Abstract
Atrial fibrillation and cerebral embolism are known to increase the risk of hemorrhagic transformation (HT). In addition, a sufficient number of collateral vessels in acute ischemic stroke can maintain the ischemic penumbra and prevent progression to the ischemic core, while an insufficient number of collateral vessels increase the HT risk after therapeutic recanalization. In this case, when the middle cerebral artery is recanalized, reperfusion injury may occur in the basal ganglia due to insufficient collateral vessels.
A 68-year-old man was admitted to the emergency room with left hemiparesis, asomatognosia, and dysarthria within 1 h of symptoms onset. Atrial fibrillation was confirmed based on the electrocardiogram test performed at the time of admission. Brain computed tomography (CT) angiography showed occlusion of the distal portion of the right middle cerebral artery (MCA) (Figure 1A). CT perfusion images showed perfusion mismatch in the right MCA territory, and intravenous tissue plasminogen activator (t-PA) was administered (Figure 1B). After performing digital subtraction angiography (DSA) for intra-arterial thrombectomy, the right MCA showed recanalization (Figure 1C). Diffusion-weighted magnetic resonance imaging (MRI) of the brain performed on the day following admission showed diffuse infarction in the right MCA territory, and the susceptibility-weighted image (SWI) revealed hemorrhagic transformation (HT) of the right lenticulostriate arterial (LSA) territory (Figure 1D,E). On the day after admission, all other neurological symptoms had recovered, except for left hemiparesis.

Figure 1.
Radiologic findings of the patient. (A) Brain computed tomography angiography revealed distal occlusion of the right middle cerebral artery. (B) Severe cerebral perfusion mismatch observed in the right hemisphere. (C) Digital subtraction angiography showed recanalization of the right middle cerebral artery. (D) Brain magnetic resonance imaging showed ischemic infarction in the right cerebral hemisphere, including the lenticulostriate artery territory. (E) Susceptibility-weighted imaging revealed hemorrhagic transformation in the lenticulostriate artery territory.
Patients with atrial fibrillation and cerebral embolism are relatively more unstable than those with atherosclerotic plaques and are at a high HT risk due to reperfusion injury [1]. Therefore, the clot often moves distally from the initial occlusion site or the artery reopens due to spontaneous lysis. In addition, a sufficient number of collateral vessels in acute ischemic stroke can maintain the ischemic penumbra and prevent progression to the ischemic core, while an insufficient number of collateral vessels can increase the HT risk after therapeutic recanalization [2]. In this patient, brain CT angiography at the time of admission showed obstruction of the distal MCA. Brain MRI performed the next day showed HT using an SWI sequence in the LSA territory of the proximal site rather than the occlusion site. This phenomenon suggests that the cardiac emboli caused by atrial fibrillation blocked the proximal portion of the MCA and occluded the entrance of the LSA initially. Following this, a spontaneous process of endogenous clot lysis occurred, and the emboli migrated to the distal MCA site [3]. Therefore, distal MCA occlusion may have been observed on brain CT angiography at the time of admission. When the occluded MCA recanalizes spontaneously, the LSA territory, which does not contain sufficient number of collateral vessels, usually suffers reperfusion injury. Recanalization of the distal MCA by intravenous t-PA administration causes further progression of reperfusion injury and HT. In contrast, the distal MCA site, which contains a sufficient number of collateral vessels, could retain the ischemic penumbra when recanalization was performed by t-PA administration, suggesting that the reperfusion injury was not severe (Figure 2) [2,3].
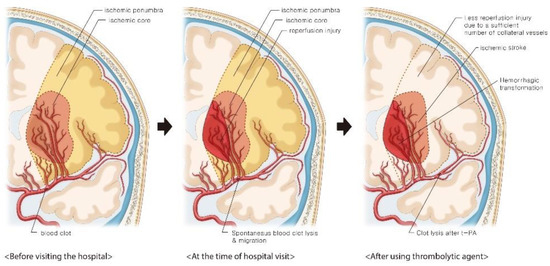
Figure 2.
Illustration of the hemorrhagic transformation after intravenous thrombolysis.
HT in the basal ganglia is likely to cause parenchymal hematoma; hence, blood pressure should be monitored in advance. When the intracranial artery is recanalized, reperfusion injury may occur in small perforating arteries. Thus, if there is spontaneous recanalization of the distal M1 occlusion, the possibility of HT in the M1 proximal segment should be considered. Of course, each case has different patient conditions (e.g., occlusion site, duration of vessel occlusion, or collateral status), so it is recommended to refer to the conclusion of this case when treating similar patients.
Author Contributions
Conceptualization, S.H.Y. and H.G.K.; methodology, C.-H.L. and H.G.K.; software, B.-S.S.; validation, B.-S.S. and H.G.K.; investigation, C.-H.L.; resources, S.H.Y.; writing—original draft preparation, S.H.Y. and C.-H.L.; writing—review and editing, H.G.K.; visualization, H.G.K.; supervision, B.-S.S.; funding acquisition, S.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Wonkwang Grant in 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethical.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Nogueira, R.G.; Gupta, R.; Jovin, T.G.; Levy, E.I.; Liebeskind, D.S.; Zaidat, O.O.; Rai, A.; Hirsch, J.A.; Hsu, D.P.; Rymer, M.M.; et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: A multicenter retrospective analysis of 1122 patients. J. Neurointerv. Surg. 2015, 7, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, O.Y.; Saver, J.L.; Kim, S.J.; Kim, G.; Chung, C.; Ovbiagele, B.; Lee, K.H.; Liebeskind, D.S.; UCLA-Samsung Stroke Collaborators. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011, 42, 2235–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.; Adams, R.D. Observations on brain embolism with special reference to the mechanism of hemorrhagic infarction. J. Neuropathol. Exp. Neurol. 1951, 10, 92–94. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).