Abstract
(1) Background: Laboratory-based molecular assays are the gold standard to detect SARS-CoV-2. In resource-limited settings, the implementation of these assays has been hampered by operational challenges and long turnaround times. Rapid antigen detection tests are an attractive alternative. Our aim is to evaluate the clinical performance of two SARS-CoV-2 rapid antigen tests during a high transmission period. (2) Methods: A total of 1277 patients seeking SARS-CoV-2 diagnosis were enrolled at four health facilities. Nasopharyngeal swabs for rapid antigen and real time PCR testing were collected for each patient. Sensitivity, specificity, positive and negative predictive values, misclassification rate, and agreement were determined. (3) Results: The overall sensitivity of Panbio COVID-19 was 41.3% (95% CI: 34.6–48.4%) and the specificity was 98.2% (95% CI: 96.2–99.3%). The Standard Q had an overall sensitivity and specificity of 45.0% (95% CI: 39.9–50.2%) and 97.6% (95% CI: 95.3–99.0%), respectively. The positive predictive value of a positive test was 93.3% and 95.4% for the Panbio and Standard Q Ag-RDTs, respectively. A higher sensitivity of 43.2% and 49.4% was observed in symptomatic cases for the Panbio and Standard Q Ag-RDTs, respectively. (4) Conclusions: Despite the overall low sensitivity, the two evaluated rapid tests are useful to improve the diagnosis of symptomatic SARS-CoV-2 infections during high transmission periods.
1. Introduction
Since the beginning of the COVID-19 pandemic, access to quality diagnosis has been key in timely clinical management of cases and disease control [1]. However, in low-income settings, access to laboratory-based diagnostic testing, including real-time reverse transcription polymerase chain reaction (RT-PCR), has been limited [2] due to lack of adequate infrastructure, complexity of equipment, challenges of sample referral system [3], shortage of reagents, and scarcity of trained personnel [4,5]. These factors often lead to long result turnaround times (TAT), which negatively impact the efficiency of infection control, especially during high transmission periods.
Point-of-care, rapid tests based on antigen detection (Ag-RDT) enable early identification of SARS-CoV-2 infection by obtaining results in 15 to 30 min [6,7], and allow for prompt clinical decision making. In turn, this reduces the demand and pressure on laboratory-based diagnostic testing. However, antigen-detection assays may have lower sensitivity when compared to laboratory-based tests that rely on the amplification of viral nucleic acid. To date, there have been few evaluations of the added value of SARS-CoV-2 Ag-RDT under field conditions in tropical settings. It is expected that specific circumstances, such as environmental conditions, COVID-19 epidemiological context, and prevalence of other relevant pathogens, among others can influence the performance of rapid tests [8,9,10,11,12,13].
We conducted an evaluation of two SARS-CoV-2 Ag-RDTs, PanbioTM and STANDARDTM Q, compared with the gold standard RT-PCR, in real life conditions during a high transmission period in Mozambique.
2. Materials and Methods
2.1. Study Setting and Participants
A cross-sectional prospective study was conducted during the second wave of high transmission in Mozambique, from January to March 2021, at four health facilities: Hospital Provincial da Matola (HPM) and Centro de Saúde de Marracuene (CSM) in Maputo Province, and Hospital Geral de Chamanculo (HGC) and Hospital Geral de Mavalane (HGM) in Maputo City. Participants aged 18 years old and above, suspected of SARS-CoV-2 infection, and contacts of confirmed cases were enrolled in the study. Recruitment into the study was based on suspected cases presented at the triage rooms. The cases and contacts were defined based on WHO guidelines [14].
For each participant, two nasopharyngeal samples were collected for testing: one was used for the Ag-RDT under evaluation and performed at the health facility of collection, while the second was shipped to the Instituto Nacional de Saúde for the RT-PCR. Only the results of the RT-PCR were issued to the participants. Sample collection and Ag RDT testing was conducted by trained and certified non-healthcare workers that had been assessed for competency by certified Trainers of Trainers [15].
2.2. Data Collection
Demographic, clinical, and epidemiological data were prospectively collected at each study site using electronic standardized forms on mobile tablets (Samsung, Suwon-si, Korea, model: TAB A 8”). Laboratory technicians that performed the RT-PCR were blinded to the results generated with the Ag-RDT. Only the results obtained in the laboratory platforms were given to the study participants. The Ag-RDT results were only used for the study purposes.
2.3. Antigen Rapid Assay testing
Two SARS-CoV-2 Ag-RDTs were evaluated, the PanbioTM COVID-19 Ag rapid test (Abbott, Jena, Germany, Ref: 41FK10 Lot: 41ADF115A) and the STANDARDTM Q COVID-19 Ag test (SD Biosensor, Suwon-si, South Korea, Ref: Q-NCOV-01G Lot: QCO3020169I), both storable at 2–30 °C. A nasopharyngeal swab with a specimen was inserted into an extraction buffer tube provided by the manufacturer, stirred the swabs for at least five times, and removed it while squeezing the sides to extract liquid from the swab. Subsequently, three to five drops were applied to the specimen well of the test device and the test result was read in 15 to 30 min. Only the results with a valid control line were considered for data analysis. The results without a control line but with a test line were considered invalid. The results without control and test lines were excluded.
2.4. Real Time PCR Testing
The nasopharyngeal swabs collected by the study staff were placed into sterile tubes containing 3 mL of viral transport media (iClean, Shenzhen, China, REF. CY-F005-20) and sent to the Instituto Nacional de Saúde on the same day of collection. At the laboratory, samples were stored at 2–8 °C for up to 24 h. Automatic SARS-CoV-2 RNA extraction and amplification was performed on the Abbott m2000 platforms (Abbott Molecular, Taipei City, Taiwan). The assay uses two sets of primers to amplify regions within the highly conserved RNA-dependent RNA polymerase (RdRp) and N genes. The automated Cobas 6800 instrument (Roche, Amadora, Portugal) that detects two genes, E gene and ORF 1ab, was used as back up for when the Abbott m2000 was out of service. In the positive results, two genes were detected. The results with only one gene detected were considered indeterminate. The Ct value was used to determine the SARS-CoV-2 viral load in the positive cases.
2.5. Statistical Methods
Two-way contingency tables were used to summarize the data and performance of the two SARS-CoV-2 Ag-RDTs compared with the gold standard. The test performances were assessed by determining their sensitivity, specificity, positive and negative predictive values, and misclassification [16]. The positive and negative predictive values were determined taking the prevalence of the RT-PCR into account.
The overall observed agreement was given by the Cohen’s kappa coefficient [17]. The statistical analysis was performed using Microsoft Excel 360 (Microsoft Co., Redmond, WA, USA), and the level of significance adopted was 0.05.
3. Results
3.1. Patient Population
A total of 1277 participants were enrolled between January and March 2021 at four health facilities: CSM (n = 298), HGM (n = 179), HGC (n = 372), and HPM (n = 428). Of these participants, 551 and 726 were used for the evaluation of the Panbio and Standard Q, respectively (Figure 1). In view of the high transmission at the time of the study, 209 (37.93%) and 371 (51.10%) individuals were RT-PCR positives, respectively. Most of the participants had Ct values below 25, 92.2% and 89.2% for those tested with the Panbio and Standard Q, respectively. The median age of participants was 38 years (range: 18–88 years old) and 54.3% (694/1277) were female. Most of the participants reported symptoms onset 7 days before presentation (76.0%) (Table 1).
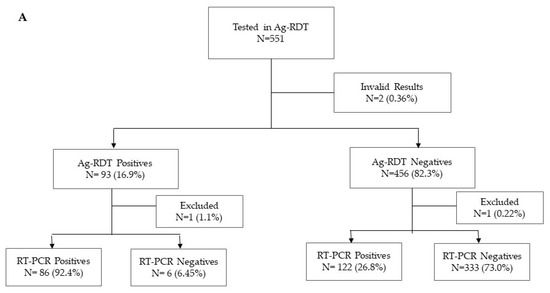
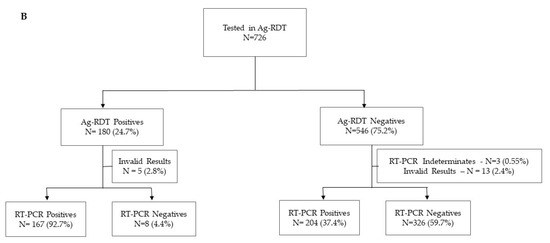
Figure 1.
Flow diagram of study participants tested by RT-PCR and PanbioTM COVID-19 Ag rapid test (A) and STANDARD Q COVID-19 Ag Test (B).

Table 1.
Characteristics of study participants.
3.2. Diagnostic Performance of PanbioTM COVID-19 Ag Rapid Test
The overall sensitivity and specificity of the Panbio Ag-RDT were 41.3% (95% CI: 34.6–48.4%) and 98.2% (95% CI: 96.2–99.3%), respectively. Higher sensitivity was observed in 43.2% (95% CI: 36.0–50.5%) of symptomatic patients when compared to 22.2% (95% CI: 6.4–47.6%) in asymptomatic participants. A sensitivity of 53.3% (95% CI: 42.6–63.7%) and 49.6% (95% CI: 41.1–58.2%) was observed in those with symptoms onset 5 and 7 days before presentation, respectively. The proportion of participants erroneously diagnosed as negative or positive was 19.0% (95% CI: 14.3–24.5%) and 20.8% (95% CI: 16.7–25.3%) in those with onset of symptoms 5 and 7 days before presentation, respectively (Table 2). In patients with symptoms onset for longer than 7 days before presentation, the sensitivity of the test was lower at 25.5% (95% CI: 14.3–39.6%), with 33.9% (95% CI: 25.3–43.3%) of the results being misclassified. The overall positive predictive value (PPV) was 93.3% (95% CI: 88.8–97.8%) and the negative predictive value (NPV) was 52.9% (95% CI: 48.6–57.1%).

Table 2.
Analytic performance of two antigen rapid assays for SARS-CoV-2 detection.
The Cohen’s k among those with symptoms onset 5 and 7 days before presentation was 0.55 (95% CI: 0.53–0.57) and 0.52 (95% CI: 0.51–0.53), respectively. In individuals without symptoms and those with symptoms onset for longer than 7 days before presentation, the Cohen’s k was 0.29 (95% CI: 0.04–0.56) and 0.25 (95% CI: 0.19–0.30), respectively.
The overall agreement between the results yielded by the RT-PCR and the Panbio Ag-RDT was good in subjects with higher viral loads, as depicted by Ct values below 16 (Table 3). In patients with lower viral loads, the overall agreement dropped to 52.7% and 11.9% for those with Ct values below 25 or higher than 26, respectively.

Table 3.
Agreement between RT-PCR positives and Ag-RDT regarding to CT values.
A total of six false positives were observed during the evaluation of the Panbio Ag-RDT. In those cases, all were symptomatic, five with symptoms onset for 7 days before presentation and three contacts of positive confirmed cases.
3.3. Diagnostic Performance of STANDARDTM Q COVID-19 Ag Test
The overall sensitivity and specificity of the Standard Q Ag-RDT were 45.0% (95% CI: 39.9–50.2%) and 97.6% (95% CI: 95.3–99.0%), respectively. Higher sensitivity was observed in 49.4% (95% CI: 43.8–55.0%) of symptomatic participants when compared to 13.3% (95% CI: 5.1–26.8%) of asymptomatic participants. A sensitivity of 61.9% (95% CI: 53.9–69.4%) and 54.4% (95% CI: 47.9–61.0%) was observed in those with symptoms onset 5 and 7 days before presentation, respectively. The proportion of participants erroneously diagnosed as negative or positive was 21.0% (95% CI: 16.6–26.0%) and 25.2% (95% CI: 21.2–29.5%) in those with onset of symptoms 5 and 7 days before presentation, respectively (Table 2). In patients with symptoms onset for longer than 7 days before presentation, the sensitivity of the test was lower at 35.2% (95% CI: 25.3–46.1%), with 44.4% (95% CI: 35.8–53.2%) of the results being misclassified. The overall positive predictive value (PPV) was 95.4% (95% CI: 92.3–98.5%) and the negative predictive value (NPV) was 66.4% (95% CI: 62.3–70.4%).
The Cohen’s k among those with symptoms onset 5 and 7 days before presentation was 0.58 (95% CI: 0.57–0.59) and 0.51 (95% CI: 0.49–0.52), respectively. In individuals without symptoms and those with symptoms onset for longer than 7 days before presentation, the Cohen’s k was 0.14 (95% CI: 0.05–0.22) and 0.24 (95% CI: 0.21–0.27), respectively.
The overall agreement between results yielded by the RT-PCR and the Standard Q Ag-RDT was good in subjects with higher viral loads as depicted by Ct values below 16 (Table 3). In patients with lower viral loads, the overall agreement dropped to 55.4% and 12.1% for those with Ct values below 25 or higher than 26, respectively.
A total of eight false positives were observed during the evaluation of the Standard Q Ag-RDT. In those cases, 75.0% were symptomatic, four with symptoms onset for 7 days before presentation and two contacts of positive confirmed cases.
4. Discussion
Timely diagnosis remains a priority in the efforts to control the spread of SARS-CoV-2. Access to laboratory-based PCR testing is challenged by the high costs of molecular tests, the complexity of laboratory-based testing procedures, and the prolonged turnaround time of results. During high transmission periods of COVID-19, positive cases have to be promptly detected for rapid clinical decision making and outbreak control [6,18].
The low-cost, ease of use, and short turnaround time of SARS-CoV-2 Ag-RDTs make them an attractive alternative to laboratory-based molecular tests [6]. Effectively, SARS-CoV-2 Ag-RDTs can contribute to improved diagnostic coverage and reduced burden of specimens’ referral logistics.
Several studies have described the performance of Ag-RDTs [9,10,11,12,13,19]. Our results demonstrated that the two Ag-RDTs, Panbio COVID-19 Ag and Standard Q COVID-19 Ag, had an overall low sensitivity. Nevertheless, the sensitivity was higher in patients who reported symptoms, especially in those with symptoms onset five or seven days before testing. Lower sensitivity (30.2% to 58.1%) has been observed in different studies. For example, Ristić et al. (2021), found an overall sensitivity of 58.1% (95% CI: 42.1–73.0), which was higher in the first five days following symptoms onset (100%, 83.3%, 66.7%, 71.4%, and 100%, for days 1 to 5, respectively) than in later days (55.6% between the 6th and 10th day) [20]. Additionally, the results obtained from Cohen’s kappa coefficient analysis showed better agreement in symptomatic patients, with symptoms 1 to 5 days before the test, thus reinforcing better applicability of both RDTs in this group of patients [21]. Another study, performed by Mboma et al. (2021), found an overall sensitivity of 30.4% (95%CI: 18.8–90.9%) in adults attending a hospital being higher at 52.9% in symptomatic cases [22]. Furthermore, Jegerlehner et al. (2021), in a different study, found an overall sensitivity of 65.3% (95% CI: 56.8–73.1) and 44.4% (95% CI 24.4–65.1) in asymptomatic cases [23]. The relatively low sensitivity observed in our study for both Ag-RDTs could be attributed to several factors, including deficient specimen collection [10], test performance, and patients’ clinical characteristics, including their viral loads [16]. Our study also observed a lower sensitivity (<35%) in asymptomatic cases, similar to those observed in other reports [22,24], that are probably associated with lower viral loads in those patients [4,20]. Evaluations in Europe and America observed a higher sensitivity of Ag-RDTs when compared to studies in Asia and Africa. Many RDTs are manufactured in Europe and America, which may affect the test performance in Asia and Africa after repeated freeze–thaw procedures during transportation [19].
Several new approaches can be used to overcome the low sensitivity of the Ag-RDT in an effort to scale-up the use of these tests for detecting, monitoring, and controlling outbreaks [24]. One, proposed to be used in low prevalence contexts, is the combined use with the RT-PCR in diagnostic algorithms [25]. Another approach, the clinical prediction rules [25], fits in high prevalence scenarios where, in absence of PCR for confirmation, symptomatic patients with negative Ag-RDT results can be clinically predicted as having COVID-19. Considering our Cohen’s kappa results in the asymptomatic patients and those with onset symptoms > 7 days, an extra caution might be required when the clinician tests these patients. Therefore, the use of the RT-PCR should be recommended.
A total of 14 false positive cases were observed on both rapid assays, with no de-mographical or clinical patterns identified. During the use of Ag-RDT for SARS-CoV-2, false positive cases can occur mostly when assay procedures are not followed correctly, when multiple specimens processing in batch mode affects the incubation times for each sample, or through cross-contamination between specimens [26]. Moreover, the time since symptoms onset and swab type can lead to false-negative results in the RT-PCR. For example, Wikramatatna et al. (2020), observed that those with symptoms onset 10 days before testing had a 25% chance of being false-negative using nasopharyngeal swabs and a 47% chance using oropharyngeal swabs [27]. If clinical suspicion of COVID-19 is high, then interpretation of RT-PCR negative results need to carefully consider the epidemiological context [28]. Most of our false positives in the Ag-RDT occurred in symptomatic individuals.
In the context of high transmission of a communicable disease, it is important that diagnostic tests with a high PPV are used. This allows for both clinical and public health decisions to be made with greater confidence. We found PPVs higher than 90% in our study population, especially among patients with symptoms. Thus, in our setting, during a high transmission period, at least 90% of those testing positive on Ag-RDTs were correctly identified as being infected with SARS-CoV-2. In many resource-limited settings, laboratory-based molecular diagnosis is not a viable alternative for efficiently detecting individuals infected with SARS-CoV-2. In these settings, Ag-RDTs are frequently the only option [29], even when or if they have a lower sensitivity. Their utilization, especially in high transmission periods, can effectively contribute to the identification of a high number of infected people, who would otherwise remain undiagnosed. For example, in a hypothetical population of 10,000 people with a positivity rate of 20%, the two evaluated Ag-RDTs would identify 826 and 900 positive cases for the Panbio and Standard Q, respectively.
5. Conclusions
Our results show that the evaluated rapid tests have great utility for SARS-CoV-2 diagnosis among symptomatic patients during high transmission periods. Laboratory-based molecular diagnosis with good sensitivity, as RT-PCR, will still be required for SARS-CoV-2 triage among contacts of positive cases, asymptomatic cases, and symptomatic patients with negative results in Ag-RDTs. Training and regular operator competency assessment must be considered for the successful implementation of Ag-RDTs in resource-limited settings.
Author Contributions
N.S.: Conceptualization, Methodology, Validation, Investigation, Writing—original draft, review and editing. J.S.: Investigation, Validation, Writing—original draft, review and editing. N.N.: Investigation, Writing—original draft, review and editing. J.C. (Jorfelia Chilaule): Investigation, Writing—original draft, review and editing. I.C.: Conceptualization, Methodology. C.M.: Investigation, Writing—review. O.L.: Conceptualization; Writing—original draft, review and editing. S.V.: Validation, Writing—review and editing. J.C. (Jane Cunningham): Writing—review and editing. I.J.: Conceptualization, Methodology, Validation, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported, in part, by the Bill & Melinda Gates Foundation #OPP1214435. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The other part of work was supported by World Health Organization (WHO), through the SARS-CoV-2 Antigen detecting rapid diagnostic test implementation projects.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional and National Health Bioethics Committees, with approval reference numbers 107/CIBS-INS/2020 of 20 December 2020 and 719/CNBS/2020 of 8 December 2020, respectively.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The datasets generated during this study are not publicly available due to the limitations of the participants’ consent forms.
Acknowledgments
We would like to acknowledge of the Virology laboratory staff and study teams at the health facilities for the patient recruitment, specimen collection and sample testing at the sites and laboratory. We also acknowledge Anésio Macicame, Júlio Rafael, Domingos Pedro and Zénia Matsinhe for assisting the study teams.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdullahi, I.N.; Emeribe, A.U.; Akande, A.O.; Ghamba, P.E.; Adekola, H.A.; Ibrahim, Y.; Dangana, A. Roles and Challenges of Coordinated Public Health Laboratory Response against COVID-19 Pandemic in Africa. J. Infect. Dev. Ctries. 2020, 14, 691–695. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Kiyaga, C.; Sendagire, H.; Joseph, E.; McConnell, I.; Grosz, J.; Narayan, V.; Esiru, G.; Elyanu, P.; Akol, Z.; Kirungi, W.; et al. Uganda’s New National Laboratory Sample Transport System: A Successful Model for Improving Access to Diagnostic Services for Early Infant HIV Diagnosis and Other Programs. PLoS ONE 2013, 8, e78609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favresse, J.; Gillot, C.; Oliveira, M.; Cadrobbi, J.; Elsen, M.; Eucher, C.; Laffineur, K.; Rosseels, C.; Van Eeckhoudt, S.; Nicolas, J.-B.; et al. Head-to-Head Comparison of Rapid and Automated Antigen Detection Tests for the Diagnosis of SARS-CoV-2 Infection. JCM 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.K.; Loh, T.P.; Wang, C.-B.; Mueller, R.; Koch, D.; Sethi, S.; Rawlinson, W.D.; Clementi, M.; Erasmus, R.; Leportier, M.; et al. IFCC Interim Guidelines on Serological Testing of Antibodies against SARS-CoV-2. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 2001–2008. [Google Scholar] [CrossRef]
- Iglὁi, Z.; Velzing, J.; Beek, J.; van Vijver, D.; van de Aron, G.; Ensing, R.; Benschop, K.; Han, W.; Boelsums, T.; Koopmans, M.; et al. Clinical Evaluation of the Roche/SD Biosensor Rapid Antigen Test with Symptomatic, Non-Hospitalized Patients in a Municipal Health Service Drive-through Testing Site. medRxiv 2020. [Google Scholar] [CrossRef]
- Bulilete, O.; Lorente, P.; Leiva, A.; Carandell, E.; Oliver, A.; Rojo, E.; Pericas, P.; Llobera, J. COVID-19 Primary Care Research Group PanbioTM Rapid Antigen Test for SARS-CoV-2 Has Acceptable Accuracy in Symptomatic Patients in Primary Health Care. J. Infect. 2021, 82, 391–398. [Google Scholar] [CrossRef]
- Haage, V.; Ferreira de Oliveira-Filho, E.; Moreira-Soto, A.; Kühne, A.; Fischer, C.; Sacks, J.A.; Corman, V.M.; Müller, M.A.; Drosten, C.; Drexler, J.F. Impaired Performance of SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Tests at Elevated and Low Temperatures. J. Clin. Virol. 2021, 138, 104796. [Google Scholar] [CrossRef]
- Fenollar, F.; Bouam, A.; Ballouche, M.; Fuster, L.; Prudent, E.; Colson, P.; Tissot-Dupont, H.; Million, M.; Drancourt, M.; Raoult, D.; et al. Evaluation of the Panbio COVID-19 Rapid Antigen Detection Test Device for the Screening of Patients with COVID-19. J. Clin. Microbiol. 2020, 59, e02589-20. [Google Scholar] [CrossRef]
- Scohy, A.; Anantharajah, A.; Bodéus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low Performance of Rapid Antigen Detection Test as Frontline Testing for COVID-19 Diagnosis. J. Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imöhl, M.; Kleines, M. Comparison of the SARS-CoV-2 Rapid Antigen Test to the Real Star SARS-CoV-2 RT PCR Kit. J. Virol. Methods 2021, 288, 114024. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wen, K.; Zhang, J.; Chen, J.; Han, C.; Chen, Y.; Wang, S.; Deng, G.; Zhou, H.; Wu, Y. Accuracy of a Nucleocapsid Protein Antigen Rapid Test in the Diagnosis of SARS-CoV-2 Infection. Clin. Microbiol. Infect. 2021, 27, 289.e1–289.e4. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.; Berenger, B.M.; Portnoy, D.; Scott, B.; Szelewicki, J.; Singh, T.; Venner, A.A.; Turnbull, L.; Pabbaraju, K.; Shokoples, S.; et al. Clinical Performance of the Abbott Panbio with Nasopharyngeal, Throat, and Saliva Swabs among Symptomatic Individuals with COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1721–1726. [Google Scholar] [CrossRef]
- WHO. WHO COVID-19: Case Definitions. WHO 2019-NCoV Surveillance Case Definition 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 (accessed on 25 April 2021).
- WHO SARS-CoV-2 Antigen Rapid Diagnostic Test Training Package. Available online: https://extranet.who.int/hslp/content/sars-cov-2-antigen-rapid-diagnostic-test-training-package (accessed on 27 August 2021).
- U.S. Food and Drug Administration Guidance for Industry and FDA Staff—Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests; US Food and Drug Administration: Silver Spring, MD, USA, 2007. Available online: https://www.fda.gov/media/71147/download (accessed on 27 August 2021).
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Strömer, A.; Rose, R.; Schäfer, M.; Schön, F.; Vollersen, A.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Performance of a Point-of-Care Test for the Rapid Detection of SARS-CoV-2 Antigen. Microorganisms 2020, 9, 58. [Google Scholar] [CrossRef]
- Khalid, M.F.; Selvam, K.; Jeffry, A.J.N.; Salmi, M.F.; Najib, M.A.; Norhayati, M.N.; Aziah, I. Performance of Rapid Antigen Tests for COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Nikolić, N.; Čabarkapa, V.; Turkulov, V.; Petrović, V. Validation of the STANDARD Q COVID-19 Antigen Test in Vojvodina, Serbia. PLoS ONE 2021, 16, e0247606. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Mboma, O.; Rieke, E.; Ahmad-Nejad, P.; Wirth, S.; Aydin, M. Diagnostic Performance of SARS-CoV-2 Rapid Antigen Test in a Large, German Cohort. Children 2021, 8, 682. [Google Scholar] [CrossRef]
- Jegerlehner, S.; Suter-Riniker, F.; Jent, P.; Bittel, P.; Nagler, M. Diagnostic Accuracy of a SARS-CoV-2 Rapid Antigen Test in Real-Life Clinical Settings. Int. J. Infect. Dis. 2021, 109, 118–122. [Google Scholar] [CrossRef]
- Torres, I.; Poujois, S.; Albert, E.; Colomina, J.; Navarro, D. Evaluation of a Rapid Antigen Test (PanbioTM COVID-19 Ag Rapid Test Device) for SARS-CoV-2 Detection in Asymptomatic Close Contacts of COVID-19 Patients. Clin. Microbiol. Infect. 2021, 27, 636.e1–636.e4. [Google Scholar] [CrossRef] [PubMed]
- Bours, M.J.L. Bayes’ Rule in Diagnosis. J. Clin. Epidemiol. 2021, 131, 158–160. [Google Scholar] [CrossRef]
- FDA. Potential for False Positive Results with Antigen Tests for Rapid Detection of SARS-CoV-2—Letter to Clinical Laboratory Staff and Health Care Providers. 2020. Available online: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory (accessed on 10 June 2021).
- Wikramaratna, P.S.; Paton, R.S.; Ghafari, M.; Lourenço, J. Estimating the False-Negative Test Probability of SARS-CoV-2 by RT-PCR. Eurosurveillance 2020, 25, 2000568. [Google Scholar] [CrossRef]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology Testing in the COVID-19 Pandemic Response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).