Maternal Steroids on Fetal Doppler Indices, in Growth-Restricted Fetuses with Abnormal Umbilical Flow from Pregnancies Complicated with Early-Onset Severe Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
3. Results
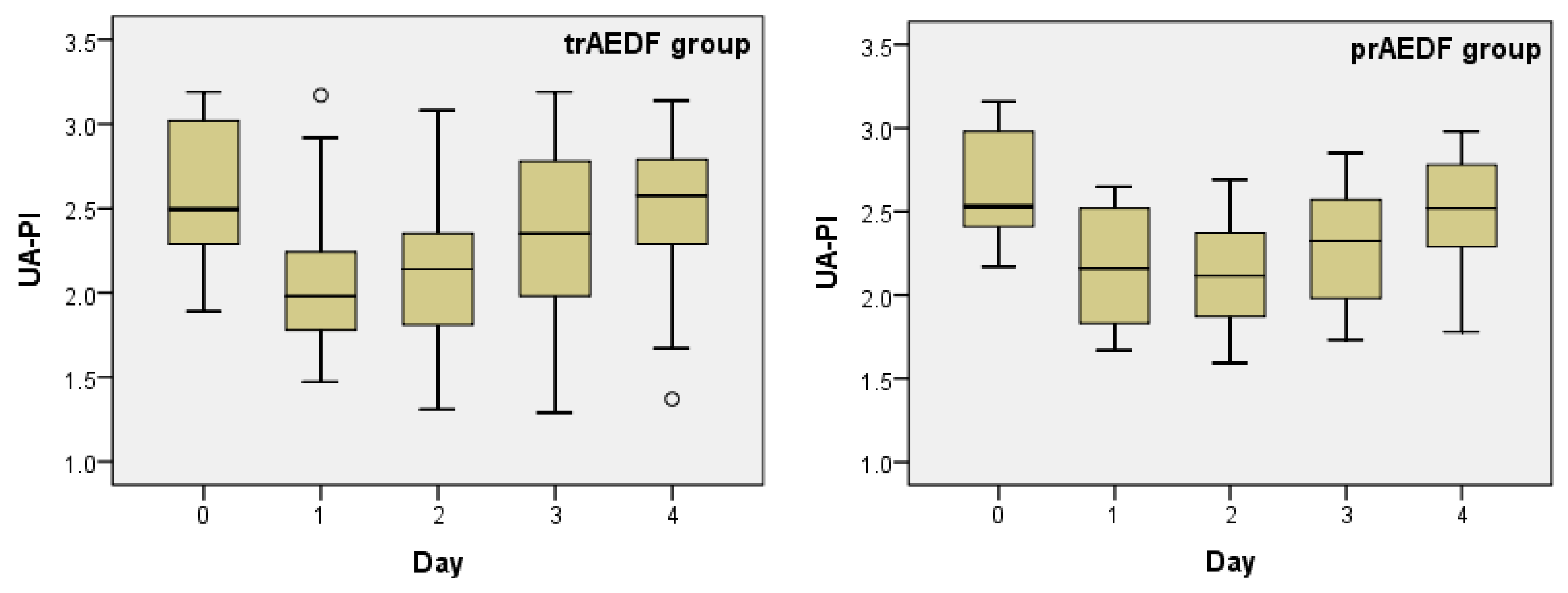
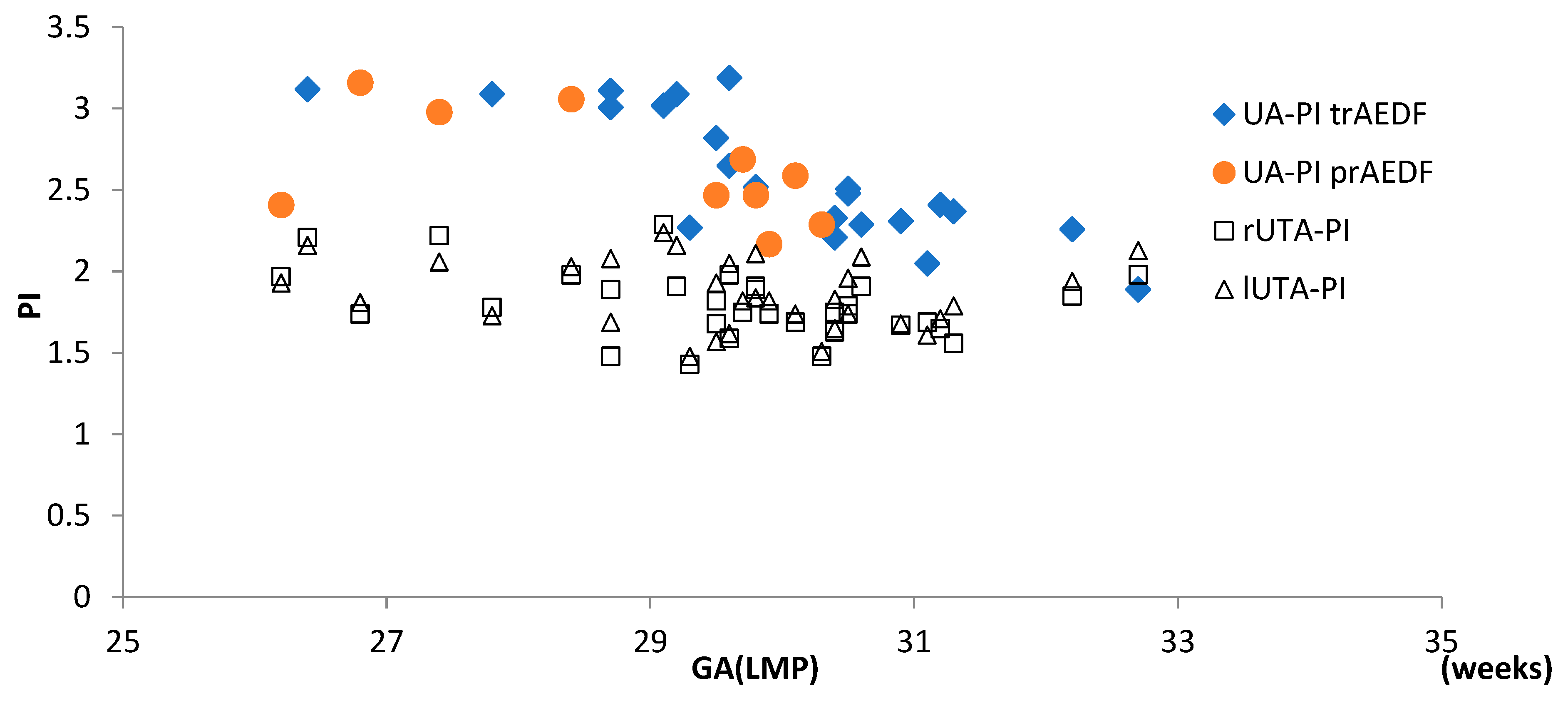
3.1. Doppler Findings on Umbilical Artery (UA)
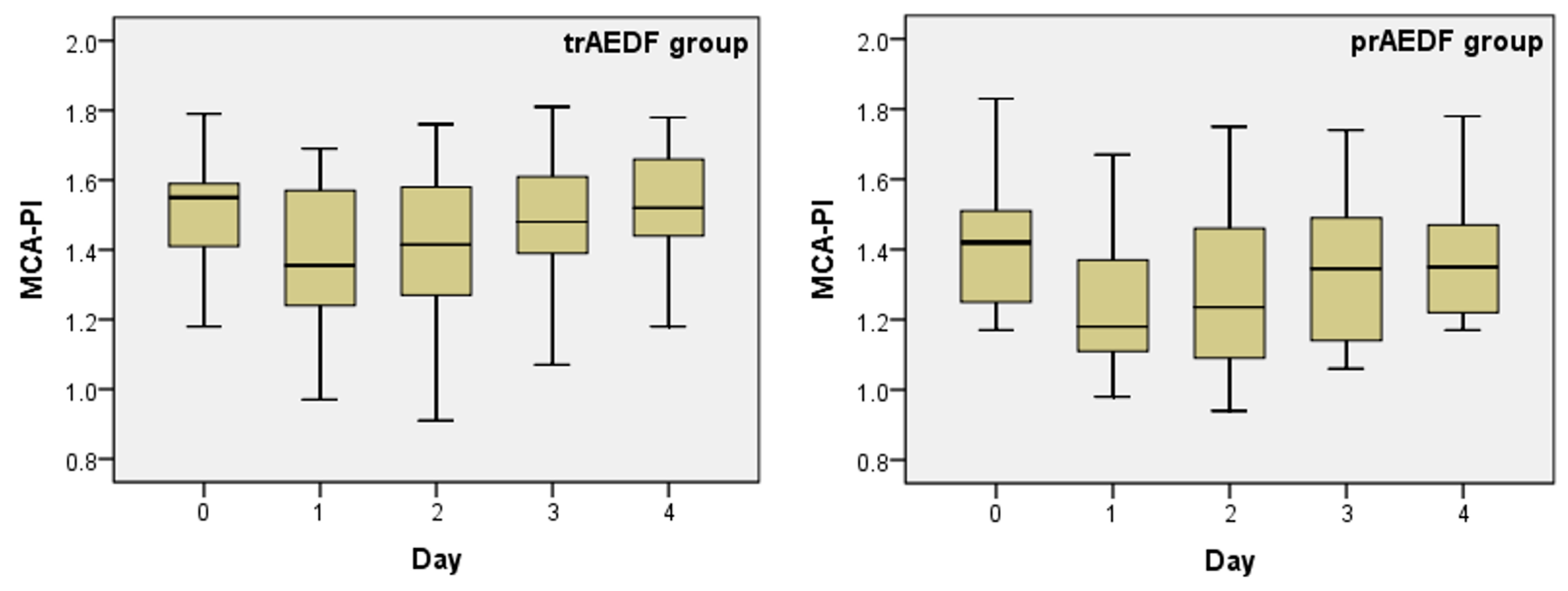
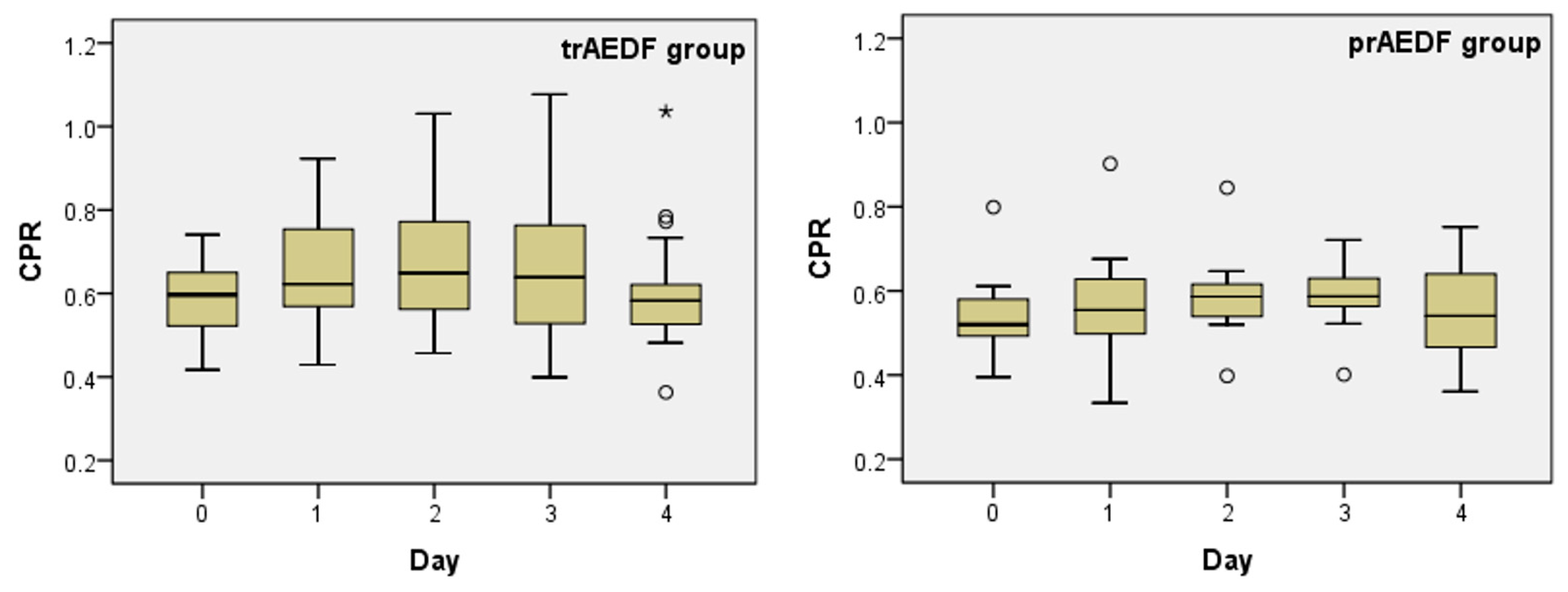
3.2. Doppler Findings on Middle Cerebral Artery (MCA) and Cerebroplacental Ratio (CPR) Evaluation
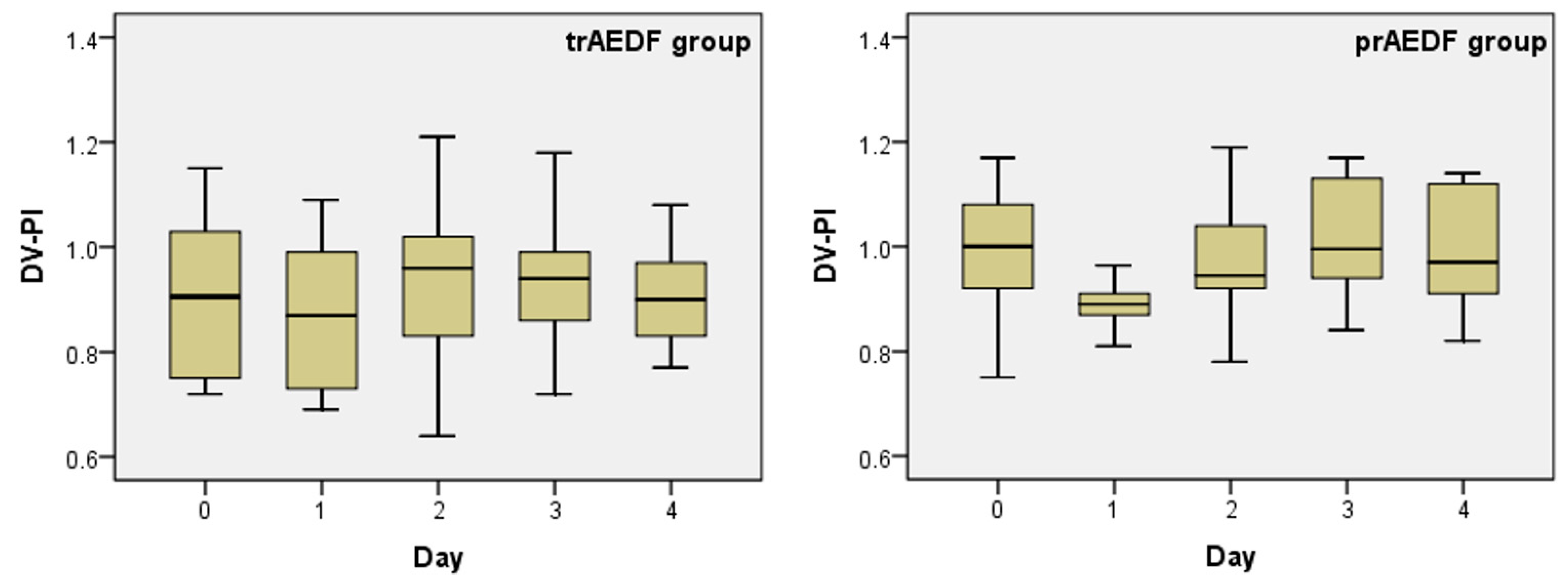
3.3. Doppler Findings on Ductus Venosus (DV)
3.4. Doppler Findings on Right and Left Uterine Arteries (r/lUTA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, D.R.; Odendaal, H.J.; Kirsten, G.F.; Smith, J.; Grove, D. Expectant management of early onset, severe preeclampsia: Perinatal outcome. Br. J. Obstet. Gynecol. 2000, 107, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar] [CrossRef] [PubMed]
- Nyflot, L.T.; Ellingsen, L.; Oian, P.; Vangen, S. Maternal deaths from hypertensive disorders: Lessons learnt. Acta Obstet. Gynecol. Scan. 2018, 97, 976–987. [Google Scholar] [CrossRef]
- Duley, L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br. Med. Bull. 2003, 67, 161–176. [Google Scholar] [CrossRef]
- Railton, A.; Allen, D.G. Management and outcome of pregnancy complicated by severe pre-eclampsia of early onset. S. Afr. Med. J. 1987, 72, 608–610. [Google Scholar]
- Figueras, F.; Gratacos, E. Stage-based approach to the management of the fetal growth restriction. Prenat. Diagn. 2014, 34, 655–659. [Google Scholar] [CrossRef]
- RCOG Green Top Guideline No. 31. 2014. Available online: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg31/ (accessed on 25 September 2022).
- Eronen, M.; Kari, A.; Pesonen, E.; Kaaja, R.; Wallgren, E.I.; Hallman, M. Value of absent or retrograde end-diastolic flow in fetal aorta and umbilical artery as a predictor of perinatal outcome in pregnancy-induced hypertension. Acta Paediatr. 1993, 82, 919–924. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.; Serra-Serra, V.; Gaffney, G.; Redman, C.W.; Hope, P.L. Neonatal outcome after pregnancy complicated by abnormal velocity waveforms in the umbilical artery. Arch. Dis. Child. 1994, 70, F84–F89. [Google Scholar] [CrossRef]
- Hartung, J.; Kalache, K.D.; Heyna, C.; Heling, K.S.; Kuhlig, M.; Wauer, R.; Bollman, R.; Chaoui, R. Outcome of 60 neonates who had ARED flow prenatally compared with a matched control group of appropriate-for-gestational age preterm neonates. Ultrasound Obstet. Gynecol. 2005, 25, 566–572. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: NICE. 2011. Available online: https://pubmed.ncbi.nlm.nih.gov/22220321/ (accessed on 17 September 2022).
- Fedunik, S.; Gaca, Z.; Malinowska, O.; Brunets, W.; Zgliczynska, M.; Wlodarczyk, M.; Wojcikiewicz, A.; Ciebiera, M. The Management of Pregnancy Complicated with the Previable Preterm and Preterm Premature Rupture of the Membranes: What about a Limit of Neonatal Viability? A Review. Diagnostics 2022, 12, 2025. [Google Scholar] [CrossRef]
- Senat, M.V.; Ville, Y. Effect of steroids on arterial Doppler in intrauterine growth retardation fetuses. Fetal. Diagn. Ther. 2000, 15, 36–40. [Google Scholar] [CrossRef]
- Müller, T.; Nanan, R.; Dietl, J. Effect of antenatal corticosteroid administration on Doppler flow velocity parameters in pregnancies with absent or reverse end-diastolic flow in the umbilical artery. Acta Obstet. Gynecol. Scand. 2003, 82, 794–796. [Google Scholar] [PubMed]
- Thuring, A.; Malcus, P.; Marsal, K. Effect of maternal betamethasone on fetal and uteroplacental blood flow velocity waveforms. Ultrasound Obstet. Gynecol. 2011, 37, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Ekin, A.; Gezer, C.; Solmaz, U.; Taner, C.E.; Ozeren, M.; Dogan, A.; Uyar, I. Effect of antenatal betamethasone administration on Doppler velocimetry of fetal and uteroplacental vessels: A prospective study. J. Perinat. Med. 2016, 44, 243–248. [Google Scholar] [CrossRef]
- Karsdorp, V.H.M.; van Vugt, J.M.G.; van Geijn, H.P.; Kostense, P.J.; Arduini, D.; Montenegro, N.; Todros, T. Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet 1994, 344, 1664–1668. [Google Scholar] [CrossRef]
- Schwartze, A.; Gembruch, U.; Krapp, M.; Katalinic, A.; Germer, U.; Axt-Fledner, R. Qualitative venous Doppler flow waveform analysis in preterm intrauterine growth-restricted fetuses with ARED flow in the umbilical artery—correlation with short-term outcome. Ultrasound Obstet. Gynecol. 2005, 25, 573–579. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Diagnostic Criteria of Severe Preeclampsia. 2013. Available online: www.acog.org/~/media/Task%20Force%20and%20Work%20Group%20Reports/public/HypertensioninPregnancy.pdf (accessed on 21 August 2022).
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rosemary, T.; Patrick, O.B.; Asma, K. Current best practice in the management of hypertensive disorders in pregnancy. Integr. Blood Press Control 2016, 9, 79–94. [Google Scholar]
- Vayssière, C.; Sentilhes, L.; Ego, A.; Bernard, C.; Cambourieu, D.; Flamant, C.; Gascoin, G.; Gaudineau, A.; Grange, G.; Houfflin-Debarge, V.; et al. Fetal growth restriction and intra-uterine growth restriction: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 193, 10–18. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; Da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Unterscheider, J. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Ciobanu, A.; Wright, A.; Syngelaky, A.; Wright, D.; Akolekar, R.; Nicolaides, K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019, 53, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Rasmussen, S.; Hanson, M.; Kiserud, T. Longitudinal reference ranges for ductus venosus flow velocities and waveform indices. Ultrasound Obstet. Gynecol. 2006, 28, 890–898. [Google Scholar] [CrossRef]
- Parra-Cordero, M.; Lees, C.; Missfelder-Lobos, H.; Seed, P.; Harris, C. Fetal arterial and venous Doppler pulsatility index and time averaged velocity ranges. Prenat. Diag. 2007, 27, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Geipel, A.; Hennemann, F.; Fimmers, R.; Willruth, A.; Lato, K.; Gembruch, U.; Berg, C. Reference ranges for Doppler assessment of uterine artery resistance and pulsatility indices in dichorionic twin pregnancies. Ultrasound Obstet. Gynecol. 2011, 37, 663–667. [Google Scholar] [CrossRef]
- Chitrit, Y.; Caubel, P.; Herrero, R.; Schwinte, A.L.; Guillaumin, D.; Boulanger, M.C. Effects of maternal dexamethasone administration on fetal Doppler flow velocity waveforms. Br. J. Obstet. Gynaecol. 2000, 107, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.; Lemancewicz, A.; Przepieść, J.; Urban, J.; Kretowska, M. Antenatal corticosteroid therapy: A comparative study of dexamethasone and betamethasone effects on fetal Doppler flow velocity waveforms. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 120, 170–174. [Google Scholar] [CrossRef]
- Sebire, N.J. Umbilical artery Doppler revisited: Pathophysiology of changes in intrauterine growth restriction revealed. Ultrasound Obstet. Gynecol. 2003, 21, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Dias, T. Fetal growth restriction—Pathophysiology, diagnosis and management of uteroplacental insufficiency before 34 weeks. Sri. Lanka J. Obstet. Gynaecol. 2012, 34, 128–130. [Google Scholar] [CrossRef]
- Adiotomre, P.N.A.; Johnstone, F.D.; Laing, I.A. Effect of absent and diastolic flow velocity in the fetal umbilical artery on subsequent outcome. Arch. Dis. Child. 1997, 76, F35–F38. [Google Scholar] [CrossRef]
- Yoon, B.H.; Lee, C.M.; Kim, S.W. An abnormal umbilical artery waveform: A strong and independent predictor of adverse perinatal outcome in patients with preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 713–721. [Google Scholar] [CrossRef]
- Simchen, M.J.; Alkazaleh, F.; Adamson, S.L.; Windrim, R.; Telford, J.; Beyene, J.; Kingdom, J. The fetal cardiovascular response to antenatal steroids in severe early-onset intrauterine growth restriction. Am. J. Obstet. Gynecol. 2004, 190, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.C.; Murila, F.; Tong, S.; Baker, L.S.; Yu, V.Y.; Wallace, E.M. Predicting perinatal outcome through changes in umbilical artery Doppler studies after antenatal corticosteroids in the growth-restricted fetus. Obstet. Gynecol. 2009, 113, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauser, J.S.; Stanek, J.; Warshak, C.; Khoury, J.; Sibai, B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am. J. Obstet. Gynecol. 2003, 189, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Korebrits, C.; Yu, D.H.; Ramirez, M.M.; Marinoni, E.; Bocking, A.D.; Challis, J.R. Antenatal glucocorticoid administration increases corticotrophin-releasing hormone in maternal plasma. Br. J. Obstet. Gynaecol. 1998, 105, 556–561. [Google Scholar] [CrossRef]
- Fahlbusch, F.B.; Ruebner, M.; Volkert, G.; Offergeld, R.; Hartner, A.; Menendez-Castro, C.; Strick, R.; Rauh, M.; Rascher, W.; Dotsch, J. Corticotropin-releasing hormone stimulates expression of leptin, 11beta-HSD2 and syncytin-1 in primary human trophoblasts. Reprod. Biol. Endocrinol. 2012, 10, 80. [Google Scholar] [CrossRef]
- Clifton, V.L.; Read, M.A.; Leitch, I.M.; Giles, W.B.; Boura, A.L.; Robinson, P.J.; Smith, R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: Involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J. Clin. Endocrinol. Metab. 1995, 80, 2888–2893. [Google Scholar]
- Hata, T.; Hashimoto, M.; Manabe, A.; Aoki, S.; Iida, K.; Masumura, S.; Miyazaki, K. Maternal and fetal nitric oxide synthesis is decreased in pregnancies with small for gestational age infants. Hum. Reprod. 1998, 13, 1070–1073. [Google Scholar] [CrossRef]
- Hampl, V.; Bibova, J.; Stranak, Z.; Wu, X.; Michelakis, E.D.; Hashimoto, K.; Archer, S.L. Hypoxic fetoplacental vasoconstriction in humans is mediated by potassium channel inhibition. Am. J. Physiol. Heart Circ. Physiol. 2002, 28, H2440–H2449. [Google Scholar] [CrossRef] [PubMed]
- Tica, O.S.; Tica, A.A.; Cojocaru, D.; Gheonea, M.; Tica, I.; Alexandru, D.O.; Cojocaru, V.; Petcu, L.C.; Tica, V.I. Dexamethasone on absent end-diastolic flow in umbilical artery, in growth restricted fetuses from early-onset preeclamptic pregnancies and the perinatal outcome. Ann. Med. 2021, 53, 1455–1463. [Google Scholar] [CrossRef]
- Odibo, A.O.; Riddick, C.; Pare, E.; Stamilio, D.M.; Macones, G.A. Cerebroplacental Doppler ratio and adverse perinatal outcomes in intrauterine growth restriction: Evaluating the impact of using gestational age-specific reference values. J. Ultrasound Med. 2005, 24, 1223–1228. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: Longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet. Gynecol. 2007, 30, 287–296. [Google Scholar] [CrossRef]
- Morales-Roselló, J.; Khalil, A.; Morlando, M.; Papageorghiou, A.; Bhide, A.; Thilaganathan, B. Changes in fetal Doppler indices as a marker of failure to reach growth potential at term. Ultrasound Obstet. Gynecol. 2014, 43, 303–310. [Google Scholar] [CrossRef]
- Monteith, C.; Flood, K.; Mullers, S.; Unterscheider, J.; Breathnach, F.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; et al. Evaluation of normalization of cerebro-placental ratio as a potential predictor for adverse outcome in SGA fetuses. Am. J. Obstet. Gynecol. 2017, 216, 285.e1–285.e6. [Google Scholar] [CrossRef]
- Dall’Asta, A.; Brunelli, V.; Prefumo, F.; Frusca, T.; Lees, C. Early onset fetal growth restriction. Matern. Health Neonatol. Perinatal. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.P.V.; Miyadahira, S.; Zugaib, M. Predicting pH at birth in absent or reversed end-diastolic velocity in the umbilical arteries. Obstet. Gynecol. 2006, 107, 1042–1048. [Google Scholar] [CrossRef]
- Bilardo, C.M.; Wolf, H.; Stigter, R.H.; Ville, Y.; Baez, E.; Visser, G.H.A.; Hecher, K. Relationship between monitoring parameters and perinatal outcome in severe, early intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2004, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Eiland, E.; Nzerue, C.; Faulkner, M. Preeclampsia. J. Pregnancy 2012, 2012, 586578. [Google Scholar] [PubMed]
- Mustafa, R.; Ahmed, S.; Gupta, A.; Venuto, R.C. A comprehensive review of hypertension in pregnancy. J. Pregnancy 2012, 2012, 105918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, L.; Sun, Q.; Wang, X.D. Expression of urocortin and corticotrophin-releasing hormone receptor-2 in patients with intrahepatic cholestasis of pregnancy. Placenta 2014, 35, e962–e968. [Google Scholar] [CrossRef] [PubMed]
| Kruskal-Wallis Test | UA-PI | MCA-PI | CPR | DV-PI | rUTA-PI | lUTA-PI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | |
| H | 20.56 | 11.35 | 10.71 | 11.35 | 6.08 | 3.32 | 3.85 | 10.80 | 1.23 | 0.196 | 1.98 | 0.32 |
| d. f. | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| p | 0.000 | 0.023 | 0.030 | 0.227 | 0.193 | 0.506 | 0.426 | 0.395 | 0.872 | 0.995 | 0.738 | 0.988 |
| Parameter | Days Compared (p) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0/1 | 0/2 | 0/3 | 0/4 | 1/2 | 1/3 | 1/4 | 2/3 | 2/4 | 3/4 | ||
| UA-PI | trAEDF | 0.001 | 0.001 | 0.091 | 0.744 | 0.841 | 0.077 | 0.002 | 0.118 | 0.003 | 0.172 |
| prAEDF | 0.010 | 0.006 | 0.079 | 0.500 | 0.896 | 0.403 | 0.055 | 0.334 | 0.041 | 0.055 | |
| MCA-PI | trAEDF | 0.030 | 0.109 | 0.782 | 0.684 | 0.465 | 0.040 | 0.006 | 0.185 | 0.045 | 0.495 |
| prAEDF | (*) | ||||||||||
| UA-PI | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||
| tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | Pr AEDF | |
| Cases | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 |
| Mean | 2.591 | 2.629 | 2.110 | 2.145 | 2.097 | 2.133 | 2.312 | 2.303 | 2.512 | 2.494 |
| Median | 2.495 | 2.530 | 1.980 | 2.160 | 2.140 | 2.115 | 2.350 | 2.325 | 2.575 | 2.520 |
| Std. Deviation | 0.397 | 0.337 | 0.453 | 0.376 | 0.452 | 0.348 | 0.506 | 0.350 | 0.457 | 0.394 |
| Minimum | 1.890 | 2.170 | 1.470 | 1.670 | 1.310 | 1.590 | 1.290 | 1.730 | 1.370 | 1.780 |
| Maximum | 3.190 | 3.160 | 3.170 | 2.650 | 3.080 | 2.690 | 3.190 | 2.850 | 3.140 | 2.980 |
| MCA-PI | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||
| tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | |
| Cases | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 |
| Mean | 1.510 | 1.414 | 1.373 | 1.246 | 1.395 | 1.289 | 1.486 | 1.357 | 1.530 | 1.397 |
| Median | 1.550 | 1.420 | 1.355 | 1.180 | 1.415 | 1.235 | 1.480 | 1.345 | 1.520 | 1.350 |
| Std. Deviation | 0.146 | 0.200 | 0.192 | 0.203 | 0.233 | 0.242 | 0.180 | 0.220 | 0.145 | 0.219 |
| Minimum | 1.180 | 1.170 | 0.970 | 0.980 | 0.910 | 0.940 | 1.070 | 1.060 | 1.180 | 1.170 |
| Maximum | 1.790 | 1.830 | 1.690 | 1.670 | 1.760 | 1.750 | 1.810 | 1.740 | 1.780 | 1.780 |
| CPR | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||
| tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | Pr AEDF | |
| Cases | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 |
| Mean | 0.588 | 0.537 | 0.655 | 0.574 | 0.688 | 0.592 | 0.656 | 0.585 | 0.605 | 0.552 |
| Median | 0.597 | 0.520 | 0.623 | 0.555 | 0.649 | 0.587 | 0.639 | 0.587 | 0.583 | 0.541 |
| Std. Deviation | 0.080 | 0.115 | 0.120 | 0.149 | 0.171 | 0.112 | 0.180 | 0.087 | 0.137 | 0.125 |
| Minimum | 0.417 | 0.395 | 0.429 | 0.334 | 0.457 | 0.398 | 0.399 | 0.401 | 0.363 | 0.361 |
| Maximum | 0.741 | 0.799 | 0.923 | 0.902 | 1.031 | 0.845 | 1.077 | 0.721 | 1.036 | 0.752 |
| DV-PI | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||
| tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | pr AEDF | tr AEDF | Pr AEDF | |
| Cases | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 | 22 | 10 |
| Mean | 0.912 | 1.000 | 0.874 | 0.894 | 0.944 | 0.968 | 0.942 | 1.025 | 0.905 | 1.001 |
| Median | 0.905 | 1.000 | 0.870 | 0.890 | 0.960 | 0.945 | 0.940 | 0.995 | 0.900 | 0.970 |
| Std. Deviation | 0.149 | 0.127 | 0.134 | 0.044 | 0.151 | 0.110 | 0.119 | 0.114 | 0.089 | 0.116 |
| Minimum | 0.720 | 0.750 | 0.690 | 0.810 | 0.640 | 0.780 | 0.720 | 0.840 | 0.770 | 0.820 |
| Maximum | 1.150 | 1.170 | 1.090 | 0.964 | 1.210 | 1.190 | 1.180 | 1.170 | 1.080 | 1.140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tica, O.S.; Tica, A.A.; Cojocaru, D.; Tica, I.; Petcu, C.L.; Cojocaru, V.; Alexandru, D.O.; Tica, V.I. Maternal Steroids on Fetal Doppler Indices, in Growth-Restricted Fetuses with Abnormal Umbilical Flow from Pregnancies Complicated with Early-Onset Severe Preeclampsia. Diagnostics 2023, 13, 428. https://doi.org/10.3390/diagnostics13030428
Tica OS, Tica AA, Cojocaru D, Tica I, Petcu CL, Cojocaru V, Alexandru DO, Tica VI. Maternal Steroids on Fetal Doppler Indices, in Growth-Restricted Fetuses with Abnormal Umbilical Flow from Pregnancies Complicated with Early-Onset Severe Preeclampsia. Diagnostics. 2023; 13(3):428. https://doi.org/10.3390/diagnostics13030428
Chicago/Turabian StyleTica, Oana Sorina, Andrei Adrian Tica, Doriana Cojocaru, Irina Tica, Cristian Lucian Petcu, Victor Cojocaru, Dragos Ovidiu Alexandru, and Vlad Iustin Tica. 2023. "Maternal Steroids on Fetal Doppler Indices, in Growth-Restricted Fetuses with Abnormal Umbilical Flow from Pregnancies Complicated with Early-Onset Severe Preeclampsia" Diagnostics 13, no. 3: 428. https://doi.org/10.3390/diagnostics13030428
APA StyleTica, O. S., Tica, A. A., Cojocaru, D., Tica, I., Petcu, C. L., Cojocaru, V., Alexandru, D. O., & Tica, V. I. (2023). Maternal Steroids on Fetal Doppler Indices, in Growth-Restricted Fetuses with Abnormal Umbilical Flow from Pregnancies Complicated with Early-Onset Severe Preeclampsia. Diagnostics, 13(3), 428. https://doi.org/10.3390/diagnostics13030428







