A New Preoperative Scoring System for Predicting Aggressiveness of Non-Functioning Pancreatic Neuroendocrine Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection and Candidate Variables
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Characeteristics of the Enrolled Patients
3.2. Univariate and Multivariate Stepwise Logistic Regression Analyses of Candidate Variables
3.3. Development of a Scoring System
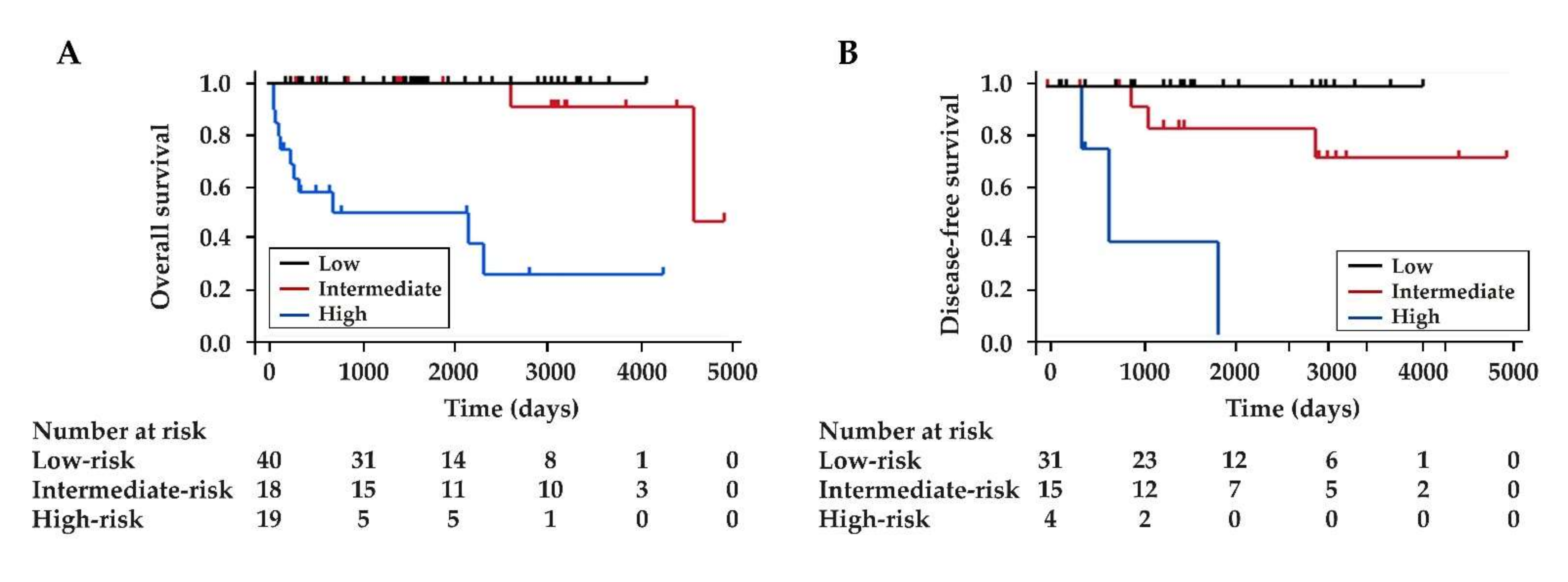
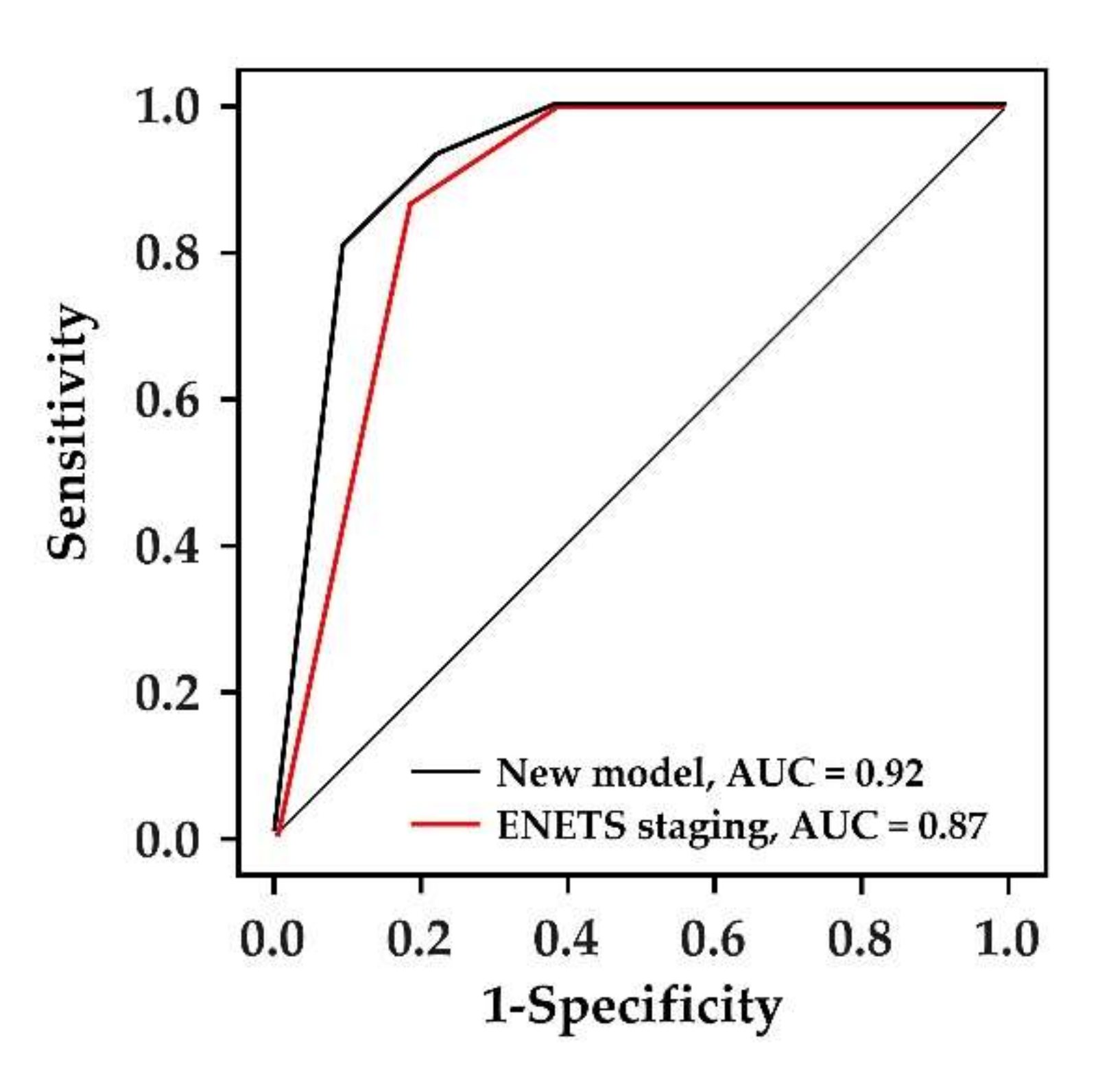
3.4. The Comparison between the New Scoring System and ENETS TNM Staging System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Kloppel, G.; et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: Are there global differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; He, C.; Sun, T.; Yan, Y.; Che, G.; Li, X.; Sun, H.; Ma, H. Trends in incidence and survival of patients with pancreatic neuroendocrine neoplasm, 1987-2016. J. Oncol. 2021, 2021, 11. [Google Scholar] [CrossRef]
- Kuo, E.J.; Salem, R.R. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann. Surg. Oncol. 2013, 20, 2815–2821. [Google Scholar] [CrossRef]
- Ji, S.; Liu, M.; Xia, H.; Ye, Z.; Xu, X.; Yu, X.; Zhuo, Q. Management of small nonfunctioning pancreatic neuroendocrine tumors: An analysis of the US surveillance, epidemiology, and end results database. Pancreas 2021, 50, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Stensbøl, A.B.; Krogh, J.; Holmager, P.; Klose, M.; Oturai, P.; Kjaer, A.; Hansen, C.P.; Federspiel, B.; Langer, S.W.; Knigge, U.; et al. Incidence, clinical presentation and trends in indication for diagnostic work-up of small intestinal and pancreatic neuroendocrine tumors. Diagnostics 2021, 11, 2030. [Google Scholar] [CrossRef]
- Ishii, T.; Katanuma, A.; Toyonaga, H.; Chikugo, K.; Nasuno, H.; Kin, T.; Hayashi, T.; Takahashi, K. Role of endoscopic ultrasound in the diagnosis of pancreatic neuroendocrine neoplasms. Diagnostics 2021, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Grimelius, L.; Hultquist, G.T.; Stenkvist, B. Cytological differentiation of asymptomatic pancreatic islet cell tumours in autopsy material. Virchows Arch. A Pathol. Anat. Histol. 1975, 365, 275–288. [Google Scholar] [CrossRef]
- Kimura, W.; Kuroda, A.; Morioka, Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig. Dis. Sci. 1991, 36, 933–942. [Google Scholar] [CrossRef]
- Bettini, R.; Partelli, S.; Boninsegna, L.; Capelli, P.; Crippa, S.; Pederzoli, P.; Scarpa, A.; Falconi, M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011, 150, 75–82. [Google Scholar] [CrossRef]
- Slagter, A.E.; Ryder, D.; Chakrabarty, B.; Lamarca, A.; Hubner, R.A.; Mansoor, W.; O’Reilly, D.A.; Fulford, P.E.; Klümpen, H.J.; Valle, J.W.; et al. Prognostic factors for disease relapse in patients with neuroendocrine tumours who underwent curative surgery. Surg. Oncol. 2016, 25, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Youn, S.; Kwon, W.; Jang, K.T.; Han, S.; Han, S.; You, Y.; Heo, J.S.; Choi, S.H.; Choi, D.W. Prognostic factors of non-functioning pancreatic neuroendocrine tumor revisited: The value of WHO 2010 classification. Ann. Hepatobiliary Pancreat Surg 2018, 22, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Partelli, S.; Maire, F.; D’Onofrio, M.; Larroque, B.; Tamburrino, D.; Sauvanet, A.; Falconi, M.; Ruszniewski, P. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2013, 98, 4784–4789. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.M.; Friedmann, P.; del Rivero, J.; Libutti, S.K.; Laird, A.M. Resection versus expectant management of small incidentally discovered nonfunctional pancreatic neuroendocrine tumors. Surgery 2016, 159, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kurita, Y.; Hara, K.; Kuwahara, T.; Mizuno, N.; Okuno, N.; Haba, S.; Okuno, M.; Natsume, S.; Senda, Y.; Kubota, K.; et al. Comparison of prognosis between observation and surgical resection groups with small sporadic non-functional pancreatic neuroendocrine neoplasms without distant metastasis. J. Gastroenterol. 2020, 55, 543–552. [Google Scholar] [CrossRef]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: Surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 4.2021, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1448 (accessed on 25 December 2021).
- Sharpe, S.M.; In, H.; Winchester, D.J.; Talamonti, M.S.; Baker, M.S. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J. Gastrointest. Surg. 2015, 19, 117–123. [Google Scholar] [CrossRef]
- Mills, L.; Drymousis, P.; Vashist, Y.; Burdelski, C.; Prachalias, A.; Srinivasan, P.; Menon, K.; Cotoi, C.; Khan, S.; Cave, J.; et al. Tumour diameter is not reliable for management of non-secreting pancreatic neuroendocrine tumours. Endocr. Connect. 2017, 6, 876–885. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Talamonti, M.S.; Tomlinson, J.S.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y.; Bentrem, D.J. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: Analysis of 3851 patients. Ann. Surg. 2008, 247, 490–500. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhan, H.; Wang, L.; Xu, J.; Zhang, G.; Zhang, Z.; Hu, S. Analysis of 100 consecutive cases of resectable pancreatic neuroendocrine neoplasms: Clinicopathological characteristics and long-term outcomes. Front. Med. 2016, 10, 444–450. [Google Scholar] [CrossRef]
- Zhou, B.; Duan, J.; Yan, S.; Zhou, J.; Zheng, S. Prognostic factors of long-term outcome in surgically resectable pancreatic neuroendocrine tumors: A 12-year experience from a single center. Oncol. Lett. 2017, 13, 1157–1164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Y.; Gao, H.; Wang, G.; Yin, L.; Xu, W.; Peng, Y.; Wu, J.; Jiang, K.; Miao, Y. A meta-analysis of prognostic factor of pancreatic neuroendocrine neoplasms. Sci. Rep. 2018, 8, 7271. [Google Scholar] [CrossRef] [PubMed]
- Genç, C.G.; Jilesen, A.P.; Partelli, S.; Falconi, M.; Muffatti, F.; van Kemenade, F.J.; van Eeden, S.; Verheij, J.; van Dieren, S.; van Eijck, C.H.J.; et al. A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann. Surg. 2018, 267, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.Y.; Lopez-Aguiar, A.G.; Switchenko, J.M.; Lipscomb, J.; Andreasi, V.; Partelli, S.; Gamboa, A.C.; Lee, R.M.; Poultsides, G.A.; Dillhoff, M.; et al. A novel validated recurrence risk score to guide a pragmatic surveillance strategy after resection of pancreatic neuroendocrine tumors: An international study of 1006 patients. Ann. Surg. 2019, 270, 422–433. [Google Scholar] [CrossRef]
- Ge, L.; Li, H.; Dong, L.; Shang, G.; Wang, W.; Li, Y.; Qi, L.; Zhao, J.; Peng, D.; Tong, G. Nomogram for predicting survival of patients with metastatic nonfunctioning pancreatic neuroendocrine tumors: A SEER based study. Medicine 2021, 100, e26347. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, W.H.; Gao, H.L.; Huang, D.; Xu, H.X.; Li, S.; Li, T.J.; Xu, S.S.; Li, H.; Long, J.; et al. A novel risk factor panel predicts early recurrence in resected pancreatic neuroendocrine tumors. J. Gastroenterol. 2021, 56, 395–405. [Google Scholar] [CrossRef]
- Canellas, R.; Burk, K.S.; Parakh, A.; Sahani, D.V. Prediction of pancreatic neuroendocrine tumor grade based on CT features and texture analysis. AJR Am. J. Roentgenol. 2018, 210, 341–346. [Google Scholar] [CrossRef]
- Nanno, Y.; Matsumoto, I.; Zen, Y.; Otani, K.; Uemura, J.; Toyama, H.; Asari, S.; Goto, T.; Ajiki, T.; Okano, K.; et al. Pancreatic duct involvement in well-differentiated neuroendocrine tumors is an independent poor prognostic factor. Ann. Surg. Oncol. 2017, 24, 1127–1133. [Google Scholar] [CrossRef]
- Yamada, S.; Fujii, T.; Suzuki, K.; Inokawa, Y.; Kanda, M.; Nakayama, G.; Sugimoto, H.; Koike, M.; Nomoto, S.; Fujiwara, M.; et al. Preoperative identification of a prognostic factor for pancreatic neuroendocrine tumors using multiphase contrast-enhanced computed tomography. Pancreas 2016, 45, 198–203. [Google Scholar] [CrossRef]
- Sallinen, V.J.; le Large, T.Y.S.; Tieftrunk, E.; Galeev, S.; Kovalenko, Z.; Haugvik, S.P.; Antila, A.; Franklin, O.; Martinez-Moneo, E.; Robinson, S.M.; et al. Prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors—A multi-institutional study. HPB 2018, 20, 251–259. [Google Scholar] [CrossRef]
- Karmazanovsky, G.; Belousova, E.; Schima, W.; Glotov, A.; Kalinin, D.; Kriger, A. Nonhypervascular pancreatic neuroendocrine tumors: Spectrum of MDCT imaging findings and differentiation from pancreatic ductal adenocarcinoma. Eur. J. Radiol. 2019, 110, 66–73. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Johnbeck, C.B.; Loft, A.; Berthelsen, A.K.; Oturai, P.; Mortensen, J.; Federspiel, B.; Langer, S.W.; Kjaer, A. 18F-FDG PET is superior to WHO grading as a prognostic tool in neuroendocrine neoplasms and useful in guiding PRRT: A prospective 10-year follow-up study. J. Nucl. Med. 2021, 62, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Boutsen, L.; Jouret-Mourin, A.; Borbath, I.; van Maanen, A.; Weynand, B. Accuracy of pancreatic neuroendocrine tumour grading by endoscopic ultrasound-guided fine needle aspiration: Analysis of a large cohort and perspectives for improvement. Neuroendocrinology 2018, 106, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Khanna, L.G.; Saqi, A.; Crapanzano, J.P.; Mitchell, J.M.; Sethi, A.; Gonda, T.A.; Kluger, M.D.; Schrope, B.A.; Allendorf, J.; et al. The role of endoscopic ultrasound-guided ki67 in the management of non-functioning pancreatic neuroendocrine tumors. Clin. Endosc. 2020, 53, 213–220. [Google Scholar] [CrossRef]
- Kloppel, G.; la Rosa, S. Ki67 labeling index: Assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018, 472, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Genç, C.G.; Falconi, M.; Partelli, S.; Muffatti, F.; van Eeden, S.; Doglioni, C.; Klümpen, H.J.; van Eijck, C.H.J.; Nieveen van Dijkum, E.J.M. Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann. Surg. Oncol. 2018, 25, 2467–2474. [Google Scholar] [CrossRef]
- Aysal, A.; Agalar, C.; Egeli, T.; Unek, T.; Oztop, I.; Obuz, F.; Sagol, O. Reconsideration of clinicopathologic prognostic factors in pancreatic neuroendocrine tumors for better determination of adverse prognosis. Endocr. Pathol. 2021, 32, 461–472. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Alhman, H.; Caplin, M.; Couvelard, A.; de Herder, W.W.; Erikssson, B.; Falchetti, A.; Falconi, M.; Komminoth, P.; et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006, 449, 395–401. [Google Scholar] [CrossRef]
- Ricci, C.; Taffurelli, G.; Campana, D.; Ambrosini, V.; Pacilio, C.A.; Pagano, N.; Santini, D.; Brighi, N.; Minni, F.; Casadei, R. Is surgery the best treatment for sporadic small (≤2 cm) non-functioning pancreatic neuroendocrine tumours? A single centre experience. Pancreatology 2017, 17, 471–477. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kamata, K.; Hara, A.; Tanaka, H.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Minaga, K.; Yamao, K.; et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for predicting the prognosis of pancreatic neuroendocrine neoplasms. Dig. Endosc. 2021, 33, 829–839. [Google Scholar] [CrossRef]
- Belousova, E.; Karmazanovsky, G.; Kriger, A.; Kalinin, D.; Mannelli, L.; Glotov, A.; Karelskaya, N.; Paklina, O.; Kaldarov, A. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: Correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin. Radiol. 2017, 72, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A scoring system to stratify curability after endoscopic submucosal dissection for early gastric cancer: “eCura System”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, T.; Hatta, W.; Hikichi, T.; Fukuda, S.; Mikami, T.; Tatsuta, T.; Nakamura, J.; Abe, Y.; Onozato, Y.; Ogata, Y.; et al. A simple prediction score for in-hospital mortality in patients with nonvariceal upper gastrointestinal bleeding. J. Gastroenterol. 2021, 56, 758–768. [Google Scholar] [CrossRef]
- Mehta, H.B.; Mehta, V.; Girman, C.J.; Adhikari, D.; Johnson, M.L. Regression coefficient-based scoring system should be used to assign weights to the risk index. J. Clin. Epidemiol. 2016, 79, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, G.A.; Ambrosetti, M.C.; Zivelonghi, C.; Lombardo, F.; Butturini, G.; Cingarlini, S.; Capelli, P.; Pozzi Mucelli, R. Solid non-functioning endocrine tumors of the pancreas: Correlating computed tomography and pathology. HPB 2017, 19, 986–991. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, H.S.; Lee, K.J.; Jang, S.I.; Lee, W.J.; Hwang, J.H.; Cho, C.M.; Park, C.H.; Han, J.; Woo, S.M.; et al. Malignant potential of small pancreatic neuroendocrine neoplasm and its risk factors: A multicenter nationwide study. Pancreatology 2021, 21, 208–214. [Google Scholar] [CrossRef]
- Crinó, S.F.; Brandolese, A.; Vieceli, F.; Paiella, S.; Conti Bellocchi, M.C.; Manfrin, E.; Bernardoni, L.; Sina, S.; D’Onofrio, M.; Marchegiani, G.; et al. Endoscopic ultrasound features associated with malignancy and aggressiveness of nonhypovascular solid pancreatic lesions: Results from a prospective observational study. Ultraschall Med. 2021, 42, 167–177. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, Y.; An, S.; Kim, S.J.; Kim, J.Y.; Kim, S.Y.; Hwang, D.W.; Park, D.H.; Lee, S.S.; Kim, S.C.; et al. Grading by the Ki-67 labeling index of endoscopic ultrasound-guided fine needle aspiration biopsy specimens of pancreatic neuroendocrine tumors can be underestimated. Pancreas 2018, 47, 1296–1303. [Google Scholar] [CrossRef]
- Unno, J.; Kanno, A.; Masamune, A.; Kasajima, A.; Fujishima, F.; Ishida, K.; Hamada, S.; Kume, K.; Kikuta, K.; Hirota, M.; et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the world health organization classification. Scand. J. Gastroenterol. 2014, 49, 1367–1374. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Rota, R.; Valenti, M.; Elio, G.; Crinò, S.F.; Manfrin, E.; Parisi, A.; Cingarlini, S.; D’Onofrio, M.; et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: A Retrospective analysis of 110 cases. Endoscopy 2020, 52, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.; Zhou, Q.Y.; Fan, B. Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e0207. [Google Scholar] [CrossRef]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.V.; Lopez-Aguiar, A.G.; Rendell, V.R.; Pokrzywa, C.; Rocha, F.G.; Kanji, Z.S.; Poultsides, G.A.; Makris, E.A.; Dillhoff, M.E.; Beal, E.W.; et al. Predictive value of chromogranin a and a pre-operative risk score to predict recurrence after resection of pancreatic neuroendocrine tumors. J. Gastrointest. Surg. 2019, 23, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Primavesi, F.; Andreasi, V.; Hoogwater, F.J.H.; Partelli, S.; Wiese, D.; Heidsma, C.; Cardini, B.; Klieser, E.; Marsoner, K.; Fröschl, U.; et al. A preoperative clinical risk score including c-reactive protein predicts histological tumor characteristics and patient survival after surgery for sporadic non-functional pancreatic neuroendocrine neoplasms: An international multicenter cohort study. Cancers 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Rizzatti, G.; Rimbaş, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G. EUS-guided radiofrequency ablation as an alternative to surgery for pancreatic neuroendocrine neoplasms: Who should we treat? Endosc. Ultrasound 2019, 8, 220–226. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A Simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]

| Variables | NF-PanNENs (n = 77) |
|---|---|
| Age, mean (SD), years | 61.1 (12.9) |
| Sex, male, n (%) | 38 (49.4) |
| Median tumor size, mm (IQR) | 18 (12–34) |
| Symptoms, yes, n (%) | 26 (33.8) |
| Tumor location, n (%) | |
| Head | 30 (39.0) |
| Body/Tail | 44 (57.1) |
| Multiple | 3 (3.9) |
| Lymph node metastasis, n (%) | 19 (24.7) |
| Distant metastasis, n (%) | 16 (20.8) |
| Tumor grade (WHO 2017), n (%) | |
| G1 | 38 (49.4) |
| G2 | 26 (33.8) |
| NET G3 | 3 (3.9) |
| NEC G3 | 10 (13.0) |
| ENETS Stage, n (%) | |
| I | 42 (54.5) |
| II | 11 (14.3) |
| III | 8 (10.4) |
| IV | 16 (20.8) |
| Treatment, n (%) | |
| Surgery | 54 (70.1) |
| Chemotherapy | 14 (18.2) |
| Surveillance | 8 (10.4) |
| Best supportive care | 1 (1.3) |
| Overall survival rate (%) | |
| 5-year OS rate | 85.9 |
| 10-year OS rate | 76.3 |
| Median follow-up period, days (IQR) | 1636 (568–3024) |
| Variables | Non-Aggressive Group (n = 46) | Aggressive Group (n = 31) | p Value |
|---|---|---|---|
| Age, mean (SD), years | 63.3 (11.5) | 57.9 (14.3) | 0.07 |
| Sex, male, n (%) | 22 (47.8) | 16 (51.6) | 0.74 |
| Median tumor size, mm (IQR) | 14 (9–18) | 38 (20–53) | <0.001 |
| Tumor grade (WHO 2017), n (%) | <0.001 | ||
| G1 | 35 (76.1) | 3 (9.7) | |
| G2 | 11 (23.9) | 15 (48.4) | |
| NET G3 | 0 (0) | 3 (9.7) | |
| NEC G3 | 0 (0) | 10 (32.3) | |
| ENETS Stage, n (%) | <0.001 | ||
| I | 40 (87.0) | 2 (6.5) | |
| II | 6 (13.0) | 5 (16.1) | |
| III | 0 (0) | 8 (25.8) | |
| IV | 0 (0) | 16 (51.6) | |
| Treatment, n (%) | <0.001 | ||
| Surgery | 37 (80.4) | 17 (54.8) | |
| Chemotherapy | 1 (2.2) | 13 (41.9) | |
| Surveillance | 8 (17.4) | 0 (0) | |
| Best supportive care | 0 (0) | 1 (3.2) | |
| Prognosis, (%) | <0.001 | ||
| 5-year OS rate | 96.8 | 70.0 | |
| 10-year OS rate | 96.8 | 50.9 | |
| Median follow-up period, days (IQR) | 1655 (287–2824) | 1395 (865–3055) | 0.19 |
| Variables | Non-Aggressive Group (n = 46) | Aggressive Group (n = 31) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (years), n (%) | 0.09 | |||
| <65 | 22 (47.8) | 21 (67.7) | 1 | |
| ≥65 | 24 (52.2) | 10 (32.3) | 0.44 (0.09–1.13) | |
| Sex, n (%) | 0.74 | |||
| Female | 24 (52.2) | 15 (48.4) | 1 | |
| Male | 22 (47.8) | 16 (51.6) | 1.16 (0.47–2.90) | |
| Symptoms, n (%) | <0.001 | |||
| No | 40 (87.0) | 11 (35.5) | 1 | |
| Yes | 6 (13.0) | 20 (64.5) | 12.12 (3.91–37.53) | |
| Tumor location, n (%) | 0.65 | |||
| Head | 16 (34.8) | 14 (45.2) | 1 | |
| Body/tail | 28 (60.9) | 16 (51.6) | 0.65 (0.60–3.94) | |
| Multiple | 2 (4.3) | 1 (3.2) | 0.57 (0.05–7.00) | |
| Number of tumors, n (%) | 0.80 | |||
| Single | 44 (95.7) | 30 (96.8) | 1 | |
| Multiple | 2 (4.3) | 1 (3.2) | 0.73 (0.06–8.45) | |
| Tumor size (mm), n (%) | <0.001 | |||
| ≤20 | 41 (89.1) | 9 (29.0) | 1 | |
| >20 | 5 (10.9) | 22 (71.0) | 20.0 (5.98–67.20) | |
| Tumor vascularity, n (%) | <0.001 | |||
| Hypervascular | 44 (95.7) | 10 (32.3) | 1 | |
| Non-hypervascular | 2 (4.3) | 21 (67.7) | 46.2 (9.28–229.91) | |
| Cystic degeneration/necrosis, n (%) | 0.002 | |||
| No | 37 (80.4) | 14 (45.2) | 1 | |
| Yes | 9 (19.6) | 17 (54.8) | 1.81 (1.81–13.78) | |
| Tumor calcification, n (%) | 0.10 | |||
| No | 45 (97.8) | 27 (87.1) | 1 | |
| Yes | 1 (2.2) | 4 (12.9) | 6.67 (0.71–62.79) | |
| MPD or CBD involvement, n (%) | 0.003 | |||
| No | 43 (93.5) | 20 (64.5) | 1 | |
| Yes | 3 (6.5) | 11 (35.5) | 7.88 (1.98–31.41) | |
| 18F-FDG PET/CT #, n (%) | 0.003 | |||
| Negative | 23 (54.8) | 5 (17.9) | 1 | |
| Positive | 19 (45.2) | 23 (82.1) | 5.57 (1.78–17.45) | |
| EUS-FNA Ki-67 LI ≥ 3%, n (%) | <0.001 | |||
| No | 38 (82.6) | 9 (29.0) | 1 | |
| Yes | 8 (17.4) | 22 (71.0) | 11.61 (3.91–34.45) | |
| EUS-FNA Ki-67 LI ≥ 5%, n (%) | <0.001 | |||
| No | 43 (93.5) | 11 (35.5) | 1 | |
| Yes | 3 (6.5) | 20 (64.5) | 26.06 (6.54–103.84) |
| Variables | OR (95% CI) | p Value | β Regression Coefficients | SE | Points | |
|---|---|---|---|---|---|---|
| Tumor size | >20 mm | 9.96 (2.05–48.46) | 0.004 | 2.30 | 0.80 | 1 |
| Tumor vascularity | Non-hypervascular | 23.23 (3.54–152.44) | 0.001 | 3.15 | 0.96 | 2 |
| EUS-FNA Ki-67 LI | ≥5% | 6.95 (1.16–41.80) | 0.034 | 1.94 | 0.92 | 1 |
| Total Points | Patients, n | Proportions of Aggressive NF-PanNENs, % |
|---|---|---|
| 0 | 40 | 7.5 (3/40) |
| 1 | 10 | 40.0 (4/10) |
| 2 | 8 | 62.5 (5/8) |
| 3 | 6 | 100 (6/6) |
| 4 | 13 | 100 (13/13) |
| Risk Groups | Total Points | Patients, n | Proportions of Aggressive NF-PanNENs, % |
|---|---|---|---|
| Low-risk | 0 | 40 | 7.5 (3/40) |
| Intermediate-risk | 1 to 2 | 18 | 50.0 (9/18) |
| High-risk | 3 to 4 | 19 | 100 (19/19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takikawa, T.; Kikuta, K.; Hamada, S.; Kume, K.; Miura, S.; Yoshida, N.; Tanaka, Y.; Matsumoto, R.; Ikeda, M.; Kataoka, F.; et al. A New Preoperative Scoring System for Predicting Aggressiveness of Non-Functioning Pancreatic Neuroendocrine Neoplasms. Diagnostics 2022, 12, 397. https://doi.org/10.3390/diagnostics12020397
Takikawa T, Kikuta K, Hamada S, Kume K, Miura S, Yoshida N, Tanaka Y, Matsumoto R, Ikeda M, Kataoka F, et al. A New Preoperative Scoring System for Predicting Aggressiveness of Non-Functioning Pancreatic Neuroendocrine Neoplasms. Diagnostics. 2022; 12(2):397. https://doi.org/10.3390/diagnostics12020397
Chicago/Turabian StyleTakikawa, Tetsuya, Kazuhiro Kikuta, Shin Hamada, Kiyoshi Kume, Shin Miura, Naoki Yoshida, Yu Tanaka, Ryotaro Matsumoto, Mio Ikeda, Fumiya Kataoka, and et al. 2022. "A New Preoperative Scoring System for Predicting Aggressiveness of Non-Functioning Pancreatic Neuroendocrine Neoplasms" Diagnostics 12, no. 2: 397. https://doi.org/10.3390/diagnostics12020397
APA StyleTakikawa, T., Kikuta, K., Hamada, S., Kume, K., Miura, S., Yoshida, N., Tanaka, Y., Matsumoto, R., Ikeda, M., Kataoka, F., Sasaki, A., Hayashi, H., Hatta, W., Ogata, Y., Nakagawa, K., Unno, M., & Masamune, A. (2022). A New Preoperative Scoring System for Predicting Aggressiveness of Non-Functioning Pancreatic Neuroendocrine Neoplasms. Diagnostics, 12(2), 397. https://doi.org/10.3390/diagnostics12020397







