Abstract
Alveolar soft part sarcoma (ASPS) is a rare malignant mesenchymal tumor mainly affecting adolescents and young adults, with a predilection for the deep soft tissues of extremities. ASPS arising in the female genital tract is extremely rare and poses a significant diagnostic challenge. We herein present two rare cases of ASPS, one occurring in the uterine corpus of a 27-year-old woman, and the other in the uterine cervix of a 10-year-old girl. We described the clinical, histological, immunophenotypical, and molecular characteristics of primary uterine ASPS. We performed immunostaining for transcription factor E3 (TFE3), human melanoma black 45 (HMB45), melan-A, desmin, pan-cytokeratin (CK), paired box 8 (PAX8), CD10, hormone receptors, and S100, and targeted RNA and DNA sequencing using commercially available cancer gene panel. In case 1, a 27-year-old woman was referred to our hospital after laparoscopic uterine myomectomy at an outside hospital. Imaging studies revealed a residual tumor in the uterine corpus. In case 2, a 10-year-old girl underwent surgical excision for the cervical mass and was diagnosed as having ASPS. She was then referred to our hospital for further management. Both patients received total hysterectomy. Histologically, they displayed characteristic histological features of ASPS. Strong nuclear TFE3 immunoreactivity, periodic acid-Schiff-positive, diastase-resistant intracytoplasmic rod-shaped crystalloids or granules, and the identification of ASPSCR1–TFE3 fusion confirmed the diagnosis of ASPS in both cases. Lack of immunoreactivity for HMB45, melan-A, desmin, pan-CK, PAX8, and S100 excluded the possibility of perivascular epithelioid cell tumor, clear cell sarcoma, metastatic renal cell carcinoma, granular cell tumor, and paraganglioma. Our observations can help pathologists make an accurate diagnosis of uterine ASPS and suggest that pathologists should include primary uterine ASPS in the differential diagnosis of uterine mesenchymal tumors.
1. Introduction
Alveolar soft part sarcoma (ASPS) is a rare malignant mesenchymal tumor that primarily affects adolescents and young adults [1,2]. ASPS comprises less than 1% of all soft tissue sarcoma cases [3]. Although this tumor tends to grow slowly and behave in a clinically indolent fashion, it has a significant risk of metastatic spread, often early in the disease course [3]. Early detection, accurate diagnosis, and appropriate management of ASPS are essential for improving the patient’s chance of survival. Prognostic factors for ASPS include tumor size, age of presentation, and the development of metastasis [4].
The female genital tract is an extremely rare site of origin for ASPS. This report presents two rare cases of ASPS, one occurring in the uterine corpus and the other in the cervix. We herein describe the clinical, histological, immunophenotypical, and molecular characteristics of primary uterine ASPS comprehensively.
2. Materials and Methods
2.1. Case Selection and Clinicopathological Data Collection
We found two uterine ASPS cases from surgical pathology archives, using the combination of keywords ‘alveolar soft part sarcoma’, ‘uterus’, ‘corpus’, ‘cervix’, ‘vagina’, and ‘vulva’. Clinical information—including age of patient at diagnosis, presenting symptom, magnetic resonance imaging (MRI) finding, positron emission tomography–computed tomography (PET-CT) finding, preoperative clinical impression, surgical procedure, postoperative treatment, postoperative recurrence and metastasis, current status, and disease-free survival period—was obtained from the electronic medical records and pathology reports. A single board-certified gynecological pathologist thoroughly reviewed all available hematoxylin and eosin (H&E)-stained slides using light microscopy. Pathological information—including the location and greatest dimension of tumor, lymphovascular space invasion (LVSI), histological growth pattern, nuclear pleomorphism, mitotic activity, and tumor cell necrosis—was collected. For case 1, the most representative slide was selected to perform immunohistochemical staining and next-generation sequencing (NGS). For case 2, in contrast, the outside unstained slides obtained from the mass excision specimen were used.
2.2. Immunohistochemical Staining
Four-micrometer-thick, formalin-fixed, paraffin-embedded (FFPE) slices were deparaffinized and rehydrated using a xylene and alcohol solution. Immunostaining was performed using automated instruments [5,6,7,8,9,10,11,12,13,14,15,16]. After antigen retrieval, the slices were incubated with the primary antibodies listed in Table 1. After chromogenic visualization, the slices were counterstained with hematoxylin. Appropriate positive and negative controls were concurrently stained to validate the staining method. Negative controls were prepared by substituting non-immune serum for primary antibodies, resulting in no detectable staining.

Table 1.
Antibodies used.
2.3. Special Staining
Four-micrometer-thick FFPE slices were stained with the periodic acid-Schiff method with diastase digestion (PAS-D) to examine the presence of coarse cytoplasmic granularity or crystalline inclusions, supporting the diagnosis of ASPS.
2.4. Nucleic Acid Extraction
Five-micrometer-thick FFPE slices were deparaffinized and rehydrated using a xylene and alcohol solution. The sections were manually microdissected under a dissecting microscope using a scalpel point dipped in ethanol. The scraped material was washed in phosphate-buffered saline and digested overnight in proteinase K at 56 °C in Buffer ATL (Qiagen, Germantown, CA, USA). DNA and RNA were isolated using the QIAamp DSP DNA FFPE Tissue Kit (Qiagen) [17,18,19,20,21]. A Qubit 4.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), a highly sensitive and accurate fluorescence-based quantitation assay, was used for sample quantitation.
2.5. NGS
NGS library preparation was performed using the extracted DNA and RNA, Ion AmpliSeq Library Preparation (Thermo Fisher Scientific), and IonChef System (Thermo Fisher Scientific) [17,18,19,20,21]. We applied the Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific), which is an amplicon-based targeted assay that enables the detection of relevant single-nucleotide variants, amplifications, gene fusions, and indels from 161 unique genes. Sequencing was performed using the IonTorrent S5 XL platform (Thermo Fisher Scientific) and positive control cell line mixtures (Horizon Discovery, Cambridge, UK). Genomic data were analyzed and alterations were detected using the IonReporter Software 5.6 (Thermo Fisher Scientific). We also manually reviewed the variant call format file and Integrated Genomic Viewer (Broad Institute, Cambridge, MA, USA). Pathogenic variants in coding regions, promoter regions, or splice variants were retained.
3. Case Presentation
3.1. Case 1: Primary ASPS of the Uterine Corpus
3.1.1. Clinical Presentation
A 27-year-old woman presented with vaginal bleeding. She underwent transvaginal ultrasonography at a local clinic, which revealed a 2.5 cm uterine mass. Laparoscopic myomectomy was performed based on the clinical impression of uterine leiomyoma. Her final pathological diagnosis was uterine ASPS. She was then referred to our hospital. Pelvic MRI revealed a suspected residual tumor at the previous myomectomy site (Figure 1A–C). PET-CT revealed increased fluorodeoxyglucose uptake within the uterine corpus. No remarkable uptake was observed elsewhere in the body. She underwent a total hysterectomy.
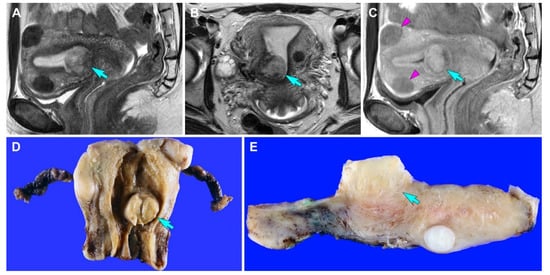
Figure 1.
Imaging and gross findings (case 1). (A,B) T2-weighted (A) sagittal and (B) axial magnetic resonance imaging (MRI) reveals a relatively well-circumscribed mass in the uterus (blue arrows). (C) Contrast-enhanced T1-weighted turbo spin-echo sagittal MRI reveals that the tumor appears to involve the superficial myometrium. The signal intensity of the uterine mass (blue arrow) is higher than that of intramural leiomyomas (purple arrowheads). (D) Grossly, a well-circumscribed mass (blue arrow) is located in the uterine corpus. (E) The cut section shows an exophytic mass (blue arrow) that appears to invade the superficial myometrium.
3.1.2. Pathological Findings
Grossly, a 2.2 cm well-circumscribed mass was identified in the lower uterine segment. The cut section showed a yellow-tan, rubbery, exophytic mass that appeared to invade the superficial myometrium (Figure 1D). A few intramural leiomyomatous nodules were also observed in the myometrial outer half (Figure 1E). Histologically, the epicenter of the tumor was located between the myometrium and endometrial stroma. The proliferative endometrial glands were unremarkable. Low-power magnification revealed that the tumor formed variable-sized cellular islands and irregularly permeated the myometrium (Figure 2A,B). Several lymphovascular spaces were closely adjacent to the tumor cells, and a few foci of LVSI were identified. Both thin fibrovascular septa and dense hyalinized stroma surrounded the sheets and nests of tumor cells (Figure 2C,D). A solid, diffuse growth with little or no intervening stroma was also noted (Figure 2E). High-power magnification demonstrated a uniform population of large polygonal cells, possessing abundant clear or vacuolated cytoplasm and distinct cell borders. The tumor cells were mildly pleomorphic. Their round-to-ovoid nuclei were centrally or eccentrically located, with bland-looking chromatin and occasional punctate nucleoli. In some areas, eosinophilic intracytoplasmic materials were located near the tumor cell nuclei (Figure 2F). No severe nuclear pleomorphism, mitotic figure, or tumor cell necrosis were observed. Based on the morphological characteristics, we considered the possibility of perivascular epithelioid cell tumor (PEComa), clear cell sarcoma (CCS), metastatic renal cell carcinoma (RCC), granular cell tumor, paraganglioma, and ASPS [22], as these tumors share similar cytological features, such as abundant clear or eosinophilic granular cytoplasm with vacuoles.
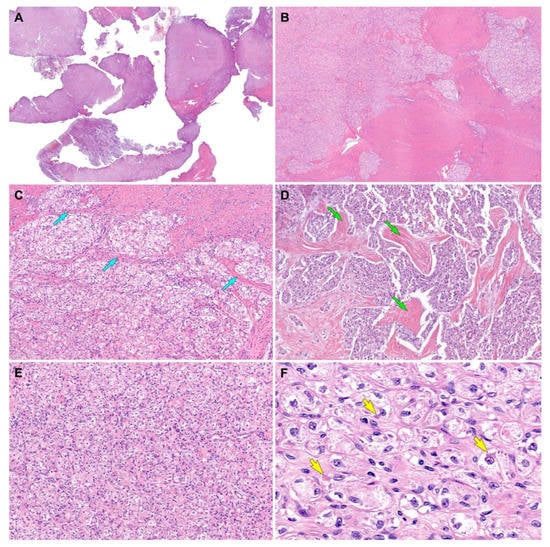
Figure 2.
Histological findings (case 1). (A) Scanning-power magnification reveals that the myomectomy specimen consists predominantly of tumor tissue infiltrating the myometrium. (B) Low-power magnification reveals that the tumor irregularly permeates the myometrium and forms variable-sized islands of tumor cells. (C) Thin fibrovascular septa (blue arrows) surround the tumor cell nests. (D) The tumor cells were organized in irregular-shaped sheets embedded in a background of dense hyalinized stroma (green arrows). (E) A solid, diffuse growth pattern with little or no intervening stroma is also noted. (F) The high-power view demonstrates large polygonal cells possessing clear or vacuolated cytoplasm and distinct cell borders. In some microscopic foci, eosinophilic intracytoplasmic materials (yellow arrows) are located closely adjacent to the nuclei. Original magnification: (A) 10×; (B) 20×; (C,D) 40×; (E) 100×; (F) 400×.
3.1.3. Results of Immunostaining and Special Staining
All tumor cells showed uniform and strong nuclear immunoreactivity for transcription factor E3 (TFE3; staining percentage, 100%; Figure 3A), a surrogate marker for ASPS chromosome region, candidate 1 (ASPSCR1)–TFE3 fusion [22,23]. PAS-D highlighted the aggregates of eosinophilic intracytoplasmic rod-shaped crystalloids (Figure 3B,C). The tumor cells also expressed CD10 (staining percentage, 70%; Figure 3D), estrogen receptor (ER; staining percentage, 30%; Figure 3E), and progesterone receptor (PR; staining percentage, 90%; Figure 3F), supporting the primary uterine origin [24]. On the other hand, the tumor cells were negative for human melanoma black 45 (HMB45; Figure 3G), melan-A (Figure 3H), desmin (Figure 3I), S100 (Figure 3J), pan-cytokeratin (pan-CK; Figure 3K), paired box 8 (PAX8; Figure 3L), and synaptophysin (Figure 3M), excluding the possibility of PEComa, CCS, metastatic RCC, granular cell tumor, and paraganglioma. MET immunostaining revealed a lack of MET-positive tumor cells (Figure 3N).
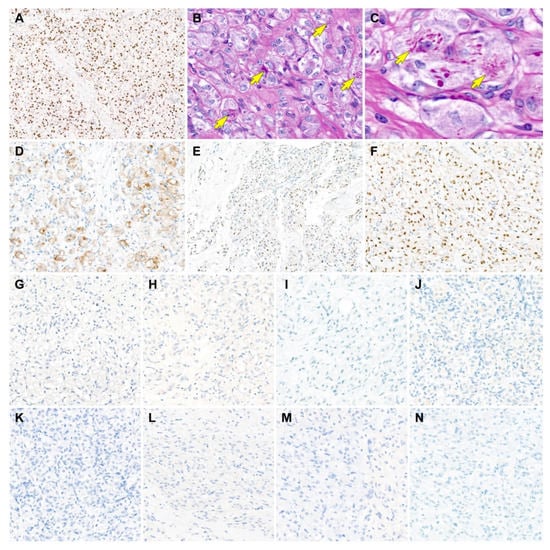
Figure 3.
Results of immunostaining and special staining (case 1). (A) The tumor cells show uniform and strong nuclear immunoreactivity for transcription factor E3 (TFE3), a surrogate marker for ASPS chromosome region, candidate 1 (ASPSCR1)–TFE3 fusion. (B,C) Periodic acid-Schiff with diastase digestion staining highlights the aggregates of eosinophilic intracytoplasmic rod-shaped crystalloids, which locate closely adjacent to the tumor cell nuclei (yellow arrows). (D–F) The tumor cells express (D) CD10, (E) estrogen receptor, and (F) progesterone receptor. (G–M) In contrast, the tumor cells are negative for (G) human melanoma black 45, (H) melan-A, (I) desmin, (J) S100, (K) pan-cytokeratin, (L) paired box 8, and (M) synaptophysin, excluding the possibility of TFE3 translocation-associated perivascular epithelioid cell tumor, clear cell sarcoma, granular cell tumor, metastatic renal cell carcinoma, and paraganglioma. (N) MET expression is absent in the tumor cells. Original magnification: (A) 60×; (B) 400×; (C) 600×; (D) 200×; (E) 40×; (F–N) 200×.
3.1.4. NGS Results
NGS analysis revealed that the tumors harbored ASPSCR1–TFE3 fusion. Lack of EWS RNA-binding protein 1 (EWSR1) translocation excluded CCS. No other pathogenic mutations or indels were detected.
3.2. Case 2: Primary ASPS of the Uterine Cervix
3.2.1. Clinical Presentation
A 10-year-old girl presented with vaginal bleeding that lasted for more than a year. Physical examination revealed a 2.9 cm cervical mass. Pelvic MRI revealed a well-circumscribed hypervascular, lobulated mass in the uterine cervix (Figure 4A). Contrast-enhanced CT also showed an enhancing cervical mass (Figure 4B). After surgical excision of the mass, she was referred to our hospital for further management of ASPS. The imaging studies after mass excision could not completely exclude the possible presence of residual tumor in the cervix. MRI detected a small cervical lesion, which was suspected to be a residual tumor. The clinicians decided to perform a total hysterectomy after discussing with the patient and her parents.
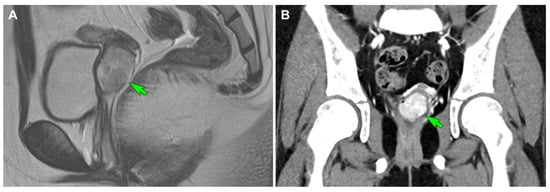
Figure 4.
Imaging findings (case 2). (A) Initial T2-weighted turbo spin-echo sagittal magnetic resonance imaging reveals a well-circumscribed hypervascular mass (green arrow) originating in the uterine cervix, with heterogeneous high signal intensity and a lobulated contour. (B) Initial contrast-enhanced computed tomography shows an enhancing mass (green arrow) abutting the uterine cervix.
3.2.2. Pathological Findings
We reviewed the outside pathology slides obtained from the mass excision specimen. Scanning-power magnification revealed a solid tumor with lobulated contour (Figure 5A). Low-power magnification showed a diffuse growth pattern without nested architecture or prominent vasculature (Figure 5B). The solid areas were highly cellular and consisted of relatively uniform tumor cells. Mildly dilated, sinusoid-like vascular channels were occasionally noted in-between solid cellular sheets. High-power magnification depicted eosinophilic intracytoplasmic globules and ample granular cytoplasm (Figure 5C). Most of the tumor cells had a centrally located, round nuclei showing mild pleomorphism and smooth nuclear membrane. Conspicuous nucleoli were rarely noted. In some areas, the discohesive tumor cells were arranged in a pseudoalveolar pattern (Figure 5D). No LVSI, tumor cell necrosis, or mitosis was detected. No residual tumor was observed in the hysterectomy specimen. A 0.8 cm small fibrotic lesion identified in the cervix exhibited post-surgical inflammation and fibrosis caused by the previous mass excision. Regarding the morphological features and the patient’s age, we considered ASPS to be the most probable diagnosis. Similar to the case 1, the differential diagnosis included PEComa, metastatic RCC, and paraganglioma. We excluded CCS and granular cell tumor based on the outside pathology report stating negative immunoreactivities for desmin and S100.
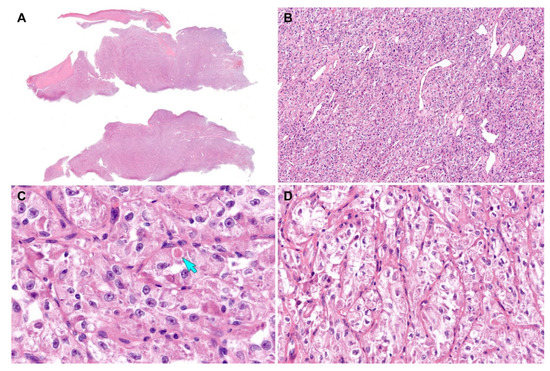
Figure 5.
Histological findings (case 2). (A) Scanning-power magnification of the mass excision specimen reveals lobulated tumor tissue. (B) Low-power magnification reveals a diffuse growth pattern without nested architecture. Mildly dilated sinusoidal vascular channels are noted. (C) High-power magnification depicts eosinophilic intracytoplasmic globules (blue arrow) and pleomorphic nuclei with conspicuous nucleoli. (D) The discohesive tumor cells are arranged in a pseudoalveolar pattern in some areas. Original magnification: (A) 10×; (B) 40×; (C) 400×; (D) 200×.
3.2.3. Results of Special Staining and Immunostaining
We performed PAS-D staining and immunostaining for HMB45, melan-A, pan-CK, CD10, and PR, using the outside unstained slides. The tumor cells were diffusely positive for TFE3 (staining percentage, 100%; Figure 6A) with moderate-to-strong staining intensity. PAS-D revealed eosinophilic granular materials within the cytoplasm (Figure 6B). PR was strongly positive in approximately 80% of the tumor cell nuclei (Figure 6C). In contrast, the tumor cells did not react with CD10 (Figure 6D), HMB45 (Figure 6E), melan-A (Figure 6F), and pan-CK (Figure 6G).
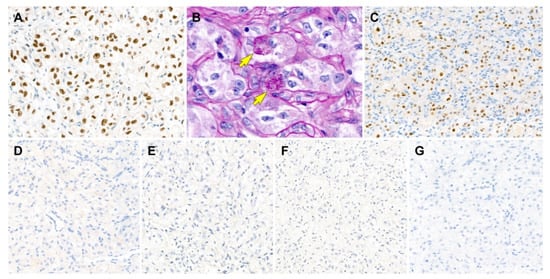
Figure 6.
Results of immunostaining and special staining (case 2). (A) The tumor cells are diffusely positive for transcription factor E3 with moderate-to-strong staining intensity. (B) Periodic acid-Schiff with diastase digestion staining reveals eosinophilic granular materials within the cytoplasm (yellow arrows). (C) Similar to case 1, progesterone receptor is strongly positive for tumor cell nuclei. (D–G) The tumor cells are negative for (D) CD10, (E) human melanoma black 45, (F) melan-A, and (G) pan-cytokeratin. Original magnification: (A) 400×; (B) 600×; (C–G) 200×.
3.2.4. NGS Results
NGS analysis revealed that the tumors harbored ASPSCR1–TFE3 fusion. Lack of EWS RNA-binding protein 1 (EWSR1) translocation excluded CCS. No other pathogenic mutations or indels were detected.
3.3. Post-Operative Follow-Up
Both patients are currently well without evidence of disease recurrence or metastasis two (case 1) and four (case 2) months postoperatively.
4. Discussion
Table 2 summarizes the clinicopathological characteristics, immunophenotypes, and molecular alterations of our cases. We found some similarities and differences between the two tumors, each of them arose in the uterine corpus and cervix, respectively. Both patients presented with vaginal bleeding. Both tumors appeared as a polypoid mass, suggesting a favorable outcome as reported in the previous literature [25]. Both tumors displayed diffuse and strong nuclear PR immunoreactivity. In contrast, CD10, an endometrial stromal marker, was positive in case 1 only. This finding raises the possibility that ASPS of the uterine corpus might have a different cell of origin from that of the cervical primary. CD10 positivity in ASPS arising from the uterine corpus supports the hypothesis that it might originate from the endometrial stromal cells [24]. PAS-D staining revealed a morphological difference of intracytoplasmic materials between them. Case 1 showed relatively well-formed, rod-shaped crystalloids, whereas in case 2 coarse granular materials were identified within the cytoplasm.

Table 2.
Summary of clinicopathological, immunophenotypical, and molecular characteristics of primary uterine alveolar soft part sarcoma.
The differential diagnosis of primary uterine ASPS includes PEComa, CCS, metastatic RCC, granular cell tumor, and paraganglioma. Differentiating ASPS from PEComa based just on morphology is sometimes challenging, as they share overlapping histological features, such as pseudoalveolar pattern and polygonal cells with clear or eosinophilic granular cytoplasm. Even though TFE3 is a surrogate marker for ASPSCR1–TFE3 fusion, strong nuclear TFE3 immunoreactivity cannot exclude the possibility of TFE3-rearranged tumors including TFE3 translocation-associated PEComa and Xp11.2 translocation RCC. Granular cell tumor can also strongly express TFE3 [25,26,27]. Therefore, additional immunostaining is mandatory to confirm the diagnosis of ASPS. Schoolmeester et al. [26] suggested HMB45, melan-A, and desmin as key markers of immunostaining panel to distinguish uterine conventional or TFE3-rearranged PEComa from ASPS. In this report, a lack of expression for all of these excluded PEComa. Absence of pan-CK and PAX8 immunoreactivity ruled out the possibility of metastatic RCC. S100 negativity excluded CCS and granular cell tumors [28].
To the best of our knowledge, 17 cases of primary uterine ASPS confirmed by either TFE3 immunostaining or molecular testing have been documented (Table 3) [25,26,27,28]. Among them, seven and eight cases were reported to arise in the uterine corpus and cervix, respectively. Two cases were reported to originate from the uterus, but a definite location was not documented. Uterine ASPS generally has a good prognosis, as only two patients developed pelvic lymph node metastasis [29] and postoperative recurrence [27], respectively. All except one patient had a small-sized (<5 cm) tumor, suggesting the association with a relatively favorable outcome compared to larger lesions in other locations [25]. However, a long-term follow-up of these cases is required to confirm the theory of uterine location being a site with favorable prognosis.

Table 3.
Summary of previously published cases of primary uterine alveolar soft part sarcoma.
Recently, as the understanding of the molecular features of ASPS—a characteristic t(X;17)(p11;q25) and its correspondent chimeric ASPSCR1–TFE3 fusion protein—has improved, attempts to develop novel drugs for ASPS are emerging [1]. Previous studies have reported that the production of ASPSCR1–TFE3 fusion proteins leads to transcriptional upregulation and increased signaling of the MET pathway [26,35]. In a previous study by Jun et al. [36], MET expression was observed in six of eight cases of TFE3-positive ASPS. However, in this report, MET was completely negative in case 1. Large-scale case studies are necessary to clarify the potential therapeutic and prognostic significances of MET immunostaining and MET-targeted therapy.
5. Conclusions
We demonstrated the clinicopathological, immunophenotypical, and genetic features of two primary ASPS arising in the uterus. Primary uterine ASPS is an extremely rare malignant mesenchymal tumor, which has several morphological mimickers. Immunostaining with a panel of antibodies including TFE3 as well as markers for melanocytic, smooth muscle, neurogenic, and epithelial lesions is necessary for the differential diagnosis. The identification of ASPSCR1–TFE3 fusion by molecular testing is helpful to confirm the diagnosis of ASPS. We believe our comprehensive and detailed analyses of primary uterine ASPS can help pathologists make an accurate histological diagnosis.
Author Contributions
Conceptualization, Y.L. and H.-S.K.; methodology, K.N.; validation, K.N. and H.Y.W.; formal analysis, Y.L. and H.-S.K.; resources, H.Y.W.; data curation, K.N. and H.Y.W.; writing—original draft preparation, Y.L. and H.Y.W.; writing—review and editing, K.N. and H.-S.K.; visualization, H.-S.K.; supervision, H.-S.K.; project administration, H.-S.K.; funding acquisition, H.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Sungkyunkwan University and the BK21 FOUR (Graduate School Innovation) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Samsung Medical Center (protocol code: 2022-01-182; date of approval: 4 February 2022).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paoluzzi, L.; Maki, R.G. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: A review. JAMA Oncol. 2019, 5, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Whiting, K.; Naous, R. Alveolar soft part sarcoma presenting as a uterine polyp: A case report. SAGE Open Med. Case Rep. 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Li, Y.; Xue, X.; Zhou, H.; Hou, L. The current management of alveolar soft part sarcomas. Medicine 2021, 100, e26805. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jacobson, A.; Harmon, D.C.; Choy, E.; Hornicek, F.J.; Raskin, K.A.; Chebib, I.A.; DeLaney, T.F.; Chen, Y.L. Prognostic factors in alveolar soft part sarcoma: A SEER analysis. J. Surg. Oncol. 2016, 113, 581–586. [Google Scholar] [CrossRef]
- Na, K.; Kim, H.S. Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am. J. Surg. Pathol. 2019, 43, 12–25. [Google Scholar] [CrossRef]
- Chang, S.; Hur, J.Y.; Choi, Y.L.; Lee, C.H.; Kim, W.S. Current status and future perspectives of liquid biopsy in non-small cell lung cancer. J. Pathol. Transl. Med. 2020, 54, 204–212. [Google Scholar] [CrossRef]
- Chang, S.; Shim, H.S.; Kim, T.J.; Choi, Y.L.; Kim, W.S.; Shin, D.H.; Kim, L.; Park, H.S.; Lee, G.K.; Lee, C.H.; et al. Molecular biomarker testing for non-small cell lung cancer: Consensus statement of the Korean Cardiopulmonary Pathology Study Group. J. Pathol. Transl. Med. 2021, 55, 181–191. [Google Scholar] [CrossRef]
- Choi, S.; Cho, J.; Lee, S.E.; Baek, C.H.; Kim, Y.K.; Kim, H.J.; Ko, Y.H. Adenocarcinoma of the minor salivary gland with concurrent MAML2 and EWSR1 alterations. J. Pathol. Transl. Med. 2021, 55, 132–138. [Google Scholar] [CrossRef]
- Jang, Y.; Jung, H.; Kim, H.N.; Seo, Y.; Alsharif, E.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Park, Y.H.; Cho, E.Y.; et al. Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast. J. Pathol. Transl. Med. 2020, 54, 95–102. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.; Choi, K.H.; Hwang, I.; Oh, Y.L. Metastatic leiomyosarcoma of the thyroid gland: Cytologic findings and differential diagnosis. J. Pathol. Transl. Med. 2021, 55, 360–365. [Google Scholar] [CrossRef]
- Choi, S.; Joo, J.W.; Do, S.I.; Kim, H.S. Endometrium-limited metastasis of extragenital malignancies: A challenge in the diagnosis of endometrial curettage specimens. Diagnostics 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Jung, Y.Y.; Kim, H.S. Serous carcinoma of the endometrium with mesonephric-like differentiation initially misdiagnosed as uterine mesonephric-like adenocarcinoma: A case report with emphasis on the immunostaining and the identification of splice site TP53 mutation. Diagnostics 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kim, B.G.; Song, S.Y.; Kim, H.S. Ovarian gynandroblastoma with a juvenile granulosa cell tumor component in a postmenopausal woman. Diagnostics 2020, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, S.; Do, S.I.; Lee, S.H.; Yoon, N.; Kim, H.S. Clinicopathological characteristics of pleomorphic high-grade squamous intraepithelial lesion of the uterine cervix: A single-institutional series of 31 cases. Diagnostics 2020, 10, 595. [Google Scholar] [CrossRef]
- Park, S.; Bae, G.E.; Kim, J.; Kim, H.S. Mesonephric-like differentiation of endometrial endometrioid carcinoma: Clinicopathological and molecular characteristics distinct from those of uterine mesonephric-like adenocarcinoma. Diagnostics 2021, 11, 1450. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.S. Primary retroperitoneal mucinous carcinoma with carcinosarcomatous mural nodules: A case report with emphasis on its histological features and immunophenotype. Diagnostics 2020, 10, 580. [Google Scholar] [CrossRef]
- Kim, S.W.; Do, S.I.; Na, K. External validation of ALK and ROS1 fusions detected using an Oncomine Comprehensive Assay. Anticancer Res. 2021, 41, 4609–4617. [Google Scholar] [CrossRef]
- Kim, H.; Na, K.; Bae, G.E.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Comprehensive immunohistochemical analyses using markers for mesonephric, endometrioid and serous tumors. Diagnostics 2021, 11, 2042. [Google Scholar] [CrossRef]
- Koh, H.H.; Park, E.; Kim, H.S. Mesonephric-like adenocarcinoma of the ovary: Clinicopathological and molecular characteristics. Diagnostics 2022, 12, 326. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Kim, H.S. Mesonephric adenocarcinoma of the vagina harboring TP53 mutation. Diagnostics 2022, 12, 119. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, S.; Kim, H.S. Extraskeletal mesenchymal chondrosarcoma of the uterus. Diagnostics 2022, 12, 643. [Google Scholar] [CrossRef]
- Tsuji, K.; Ishikawa, Y.; Imamura, T. Technique for differentiating alveolar soft part sarcoma from other tumors in paraffin-embedded tissue: Comparison of immunohistochemistry for TFE3 and CD147 and of reverse transcription polymerase chain reaction for ASPSCR1-TFE3 fusion transcript. Hum. Pathol. 2012, 43, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Lal, P.; Hutchinson, B.; Lui, M.Y.; Reuter, V.E.; Ladanyi, M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: A sensitive and specific immunohistochemical assay. Am. J. Surg. Pathol. 2003, 27, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, S.; Minato, H.; Kobayashi, M.; Ueda, Y.; Oda, Y.; Hashimoto, S.; Inoue, M. Alveolar soft part sarcoma of the endometrium with expression of CD10 and hormone receptors. APMIS 2007, 115, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Vishwajeet, V.; Elhence, P.; Singh, P.; Ghuman, N.K. Alveolar soft part sarcoma of uterine corpus in a young female: A case report with review of literature. Int. J. Gynecol. Pathol. 2021, 40, 272–277. [Google Scholar] [CrossRef]
- Schoolmeester, J.K.; Carlson, J.; Keeney, G.L.; Fritchie, K.J.; Oliva, E.; Young, R.H.; Nucci, M.R. Alveolar soft part sarcoma of the female genital tract: A morphologic, immunohistochemical, and molecular cytogenetic study of 10 cases with emphasis on its distinction from morphologic mimics. Am. J. Surg. Pathol. 2017, 41, 622–632. [Google Scholar] [CrossRef]
- Ryu, A.; Mun, S.T.; Lee, H.J.; Kim, N.S. Recurrent alveolar soft part sarcoma of the uterine cervix. J. Obstet. Gynaecol. 2017, 37, 1099–1101. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Liu, Y.; Liu, C. Alveolar soft part sarcoma of uterine cervix in a postmenopausal woman: A case report and review of literature. Int. J. Clin. Exp. Pathol. 2017, 10, 9812–9815. [Google Scholar]
- Zhang, L.L.; Tang, Q.; Wang, Z.; Zhang, X.S. Alveolar soft part sarcoma of the uterine corpus with pelvic lymph node metastasis: Case report and literature review. Int. J. Clin. Exp. Pathol. 2012, 5, 715–719. [Google Scholar]
- Roma, A.A.; Yang, B.; Senior, M.E.; Goldblum, J.R. TFE3 immunoreactivity in alveolar soft part sarcoma of the uterine cervix: Case report. Int. J. Gynecol. Pathol. 2005, 24, 131–135. [Google Scholar] [CrossRef]
- Williams, A.; Bartle, G.; Sumathi, V.P.; Meis, J.M.; Mangham, D.C.; Grimer, R.J.; Kindblom, L.G. Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: Useful diagnostic tools in cases with unusual histological features. Virchows Arch. 2011, 458, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Ichikawa, R.; Ishii, R.; Oe, S.; Kato, R.; Kobayashi, Y.; Kuroda, M.; Udagawa, Y. A case of primary alveolar soft part sarcoma of the uterine cervix and a review of the literature. Int. J. Clin. Oncol. 2011, 16, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J. Alveolar soft part sarcoma of the uterine cervix: A case report and review of the literature. Korean J. Pathol. 2014, 48, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jiang, W.; He, Y.; Li, L. Primary alveolar soft part sarcoma of the uterine cervix: A case report and literature review. Int. J. Clin. Exp. Pathol. 2014, 7, 8223–8226. [Google Scholar]
- Tsuda, M.; Davis, I.J.; Argani, P.; Shukla, N.; McGill, G.G.; Nagai, M.; Saito, T.; Lae, M.; Fisher, D.E.; Ladanyi, M. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007, 67, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Jun, H.J.; Lee, J.; Lim, D.H.; Park, J.O.; Ahn, G.; Seo, S.W.; Sung, K.S.; Lim, D.H.; Yoo, K.H.; Choi, Y.L. Expression of MET in alveolar soft part sarcoma. Med. Oncol. 2010, 27, 459–465. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).