outside the Cells? Viruses 2020, 12, 434. [Google Scholar] [CrossRef]
- Sasaki-Tanaka, R.; Masuzaki, R.; Okamoto, H.; Shibata, T.; Moriyama, M.; Kogure, H.; Kanda, T. Drug Screening for Hepatitis A Virus (HAV): Nicotinamide Inhibits c-Jun Expression and HAV Replication. J. Virol. 2023, 97, e01987-22. [Google Scholar] [CrossRef]
- Primadharsini, P.P.; Nagashima, S.; Nishiyama, T.; Takahashi, M.; Murata, K.; Okamoto, H. Development of Recombinant Infectious Hepatitis E Virus Harboring the NanoKAZ Gene and Its Application in Drug Screening. J. Virol. 2022, 96, e01906-21. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Lim, S.-G.; Plesniak, R.; Tsuji, K.; Janssen, H.L.A.; Pojoga, C.; Gadano, A.; Popescu, C.P.; Stepanova, T.; Asselah, T.; et al. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N. Engl. J. Med. 2022, 387, 1957–1968. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Zhang, J.; Tang, T.; Kong, Y.; Ye, F.; Zhang, X.; Liu, X.; Lin, S. Thymosin A1 Treatment Reduces Hepatic Inflammation and Inhibits Hepatocyte Apoptosis in Rats with Acute Liver Failure. Exp. Ther. Med. 2018, 15, 3231–3238. [Google Scholar] [CrossRef] [PubMed]
- Shokravi, S.; Borisov, V.; Zaman, B.A.; Niazvand, F.; Hazrati, R.; Khah, M.M.; Thangavelu, L.; Marzban, S.; Sohrabi, A.; Zamani, A. Mesenchymal Stromal Cells (MSCs) and Their Exosome in Acute Liver Failure (ALF): A Comprehensive Review. Stem Cell Res. Ther. 2022, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.C.; Danziger-Isakov, L. Respiratory Viral Infections in Solid Organ and Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 707–726. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, A. Intensive Care Management of Acute Liver Failure: Considerations While Awaiting Liver Transplantation. J. Clin. Transl. Hepatol. 2019, 7, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Saluja, V.; Sharma, A.; Pasupuleti, S.S.; Mitra, L.G.; Kumar, G.; Agarwal, P.M. Comparison of Prognostic Models in Acute Liver Failure: Decision Is to Be Dynamic. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2019, 23, 574–581. [Google Scholar] [CrossRef]
- Stahl, K.; Hadem, J.; Schneider, A.; Manns, M.P.; Wiesner, O.; Schmidt, B.M.W.; Hoeper, M.M.; Busch, M.; David, S. Therapeutic Plasma Exchange in Acute Liver Failure. J. Clin. Apheresis 2019, 34, 589–597. [Google Scholar] [CrossRef]
- Saliba, F.; Camus, C.; Durand, F.; Mathurin, P.; Letierce, A.; Delafosse, B.; Barange, K.; Perrigault, P.F.; Belnard, M.; Ichaï, P.; et al. Albumin Dialysis with a Noncell Artificial Liver Support Device in Patients with Acute Liver Failure: A Randomized, Controlled Trial. Ann. Intern. Med. 2013, 159, 522–531. [Google Scholar] [CrossRef]
- Sponholz, C.; Matthes, K.; Rupp, D.; Backaus, W.; Klammt, S.; Karailieva, D.; Bauschke, A.; Settmacher, U.; Kohl, M.; Clemens, M.G.; et al. Molecular Adsorbent Recirculating System and Single-Pass Albumin Dialysis in Liver Failure—A Prospective, Randomised Crossover Study. Crit. Care 2016, 20, 2. [Google Scholar] [CrossRef]
- Ogura, Y.; Kabacam, G.; Singhal, A.; Moon, D.-B. The Role of Living Donor Liver Transplantation for Acute Liver Failure. Int. J. Surg. 2020, 82, 145–148. [Google Scholar] [CrossRef]
- Lee, C.A.; Sinha, S.; Fitzpatrick, E.; Dhawan, A. Hepatocyte Transplantation and Advancements in Alternative Cell Sources for Liver-Based Regenerative Medicine. J. Mol. Med. Berl. Ger. 2018, 96, 469–481. [Google Scholar] [CrossRef]
- Campsen, J.; Blei, A.T.; Emond, J.C.; Everhart, J.E.; Freise, C.E.; Lok, A.S.; Saab, S.; Wisniewski, K.A.; Trotter, J.F. Outcomes of Living Donor Liver Transplantation for Acute Liver Failure: The Adult-to-Adult Living Donor Liver Transplantation Cohort Study. Liver Transplant. 2008, 14, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, G. Living Donor Liver Transplantation in India. Hepatobiliary Surg. Nutr. 2016, 5, 127–132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).