Abstract
Given the increased risk of cardiovascular events associated with resistant hypertension, predictive cardiovascular prognosis is extremely important. Ambulatory blood pressure monitoring (ABPM) is mandatory for resistant hypertension diagnosis, but its use for prognosis is scarce. This observational longitudinal study included 258 patients (mean age of 60.4 ± 11.2 years; 61.2% male), who underwent 24 h ABPM in a hypertension unit from 1999 to 2019. The outcomes were global cardiovascular events (cerebrovascular, coronary, and other cardiovascular events). The mean follow-up period was 6.0 ± 5.0 years. Sixty-eight cardiovascular events (61 nonfatal) were recorded. Patients who experienced cardiovascular events were generally older, with higher rates of chronic kidney disease and prior cardiovascular events. The 24 h systolic blood pressure (hazard ratio 1.44; 95% CI 1.10–1.88), night systolic blood pressure (1.35; 95% CI 1.01–1.80), and 24 h pulse pressure (2.07; 95% CI 1.17–3.67) were independent predictors of global cardiovascular events. Multivariate Cox analysis revealed a higher risk of future cardiovascular events, particularly in patients with a 24 h daytime and nighttime pulse pressure > 60 mm Hg with respective hazard ratios of 1.95; 95% CI 1.01–3.45; 2.15; 95% CI 1.21–3.83 and 2.07; 95% CI 1.17–3.67. In conclusion, APBM is a fundamental tool not only for the diagnosis of resistant hypertension, but also for predicting future cardiovascular events.
1. Introduction
Cardiovascular (CV) and cerebrovascular events are mostly caused by arterial hypertension. Epidemiological studies report a 7.1 million-death incidence rate annually, with ischemic illness accounting for 49% of deaths and cerebrovascular disease for 69% of deaths globally [1]. The worst outcome is associated with resistant hypertension (RH). It is expected that the prevalence of RH will increase in the coming years due to the increase in the world population, obesity, and the prevalence of diabetes mellitus (DM) [2,3,4]. Independent of BP control, RH is associated with a higher risk of CV disease and all-cause mortality [2,3,5,6]. Patients with RH also have a higher prevalence of end organ damage [7], DM, chronic kidney disease (CKD) patients [2,3,5], and obesity [8,9,10]. The estimated prevalence of RH among treated arterial hypertension patients is approximately 10–18% [2,5,11]. However, prevalence rates range from 5–30% in patients with treated hypertension, due to the different definitions of RH used [5,12]. Resistant hypertension is defined as uncontrolled blood pressure (BP), despite the administration of optimum doses of three first-line classes of antihypertensive drugs, including a diuretic (renin-angiotensin system blockers, calcium-channel blockers, and thiazide diuretics) or adequate BP control requiring four or more antihypertensive drugs from different classes [13]. According to the European Society of Hypertension, RH is commonly diagnosed based on office BP that should be confirmed by ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) [14]. ABPM is key, given it removes the often-observed white coat effect, thus creating a more real and homogeneous sample [15]. In the study by Sierra et al. (2011), a group of over 68,000 treated hypertensive individuals included in the Spanish ABPM registry were analyzed. Based on office measurements, the prevalence of RH was 14.8% among treated hypertensives and 12.0% when only patients with BP ≥140/90 mmHg were included (i.e., excluding patients with normal BP but treated with ≥4 antihypertensive drugs). However, after the assessment of the ABPM data, the prevalence of RH changed dramatically. Surprisingly, 37.2% of the originally identified RH patients had ‘white-coat RH’ (24 h systolic blood pressure (SBP)/diastolic blood pressure (DBP) < 130/80 mmHg). Whereas, only 62.5% had true RH (24 h SBP/DBP ≥ 130/80 mmHg) [16]. Similar results were reported by Parati et al. (2014), who found that less than 40% of the patients fulfilled the diagnostic criteria for RH based on ABPM data, compared to office BP due to the ‘white coat effect’ [15] This difference in the use of ABPM data or office reduces the comparability of these studies’ results [15,17,18,19,20,21,22,23]. Besides the stated importance of ABPM data for the diagnosis of RH, it is also an important tool for CV prognosis in patients with hypertension [14,15]. Some studies have found predictive value in ABPM for the onset of target organ damage and future CV events. Furthermore, future CV events were associated with higher daytime ABPM values [17,24,25], higher nighttime BP levels [26,27], and more specifically, anomalous nighttime dipping patterns [24,27]. In addition, pulse pressure (PP), (the difference between SBP and DBP values) is also a well-established risk factor for CV events among the hypertensive population [28,29,30]. In middle-aged and older people, PP is increased and has additional adverse prognostic significance. Specifically, in older adults, a PP greater than 60 mmHg is indicative of CV risk [14]. However, its value for patients with a PP greater than 60 mmHg is yet to be determined. In this study, we aimed to determine which variables were the most accurate predictors of future cardiovascular events when the diagnosis of RH was based on ABPM data, excluding the “white coat effect”.
2. Materials and Methods
2.1. Study Design and Patients
This retrospective study included patients aged 18 years or older who underwent 24 h ABPM between 1999 and 2019 in the hypertensive ambulatory unit of the Centro Hospitalar do Baixo Vouga Aveiro, Portugal. All included patients had a mean 24 h SBP of ≥130 mmHg or daytime SBP ≥ 135 mmHg during the day, while taking maximally tolerated doses of at least three antihypertensive agents, including a diuretic [6], or controlled BP with four or more antihypertensive agents [31]. Secondary hypertension was an exclusion criterion. The ethics committee of the Centro Hospitalar do Baixo Vouga Aveiro approved the study (N/Ref. 073619, 21 September 2016). All procedures were conducted in accordance with the Helsinki Declaration.
2.2. Clinical Data
With the 24 h ABPM data, age, sex, body mass index (BMI), and a list of antihypertensive drugs in use were collected. In addition, the medical files of all patients were consulted to collect data regarding CV risk factors, such as DM, smoking history, history of previous CV events, blood glucose, dyslipidaemia, serum creatinine, presence of proteinuria, and low-density lipoproteins cholesterol (LDL) and echocardiogram. Glomerular filtration rate (GFR) was calculated according to the Cockcroft–Gault equation, and CKD was assumed if the patient had GFR <60 mL/min/1.73 m2, according to the 2021 kidney disease guidelines [31]
2.3. Events
The type and date of the events were collected from the hospital’s records.
CV events were subdivided in coronary events (myocardial infarction, coronary angioplasty, coronary by-pass, angina pectoris), sudden death, acute heart failure requiring hospitalization, cerebrovascular events (ischemic and hemorrhagic strokes, transient ischemic attack), and peripheral arterial disease. When more than one event occurred in the same patient, only the first event was considered and at that moment, for the purposes of this study, the follow-up period was considered to have ended. Then, events were classified into fatal and nonfatal. For all deaths, the cause of death was confirmed by consulting hospital records or death certificates and classified as CV or other. When the cause of death was not specified, it was recorded as undetermined. Whenever patients did not present any documented event in the medical file, the follow up ended with the last registered assessment.
2.4. ABPM
ABPM was performed using the Spacelabs 90,207 device. All patients underwent 24 h BP monitoring on a weekday, with measurements on the nondominant arm every 15 min during the day and every 30 min at night. Recordings with >70% valid data were accepted, with >20 valid readings while awake with at least 2 valid readings per hour and at >7 valid readings while asleep with at least 1 valid reading per hour. The patient should not exercise vigorously. At the time of inflation, the patient should stop moving and keep the arm relaxed [15]. The nocturnal SBP dipping (%) was calculated as 100 × [1 − sleep SBP/awake SBP ratio]. According to this, patients were classified as extreme dippers (SBP decline >20%), dippers (SBP decline more than 10% and less than 20%), nondippers (SBP decline between 0 and 10%), and risers (increase in SBP during nighttime) [32]. PP was defined as the difference between systolic and diastolic values [14,15,33].
2.5. Statistical Analysis
Statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation. Differences between groups were assessed by parametric (t-test) or equivalent nonparametric tests as appropriate. Differences in proportions were assessed using the chi-square test. Long-term cumulative survival curves in PP, with a cut off of 60 mmHg, were estimated using the Kaplan–Meier method, and comparisons between the two groups were made using a log-rank test. The effects of prognostic factors on survival were assessed using hazard ratios (HR) determined by univariate and multivariate regression analysis. First, ABPM variables (SBP, DBP and PP) for the 24 h period, daytime and nighttime, as well as night-to-day ratios were assessed in univariate Cox regression analysis. Those showing significant associations were then entered in a model including confounding variables (age, sex, body mass index, DM, previous CV event) for the multivariate Cox analysis.
For ABPM variables, a 1 standard deviation (SD) increment was used to report HR (95% confidence interval). Statistical significance was defined as a two-sided p value < 0.05.
3. Results
Between 1999 and December 2019, ABPM was performed in 4501 patients. Of these, 258 (5.7%) were patients with RH. Most patients were male (n = 158, 61.2%), with a mean age of 60.4 ± 11.2 years. The mean follow-up period was 6.0 ± 5.0 years. All patients were taking three or more antihypertensive drugs at the highest tolerated dose, with a mean number of 4.1 ± 0.8 drugs. Eighty-nine patients (34.4%) had a previous CV event, and most presented CV risk factors (dyslipidemia, 74.2%; obesity, 45.2%; DM, 45.2%; CKD, 31.3%). Table 1 presents patients characteristics.

Table 1.
Patient characteristics.
Of the 68 CV events, 25 were cerebrovascular events (20 ischemic strokes and 5 hemorrhagic strokes), 20 coronary events, and 22 other CV events (16 acute heart failures, 6 peripheral arterial disease) and 1 sudden death (Table 2). Regarding the 18 deaths during follow up, 7 were considered CV events (2 ischemic cerebrovascular events, 3 coronary events, 1 acute heart failure, and 1 sudden death), and 11 had nonCV causes (8 unknown causes, 1 cancer, and 2 infectious causes).

Table 2.
Cardiovascular event characterization.
Table 3 compares patients with no CV events during follow up to those who experienced CV events. Patients with CV events were older (63.2 vs. 59.3 years, p < 0.05) and had a lower GFR (63.1 ± 32.9 vs. 74.7 ± 33.2 mL/min/m2, p < 0.05). Patients with CV events also had a significantly higher prevalence of previous cardiovascular events, (52.9% vs. 27.7%, p < 0.05) and CKD (52.5% vs. 31.2%, p < 0.05), compared to patients with no CV events.

Table 3.
Comparison between the group with events vs. group without event.
The 24 h SBP (138.9 ± 16.4 vs. 132.8 ± 16.1 mmHg, p < 0.05), daytime SBP (143.3 ± 16.1 vs. 137.7 ± 17 mmHg, p < 0.05), and nighttime SBP (130.1 ± 17.6 vs. 123.7 ± 17.9 mmHg, p < 0.05), were higher in patients with events. Likewise, 24 h PP (62.3 ± 14.7 vs. 55.4 ± 12.9 mmHg, p < 0.05), daytime PP (63.0 ± 14.9 vs. 56.2 ± 13.6 mmHg, p < 0.05), and nighttime PP (60.8 ± 13.8 vs. 53.8 ± 13.0 mmHg) were also higher in patients who experienced a CV event, compared to patients with no events.
Figure 1 presents the Kaplan–Meier survival curve free of events for the cut off of ambulatory PP > 60 mmHg. Patients with a 24 h PP > 60 mmHg (log rank 12.1; p < 0.001), daytime PP > 60 mmHg (log rank 13.5; p < 0.001), and nighttime PP > 60 mmHg (log rank 19.3; p < 0.001) presented lower survival.
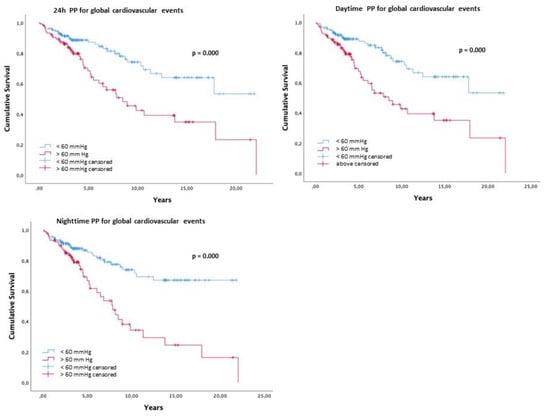
Figure 1.
Kaplan–Meier survival curve free of events for the cut off of 24 h, daytime and nighttime PP > 60 mmHg for global cardiovascular events.
For the univariate Cox analysis, the confounding factors age, sex, BMI, diabetes, previous CV events, the number of antihypertensive medications, the ejection fraction of the left ventricle, LDL, glycemia, and creatinine were evaluated. Only age, gender, BMI, diabetes mellitus, and previous cardiovascular events were statistically significant and considered for the univariate and multivariate Cox analyses (Table 4).

Table 4.
Univariate and multivariate Cox analysis for cardiovascular events, adjusted for confounding variables (age, gender, body mass index, diabetes, and previous cardiovascular events).
In the univariate Cox analysis, the 24 h SBP (HR 1.37, CI 1.08–1.72) and night SBP (HR 1.45, CI 1.13–1.88) were predictors of CV events. All PP variables, namely 24 h PP (HR 1.51, CI 1.20–1.59), daytime PP (HR 1.44, CI 1.13–1.82), and nighttime PP (HR 1.45, CI 1.13–1.80) were associated with CV events. Furthermore, 24 h PP > 60 mmHg, daytime PP > 60 mmHg, and nighttime PP > 60 mmHg were also significant predictors of CV events (Table 4).
In a multivariate Cox analysis, the 24 h SBP (HR 1.44, CI 1.10–1.88), nighttime SBP (HR 1.35, CI 1.01–1.80), and the 24 h PP (HR 1.39, CI 1.02–1.89) were independent predictors of CV events. When the PP > 60 mmHg was considered in the multivariate Cox, the 24 h PP > 60 mmHg (HR 1.95, CI 1.01–3.45), daytime PP > 60 mmHg (HR 2.15, CI 1.21–3.82), and nighttime PP > 60 mmHg (HR 2.07, CI 1.17–3.67) were significant predictors of CV events (Table 4)
4. Discussion
The present study was to determine which variables were the most accurate predictors of future cardiovascular events when the diagnosis of RH was based on ABPM data, excluding “white coat resistant hypertension”. The main findings are: (1) patients who experienced CV events were older, male, more likely to have experienced previous CV events, and with lower creatinine clearance; (2) the 24 h SBP, daytime SBP, and nighttime SBP are predictors of CV events (both fatal and nonfatal), but not DBP values; and (3) the cut off value of PP > 60 mmHg was associated with a higher risk of CV events. It is well documented that RH is associated with a higher CV risk (older age, male, obesity, DM, CKD) and worse outcomes [2,3,5,6,34,35,36] A retrospective study involving more than 200,000 participants observed a higher prevalence of coronary events (24%), strokes (14%), and heart failure (46%) in RH patients than in other types of patients with hypertension [6]. Smith et al. (2014) reported that patients with true RH may have a greater BP burden over time, contributing to a higher CV risk, compared to those with controlled arterial hypertension. Therefore, RH seems to be a more important prognostic factor than BP control [2]. This is most likely because we are treating a population with a higher risk. However, we cannot discount the significance of controlling BP, since it reduces the absolute CV risk and delays or even prevents the development of organ lesions, such as CKD, which further increase the CV risk in such patients [37]. Contrary to previous research [31,33], a greater number of cerebrovascular events were observed in the present study than acute myocardial infarction. This may be explained by a higher annual incidence of cerebrovascular events in Portugal, compared to the incidence of coronary events [38]. Our findings demonstrate that the 24 h SBP, nighttime SBP, and PP values present the most important prognostic value. In fact, if we compare the SBP and DBP values in the group with events and without events, we find that the difference is verified in the SBP values and not the DBP values. Therefore, we may assume that the discriminative value of the PP will come at the expense of the variation in systolic values. BP changes with age [39]. SBP increases after 40 years of age, and DBP declines after 50 years of age, thus increasing PP [40]. The average age of patients with CV events in this study was 63.2 ± 10.6 years, so it is not surprising that the predictive value of BP is primarily based on SBP values, which will also be reflected in PP. Other studies analyzed the predictive value of ABPM in relation to future CV events in patients with RH [25,41,42,43,44,45,46,47]. Predictive value was reported for SBP values [25,34,42], nighttime SBP [25,41], and daytime SBP [24,41]. Abnormal SBP dipping patterns was also linked to a worst prognosis [44], while no correlation was found for morning surge [48]. One study also found predictive value in DBP values [17]. Finally, the analysis of PP was associated with potential CV events [43]. Those who found significance in SBP values [25,41,42] have a mean age similar to our study, while those who found significance in DBP values [17] were 10 years younger, with a median age of 50 years. With age, the change in BP and PP may be associated with the age-related disruption of the orderly arrangement of elastic lamella in the aorta and central elastic arteries, which stiffens the aorta [39]. In our study, the Kaplan–Meier survival curve clearly demonstrated that patients with a PP >60 mmHg, had worse event survival. PP determined by ABPM data is a well-defined risk factor for CV events [28,30,49]. The European Society of Cardiology/European Society of Hypertension 2018 guidelines considered PP > 60 mmHg as an important marker of worst CV outcome for patients over 60 years [14,40]. According to pathophysiology, PP may be responsible for muscular overload in vessels [50], resulting in stiffer arteries [40] due to an increase in collagen content and a decrease in elastin fibers. This activates the renin-angiotensin system, increasing the inflammation pathway, promoting organ damage, and increasing vessel stiffness, thus becoming a vicious cycle [40]. In 2016, Salles and Cardoso [27] summarized all the longitudinal studies in patients with RH that analyzed the prognostic value of ABPM for future CV events. The authors concluded that nighttime BP and the nondipping pattern were the most significant [27]. In 2021, an analysis of a cohort of 1276 patients with RH over eight three-year periods found that ABPM was the best prognostic marker for morbidity and CV mortality [51]. In both the stated studies, PP was not examined. Lempiäinen et al. [52] studied 1045 arterial hypertension patients undergoing ABPM (56.7% with a CV event). According to their findings, middle-aged people’s high nighttime PP was the most accurate ambulatory BP indicator of CV and all-cause mortality [52]. The same was noted in studies on populations with essential hypertension [28,29]. Our results support the predictive value of PP and the cut off PP > 60 mmHg as a useful tool for the prediction of new CV events in patients with true RH.
5. Study Limitations
This retrospective study has some limitations that deserve attention. Firstly, the sample size was relatively small and mainly comprised white/Caucasian patients from a limited geographic region, making these results difficult to generalize for other populations. Secondly, there was a male predominance (approximately 60% of the patients were male). Thirdly, the medications were self-reported at the time of ABPM, which may be inaccurate. In addition, medications can differ between ABPM and follow up. Lastly, follow up was discontinued in 11 patients due to deaths from nonCV events; furthermore, the cause of death was unidentified in six of these patients (absence of cause of death registration in the electronic process). Some of these patients could have died because of a CV event. Taken together, these data can lead to underestimating the outcome of this analysis.
6. Conclusions
In the present study, ABPM was essential to obtain a diagnosis of true RH. The SBP and PP are the most important ABPM values for predicting CV events, particularly the 24 h SBP and 24 h PP. For the first time, PP > 60 mmHg was identified as an indispensable tool for determining the prognosis of new CV events in patients with true RH.
Author Contributions
Conceptualization, J.M.B. and S.L.; Methodology, J.M.B. and F.G.P.; Formal analysis, F.G.P.; Investigation, J.M.B. and F.G.P.; Resources, J.M.B. and L.F.; Writing—original draft, S.L.; Writing—review & editing, J.M.B. and S.L.; Supervision, J.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Centro Hospitalar do Baixo Vouga (ref. 073619, 21 September 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lazaridis, A.A.; Sarafidis, P.A.; Ruilope, L.M. Ambulatory Blood Pressure Monitoring in the Diagnosis, Prognosis, and Management of Resistant Hypertension: Still a Matter of Our Resistance? Curr. Hypertens. Rep. 2015, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Gong, Y.; Handberg, E.; Messerli, F.H.; Bakris, G.L.; Ahmed, A.; Bavry, A.A.; Pepine, C.J.; Cooper-Dehoff, R.M. Predictors and Outcomes of Resistant Hypertension among Patients with Coronary Artery Disease and Hypertension. J. Hypertens. 2014, 32, 635. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarski, K.R.; Sozio, S.M.; Chen, J.; Sang, Y.; Shafi, T. Resistant Hypertension and Cardiovascular Disease Mortality in the US: Results from the National Health and Nutrition Examination Survey (NHANES). BMC Nephrol. 2019, 20, 138. [Google Scholar] [CrossRef]
- Egan, B.M.; Li, J.; Hutchison, F.N.; Ferdinand, K.C. Hypertension in the United States, 1999 to 2012: Progress toward Healthy People 2020 Goals. Circulation 2014, 130, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Nyaga, U.F.; Sime, P.S.; Francis, I.; Bigna, J.J. Global Prevalence of Resistant Hypertension: A Meta-Analysis of Data from 3.2 Million Patients. Heart 2019, 105, 98–105. [Google Scholar] [CrossRef]
- Daugherty, S.L.; Powers, J.D.; Magid, D.J.; Tavel, H.M.; Masoudi, F.A.; Margolis, K.L.; O’Connor, P.J.; Selby, J.V.; Ho, P.M. Incidence and Prognosis of Resistant Hypertension in Hypertensive Patients. Circulation 2012, 125, 1635–1642. [Google Scholar] [CrossRef]
- Oliveras, A.; De La Sierra, A. Resistant Hypertension: Patient Characteristics, Risk Factors, Co-Morbidities and Outcomes. J. Hum. Hypertens. 2014, 28, 213–217. [Google Scholar] [CrossRef]
- Acharya, T.; Tringali, S.; Singh, M.; Huang, J. Resistant Hypertension and Associated Comorbidities in a Veterans Affairs Population. J. Clin. Hypertens. 2014, 16, 741–745. [Google Scholar] [CrossRef]
- Holecki, M.; Duława, J.; Chudek, J. Resistant Hypertension in Visceral Obesity. Eur. J. Intern. Med. 2012, 23, 643–648. [Google Scholar] [CrossRef]
- Bakhtar, O.; Ference, B.A.; Levy, P.D.; Nasser, S.A.; Hedquist, L.; Flack, J.M. The Relationship of Resistant Hypertension and Treatment Outcomes with Total Compliance and Brain Natriuretic Peptide in an African American Hypertensive Cohort. J. Clin. Hypertens. 2011, 13, 618–622. [Google Scholar]
- Shalaeva, E.V.; Messerli, F.H. What Is Resistant Arterial Hypertension? Blood Press. 2023, 32, 2185457. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; Booth, J.N.; Oparil, S.; Irvin, M.R.; Shimbo, D.; Lackland, D.T.; Howard, G.; Safford, M.M.; Muntner, P. Refractory Hypertension: Determination of Prevalence, Risk Factors, and Comorbidities in a Large, Population-Based Cohort. Hypertension 2014, 63, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Blonsky, R.; Pohl, M.; Nally, J.V.; Thomas, G. 2017 ACC/AHA Hypertension Guidelines: Toward Tighter Control. Cleve Clin. J. Med. 2018, 85, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension Practice Guidelines for Ambulatory Blood Pressure Monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- de la Sierra, A.; Segura, J.; Banegas, J.R.; Gorostidi, M.; de la Cruz, J.J.; Armario, P.; Oliveras, A.; Ruilope, L.M. Clinical Features of 8295 Patients with Resistant Hypertension Classified on the Basis of Ambulatory Blood Pressure Monitoring. Hypertension 2011, 57, 898–902. [Google Scholar] [CrossRef]
- Redon, J.; Campos, C.; Narciso, M.L.; Rodicio, J.L.; Pascual, J.M.; Ruilope, L.M. Prognostic Value of Ambulatory Blood Pressure Monitoring in Refractory Hypertension. Hypertension 1998, 31, 712–718. [Google Scholar] [CrossRef]
- Brown, M.A.; Buddle, M.L.; Martin, A. Is Resistant Hypertension Really Resistant? Am. J. Hypertens. 2001, 14, 1263–1269. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Bloch, K.V.; Da Rocha Nogueira, A.; Salles, G.F. True Resistant Hypertension: Is It Possible to Be Recognized in the Office? Am. J. Hypertens. 2005, 18, 1534–1540. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Bloch, K.V.; Nogueira, A.R.; Salles, G.F. Twenty-Four Hour Ambulatory Blood Pressure Monitoring Pattern of Resistant Hypertension. Blood Press. Monit. 2003, 8, 181–185. [Google Scholar] [CrossRef]
- Veglio, F.; Rabbia, F.; Riva, P.; Martini, G.; Cat Genova, G.; Milan, A.; Paglieri, C.; Carra, R.; Chiandussi, L. Ambulatory Blood Pressure Monitoring and Clinical Characteristics of the True and White-Coat Resistant Hypertension. Clin. Exp. Hypertens. 2001, 23, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hernández-DelRey, R.; Armario, P.; Martin-Baranera, M.; Sánchez, P.; Cárdenas, G.; Pardell, H. Target-Organ Damage and Cardiovascular Risk Profile in Resistant Hypertension. Influence of the White-Coat Effect. Blood Press. Monit. 1998, 3, 331–337. [Google Scholar]
- Mezzetti, A.; Pierdomenico, S.D.; Costantini, F.; Romano, F.; Bucci, A.; Di Gioacchino, M.; Cuccurullo, F. White-Coat Resistant Hypertension. Am. J. Hypertens. 1997, 10, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Magnanini, M.M.F.; Nogueira, A.D.R.; Carvalho, M.S.; Bloch, K. V Ambulatory Blood Pressure Monitoring and Cardiovascular Risk in Resistant Hypertensive Women. Arq. Bras. Cardiol. 2009, 92, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Lapenna, D.; Bucci, A.; Di Tommaso, R.; Di Mascio, R.; Manente, B.M.; Caldarella, M.P.; Neri, M.; Cuccurullo, F.; Mezzetti, A. Cardiovascular Outcome in Treated Hypertensive Patients with Responder, Masked, False Resistant, and True Resistant Hypertension. Am. J. Hypertens. 2005, 18, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- de la Sierra, A.; Banegas, J.R.; Segura, J.; Gorostidi, M.; Ruilope, L.M. Ambulatory Blood Pressure Monitoring and Development of Cardiovascular Events in High-Risk Patients Included in the Spanish ABPM Registry: The CARDIORISC Event Study. J. Hypertens. 2012, 30, 713–719. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Salles, G.F. Prognostic Importance of Ambulatory Blood Pressure Monitoring in Resistant Hypertension: Is It All That Matters? Curr. Hypertens. Rep. 2016, 18, 85. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Pede, S.; Porcellati, C. Ambulatory Pulse Pressure: A Potent Predictor of Total Cardiovascular Risk in Hypertension. Hypertension 1998, 32, 983–988. [Google Scholar] [CrossRef]
- Kollias, A.; Stergiou, G.S.; Dolan, E.; O’Brien, E. Ambulatory Arterial Stiffness Index: A Systematic Review and Meta-Analysis. Atherosclerosis 2012, 224, 291–301. [Google Scholar] [CrossRef]
- Safar, M.E. Epidemiological Aspects of Pulse Pressure and Arterial Stiffness. J. Hypertens. Suppl. 1999, 17, S37–S40. [Google Scholar]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. Executive Summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. Linee guida ESC/ESH 2018 per la diagnosi e il trattamento dell’ipertensione arteriosa. Task Force per la Diagnosi e il Trattamento dell’Ipertensione Arteriosa della Società Europea di Cardiologia (ESC) e della Società Europea dell’Ipertensione Arteriosa (ESH) [2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH)]. G. Ital. Cardiol. 2018, 19 (Suppl. S1), 3S–73S. (In Italian) [Google Scholar] [CrossRef]
- Stergiou, G.S.; Palatini, P.; Asmar, R.; Bilo, G.; De La Sierra, A.; Head, G.; Kario, K.; Mihailidou, A.; Wang, J.; Mancia, G.; et al. Blood Pressure Monitoring: Theory and Practice. European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability Teaching Course Proceedings. Blood Press. Monit. 2018, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.L.; Leite, N.C.; Bacan, G.; Ataíde, D.S.; Gorgonio, L.K.C.; Salles, G.F. Prognostic Importance of Resistant Hypertension in Patients with Type 2 Diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetes Care 2020, 43, 219–227. [Google Scholar] [CrossRef]
- Sim, J.J.; Bhandari, S.K.; Shi, J.; Reynolds, K.; Calhoun, D.A.; Kalantar-Zadeh, K.; Jacobsen, S.J. Comparative Risk of Renal, Cardiovascular, and Mortality Outcomes in Controlled, Uncontrolled Resistant, and Nonresistant Hypertension. Kidney Int. 2015, 88, 622–632. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Salles, G.F. Refractory Hypertension and Risks of Adverse Cardiovascular Events and Mortality in Patients with Resistant Hypertension: A Prospective Cohort Study. J. Am. Heart Assoc. 2020, 9, e017634. [Google Scholar] [CrossRef]
- Smith, S.M. Resistant Hypertension and Susceptible Outcomes: Exploring the Benefits of Aggressive Blood Pressure Control. J. Clin. Hypertens. 2016, 18, 40. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies (2021); Series: State of Health in the EU; 2021. Available online: https://health.ec.europa.eu/system/files/2021-12/2021_chp_pt_english.pdf (accessed on 14 May 2023).
- O’Rourke, M.F. Isolated Systolic Hypertension, Pulse Pressure, and Arterial Stiffness as Risk Factors for Cardiovascular Disease. Curr. Hypertens. Rep. 1999, 1, 204–211. [Google Scholar] [CrossRef]
- Gavish, B.; Bursztyn, M. Ambulatory Pulse Pressure Components: Concept, Determination and Clinical Relevance. J. Hypertens. 2019, 37, 765–774. [Google Scholar] [CrossRef]
- Salles, G.F.; Cardoso, C.R.L.; Muxfeldt, E.S. Prognostic Influence of Office and Ambulatory Blood Pressures in Resistant Hypertension. Arch. Intern. Med. 2008, 168, 2340–2346. [Google Scholar] [CrossRef]
- Ríos, M.T.; Domínguez-Sardiña, M.; Ayala, D.E.; Gomara, S.; Sineiro, E.; Pousa, L.; Callejas, P.A.; Fontao, M.J.; Fernández, J.R.; Hermida, R.C. Prevalence and Clinical Characteristics of Isolated-Office and True Resistant Hypertension Determined by Ambulatory Blood Pressure Monitoring. Chronobiol. Int. 2013, 30, 207–220. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Fiszman, R.; Castelpoggi, C.H.; Salles, G.F. Ambulatory Arterial Stiffness Index or Pulse Pressure: Which Correlates Better with Arterial Stiffness in Resistant Hypertension? Hypertens. Res. 2008, 31, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Muxfeldt, E.S.; Cardoso, C.R.L.; Salles, G.F. Prognostic Value of Nocturnal Blood Pressure Reduction in Resistant Hypertension. Arch. Intern. Med. 2009, 169, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Muxfeldt, E.S.; Salles, G.F. Pulse Pressure or Dipping Pattern: Which One Is a Better Cardiovascular Risk Marker in Resistant Hypertension? J. Hypertens. 2008, 26, 878–884. [Google Scholar] [CrossRef]
- De Nicola, L.; Gabbai, F.B.; Agarwal, R.; Chiodini, P.; Borrelli, S.; Bellizzi, V.; Nappi, F.; Conte, G.; Minutolo, R. Prevalence and Prognostic Role of Resistant Hypertension in Chronic Kidney Disease Patients. J. Am. Coll. Cardiol. 2013, 61, 2461–2467. [Google Scholar] [CrossRef]
- Irvin, M.R.; Booth, J.N.; Sims, M.; Bress, A.P.; Abdalla, M.; Shimbo, D.; Calhoun, D.A.; Muntner, P. The Association of Nocturnal Hypertension and Nondipping Blood Pressure with Treatment-Resistant Hypertension: The Jackson Heart Study. J. Clin. Hypertens. 2018, 20, 438–446. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Salles, G.F. Associations of the Nocturnal Blood Pressure Fall and Morning Surge with Cardiovascular Events and Mortality in Individuals with Resistant Hypertension. J. Hypertens. 2021, 39, 1177–1187. [Google Scholar] [CrossRef]
- Li, Y.; Dolan, E.; Wang, J.-G.; Thijs, L.; Zhu, D.-L.; Staessen, J.A.; O’Brien, E.; Stanton, A. Ambulatory Arterial Stiffness Index: Determinants and Outcome. Blood Press. Monit. 2006, 11, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Smulyan, H. Coronary Ischemic Disease, Arterial Stiffness, and Pulse Pressure. Am. J. Hypertens. 2004, 17, 724–726. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Salles, G.C.; Salles, G.F. Prognostic Importance of On-Treatment Clinic and Ambulatory Blood Pressures in Resistant Hypertension: A Cohort Study. Hypertension 2020, 75, 1184–1194. [Google Scholar] [CrossRef]
- Lempiäinen, P.A.; Ylitalo, A.; Huikuri, H.; Kesäniemi, Y.A.; Ukkola, O.H. Nighttime Ambulatory Pulse Pressure Predicts Cardiovascular and All-Cause Mortality among Middle-Aged Participants in the 21-Year Follow-Up. J. Clin. Hypertens. 2021, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).