Minimally Invasive Tissue Sampling via Post Mortem Ultrasound: A Feasible Tool (Not Only) in Infectious Diseases—A Case Report
Abstract
1. Introduction
Case Report—Clinical History
2. Materials and Methods
3. Results
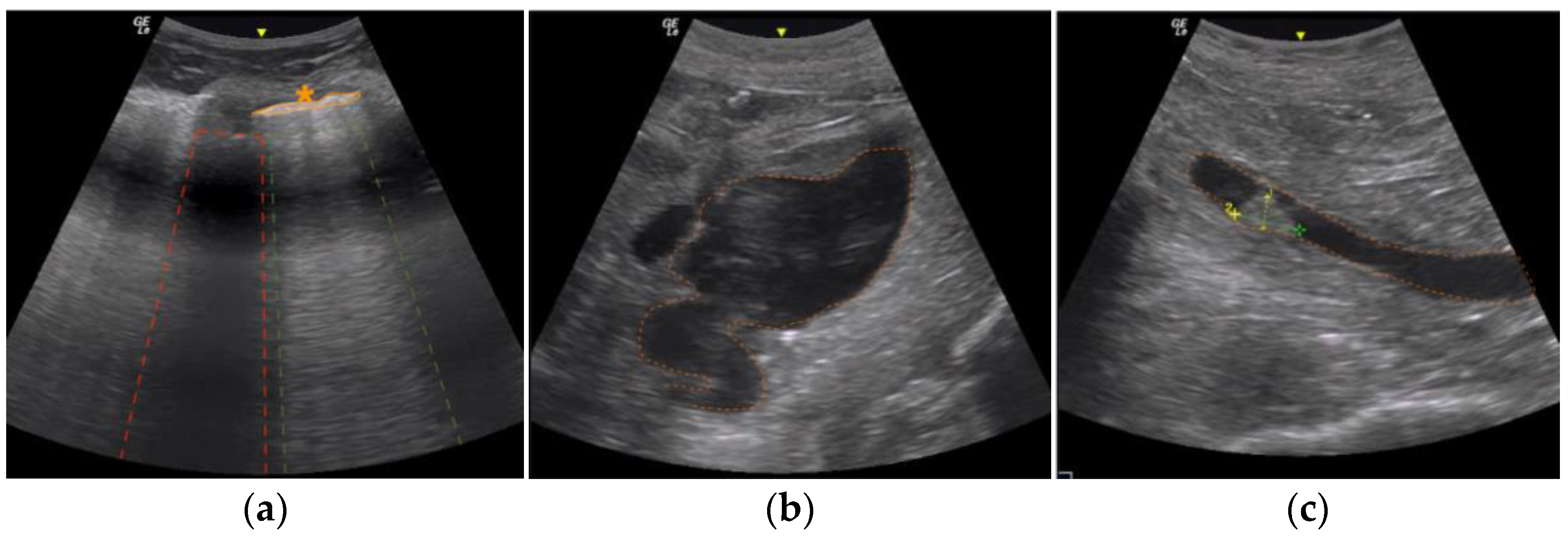
3.1. Post Mortem Ultrasound Findings
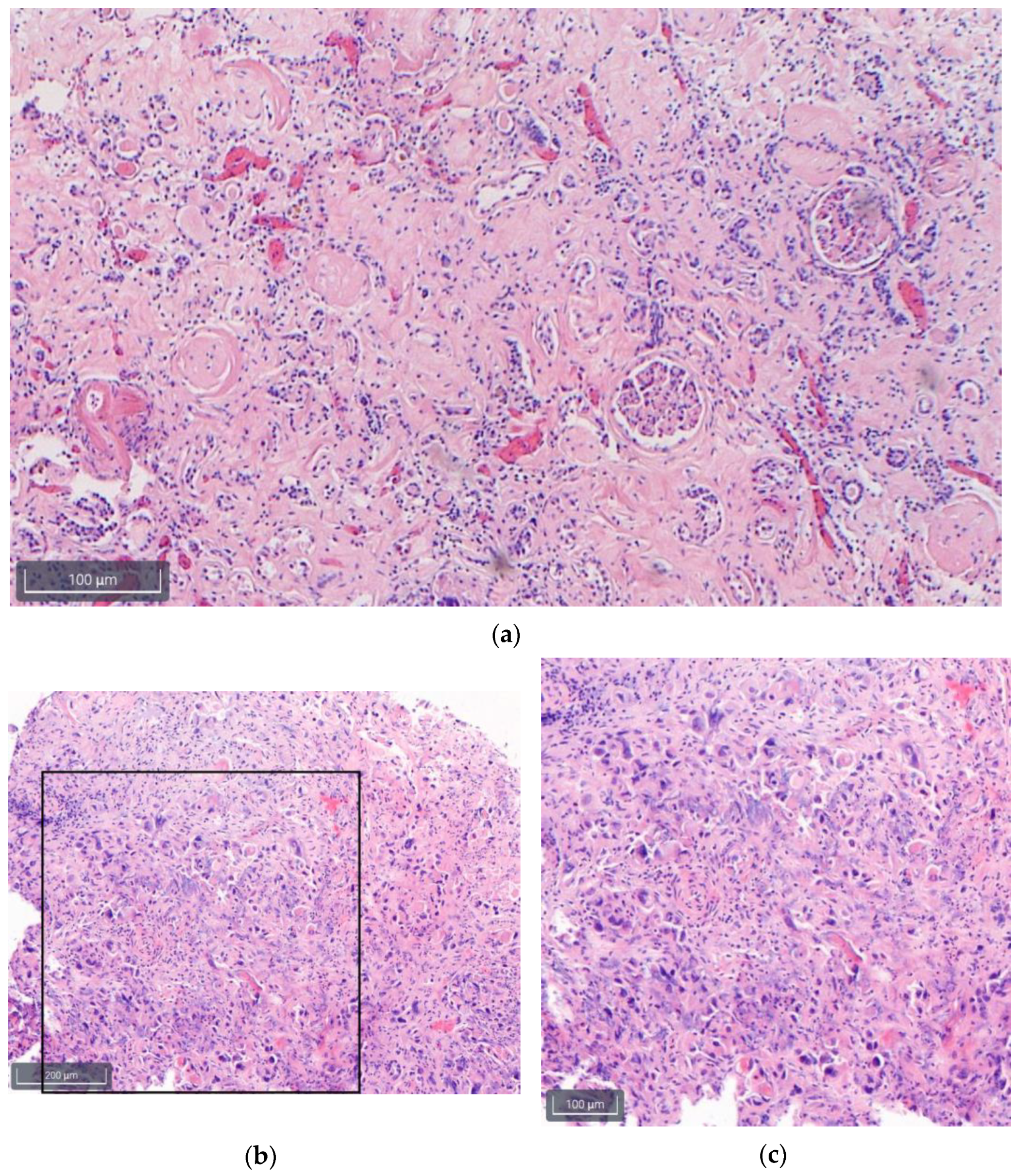
3.2. Histology Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blokker, B.M.; Weustink, A.C.; Wagensveld, I.M.; von der Thüsen, J.H.; Pezzato, A.; Dammers, R.; Bakker, J.; Renken, N.S.; Bakker, M.A.D.; van Kemenade, F.J.; et al. Conventional Autopsy versus Minimally Invasive Autopsy with Postmortem MRI, CT, and CT-guided Biopsy: Comparison of Diagnostic Performance. Radiology 2018, 289, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Marimon, L.; Ismail, M.R.; Carrilho, C.; Fernandes, F.; Ferrando, M.; Castillo, P.; Rodrigo-Calvo, M.T.; Guerrero, J.; Ortiz, E.; et al. Minimally Invasive Autopsy Practice in COVID-19 Cases: Biosafety and Findings. Pathogens 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Loibner, M.; Langner, C.; Regitnig, P.; Gorkiewicz, G.; Zatloukal, K. Biosafety Requirements for Autopsies of Patients with COVID-19: Example of a BSL-3 Autopsy Facility Designed for Highly Pathogenic Agents. Pathobiology 2021, 88, 37–45. [Google Scholar] [CrossRef]
- Fitzek, A.; Schädler, J.; Dietz, E.; Ron, A.; Gerling, M.; Kammal, A.L.; Lohner, L.; Falck, C.; Möbius, D.; Goebels, H.; et al. Prospective postmortem evaluation of 735 consecutive SARS-CoV-2-associated death cases. Sci. Rep. 2021, 11, 19342. [Google Scholar] [CrossRef] [PubMed]
- Union. POotE. Commission Directive (EU) 2020/739 of 3 June 2020 Amending Annex III to Directive 2000/54/EC of the European Parliament and of the COUNCIL as Regards the Inclusion of SARS-CoV-2 in the List of Biological Agents Known to Infect Humans and Amending Commission Directive (EU) 2019/1833. Publications Office of the EU. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020L0739 (accessed on 10 October 2022).
- Chui, P.; Chong, P.; Chong, B.; Wagener, S. Mobile Biosafety Level-4 Autopsy Facility—An Innovative Solution. Appl. Biosaf. 2007, 12, 238–244. [Google Scholar] [CrossRef]
- Autsch, A.; Ihle, H.; Kleemann, S.; Sanft, J.; Hahnemann, M.; Hubig, M.; Philipp, M.; Bauer, M.; Deinhardt-Emmer, S.; Gaßler, N.; et al. SARS-CoV-2-associated fatalities within the first year of the COVID-19 pandemic: An autopsy study. Rechtsmedizin 2023, 33, 262–268. [Google Scholar] [CrossRef]
- Kniep, I.; Heinemann, A.; Edler, C.; Sperhake, J.P.; Püschel, K.; Ondruschka, B.; Schröder, A.S. COVID-19 lungs in post-mortem computed tomography. Rechtsmedizin 2021, 31, 145–147. [Google Scholar] [CrossRef]
- Machnicki, S.; Patel, D.; Singh, A.; Talwar, A.; Mina, B.; Oks, M.; Makkar, P.; Naidich, D.; Mehta, A.; Hill, N.S.; et al. The usefulness of chest CT imaging in patients with suspected or diagnosed COVID-19: A review of literature. Chest 2021, 160, 652–670. [Google Scholar] [CrossRef]
- Servadei, F.; Mauriello, S.; Scimeca, M.; Caggiano, B.; Ciotti, M.; Anemona, L.; Montanaro, M.; Giacobbi, E.; Treglia, M.; Bernardini, S.; et al. Persistence of SARS-CoV-2 viral RNA in nasopharyngeal swabs after death: An observational study. Microorganisms 2021, 9, 800. [Google Scholar] [CrossRef]
- Aiello, F.; Ciotti, M.; Afflitto, G.G.; Rapanotti, M.C.; Caggiano, B.; Treglia, M.; Grelli, S.; Bernardini, S.; Mauriello, S.; Nucci, C.; et al. Post-mortem RT-PCR assay for SARS-CoV-2 RNA in COVID-19 patients’ corneal epithelium, conjunctival and nasopharyngeal swabs. J. Clin. Med. 2021, 10, 4256. [Google Scholar] [CrossRef]
- Casagrande, M.; Fitzek, A.; Spitzer, M.S.; Püschel, K.; Glatzel, M.; Krasemann, S.; Nörz, D.; Lütgehetmann, M.; Pfefferle, S.; Schultheiss, M. Presence of SARS-CoV-2 RNA in the cornea of viremic patients with COVID-19. JAMA Ophthalmol. 2021, 139, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.S.; Edler, C.; Ondruschka, B.; Püschel, K.; Schädler, J.; Heinemann, A.; Heinrich, F.; Lütgehetmann, M.; Pfefferle, S.; Aepfelbacher, M.; et al. The handling of SARS-CoV-2 associated deaths—Infectivitiy of the body. Forensic. Sci. Med. Pathol. 2021, 17, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, F.; Schröder, A.S.; Gerberding, A.-L.; Gerling, M.; Langenwalder, F.; Lange, P.; Heinemann, A.; Bibiza-Freiwald, E.; Nörz, D.S.; Aepfelbacher, M.; et al. Postmortem antigen-detecting rapid diagnostic tests to predict infectivity of SARS-CoV-2-associated deaths. Emerg. Infect. Dis. 2022, 28, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Costache, M.; Lazaroiu, A.M.; Contolenco, A.; Costache, D.; George, S.; Sajin, M.; Patrascu, O.M. Clinical or postmortem? The importance of the autopsy; a retrospective study. Maedica 2014, 9, 261–265. [Google Scholar]
- Shieh, W.-J.; Blau, D.M.; Denison, A.M.; DeLeon-Carnes, M.; Adem, P.; Bhatnagar, J.; Sumner, J.; Liu, L.; Patel, M.; Batten, B.; et al. 2009 pandemic influenza A (H1N1): Pathology and pathogenesis of 100 fatal cases in the United States. Am. J. Pathol. 2010, 177, 166–175. [Google Scholar] [CrossRef]
- Bassat, Q.; Castillo, P.; Martínez, M.J.; Jordao, D.; Lovane, L.; Hurtado, J.C.; Nhampossa, T.; Ritchie, P.S.; Bandeira, S.; Sambo, C.; et al. Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in Mozambique: An observational study. PLoS Med. 2017, 14, e1002317. [Google Scholar] [CrossRef]
- Castillo, P.; Martínez, M.J.; Ussene, E.; Jordao, D.; Lovane, L.; Ismail, M.R.; Carrilho, C.; Lorenzoni, C.; Fernandes, F.; Bene, R.; et al. Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study. PLoS Med. 2016, 13, e1002171. [Google Scholar] [CrossRef]
- Blum, L.S.; Karia, F.P.; Msoka, E.F.; Mwanga, M.O.; Crump, J.A.; Rubach, M.P. An In-Depth Examination of Reasons for Autopsy Acceptance and Refusal in Northern Tanzania. Am. J. Trop. Med. Hyg. 2020, 103, 1670–1680. [Google Scholar] [CrossRef]
- Hoyert, D.L. The changing profile of autopsied deaths in the United States, 1972–2007. NCHS Data Brief 2011, 67, 1–8. [Google Scholar]
- Pfefferle, S.; Reucher, S.; Nörz, D.; Lütgehetmann, M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Eurosurveillance 2020, 25, 2000152. [Google Scholar] [CrossRef]
- Kiefl, D.; Eisenmann, S.; Michels, G.; Schmid, M.; Ludwig, C.; Pin, M.; Glöckner, E.; Petersen, P.-F.; Damjanovic, D.; Schellhaas, S.; et al. German recommendations on lung and thoracic ultrasonography in patients with COVID-19. Med. Klin. Intensiv. Notfmed. 2020, 115, 654–667. [Google Scholar] [CrossRef]
- Dettmeyer, R.B. Forensic Histopathology: Fundamentals and Perspectives, 2nd ed.; Springer International Publishing: Cham, Germany, 2018; pp. 401–405. [Google Scholar]
- Sebire, N.J.; Weber, M.A.; Thayyil, S.; Mushtaq, I.; Taylor, A.; Chitty, L.S. Minimally invasive perinatal autopsies using magnetic resonance imaging and endoscopic postmortem examination (“keyhole autopsy”): Feasibility and initial experience. J. Matern. Fetal Neonatal Med. 2012, 25, 513–518. [Google Scholar] [CrossRef]
- Dirnhofer, R.; Jackowski, C.; Vock, P.; Potter, K.; Thali, M.J. VIRTOPSY: Minimally invasive, imaging-guided virtual autopsy. Radiographics 2006, 26, 1305–1333. [Google Scholar] [CrossRef]
- Neidhardt, M.; Gerlach, S.; Mieling, R.; Laves, M.-H.; Weib, T.; Gromniak, M.; Fitzek, A.; Mobius, D.; Kniep, I.; Ron, A.; et al. Robotic Tissue Sampling for Safe Post-Mortem Biopsy in Infectious Corpses. IEEE Trans. Med. Robot. Bionics 2022, 4, 94–105. [Google Scholar] [CrossRef]
- Tole, N.M.; Ostensen, H.; World Health Organization. Diagnostic Imaging and Laboratory Technology Team. In Basic Physics of Ultrasonic Imaging; Tole, N.M., Ostensen, H., Eds.; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Charlier, P.; Chaillot, P.-F.; Watier, L.; Ménétrier, M.; Carlier, R.; Cavard, S.; Hervé, C.; de la Grandmaison, G.L.; Huynh-Charlier, I. Is post-mortem ultrasonography a useful tool for forensic purposes? Med. Sci. Law 2013, 53, 227–234. [Google Scholar] [CrossRef]
- Brahee, D.D.; Ogedegbe, C.; Hassler, C.; Nyirenda, T.; Hazelwood, V.; Morchel, H.; Patel, R.S.; Feldman, J. Body Mass Index and Abdominal Ultrasound Image Quality:A Pilot Survey of Sonographers. J. Diagn. Med. Sonogr. 2013, 29, 66–72. [Google Scholar] [CrossRef]
- Bassat, Q.; Ordi, J.; Vila, J.; Ismail, M.R.; Carrilho, C.; Lacerda, M.; Munguambe, K.; Odhiambo, F.; Lell, B.; Sow, S.; et al. Development of a post-mortem procedure to reduce the uncertainty regarding causes of death in developing countries. Lancet Glob. Health 2013, 1, e125–e126. [Google Scholar] [CrossRef]
- Paganelli, C.R.; Goco, N.J.; McClure, E.M.; Banke, K.K.; Blau, D.M.; Breiman, R.F.; Menéndez, C.; Rakislova, N.; Bassat, Q. The evolution of minimally invasive tissue sampling in postmortem examination: A narrative review. Glob. Health Action 2020, 13, 1792682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terence Azeke, A.; Schädler, J.; Ondruschka, B.; Steurer, S.; Möbius, D.; Fitzek, A. Minimally Invasive Tissue Sampling via Post Mortem Ultrasound: A Feasible Tool (Not Only) in Infectious Diseases—A Case Report. Diagnostics 2023, 13, 2643. https://doi.org/10.3390/diagnostics13162643
Terence Azeke A, Schädler J, Ondruschka B, Steurer S, Möbius D, Fitzek A. Minimally Invasive Tissue Sampling via Post Mortem Ultrasound: A Feasible Tool (Not Only) in Infectious Diseases—A Case Report. Diagnostics. 2023; 13(16):2643. https://doi.org/10.3390/diagnostics13162643
Chicago/Turabian StyleTerence Azeke, Akhator, Julia Schädler, Benjamin Ondruschka, Stefan Steurer, Dustin Möbius, and Antonia Fitzek. 2023. "Minimally Invasive Tissue Sampling via Post Mortem Ultrasound: A Feasible Tool (Not Only) in Infectious Diseases—A Case Report" Diagnostics 13, no. 16: 2643. https://doi.org/10.3390/diagnostics13162643
APA StyleTerence Azeke, A., Schädler, J., Ondruschka, B., Steurer, S., Möbius, D., & Fitzek, A. (2023). Minimally Invasive Tissue Sampling via Post Mortem Ultrasound: A Feasible Tool (Not Only) in Infectious Diseases—A Case Report. Diagnostics, 13(16), 2643. https://doi.org/10.3390/diagnostics13162643






