The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review
Abstract
1. Introduction
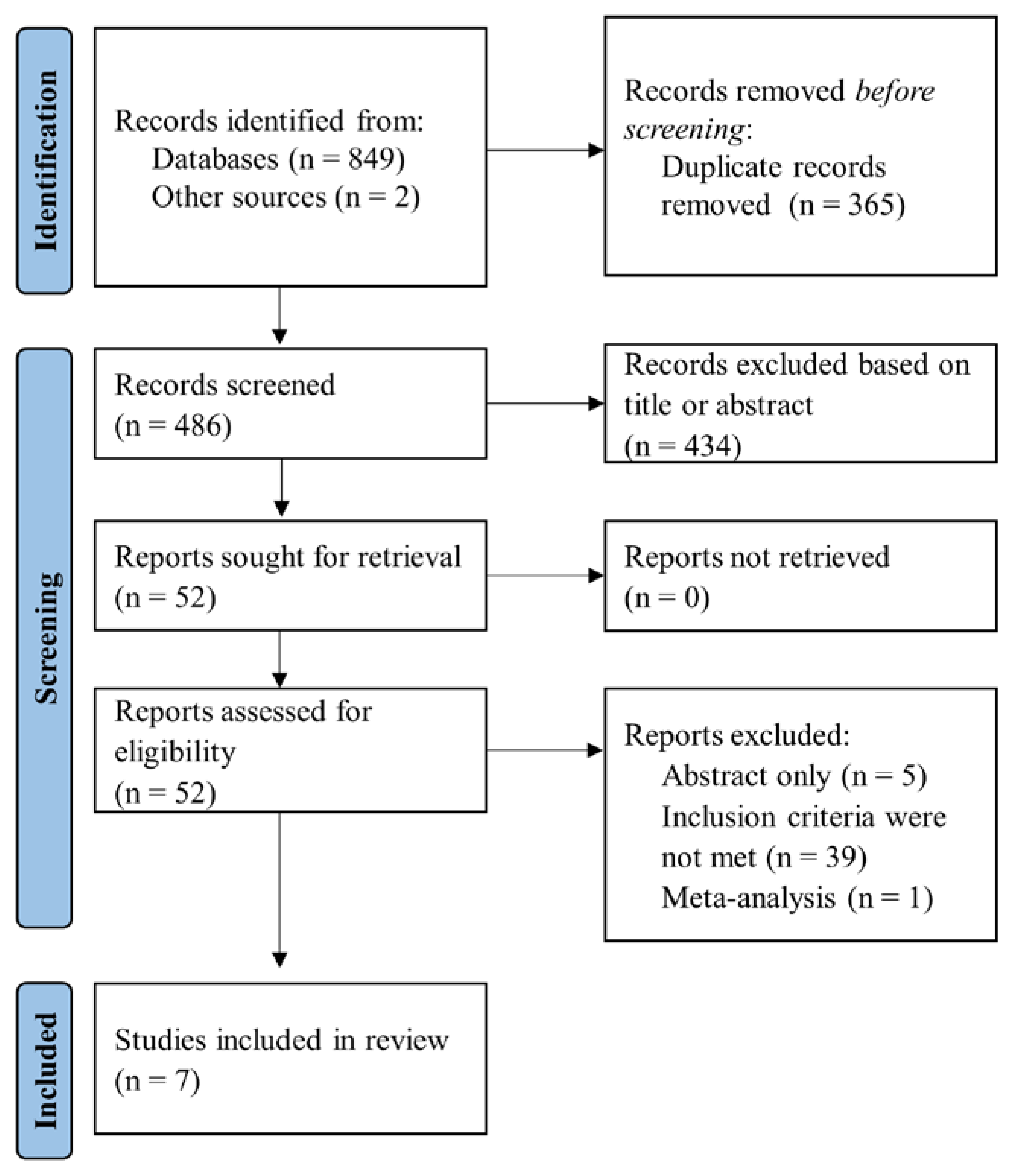
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria and Outcomes
2.3. Data Collection
2.4. Quality Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New Insights into the Role of Iron in Inflammation and Atherosclerosis. EBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Ellervik, C.; Marott, J.L.; Tybjærg-Hansen, A.; Schnohr, P.; Nordestgaard, B.G. Total and Cause-Specific Mortality by Moderately and Markedly Increased Ferritin Concentrations: General Population Study and Metaanalysis. Clin. Chem. 2014, 60, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Biddulph, J.P.; Rafnsson, S.B.; Trivella, M.; Nihoyannopoulos, P.; Demakakos, P. The Association of Ferritin with Cardiovascular and All-Cause Mortality in Community-Dwellers: The English Longitudinal Study of Ageing. PLoS ONE 2017, 12, e0178994. [Google Scholar] [CrossRef]
- Erdem, E.; Karatas, A.; Ecder, T. The Relationship between Serum Ferritin Levels and 5-Year All-Cause Mortality in Hemodialysis Patients. Blood Purif. 2022, 51, 55–61. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yokoyama, K.; Yokoo, T.; Shigematsu, T.; Iseki, K.; Tsubakihara, Y. The Different Association between Serum Ferritin and Mortality in Hemodialysis and Peritoneal Dialysis Patients Using Japanese Nationwide Dialysis Registry. PLoS ONE 2015, 10, e0143430. [Google Scholar] [CrossRef]
- Bai, Z.W.C.; Xing, Y.; Shao, B. Serum Ferritin and the Risk of Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis of Cross-Sectional Studies. Biomed. Environ. Sci. 2021, 34, 623–631. [Google Scholar]
- Sung, K.C.; Kang, S.M.; Cho, E.J.; Park, J.B.; Wild, S.H.; Byrne, C.D. Ferritin Is Independently Associated with the Presence of Coronary Artery Calcium in 12,033 Men. Arter. Thromb. Vasc. Biol. 2012, 32, 2525–2530. [Google Scholar] [CrossRef]
- Fischer, M.L.; Nordestgaard, B.G.; Schnohr, P.; Ellervik, C. Increased Ferritin Concentration and Risk of Atrial Fibrillation and Heart Failure in Men and Women: Three Studies of the Danish General Population Including 35799 Individuals. Clin. Chem. 2019, 65, 180–188. [Google Scholar]
- Knuiman, M.W.; Divitini, M.L.; Olynyk, J.K.; Cullen, D.J.; Bartholomew, H.C. Serum Ferritin and Cardiovascular Disease: A 17-Year Follow-up Study in Busselton, Western Australia. Am. J. Epidemiol. 2003, 158, 144–149. [Google Scholar] [CrossRef]
- Holay, M.P.; Choudhary, A.A.; Suryawanshi, S.D. Serum Ferritin-a Novel Risk Factor in Acute Myocardial Infarction. Indian Heart J. 2012, 64, 173–177. [Google Scholar] [CrossRef]
- Reinhold, J.; Papadopoulou, C.; Baral, R.; Vassiliou, V.S. Iron Deficiency for Prognosis in Acute Coronary Syndrome—A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2021, 328, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.; Yang, Z.; Huang, D.; Weng, H.; Zeng, X. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Arroyo-Ucar, E.; Avanzas, P. Serum Ferritin Deficiency and Major Adverse Cardiovascular Events after Primary Percutaneous Coronary Intervention in Patients with St-Elevation Myocardial Infarction without Anemia. Int. J. Cardiol. 2013, 168, 4914–4916. [Google Scholar] [CrossRef]
- Cosentino, N.; Campodonico, J.; Pontone, G.; Guglielmo, M.; Trinei, M.; Sandri, M.T.; Riggio, D.; Baggiano, A.; Milazzo, V.; Moltrasio, M.; et al. Iron Deficiency in Patients with St-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Int. J. Cardiol. 2020, 300, 14–19. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Q.; Chen, G.; Ye, D.; Xu, W. Impaired Renal Function and Abnormal Level of Ferritin Are Independent Risk Factors of Left Ventricular Aneurysm after Acute Myocardial Infarction: A Hospital-Based Case-Control Study. Medicine 2018, 97, e12109. [Google Scholar] [CrossRef]
- Suzuki, T.; Toba, K.; Kato, K.; Ozawa, T.; Higasimura, M.; Kitajima, T.; Oda, H.; Tsuchida, K.; Tomosugi, N.; Saitoh, H.; et al. Serum Ferritin Levels Adversely Affect Cardiac Function in Patients with St-Elevation Myocardial Infarction Who Underwent Successful Percutaneous Coronary Intervention. Int. J. Cardiol. 2012, 167, 286–288. [Google Scholar] [CrossRef]
- Basu, A.; Seth, S.; Arora, K.; Bansal, N. Levels of Thyroid Hormone, Ferritin and Testosterone in Acute Myocardial Infarction (Ami) Patients in North India. J. Cardiovasc. Dis. Res. 2014, 5, 15–21. [Google Scholar] [CrossRef][Green Version]
- Singh, S. Short Term Prognostic Value of Serum Ferritin on Acute Myocardial Infarction. Int. J. Med. Biomed. Stud. 2021, 5, 40–42. [Google Scholar]
- Malthesh, M.; Gosavi, S.; Shastry, S.; Rajesh, R.; Vaishnav, P.; Maruthi, M. Relationship of Acute St-Elevation Myocardial Infarction with Hs-Crp and Serum Iron Profile in Southern India: A Cross-Sectional Study. J. Clin. Diagn. Res. 2020, 14, OC05–OC08. [Google Scholar] [CrossRef]
- Rizzo, C.; Carbonara, R.; Ruggieri, R.; Passantino, A.; Scrutinio, D. Iron Deficiency: A New Target for Patients with Heart Failure. Front. Cardiovasc. Med. 2021, 8, 908. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 Esc Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (Esc) with the Special Contribution of the Heart Failure Association (Hfa) of the Esc. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 Esc Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with St-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with St-Segment Elevation of the European Society of Cardiology (Esc). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 Esc Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent St-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Pourmoghaddas, A.; Sanei, H.; Garakyaraghi, M.; Esteki-Ghashghaei, F.; Gharaati, M. The Relation between Body Iron Store and Ferritin, and Coronary Artery Disease. ARYA Atheroscler. 2014, 10, 32–36. [Google Scholar]
- Khalili, A.; Ghorbanihaghjo, A.; Rashtchizadeh, N.; Gaffari, S. Association between Serum Ferritin and Circulating Oxidized Low-Density Lipoprotein Levels in Patients with Coronary Artery Disease. J. Cardiovasc. Thorac. Res. 2012, 4, 1–4. [Google Scholar]
- Khan, A.; Khan, W.M.; Ayub, M.; Humayun, M.; Haroon, M. Ferritin Is a Marker of Inflammation Rather Than Iron Deficiency in Overweight and Obese People. J. Obes. 2016, 2016, 1937320. [Google Scholar] [CrossRef]
| Author, Year | Design | Patients No | Age, Mean/Median ± SD | Setting | Parameters | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|---|
| Dominguez-Rodriguez et al., 2013 | Observational, single centre | 258 | 64 ± 13—patients with MACE | Patients with STEMI treated with primary PCI within 6 h from symptoms onset | Low ferritin concentration (one assessment) | MACE (cardiovascular death, non-fatal myocardial infarction, re-admission for unstable angina) | 30-days |
| 65 ± 12—patients without MACE | |||||||
| Cosentino et al., 2020 | Observational, single centre, prospective | 420 | 65 ± 12 | Consecutive patients with STEMI treated with primary PCI | Low ferritin values (<100 µg/L) or transferrin saturation < 20% | Composite of in-hospital mortality and Killip class ≥ 3 | In-hospital |
| Feng et al., 2018 | Observational, single centre, case-control | 60—cases (AMI with LVA) | 67.3 ± 11.8—patients with LVA | Patients with AMI with LVA | Patients were divided in three groups according to ferritin values: group 1 (<7.0 ng/mL), group 2 (7.0–323.0 ng/mL), group 3 (>323.0 ng/mL) | LVA formation | - |
| 133—controls (AMI without LVA) | 66.03 ± 12.83—patients without LVA | ||||||
| Suzuki et al., 2012 | Observational, prospective | 53 | 66 | Patients with STEMI with successful PCI within 24 h from symptoms onset | Ferritin levels were stratified in tertiles: <100 ng/mL, 100–200 ng/mL, >200 ng/mL | LVEF at baseline and during follow-up | 6 months |
| Basu et al., 2014 | Observational, cross-sectional, single centre | 43—patients with AMI | 50.7 ± 15.6—patients with AMI | Male patients with AMI | Ferritin concentration | LVEF < 35% vs. LVEF 35–50% | - |
| 40—patients without AMI | 47.9 ± 18.3—patients without AMI | ||||||
| Singh et al., 2021 | Observational | 150 | - | Patients with STEMI (n = 50), NSTEMI (n = 50) and healthy controls (n = 50) | Ferritin levels were stratified in tertiles: <120 ng/mL, 120–220 ng/mL, >220 ng/mL | (a) LVEF (b) Killip class (c) mortality (d) recurrent angina | In-hospital |
| Malthesh et al., 2020 | Observational, cross-sectional | 45 | 63.4 ± 11.8 | Patients with STEMI | Ferritin concentration | (a) duration of hospitalization (b) mortality | In-hospital |
| Study | Outcomes | Results | |
|---|---|---|---|
| Dominguez-Rodriguez, 2013 | MACE at 30-days | Low ferritin values | |
| OR 1.003 (95% CI, 1.001–1.006)—multivariate analysis | p = 0.01 | ||
| AUC 0.65 (95% CI, 0.562–0.753) | p = 0.001 | ||
| Ferritin cut-off 83 ng/mL: sensitivity 86%, specificity 69% | |||
| Cosentino, 2019 | Composite of in-hospital mortality and Killip class ≥ 3 | Ferritin < 100 µg/L or transferrin saturation < 20% | |
| Unadjusted OR 0.48 (95% CI, 0.28–0.87) | p = 0.01 | ||
| Adjusted OR 0.50 (95% CI, 0.27–0.93) | p = 0.02 | ||
| Feng et al., 2018 | LVA formation | Low or high levels of ferritin | |
| OR 1.151 (95% CI, 1.050–1.252)—adjusted for multiple variables | p = 0.042 | ||
| Suzuki et al., 2012 | LVEF at baseline | No significant differences were observed across ferritin concentrations | |
| LVEF decline during follow-up | LVEF decline was accentuated in patients with ferritin > 200 ng/mL vs. patients with ferritin < 100 ng/mL | p < 0.01 | |
| LVEF decline was accentuated in patients with ferritin > 200 ng/mL vs. patients with ferritin 100–200 ng/mL | p < 0.05 | ||
| Basu et al., 2014 | LVEF < 35% vs. LVEF 35–50% | Ferritin 211.38 ± 52.66 ng/mL vs. ferritin 167.27 ± 30.05 ng/mL | p = 0.002 |
| Singh et al., 2021 | Ferritin < 120 ng/mL vs. ferritin 120–220 ng/mL vs. ferritin > 220 ng/mL | ||
| LVEF < 35% | 2 patients vs. 4 patients vs. 9 patients | p = 0.01 | |
| Recurrent angina | 2 patients vs. 10 patients vs. 8 patients | p = 0.09 | |
| Heart failure | 4 patients vs. 6 patients vs. 8 patients | p = 0.1 | |
| Death | 1 patient vs. 2 patients vs. 5 patients | p = 0.03 | |
| Malthesh et al., 2020 | Duration of hospitalization | Spearman’s R coefficient 0.38 | p = 0.01 |
| Mortality | Ferritin 214.2 ± 157.3 µg/dL (deceased patients) vs. ferritin 126.6 ± 93.0 µg/dL (survivors) | p = 0.15 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, C.; Floria, M.; Popa, I.V.; Burlacu, A. The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review. Diagnostics 2022, 12, 476. https://doi.org/10.3390/diagnostics12020476
Brinza C, Floria M, Popa IV, Burlacu A. The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review. Diagnostics. 2022; 12(2):476. https://doi.org/10.3390/diagnostics12020476
Chicago/Turabian StyleBrinza, Crischentian, Mariana Floria, Iolanda Valentina Popa, and Alexandru Burlacu. 2022. "The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review" Diagnostics 12, no. 2: 476. https://doi.org/10.3390/diagnostics12020476
APA StyleBrinza, C., Floria, M., Popa, I. V., & Burlacu, A. (2022). The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review. Diagnostics, 12(2), 476. https://doi.org/10.3390/diagnostics12020476








