Conservative Management and Ultrasound Follow-Up of Parasitic Myoma: Our Experience and Literature Review
Abstract
1. Introduction
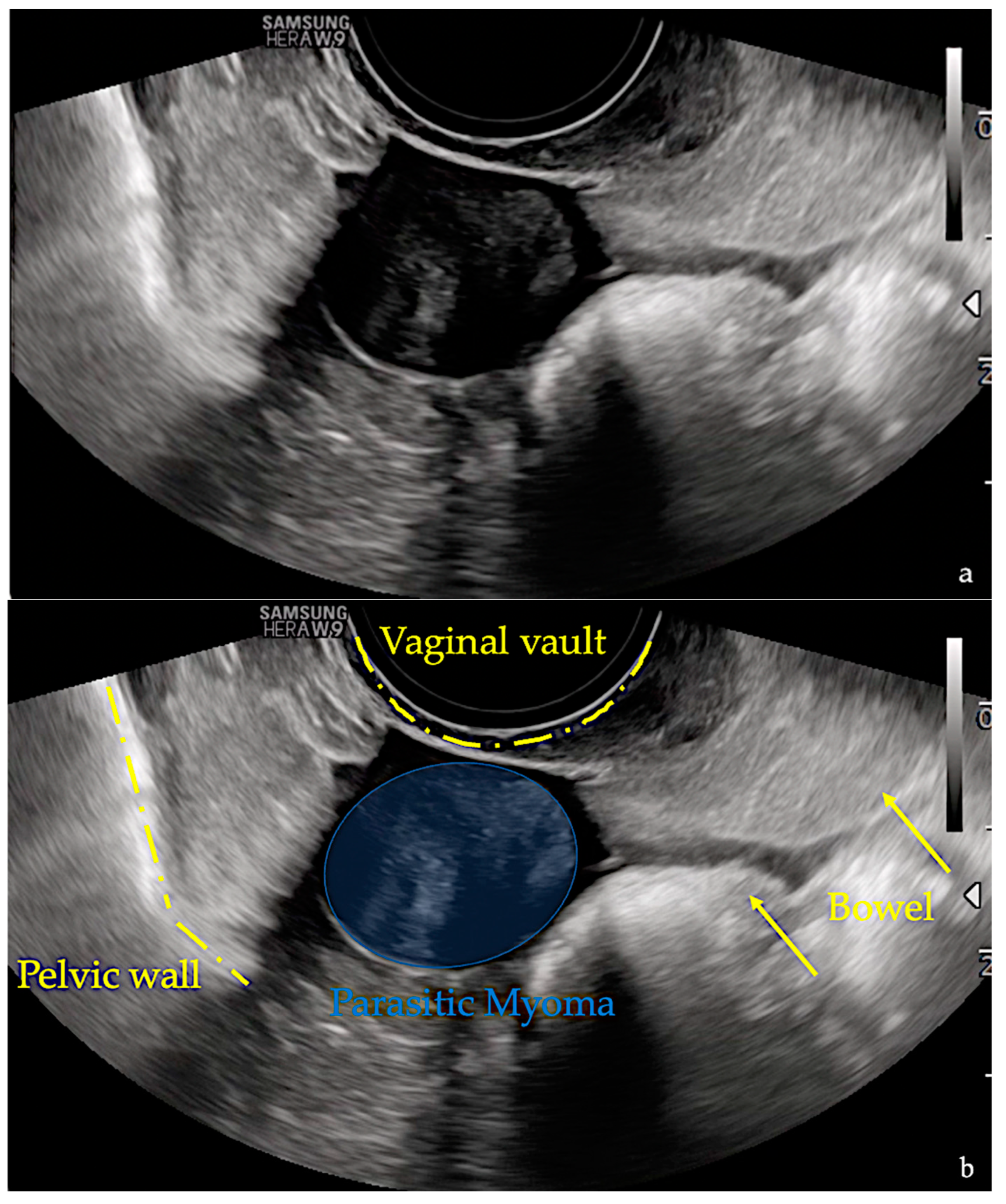
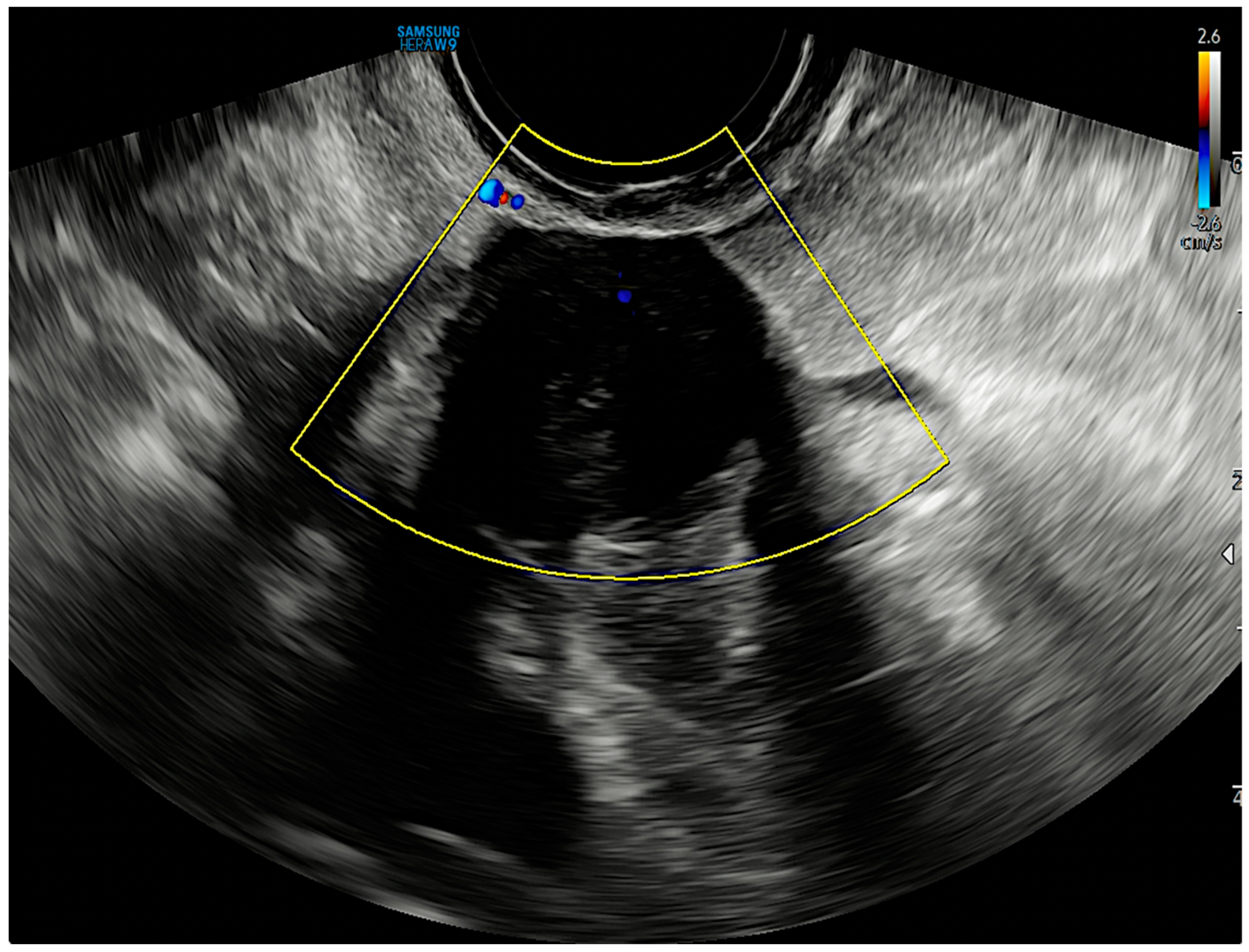
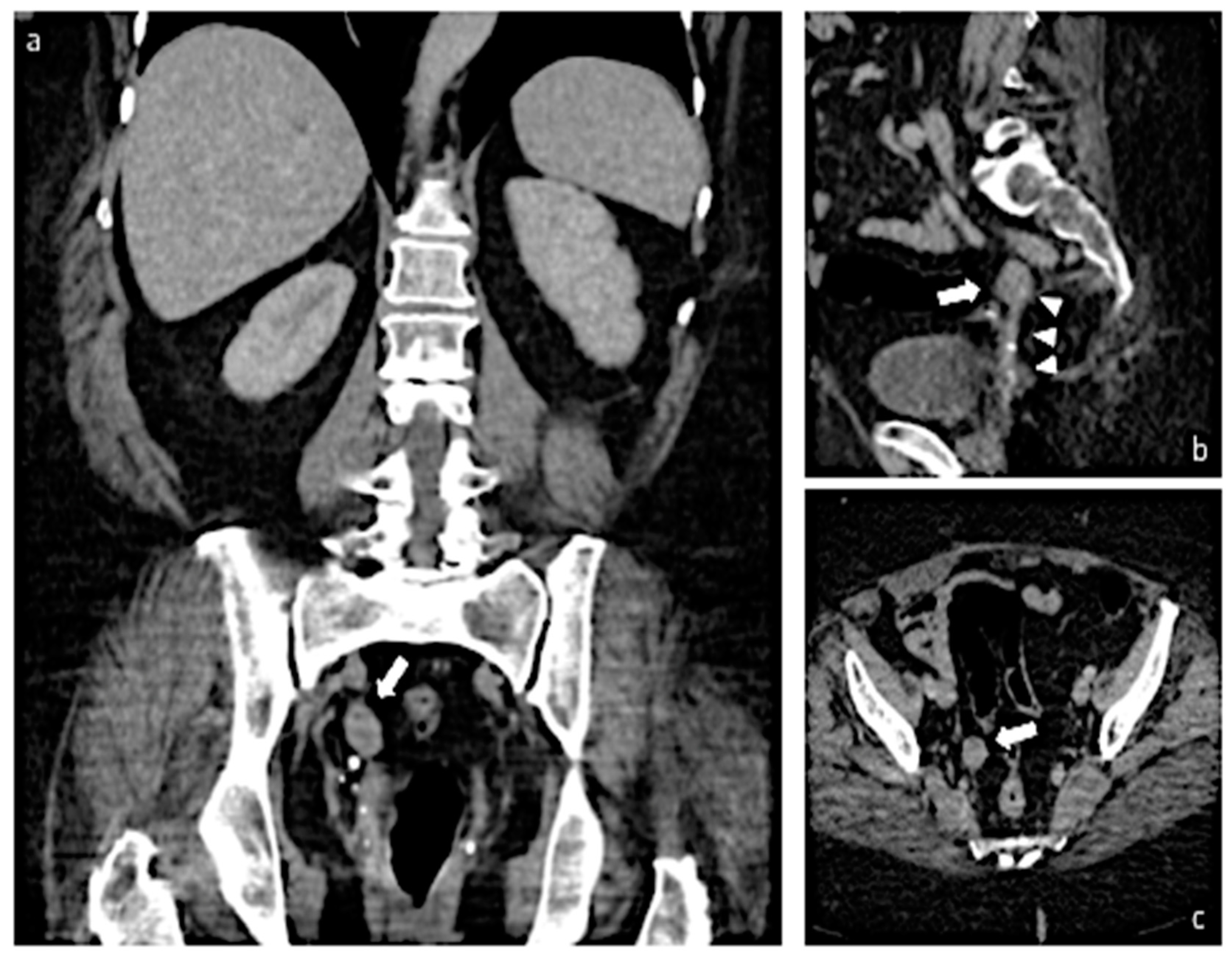
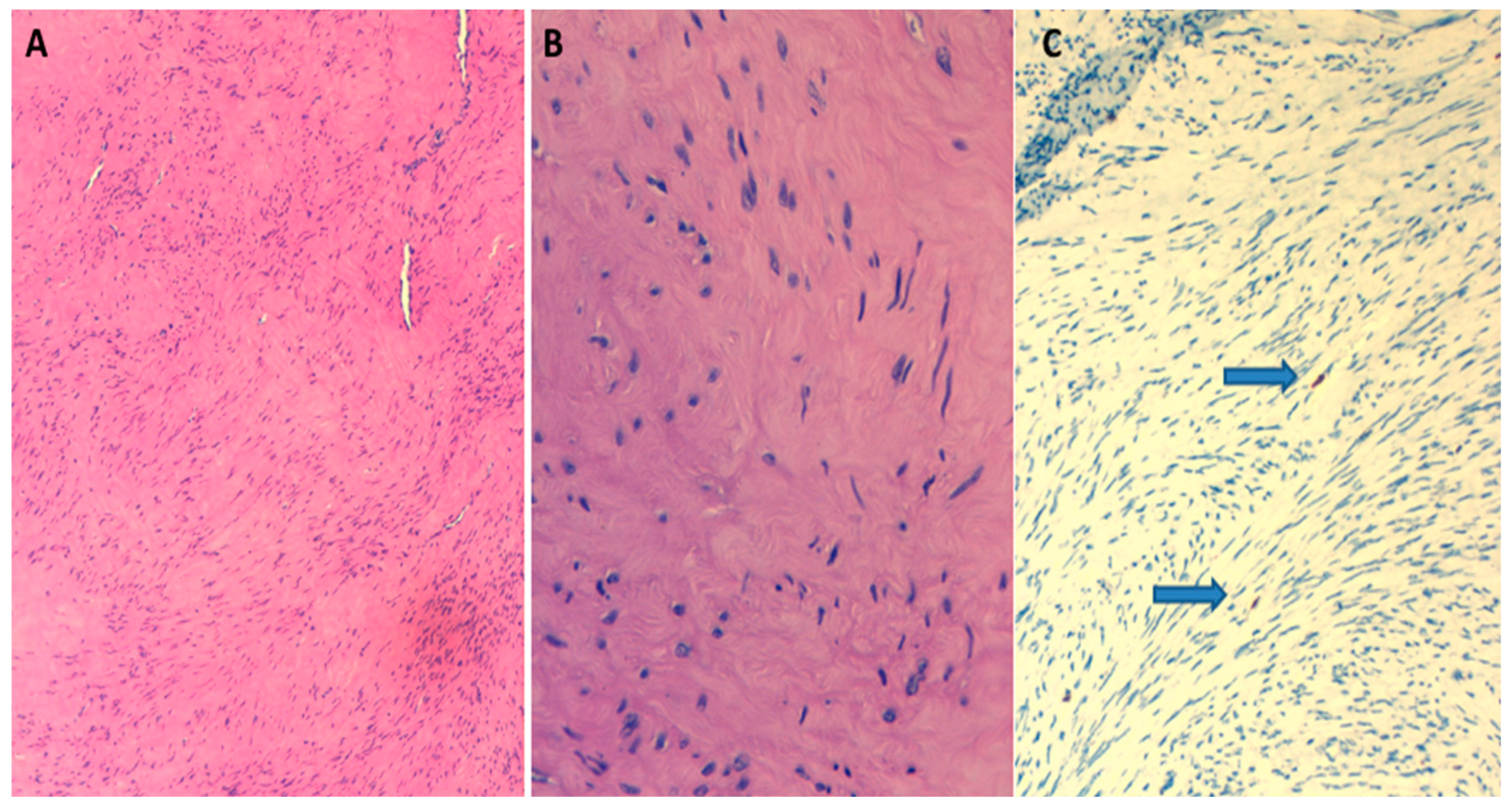
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, E.; Cookson, C.; Gandolfo, R.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. Int. J. Obstet. Gynaecol. 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.; Broder, M.S.; Fraser, I.S.; for the FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynecol. Obstet. 2011, 113, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.A.; Cullen, T.S. Myomata of the Uterus; WB Saunders: Philadelphia, PA, USA, 1909. [Google Scholar]
- Brody, S. Parasitic fibroid. Am. J. Obstet. Gynecol. 1953, 65, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Singh, V. A Curious Case of Parasitic Fibroid in a Postmenopausal Woman. Cureus 2022, 14, e25048. [Google Scholar] [CrossRef]
- Van Der Meulen, J.F.; Pijnenborg, J.; Boomsma, C.M.; Verberg, M.F.G.; Geomini, P.M.A.J.; Bongers, M.Y. Parasitic myoma after laparoscopic morcellation: A systematic review of the literature. Int. J. Obstet. Gynaecol. 2015, 123, 69–75. [Google Scholar] [CrossRef]
- Brieger, G.; MacGibbon, A.; Peat, B. Torsion of a Parasitic Fibroid. Aust. N. Z. J. Obstet. Gynaecol. 1995, 35, 224–225. [Google Scholar] [CrossRef]
- Sofoudis, C.; Trouvas, D.; Zioris, K. Torsion of intestinal parasitic myoma after laparoscopic morcellation: A case report. J. Surg. Case Rep. 2020, 2020, rjaa032. [Google Scholar]
- Cho, I.A.; Baek, J.C.; Park, J.K.; Song, D.H.; Kim, W.J.; Lee, Y.K.; Shin, J.K.; Choi, W.J.; Lee, S.A.; Lee, J.H.; et al. Torsion of a parasitic myoma that developed after abdominal myomectomy. Obstet. Gynecol. Sci. 2016, 59, 75–78. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Francis, N.; Oyawoye, O.; Chander, B. Secondary amenorrhoea with high inhibin B level caused by parasitic ovarian leiomyoma. Gynecol. Endocrinol. 2009, 26, 93–95. [Google Scholar] [CrossRef]
- Fasih, N.; Prasad Shanbhogue, A.K.; Macdonald, D.B.; Fraser-Hill, M.A.; Papadatos, D.; Kielar, A.Z.; Doherty, G.P.; Walsh, C.; McInnes, M.; Atri, M. Leiomyomas beyond the Uterus: Unusual Locations, Rare Manifestations. RadioGraphics 2008, 28, 1931–1948. [Google Scholar] [CrossRef]
- Takeda, A.; Koike, W.; Watanabe, K. Multimodal imaging for diagnosis and management of parasitic peritoneal myoma with myxoid degeneration after laparoscopic-assisted myomectomy with electric power morcellation. J. Obstet. Gynaecol. Res. 2018, 44, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in gynecological disease (15): Clinical and ultrasound characteristics of uterine sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Micha, G.; Galatis, D.G.; Kalopita, K.; Strongylos, A.; Benekos, C.; Kalaitzi, K.; Karachalios, P.-K.; Anifantaki, F.; Dalivigkas, I.; Gripiotis, I.; et al. Unusual Case of a Parasitic Extra-Uterine Leiomyoma Presenting With Lower Abdominal Pain. Cureus 2022, 14, e30141. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Lee, M.H.; Wei, Y.C.; Hsu, Y.H.; Hong, M.K. Iatrogenic Parasitic Myoma with Two Recurrence Times after Subsequent My-omectomy: A Rare and Complicated Case Report. Gynecol. Minim. Invasive Ther. 2020, 9, 154–158. [Google Scholar]
- Ostrzenski, A. Uterine leiomyoma particle growing in an abdominal-wall incision after laparoscopic retrieval. Obstet. Gynecol. 1997, 89, 853–854. [Google Scholar] [CrossRef]
- Cucinella, G.; Granese, R.; Calagna, G.; Somigliana, E.; Perino, A. Parasitic myomas after laparoscopic surgery: An emerging com-plication in the use of morcellator? Description of four cases. Fertil. Steril. 2011, 96, e90–e96. [Google Scholar] [CrossRef]
- Lu, B.; Xu, J.; Pan, Z. Iatrogenic parasitic leiomyoma and leiomyomatosis peritonealis disseminata following uterine morcellation. J. Obstet. Gynaecol. Res. 2016, 42, 990–999. [Google Scholar] [CrossRef]
- Pezzuto, A.; Pontrelli, G.; Ceccaroni, M.; Ferrari, B.; Nardelli, G.; Minelli, L. Case report of asymptomatic peritoneal leiomyomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 148, 205–206. [Google Scholar] [CrossRef]
- Takeda, A.; Imoto, S.; Mori, M.; Yamada, J.; Nakamura, H. Rapid growth of parasitic myoma in early pregnancy: Previously un-described manifestation of a rare disorder after laparoscopic-assisted myomectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 162, 117–118. [Google Scholar] [CrossRef]
- Al-Talib, A.; Tulandi, T. Pathophysiology and Possible Iatrogenic Cause of Leiomyomatosis Peritonealis Disseminata. Gynecol. Obstet. Investig. 2010, 69, 239–244. [Google Scholar] [CrossRef]
- Darii, N.; Anton, E.; Doroftei, B.; Ciobica, A.; Maftei, R.; Anton, S.C.; Mostafa, T. Iatrogenic parasitic myoma and iatrogenic adeno-myoma after laparoscopic morcellation: A mini-review. J. Adv. Res. 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stabile, G.; Zinicola, G.; Romano, F.; Laganà, A.S.; Pozzolo, C.D.; Ricci, G. Pelvic mass, ascites, hydrothorax: A malignant or benign condition? Meigs syndrome with high levels of CA 125. Menopausal Rev. 2021, 20, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Di Legge, A.; Pollastri, P.; Mancari, R.; Ludovisi, M.; Mascilini, F.; Franchi, D.; Jurkovic, D.; Coccia, M.E.; Timmerman, D.; Scambia, G.; et al. Clinical and ultrasound characteristics of surgically removed adnexal lesions with largest diameter ≤ 2.5 cm: A pictorial essay. Ultrasound Obstet. Gynecol. 2017, 50, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Aust, T.; Gale, P.; Cario, G.; Robertson, G. Bowel resection for iatrogenic parasitic fibroids with preoperative investigations sug-gestive of malignancy. Fertil. Steril. 2011, 96, e1–e3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.; Pelaccia, E.; Di Florio, C.; Palumbo, P.; Sollima, L.; Ludovisi, M.; Guido, M. Conservative Management and Ultrasound Follow-Up of Parasitic Myoma: Our Experience and Literature Review. Diagnostics 2023, 13, 1818. https://doi.org/10.3390/diagnostics13101818
Bruno M, Pelaccia E, Di Florio C, Palumbo P, Sollima L, Ludovisi M, Guido M. Conservative Management and Ultrasound Follow-Up of Parasitic Myoma: Our Experience and Literature Review. Diagnostics. 2023; 13(10):1818. https://doi.org/10.3390/diagnostics13101818
Chicago/Turabian StyleBruno, Matteo, Erika Pelaccia, Christian Di Florio, Pierpaolo Palumbo, Laura Sollima, Manuela Ludovisi, and Maurizio Guido. 2023. "Conservative Management and Ultrasound Follow-Up of Parasitic Myoma: Our Experience and Literature Review" Diagnostics 13, no. 10: 1818. https://doi.org/10.3390/diagnostics13101818
APA StyleBruno, M., Pelaccia, E., Di Florio, C., Palumbo, P., Sollima, L., Ludovisi, M., & Guido, M. (2023). Conservative Management and Ultrasound Follow-Up of Parasitic Myoma: Our Experience and Literature Review. Diagnostics, 13(10), 1818. https://doi.org/10.3390/diagnostics13101818






