PVR—A Prognostic Biomarker Correlated with Immune Cell Infiltration in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Material and Methods
2.1. The Levels of PVR mRNA in Various Human Tumors
2.2. PVR Expression in Tumor Tissues Collected from HCC Patients
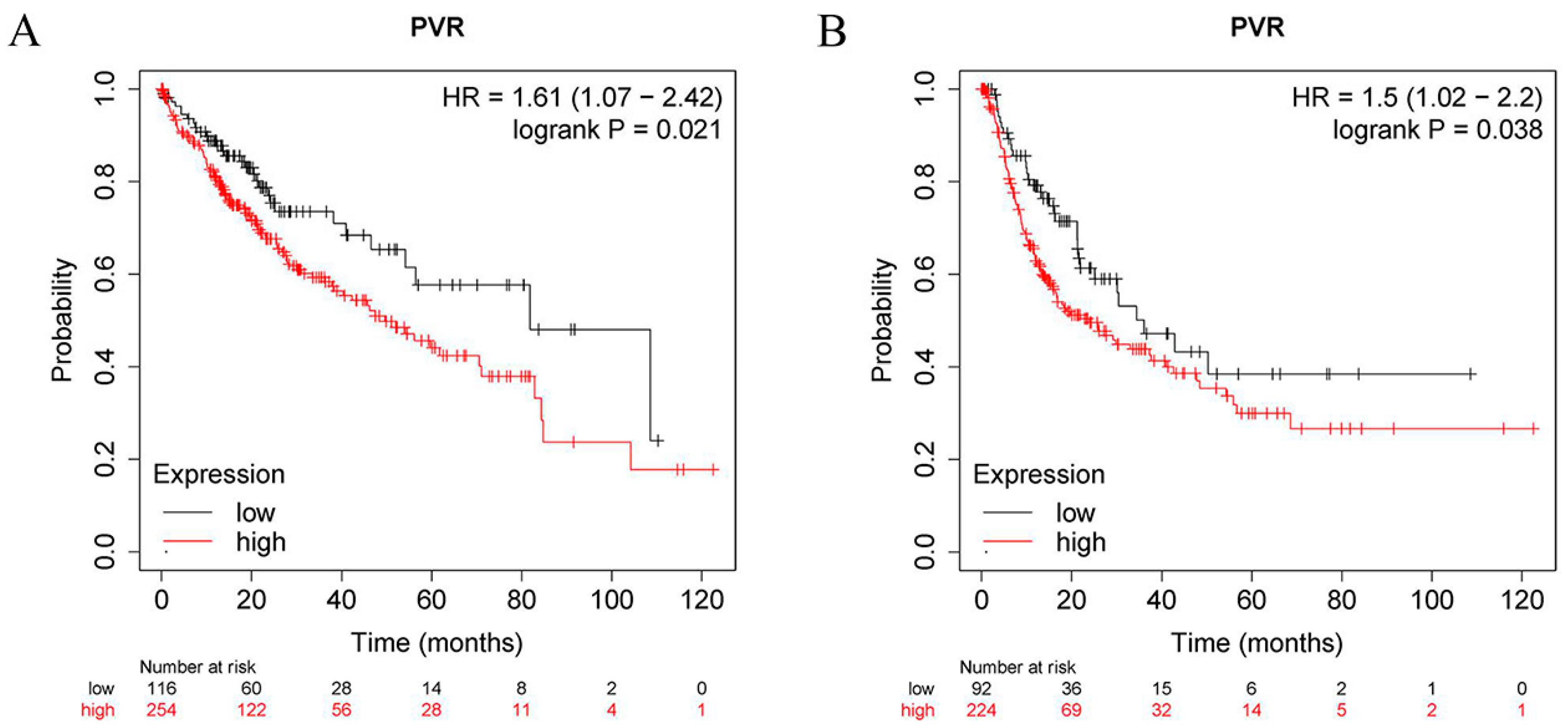
2.3. Prognostic Analysis of PVR in HCC Patients
2.4. PPI Network and GO/KEGG Analysis
2.5. Immune Landscape of PVR in HCC
3. Results
3.1. PVR Was Upregulated in Pan-Cancer including HCC
3.2. Prognostic Significance of PVR Expression in HCC
3.3. Association of PVR Expressions with Clinical Characteristics of HCC Patients
3.4. Correlation and PVR-Related Gene Enrichment Analysis
3.5. Relationship between PVR Expression and Immune Scores
3.6. Relationship between PVR Expression and Immune Cell Infiltration
3.7. Correlation between PVR Expression Levels and Immune Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, A.; Bai, Y.; Lin, J.; Yang, X.; Wang, D.; Yang, X.; Jiang, Y.; Zhao, H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019, 42, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, K.; Chen, Y.; Zhu, M.; Li, M. Role of Alpha-fetoprotein in hepatocellular carcinoma drug resistance. Curr. Med. Chem. 2021, 28, 1126–1142. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Chaudhary, B.; Elkord, E. Regulatory T Cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Sasidharan, N.V.; Elkord, E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell. Biol. 2018, 96, 21–33. [Google Scholar] [CrossRef]
- Kakunaga, S.; Ikeda, W.; Shingai, T.; Fujito, T.; Yamada, A.; Minami, Y.; Imai, T.; Takai, Y. Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras-Raf-MEK-ERK signaling. J. Biol. Chem. 2004, 279, 36419–36425. [Google Scholar] [CrossRef]
- Oda, T.; Ohka, S.; Nomoto, A. Ligand stimulation of CD155alpha inhibits cell adhesion and enhances cell migration in fibroblasts. Biochem. Biophys. Res. Commun. 2004, 319, 1253–1264. [Google Scholar] [CrossRef]
- Lange, R.; Peng, X.; Wimmer, E.; Lipp, M.; Bernhardt, G. The poliovirus receptor CD155 mediates cell-to-matrix contacts by specifically binding to vitronectin. Virology 2001, 285, 218–227. [Google Scholar] [CrossRef]
- Reymond, N.; Imbert, A.-M.; Devilard, E.; Fabre, S.; Chabannon, C.; Xerri, L.; Farnarier, C.; Cantoni, C.; Bottino, C.; Moretta, A.; et al. DNAM-1 and PVR Regulate Monocyte Migration through Endothelial Junctions. J. Exp. Med. 2004, 199, 1331–1341. [Google Scholar] [CrossRef]
- Braun, M.; Aguilera, A.R.; Sundarrajan, A.; Corvino, D.; Stannard, K.; Krumeich, S.; Das, I.; Lima, L.G.; Guzman, L.G.M.; Li, K.; et al. CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8+ T Cells. Immunity 2020, 53, 805–823.e15. [Google Scholar] [CrossRef]
- Liu, L.; You, X.; Han, S.; Sun, Y.; Zhang, J.; Zhang, Y. CD155/TIGIT, a novel immune checkpoint in human cancers (Review). Oncol. Rep. 2021, 45, 835–845. [Google Scholar] [CrossRef]
- Molfetta, R.; Zitti, B.; Lecce, M.; Milito, N.D.; Stabile, H.; Fionda, C.; Cippitelli, M.; Gismondi, A.; Santoni, A.; Paolini, R. CD155: A multi-functional molecule in tumor progression. Int. J. Mol. Sci. 2020, 21, 922. [Google Scholar] [CrossRef]
- Nakai, R.; Maniwa, Y.; Tanaka, Y.; Nishio, W.; Yoshimura, M.; Okita, Y.; Ohbayashi, C.; Satoh, N.; Ogita, H.; Takai, Y.; et al. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010, 101, 1326–1330. [Google Scholar] [CrossRef]
- Kinugasa, M.; Amano, H.; Satomi-Kobayashi, S.; Nakayama, K.; Miyata, M.; Kubo, Y.; Nagamatsu, Y.; Kurogane, Y.; Kureha, F.; Yamana, S.; et al. Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ. Res. 2012, 110, 716–726. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Zhao, K.; Ma, Z.; Zhang, W. Comprehensive Analysis to Identify SPP1 as a Prognostic Biomarker in Cervical Cancer. Front. Genet. 2022, 12, 732822. [Google Scholar] [CrossRef] [PubMed]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 2015, 35, 2287–2297. [Google Scholar] [PubMed]
- Liu, L.; Wang, Y.; Geng, C.; Wang, A.; Han, S.; You, X.; Sun, Y.; Zhang, J.; Lu, W.; Zhang, Y. CD155 promotes the progression of cervical cancer cells through AKT/mTOR and NF-κB pathways. Front. Oncol. 2021, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, B.; Gao, J.; Xin, N.; Wang, W.; Song, X.; Shao, Y.; Zhao, C. CD155 knockdown promotes apoptosis via AKT/Bcl-2/Bax in colon cancer cells. J. Cell. Mol. Med. 2018, 22, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.L.; Zhang, C.Y.; Zheng, W.J.; Xian, J.R.; Yang, W.J.; Liu, T.; Chen, W.; Li, T.; Wang, B.L.; Pan, B.S.; et al. CD155/SRC complex promotes hepatocellular carcinoma progression via inhibiting the p38 MAPK signalling pathway and correlates with poor prognosis. Clin. Transl. Med. 2022, 12, e794. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Tang, T.; Liang, T. Identification of tumor antigens and immune subtypes of pancreatic adenocarcinoma for mRNA vaccine development. Mol. Cancer 2021, 20, 44. [Google Scholar] [CrossRef]
- Mahnke, K.; Enk, A.H. TIGIT-CD155 Interactions in melanoma: A novel co-inhibitory pathway with potential for clinical intervention. J. Investig. Dermatol. 2016, 136, 9–11. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Blake, S.J.; Dougall, W.C.; Miles, J.J.; Teng, M.W.; Smyth, M.J. Molecular pathways: Targeting CD96 and TIGIT for cancer immunotherapy. Clin. Cancer Res. 2016, 22, 5183–5188. [Google Scholar] [CrossRef]
- Lepletier, A.; Madore, J.; O’Donnell, J.S.; Johnston, R.L.; Li, X.-Y.; McDonald, E.; Ahern, E.; Kuchel, A.; Eastgate, M.; Pearson, S.-A.; et al. Tumor CD155 expression is associated with resistance to anti-PD1 immunotherapy in metastatic melanoma. Clin. Cancer. Res. 2020, 26, 3671–3681. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sanders, C.; Hou, J.; Li, X.-Y.; Ferrone, S.; et al. IL15 Stimulation with TIGIT blockade reverses CD155-mediated NK-Cell dysfunction in melanoma. Clin. Cancer. Res. 2020, 26, 5520–5533. [Google Scholar] [CrossRef]
- Sarhan, D.; Cichocki, F.; Zhang, B.; Yingst, A.; Spellman, S.R.; Cooley, S.; Verneris, M.R.; Blazar, B.R.; Miller, J.S. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res. 2016, 76, 5696–5706. [Google Scholar] [CrossRef]
- Chiu, D.K.-C.; Yuen, V.W.-H.; Cheu, J.W.-S.; Wei, L.L.; Ting, V.; Fehlings, M.; Sumatoh, H.; Nardin, A.; Newell, E.W.; Ng, I.O.-L.; et al. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology 2020, 159, 609–623. [Google Scholar] [CrossRef]






| Characteristic | Low expression of PVR | High Expression of PVR | p Value |
|---|---|---|---|
| n | 187 | 187 | |
| Gender, n (%) | 0.825 | ||
| Female | 59 (15.8%) | 62 (16.6%) | |
| Male | 128 (34.2%) | 125 (33.4%) | |
| Age, n (%) | 0.961 | ||
| ≤60 | 89 (23.9%) | 88 (23.6%) | |
| >60 | 97 (26.0%) | 99 (26.5%) | |
| Race, n (%) | 0.760 | ||
| Asian | 76 (21.0%) | 84 (23.2%) | |
| Black or African American | 8 (2.2%) | 9 (2.5%) | |
| White | 95 (26.2%) | 90 (24.9%) | |
| T stage, n (%) | 0.031 | ||
| T1 + T2 | 146 (39.5%) | 131(35.4%) | |
| T3 + T4 | 37 (10.0%) | 56 (15.1%) | |
| N stage, n (%) | 0.628 | ||
| N0 | 117 (45.3%) | 137 (53.1%) | |
| N1 | 1 (0.4%) | 3 (1.2%) | |
| M stage, n (%) | 0.337 | ||
| M0 | 122 (44.9%) | 146 (53.7%) | |
| M1 | 3 (1.1%) | 1 (0.4%) | |
| Pathologic stage, n (%) | 0.043 | ||
| Stage I + Stage II | 139 (39.7%) | 121 (34.6%) | |
| Stage III + Stage IV | 37 (10.6%) | 53 (15.1%) | |
| Histologic grade, n (%) | 0.778 | ||
| G1 | 30 (8.1%) | 25 (6.8%) | |
| G2 | 90 (24.4%) | 88 (23.8%) | |
| G3 | 59 (16%) | 65 (17.6%) | |
| G4 | 5 (1.4%) | 7 (1.9%) | |
| Child–Pugh grade, n (%) | 0.910 | ||
| A | 108 (44.8%) | 111 (46.1%) | |
| B | 10 (4.1%) | 11 (4.6%) | |
| C | 1 (0.4%) | 0 (0%) | |
| Vascular invasion, n (%) | 0.561 | ||
| No | 103 (32.4%) | 105 (33%) | |
| Yes | 59 (18.6%) | 51 (16%) |
| Description | Gene Markers | None | Purity | ||
|---|---|---|---|---|---|
| Core | p | Core | p | ||
| CD8+ T cell | CD8A | −0.045 | 0.389 | 0.056 | 0.300 |
| CD8B | −0.088 | 0.091 | −0.001 | 0.991 | |
| T cell (general) | CD3D | −0.071 | 0.172 | 0.001 | 0.982 |
| CD3E | −0.073 | 0.162 | 0.035 | 0.521 | |
| CD2 | −0.064 | 0.220 | 0.037 | 0.494 | |
| B cell | CD19 | −0.055 | 0.292 | 0.015 | 0.784 |
| CD79A | −0.064 | 0.217 | 0.026 | 0.630 | |
| Monocyte | CD86 | 0.022 | 0.670 | 0.136 | * |
| CSF1R | −0.007 | 0.891 | 0.112 | * | |
| TAM | CCL2 | 0.003 | 0.960 | 0.098 | 0.068 |
| CD68 | 0.027 | 0.600 | 0.103 | 0.057 | |
| IL10 | 0.005 | 0.929 | 0.102 | 0.058 | |
| M1 Macrophage | iNOS | 0.143 | ** | 0.161 | ** |
| IRF5 | 0.228 | *** | 0.232 | *** | |
| PTGS2 | 0.115 | * | 0.234 | *** | |
| M2 Macrophage | CD163 | 0.045 | 0.388 | 0.171 | ** |
| VSIG4 | 0.092 | * | 0.214 | *** | |
| MS4A4A | 0.002 | 0.969 | 0.125 | * | |
| Natural killer cell | KIR2DL1 | −0.043 | 0.406 | −0.036 | 0.505 |
| KIR2DL3 | −0.051 | 0.331 | −0.019 | 0.722 | |
| KIR2DL4 | −0.034 | 0.517 | 0.017 | 0.756 | |
| KIR3DL1 | −0.037 | 0.475 | −0.011 | 0.844 | |
| KIR3DL2 | −0.056 | 0.286 | −0.011 | 0.840 | |
| KIR3DL3 | −0.087 | 0.094 | −0.066 | 0.224 | |
| KIR2DS4 | −0.021 | 0.687 | −0.006 | 0.912 | |
| Dendritic cell | HLA-DPB1 | −0.082 | 0.114 | 0.010 | 0.859 |
| HLA-DQB1 | −0.071 | 0.170 | 0.011 | 0.837 | |
| HLA-DRA | −0.055 | 0.292 | 0.045 | 0.400 | |
| HLA-DPA1 | −0.053 | 0.307 | 0.054 | 0.317 | |
| BDCA−1(CD1C) | −0.010 | 0.844 | 0.053 | 0.328 | |
| BDCA−4(NRP1) | 0.143 | ** | 0.177 | ** | |
| CD11c(ITGAX) | 0.116 | * | 0.237 | *** | |
| Th1 | TBX21 | −0.088 | 0.089 | 0.009 | 0.866 |
| STAT4 | 0.097 | 0.061 | 0.145 | ** | |
| STAT1 | 0.165 | ** | 0.224 | *** | |
| TNF | 0.040 | 0.442 | 0.165 | * | |
| INF-α(IL28B) | −0.037 | 0.472 | 0.061 | 0.260 | |
| Th2 | GATA3 | −0.006 | 0.905 | 0.110 | * |
| STAT6 | 0.160 | ** | 0.183 | *** | |
| STAT5A | 0.135 | ** | 0.206 | *** | |
| IL13 | 0.088 | 0.092 | 0.119 | * | |
| Tfh | BCL6 | 0.147 | ** | 0.158 | ** |
| IL21 | −0.065 | 0.210 | −0.038 | 0.483 | |
| Th17 | STAT3 | 0.234 | *** | 0.307 | *** |
| IL17A | −0.021 | 0.689 | −0.025 | 0.649 | |
| Treg | FOXP3 | 0.116 | * | 0.149 | *** |
| CCR8 | 0.149 | ** | 0.238 | *** | |
| STAT5B | 0.325 | *** | 0.332 | *** | |
| TGFB1 | 0.058 | 0.264 | 0.122 | * | |
| T cell exhaustion | PD−1 | −0.032 | 0.542 | 0.146 | ** |
| CTLA4 | −0.020 | 0.700 | 0.058 | 0.281 | |
| LAG3 | −0.030 | 0.568 | 0.014 | 0.798 | |
| TIM3 | 0.042 | 0.417 | 0.162 | ** | |
| GZMB | −0.119 | * | −0.046 | 0.394 | |
| Neutrophils | CD66b (CEACAM8) | 0.089 | 0.088 | 0.107 | * |
| CD11b (ITGAM) | 0.065 | 0.213 | 0.134 | * | |
| CCR7 | −0.065 | 0.214 | 0.052 | 0.338 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-F.; Quan, B.; Li, M.; Zhang, F.; Hu, K.-S.; Yin, X. PVR—A Prognostic Biomarker Correlated with Immune Cell Infiltration in Hepatocellular Carcinoma. Diagnostics 2022, 12, 2953. https://doi.org/10.3390/diagnostics12122953
Liu W-F, Quan B, Li M, Zhang F, Hu K-S, Yin X. PVR—A Prognostic Biomarker Correlated with Immune Cell Infiltration in Hepatocellular Carcinoma. Diagnostics. 2022; 12(12):2953. https://doi.org/10.3390/diagnostics12122953
Chicago/Turabian StyleLiu, Wen-Feng, Bing Quan, Miao Li, Feng Zhang, Ke-Shu Hu, and Xin Yin. 2022. "PVR—A Prognostic Biomarker Correlated with Immune Cell Infiltration in Hepatocellular Carcinoma" Diagnostics 12, no. 12: 2953. https://doi.org/10.3390/diagnostics12122953
APA StyleLiu, W.-F., Quan, B., Li, M., Zhang, F., Hu, K.-S., & Yin, X. (2022). PVR—A Prognostic Biomarker Correlated with Immune Cell Infiltration in Hepatocellular Carcinoma. Diagnostics, 12(12), 2953. https://doi.org/10.3390/diagnostics12122953






