Abstract
The microbiome is vital for the proper function of the gastrointestinal tract (GIT) and the maintenance of overall wellbeing. Gut ischemia may lead to disruption of the intestinal mucosal barrier, resulting in bacterial translocation. In this systematic review, according to PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines, we constructed a search query using the PICOT (Patient, Intervention, Comparison, Outcome, Time) framework. Eligible studies reported in PubMed, up to April 2021 were selected, from which, 57 publications’ data were included. According to these, escape of intraluminal potentially harmful factors into the systemic circulation and their transmission to distant organs and tissues, in utero, at birth, or immediately after, can be caused by reduced blood oxygenation. Various factors are involved in this situation. The GIT is a target organ, with high sensitivity to ischemia–hypoxia, and even short periods of ischemia may cause significant local tissue damage. Fetal hypoxia and perinatal asphyxia reduce bowel motility, especially in preterm neonates. Despite the fact that microbiome arouse the interest of scientists in recent decades, the pathophysiologic patterns which mediate in perinatal hypoxia/asphyxia conditions and gut function have not yet been well understood.
1. Introduction
The human body hosts about 100 trillion microorganisms, called microbiota, which are involved in multiple functions, such as vitamin synthesis, bile salt metabolism, fiber, mucus and fatty acid catabolism, regulation of inflammation and homeostasis of the immune system [1]. They colonize the skin, the mammary glands, the saliva and oral mucosa, the conjunctiva, the airway, the urogenital system and the GIT. The genome of the microbiota is called the microbiome and it has special characteristics, such as its own weight, genetic and cellular content and its own metabolic activity [1,2]. It begins to be established in utero by the maternal microbial flora. Microbial communities are isolated in healthy embryos in the uterus, umbilical cord blood, amniotic fluid, placenta, and embryonic membranes. The microbiome in neonates varies in composition and diversity, taking the adult type at 3–5 years of age [2,3,4].
In a healthy host, the microbiota plays a vital role in maintaining proper bowel function and good health in general. Changes in the microbiota’s composition can adversely affect the normal immune and metabolic pathways and the general well-being of the host. In other words, there is a dynamic relationship that can be transformed from ‘symbiotic’ to ‘dysbiotic’ and life-threatening [5,6,7]. Many factors are causally related to these fundamental changes such as genetic background, maternal diet prior to and during pregnancy, maternal BMI during pregnancy, the adequacy of nutrients’ intake, maternal microbiome, administration of probiotics, mode of delivery, gestational age, perinatal stress, administration of antibiotics to the mother during pregnancy and to the baby early in neonatal life, infections during pregnancy and during the perinatal or neonatal period, the newborn’s diet (breastfeeding, cow’s milk, mixed diet), and finally other environmental factors such as temperature, humidity, pH and oxygen levels in the tissues [8,9,10].
Intestinal mucosa acts as an anatomical and functional barrier (IMB) and plays an important role in preventing the leak of intestinal bacteria and their breakdown products to extra-enteric tissues. In pathological conditions, potentially harmful intraluminal agents escape to the mesenteric lymph nodes and to nearby and distant organs and tissues, and this results in dysfunction of many organs and even death. This phenomenon is called bacterial translocation [11,12,13].
The aim of this study is to systematically investigate whether asphyxia contributes to microbial translocation in neonates.
2. Materials and Methods
2.1. Study Design
This study was performed following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [14]. Eligible studies reported in PubMed, up to the study data collection time point (April 2021) were selected as being potentially eligible to be included. Only studies published in the English language were selected and there was a restriction on publication year, i.e., only publications after 1 January 1990 were requested; moreover no restrictions on publication type were posed. This was due to the lack of relevant information from previous years, as it is a field that has aroused the interest of scientists only in recent decades.
2.2. Search Question Formation
The search query was structured according to the PICOT (Population-Intervention-Comparison-Outcome-Time) framework [15]. After defining and joining PICO individual parts, the final question was issued in PubMed. The individual PICO components are depicted in Table 1; note that MeSH (Medical Subject Headings) terms were not extensively used and instead keywords were searched in abstract and title, as many publications do not use defined and appropriate MeSH terms.

Table 1.
Individual PICO Components.
Structured query took place according to the PICO framework. The final query issued in PubMed was the synthesis of the individual components appearing in the last table row. Asterisks denote extension of the search terms with any other additional characteristics.
This systematic search returned 2320 publications; additional publications not found by the PICO question but known to the authors were included (n = 41) (Figure 1). Two researchers (DIM and AP) reviewed all search results independently (screening process). The review was based on titles and abstracts and irrelevant studies were included for the subsequent stage of full text review. In case of disagreements the opinion of a third researcher (TB) was requested. Other researchers participated in the data extraction stages. Figure 1 depicts the various steps of data collection and selection process and Figure 2 the initial publications for each year. Finally 57 publications were found eligible for inclusion. Notably the number of publications has an increasing trend in the last decade (Figure 2) indicative that the study subject has become more interesting to the scientific community. As no quantitative results were reported in the majority of the studies, and the focus of this review is under various conditions (i.e., hypoxia/asphyxia, ischemia/reperfusion injury of the intestinal barrier, and necrotizing enterocolitis), this study does not report meta-analysis results such as pooled effects or heterogeneity indexes, such as Cochran’s Q measure or the I2. However, in order to ensure the quality of the included studies, articles published in PubMed listed journals were used, but conference proceedings were avoided.
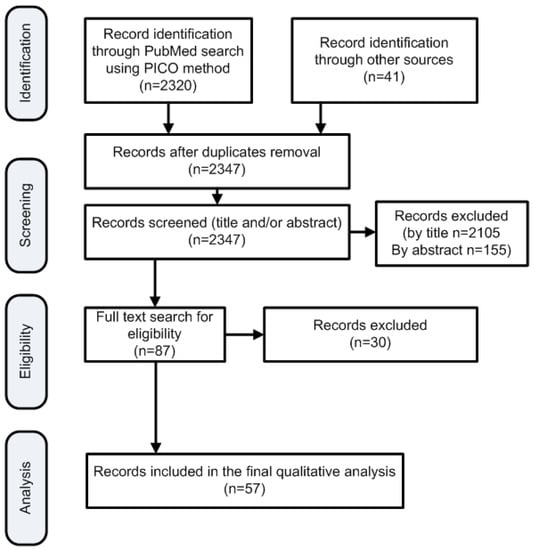
Figure 1.
Flowchart of the search strategy according to the PRISMA framework.
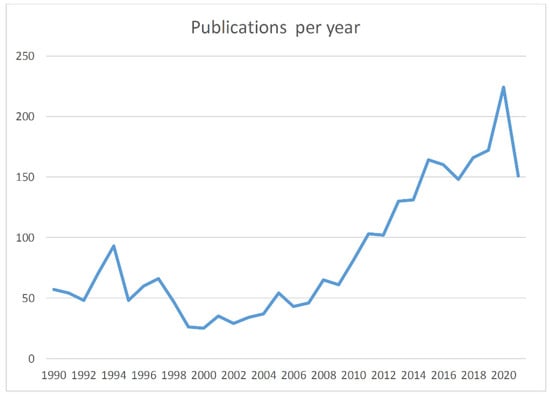
Figure 2.
Publications by year. For 2021 data, this goes up to the end of October.
3. Results
3.1. Microbial Translocation in Hypoxic/Asphyxiating Conditions
The transition from intrauterine to extrauterine life usually occurs without problems. However, 10% of newborns need support in this process, while 1% require specialized intervention [16]. Perinatal asphyxia is defined as the lack of oxygen that may occur around delivery, leading to a reduction in the diffusion of oxygen into multiple organs and tissues. It is one of the leading causes of neonatal mortality in the first week of life. Various perinatal events may cause perinatal asphyxia. According to the World Health Organization (WHO), about four million newborns worldwide develop perinatal asphyxia each year, and one million babies die from it. These numbers correspond to 38% of all deaths of children under 5 years of age. In low-income countries, 23% of all neonatal deaths are attributed to perinatal asphyxia [17,18].
Asphyxia and hypoxia are two similar terms which are used in physiology to describe an inadequate supply of oxygen to cells and tissues. The main difference between asphyxia and hypoxia is that asphyxia is caused by an injury or obstruction of the airway passages whereas hypoxia is caused by insufficient delivery, uptake or utilization of oxygen by the body’s tissues. The clinical definition of neonatal hypoxic ischemic injury is “asphyxia of the umbilical blood supply to the human fetus occurring at 36 gestational weeks or later”. Neonatal hypoxia ischemia is synonymous with hypoxic-ischemic encephalopathy occurring in the term infant. The diagnostic criteria for neonatal hypoxic ischemia are based on a set of markers demonstrated to correlate with clinical outcome. These include 5-min Apgar score of less than 5, need for delivery room intubation or CPR, umbilical cord arterial pH less than 7.00 and abnormal neurological signs, such as hypotonic muscles or lack of sucking reflex [19]. Asphyxia causes disturbance in gas exchange, resulting in hypoxemia and hypercapnia. The combination of reduced oxygen supply (hypoxia) and reduced blood supply (ischemia) leads to dysfunction of all neonatal organs, such as the myocardium, lungs and GIT, and causes nerve cell death and brain damage. The pathogenetic mechanisms are still not completely understood and there is no cure considered to be gold standard. Metabolomics technology has been able to describe perinatal pathological conditions and to demonstrate useful means of monitoring, evaluating, and identifying potential biomarkers associated with asphyxia episodes [20,21].
An in vivo study in a murine model of obstructive sleep apnea, published in 2015, investigated if intermittent hypoxia, which resembles one of the hallmarks of obstructive sleep apnea, leads to modified fecal microbiome; a higher occurrence of Firmicutes and a smaller occurrence of Bacteroidetes and Proteobacteria phyla than controls was reported. Fecal microbiota composition and diversity were altered as a consequence of intermittent hypoxia, suggesting that physiological interplays between host and gut microbiota could be deregulated in similar situations [22].
Zhdanov et al., in 2016, studied in rats the effect of reduced tissue oxygenation on inflammation of the intestinal mucosa. Mice with colitis had increased inflammatory markers and increased measured oxygen in the upper layers of the mucosa, without affecting the architecture of the epithelium, but with concomitant partial activation of Hypoxia Induced Factor 1 and adverse effects on pyruvate dehydrogenase, suggestive of reduced mitochondrial respiration. Colonic inflammation is linked to decreased tissue oxygenation, and significantly affects gut homeostasis. Even though there is crosstalk between O2 consumption and supply in the inflamed tissues, the mechanisms are not fully understood. The methods used in this animal model demonstrated new horizons for the study of human diseases associated with hypoxia and inflammatory response of the intestinal mucosa [23]. Table 2 summarizes studies relevant to asphyxia and microbial translocation.

Table 2.
Systemic research results for Microbial Translocation in Neonatal Hypoxic/Asphyxiating conditions.
3.2. The Impact of Ischemia/Reperfusion(I/R) Injury on the Intestinal Barrier Function
Küçükaydin et al. investigated bacterial transposition after experimental ischemic damage and reperfusion of the intestinal mucosa in rats, using blood samples from the superior mesenteric artery and portal vein as well as tissue fragments from the final ileum and mesenteric lymph nodes [23]. Polymerase Chain Reaction in these samples showed genetic material of E. coli, and histological studies of the same samples suggested that intestinal ischemia-reperfusion damage may result in bacterial transmission. Several studies, in in vivo model systems, demonstrated that I/R can increase intestinal mucosal permeability, further bacterial translocation, and trigger gut cytokine production. Despite the cellular heterogeneity of the gut, nonetheless the direct effects of hypoxia/reoxygenation on intestinal epithelial cells was investigated in an in vitro model. Increased monolayer permeability to phenol red, increased E. coli bacterial translocation, and decreased transepithelial electrical resistance, showed that hypoxia/reoxygenation can directly impair cellular function [24].
Sun et al. suggested that both endothelial and epithelial barrier integrity is harmed in the early phase after I/R, and that the epithelial barrier more adequately regulates macromolecular leak compared with the endothelial barrier. I/R impairs the intestinal barrier by causing tissue hypoxia and by activating the phagocytic system and irritating barrier damage, which finally may result in bacterial translocation and remote organ dysfunction [25].
More recently, a team of investigators interested in the relationship between hypoxia and I/R injury looked for the occurrence of bacterial translocation after cardiac arrest, followed or not by successful resuscitation, a field that has not yet been adequately studied. Gkiza et al. studied two groups of pig, where in the first only minimal aseptic interventions were done, while in the second ventricular fibrillation and finally obstruction was performed. The researchers advocated the existence of bacterial transmission through the mechanism of intestinal I/R damage due to cardiac arrest and they also suggested, for the first time, that cardiopulmonary arrest may lead to systemic inflammation soon after successful resuscitation [26] (Table 3).

Table 3.
The intestinal barrier function and I/R injury.
3.3. Necrotizing Enterocolitis and Translocation
Table 4 summarizes the published work relevant to NEC and bacterial translocation. The GIT is particularly vulnerable to ischemic damage. Even short periods of ischemia may cause significant local tissue damage. Fetal hypoxia and perinatal asphyxia act by reducing bowel motility, especially in preterm infants. Previous studies in animal model systems showed that oxygen administration during airway management prevents hypoxemia, intestinal harm, and bacterial translocation [28]. As it becomes obvious from recent literature, ischemic preconditioning seems to be beneficial for the human heart and the liver. Prospective controlled studies in humans, including ischemic preconditioning of the intestine, are lacking [29].
Necrotizing enterocolitis is an inflammatory bowel disease that affects newborns, especially premature ones, with 15–30% mortality and 10–50% long-term morbidity [30]. Survivors suffer from short-term gastrointestinal complications (feeding difficulties), as well as long-term complications (reduced growth, possible liver disease due to parenteral nutrition, and short bowel syndrome) [30,31]. Less than 10% of all NEC cases involve full-term newborns [32]. The gestational age is inversely proportional to the time of onset of NEC, i.e., premature infants develop the disease later in life, while full-term babies appear to get sick earlier. The pathogenesis remains unclear, but it is believed that in premature babies, bacterial invasion and activation of the inflammatory process occur with possible subsequent cell necrosis, due to dysfunction or immaturity of the intestinal barrier. In full term infants, different clinical conditions were identified as possible risk factors, including perinatal hypoxia/asphyxia, certain congenital heart diseases, polycythemia/thrombotic conditions, endocrine diseases and perinatal sepsis [33].
The distal ileum and the proximal colon, are the most commonly affected parts of the intestine in neonates with necrotizing enterocolitis [34]. Current data suggest that intestinal microbial dysbiosis precedes NEC in premature infants. However, further research, with large prospective studies and standard methodology is needed to understand the significance of the changes that occur in the microbiome during the early postnatal period, in order to assess the reproducibility of the available data on the issue [35,36].
The pathophysiology of NEC is multifactorial. Ischemic injury of the intestinal mucosa is caused by the “diving reflex”, where peripheral vasoconstriction and redistribution of blood from the non-vital to vital organs such as heart, brain and adrenal glands occur in response to the drop in blood pressure during asphyxia. This type of ischemic bowel injury is one of the pathogenetic mechanisms of NEC, secondary to infection or asphyxia. Due to hypoxic injury, production of nitric oxide, which is necessary to maintain the proper perfusion of the intestine, is reduced, with a consequent reduction in the resistance of the intestinal vessels [37,38]. The increased bacterial translocation by the disturbed intestinal epithelium activates endogenous signaling, such as the platelet activating factor and the tissue necrosis membrane, inducing the cataract of inflammation. What remains to be clarified is the exact point where the various aforementioned clinical factors play a role. For example, is intestinal feeding, bacteria, or hypoxic damage simply the initiator of this pathway? Studies have focused on these factors, as they may be involved in the etiology of NEC [39].
The GIT is supported by a rich and multiplex underlying vasculature. As a result, the intestinal epithelial cell layer is susceptible to damage associated with decreased blood flow. The resulting hypoxia is a consequence of both diminished perfusion and increased metabolism within the mucosa. The metabolic shift may result in “cytopathic hypoxia,” a type of mitochondrial dysfunction which leads to reduced intracellular oxygen and ATP availability. Even though, this type of damage constitutes a risk to the epithelial function by restricting harmful luminal entities, recent data have suggested that the intestinal epithelium is equipped with hypoxia-inducible adaptive mechanisms, which sustain barrier function under conditions of such a dysfunction. Compared with other mucosal surfaces, intestinal epithelial cells seem to be uniquely resistant to disruption by hypoxia. Such observations may relate to the fact that intestinal epithelial cells are conditioned to a lower pO2 than other tissues [40].
Despite the fact that there is strong correlation between barrier breakdown and bacterial translocation, the molecular mechanisms of bacterial translocation from the lumen of the GIT to the bloodstream are not well understood. Although there is an increase in translocated bacteria with hypoxia, most evidence suggests that overall integrity of the intestinal epithelium remains intact, even in relatively severe hypoxia. This finding may indicate that bacterial translocation during intestinal inflammation is a consequence of increased transcellular bacterial movement, rather than a breakdown of epithelial integrity [41].
In a recent study of a pig cardiopulmonary resuscitation model, the authors attempted to highlight the metabolic profile in plasma samples from asphyxiated animals and animals with ventricular fibrillation. The metabolic profile of the two groups differed significantly during cardiac arrest and during the resuscitation phase. Animals with the worst outcome had overproduction of the electro-coenzyme A (Krebs cycle), suggesting a possible prognostic role for this metabolite [42]. The intestinal mucosa becomes ‘injured’, its structure alters, the intestinal barrier is weakened, microbial populations move through the disturbed intestinal barrier, and mediators of inflammation are released. The corresponding part of the GIT ‘dies’, while the initial local damage leads to a generalized response of the body as various microorganisms and endotoxins travel through the blood to peripheral tissues and organs (bacterial translocation), with potentially life-threatening consequences, such as sepsis and multiple organ dysfunction syndrome. The various bacterial strains enter the systemic circulation via the intestinal venous system and the portal vein, or through the intestinal lymphatic route [43,44,45].

Table 4.
The relationship between gut microbiota, intestinal barrier function and microbial translocation and necrotizing enterocolitis.
Table 4.
The relationship between gut microbiota, intestinal barrier function and microbial translocation and necrotizing enterocolitis.
| Authors | Year | Topic | Outcome |
|---|---|---|---|
| Ali Nayci et al. [28] | 2006 | Oxygen supplementation during airway instrumentation improves intestinal barrier dysfunction | Oxygen prevents hypoxemia, intestinal damage, and bacterial translocation |
| Mallick et al.[29] | 2004 | I/R injury of the intestine and protective strategies against injury | Prospective controlled studies in humans involving ischemic preconditioning of the intestine are lacking |
| Berman et al. [31] | 2011 | NEC: an update | Most frequent long-term complications include short bowel syndrome, abnormal growth, neurodevelopmental delay. |
| Ostlie et al. [32] | 2003 | NEC in full-term infants | 10% of cases of NEC are full term infants |
| Short et al. [33] | 2014 | Late onset of NEC in the full-term infant is associated with increased mortality: results from a two-center analysis | The gestational age is inversely proportional to the time of onset of NEC. Possible risk factors include perinatal hypoxia/asphyxia, congenital heart diseases etc. |
| Iben et al. [34] | 2011 | NEC | The distal ileum and the proximal colon, are the most commonly affected in NEC |
| Patole et al. [35] | 2017 | Microbiota and NEC | Microbial dysbiosis has been implicated in pathogenesis of NEC |
| Pammi et al. [36] | 2017 | Intestinal dysbiosis in preterm infants preceding NEC: a systematic review and meta-analysis | Further research is needed in order to assess the reproducibility of the available data on the issue |
| Grishin et al. [37] | 2016 | Roles of nitric oxide and intestinal microbiota in the pathogenesis of NEC | Nitric oxide plays a prominent role in the intestinal barrier damage by inducing enterocyte apoptosis and inhibiting the epithelial restitution processes |
| Stevenson et al. [38] | 2006 | Historical perspectives: NEC an inherited or acquired condition? | Intestinal mucosa ischemic injury caused by the “diving reflex” occurs in response to the decrease of blood pressure during perinatal asphyxia |
| Keely et al. [39] | 2010 | Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia | It remains to be determined whether HIF-mediated, PAFr-dependent bacterial translocation represents a physiological clearance mechanism or rather serves as a pathophysiologic mechanism whereby bacteria exploit PAFr as a route of entry |
| Glover et al. [40] | 2016 | Oxygen metabolism and barrier regulation in the intestinal mucosa | In the intestine, baseline pO2 levels are uniquely low due to counter-current blood flow and the presence of large numbers of bacteria and this mechanism contributes to the gut mucosa homeostasis |
| Tugtekin et al. [41] | 2001 | Increased ileal-mucosal-arterial PCO2 gap is associated with impaired villus microcirculation in endotoxic pigs | Increased ileal-mucosal-arterial delta PCO2 during porcine endotoxemia is related to impaired villus microcirculation |
| Varvarousis et al. [42] | 2017 | Metabolomics profiling reveals different patterns in an animal model of asphyxial and dysrhythmic cardiac arrest | Succinate overproduction was observed in the animals with the worse outcome, suggesting a potential prognostic role for this metabolite |
| Lim et al. [43] | 2015 | Pathogenesis of NEC | Opportunistic pathogens breach the gut barrier and incite an inflammatory response that leads to overproduction of inflammatory mediators which exacerbate the initial mucosal injury and also suppress the intestinal repair mechanisms |
| Hackam et al. [44] | 2013 | Mechanisms of gut barrier failure in the pathogenesis of NEC: Toll-like receptors throw the switch | Activation of the receptor for bacterial endotoxin, TLR4, is required for the development of intestinal barrier failure leading to NEC |
| Patel et al. [45] | 2015 | Intestinal microbiota and its relationship with NEC | Shifting the balance of intestinal microbiota from a pathogenic to protective complement of bacteria can protect the gut from inflammation and subsequent injury that leads to NEC |
3.4. Bacterial Endotoxins
Nine publications related to bacterial endotoxins were identified (Table 5). The term “endotoxin” is synonymous, and is used interchangeably with, the term lipopolysaccharide (LPS). LPS, which comes exclusively from Gram-negative bacteria, has been the gold standard in the study of the basic mechanisms of sepsis for years [45]. According to the U.S. National Library of Medicine, using the term endotoxin we describe “toxins closely linked to the cytoplasm or cell wall of microorganisms, which are not readily distributed in the culture medium but are released by the cell solution.” Endotoxins cause fever and, in larger doses, shock and death. They also provoke an inflammatory response through interaction with high-affinity receptors on leukocytes [45]. The increase in plasma LPS levels disrupts the intestinal mucosal barrier and the transfer of bacteria and their components into the circulation. High levels of LPS are seen in systemic sepsis and in NEC. Intestinal barrier function is affected through different pathways, including direct effects on the repression of intestinal restitution, and indirect effects such as by promoting the release of signaling molecules from enterocytes, including NO and interferon (IFN)-γ. Endotoxin signaling in enterocytes is mainly mediated by a receptor called toll-like receptor 4 (TLR4) and myeloid differentiation factor 2 (MD-2) [46,47]. Wolfs et al., using immunochemistry in the normal and inflamed ileum of neonates and adults, reported that the absence of MD-2 in the immature neonatal gut suggests impaired LPS sensing, which could predispose neonates to NEC upon microbial colonization of the immature intestine. The obvious expression of MD-2 by Paneth cells supports the censorious idea that these cells respond to luminal bacterial products in order to maintain homeostasis with the intestinal microbiota in vivo [48].

Table 5.
Bacterial lipopolysaccharides (LPS/Endotoxins) and their role in the inflammatory pathway of NEC.
Systemic stress causes breakdown in the intestinal mucosal barrier, leading to translocation of bacteria and endotoxin and the initiation of a signaling response within the enterocyte. Enterocyte signaling plays a principle role in the pathogenesis of NEC by the following mechanisms: (1) Local villus enterocytes produce nitric oxide, which increases in enterocyte apoptosis and impaired multiplication, (2) Translocation of endotoxin causes a Phosphoinositide 3-kinase -dependent activation of Ras homolog family member A-GTPase within the enterocyte leading to decreased enterocyte migration and impaired restitution, (3) Dysregulated by endotoxin, sodium–proton exchange makes the enterocyte monolayer more susceptible to damage in an acidic microenvironment characteristic of systemic sepsis, (4) Endotoxin, finally, is associated with a mitogen-activated protein kinase p38-dependent release of the pro-inflammatory molecule Cyclooxygenase-2 by the enterocyte, which intensifies systemic inflammatory response [49].
Sodium/proton exchangers (NHE), present at the basolateral and apical surfaces of enterocytes, are essential for the preservation of enterocyte activity during extracellular acidosis. Necrotizing enterocolitis is characterized by systemic hypoperfusion, metabolic acidosis, and the apical to basolateral translocation of LPS. Cetin, Dunklebarger et al. hypothesized that LPS differentially impairs NHE activity at the basolateral and apical zones of enterocytes, leading to cellular acidification, and investigated the mechanisms involved. Experimental NEC was induced in newborn rats using a combination of gavage feeds and hypoxia. Results of Western blot analysis and confocal microscopy in the presence or absence of LPS suggest that LPS selectively diminishes basolateral NHE1 but not apical NHE3, leading to cytoplasmic acidification during extracellular acidosis. This could damage enterocyte function after LPS translocation and also suggests a mechanism leading to barrier disruption in NEC [50].
Experimental necrotizing enterocolitis (NEC) is characterized by circulating LPS and impaired enterocyte migration. Cetin, Dunklebarger et al. reported that enterocyte migration is inhibited by LPS through increased expression and function of alpha 3- and beta 1-integrins and suggested that modulation of enterocyte migration via integrins may provide novel insights into the pathogenesis of NEC, in which intestinal restitution is impaired [51].
In the course of several years, research has tried to crack the case between hypoxia or asphyxia and LPS at a molecular level. Corcoran & O’Neill described the induction of Hypoxia Induced Factor 1a (HIF1a) by LPS-activated macrophages, which is of fundamental importance in glycolysis and the induction of pro-inflammatory genes, particularly that of interleukin-1 (IL-1). LPS seems to affect other key points in the inflammatory sequelae, promoting or inhibiting other metabolites [52]. Yet these mechanisms continue to be poorly understood.
4. Discussion
Data of bacteria involved in the pathogenesis of NEC is limited by the infant’s fragility, the restriction of analysis to feces, the use of culture-based methods, and the lack of clinically-relevant animal models.
Perinatal asphyxia is a complex phenomenon that affects the health status of mammals at multiple levels, and although researchers have been largely concerned over recent years, there are not yet enough studies of animal models that examine the association between microbial translocation induced by hypoxemia and tissue damage as a result of ischemia or ischemia and subsequent reperfusion.
There is also insufficient data from clinical or laboratory tests related to the subject under study. There are studies in animal models, mainly rats, mice, rabbits, quails, piglets, and non-human primates, but there is still lack of information about perinatal and neonatal age in humans. These studies provide important knowledge, but leave many questions unanswered about the pathogenetic mechanisms of life-threatening conditions associated with perinatal asphyxia, especially at the microbial level. Most research data focus on the effect of hypoxia/asphyxia on the brain or gut–brain axis, with an extensive analysis of the pathophysiology of ischemic brain injury. In contrast, data on the effect of these conditions on other organs, such as GIT, liver, spleen, lungs and systemic inflammation, are scarce.
Due to biochemical complexities beyond the scope of studies in single-cell cultures, animal models are essential to understand the mechanisms involved in conditions that resemble the pathophysiology of NEC and the effects of inflammation on the immature intestinal tract [55,56,57], as shown in Table 6.

Table 6.
Investigation of pathogenetic mechanisms of NEC and other common GIT diseases of neonates associated with microbiota and the use of different research methods.
A fundamental approach to this is the use of in vivo experimental neonatal rodents and pigs. Much of the experimental evidence derives from models in rodents, and show the protective effect of breast milk and the role of specific molecular mechanisms involved in premature innate immune and intestinal injury response. This type of model tests how genetic disruption of specific genes alters the NEC phenotype. More recently, pigs have emerged as an animal model of NEC and are used to establish the role of bacterial colonization, prematurity, parenteral nutrition and antibiotic therapy [58]. The pig is the closest animal to the human, in terms of both anatomy and physiology, and therefore it allows simulation and projection of findings for humans. The hemodynamic parameters in neonatal piglets (heart rate and blood pressure) are also comparable to those of humans. In addition, the pathophysiology of the response to neonatal asphyxia is similar, which consolidates the role of the pig as an experimental animal in the study of the effects of the latter. Furthermore, the similarities between pigs and humans extend to the size of various organs and immune mechanisms [59].
The purpose of this review was mainly to highlight: (a) the effect of a systemic situation (asphyxia) on a system/tissue and the human body as a whole (due to the bacterial translocation, if any); (b) the study of existing data on physiology–pathophysiology. Given the complexity of the effect of asphyxia and the constant interaction between cells and systems of a living multicellular organism, mediated by endogenous chemicals and signaling molecules, it would be virtually impossible for an in vitro model to offer conclusions.
The major limitation of this study is the lack of a systematic analysis of the studies’ quality, and notably the majority of the papers involved in this study are reviews (non-systematic). Moreover, there are numerous studies presenting results of in vitro experiments and experiments on animal models, which are considered of low evidence in the evidence based medicine pyramid. In Appendix A studies involved in this systematic review are presented along with the study type and the level of evidence according to the evidence based medicine pyramid [61]. Another issue of our study, and in general studies of this type, due to ethical reasons, is that the vast majority are either in vitro or based on animal models, and therefore are certainly pre-clinical and, moreover, are considered of low evidential quality. Notably, among the analyzed studies, only one was on human subjects [50]. To our knowledge, this is the first review performed in a systematic manner.
5. Conclusions
In conclusion, it is clear that further scientific studies using multicellular organisms are needed. Each animal has distinct advantages and disadvantages related to its viability, body size, genetic differences, and cost. The choice of the kind of animal model is strongly influenced by the scientific question that the researchers seek to answer [60]. As analyzed above, pigs seem to be a sufficient option in this case, although no model may perfectly mimic human diseases such as NEC. Thus, it will be possible partially to generalize our conclusions on the human body, where communication between different cell and tissue types is complex and cannot be artificially simulated at present.
Author Contributions
Conceptualization, N.I., T.X., D.-I.M.; methodology, D.-I.M., A.P., Z.I., T.B., G.K.; data curation, D.-I.M., A.P.; Formal analysis: D.-I.M., R.S.; investigation: D.-I.M., Z.I., G.K., C.S.; writing—original draft preparation, D.-I.M., A.P., N.I., T.X., R.S., G.K., T.B., Z.I., C.S.; writing—review and editing, N.I., T.X., T.B.; visualization, A.P.; supervision, N.I., T.X., C.S.; project administration, N.I., T.X.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Evaluation of Studies Quality/Level of Evidence According to the Evidence Based Medicine Pyramid

Table A1.
Systemic research results on Microbial Translocation in Neonatal Hypoxic/Asphyxiating conditions.
Table A1.
Systemic research results on Microbial Translocation in Neonatal Hypoxic/Asphyxiating conditions.
| Authors | Year | Study Type | Level of Evidence |
|---|---|---|---|
| International Liaison Committee on Resuscitation [16] | 2005 | Guideline | High |
| Fattuoni et al. [17] | 2015 | Review | High |
| Aslam et al. [18] | 2014 | Retrospective Case control study | Medium |
| Antonucci et al. [20] | 2014 | Review | High |
| Rainaldi et al. [21] | 2016 | Review | High |
| Moreno et al. [22] | 2016 | RCT on mice (10 and 10) | Medium |

Table A2.
Intestinal barrier function and I/R injury.
Table A2.
Intestinal barrier function and I/R injury.
| Authors | Year | Study Type | Level of Evidence |
|---|---|---|---|
| Küçükaydin et al. [24] | 2000 | RCT on mice (20, 20 and d20) | Medium |
| Xu et al. [25] | 1999 | In vitro study on cell cultures | Low |
| Sun et al. [26] | 2000 | RCT on mice | Medium |
| Gkiza et al. [27] | 2012 | Case control study on piglets (24 and 22) | Medium |

Table A3.
The relationship between gut microbiota, intestinal barrier function and microbial translocation and necrotizing enterocolitis.
Table A3.
The relationship between gut microbiota, intestinal barrier function and microbial translocation and necrotizing enterocolitis.
| Authors | Year | Study Type | Level of Evidence |
|---|---|---|---|
| Ali Nayci et al. [28] | 2006 | RCT on mice (15 20 and 20) | Medium |
| Mallick et al. [29] | 2004 | Review | High |
| Berman et al. [31] | 2011 | Seminar paper/educational | High |
| Ostlie et al. [32] | 2003 | Retrospective case control study | Medium |
| Short et al. [33] | 2014 | Retrospective review | Medium |
| Iben et al. [34] | 2011 | Book chapter | High |
| Patole et al. [35] | 2017 | Review | High |
| Pammi et al. [36] | 2017 | Systematic review and meta-analysis | High |
| Grishin et al. [37] | 2016 | Review | High |
| Stevenson et al. [38] | 2006 | Personal insight | Low |
| Keely et al. [39] | 2010 | In vitro study on cell cultures | Low |
| Glover et al. [40] | 2016 | Review | High |
| Tugtekin et al. [41] | 2001 | RCT on domestic pigs (12 with toxin and 10 without) | Medium |
| Varvarousis et al. [42] | 2017 | RCT on swine (10: asphyxial cardiac arrest and 10: ventricular fibrillation cardiac arrest) | Medium |
| Lim et al. [43] | 2015 | Review | High |
| Hackam et al. [44] | 2013 | Review (description of current research) | High |
| Patel et al. [45] | 2015 | Review | High |

Table A4.
Bacterial lipopolysaccharides (LPS/Endotoxins) and their role in the inflammatory pathway of NEC.
Table A4.
Bacterial lipopolysaccharides (LPS/Endotoxins) and their role in the inflammatory pathway of NEC.
| Authors | Year | Study Type | Level of Evidence |
|---|---|---|---|
| Beutler et al. [46] | 2003 | Review | High |
| Jiang et al. [47] | 1995 | Experiment on 37 rats | Low |
| Yao et al. [48] | 1995 | RCT on rats | Medium |
| Anand et al. [49] | 2007 | Review | High |
| Wolfs et al. [50] | 2010 | Specimens obtained from the ileum of infants and adults and stored tissue material | Medium |
| Hackam et al. [51] | 2005 | Review | High |
| Cetin et al. [52] | 2004 | Cell culture | Low |
| Qureshi et al. [53] | 2005 | Experiment on rats | Low |
| Corcoran et al. [54] | 2016 | Review | High |

Table A5.
Investigation of pathogenetic mechanisms of NEC and other common GIT diseases of neonates associated with microbiota and the use of different research methods.
Table A5.
Investigation of pathogenetic mechanisms of NEC and other common GIT diseases of neonates associated with microbiota and the use of different research methods.
| Authors | Year | Study Type | Level of Evidence |
|---|---|---|---|
| Ares et al. [55] | 2018 | Review on animal models | High |
| Azcarate-Peril et al. [56] | 2011 | Study on 23 piglets | High |
| Berthe C Oosterloo et al. [57] | 2014 | Review on piglet models | High |
| Aroni et al. [58] | 2012 | Study on 10 piglets | Low |
| Sangild et al. [59] | 2013 | Review on piglet animal models | High |
| Barré-Sinoussi et al. [60] | 2015 | Opinion | Low |
References
- DiBartolomeo, M.E.; Claud, E.C. The Developing Microbiome of the Preterm Infant. Clin. Ther. 2016, 38, 733–739. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Isolauri, E.; Salminen, S. Gut microbiota: A source of novel tools to reduce the risk of human disease? Pediatric Res. 2015, 77, 182–188. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Sodhi, P.; Fiset, P. Necrotizing enterocolitis. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 1–4. [Google Scholar] [CrossRef]
- Collado, M.C.; Cernada, M.; Bauerl, C.; Vento, M.; Perez-Martinez, G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012, 3, 352–365. [Google Scholar] [CrossRef]
- Grzeskowiak, L.; Collado, M.C.; Mangani, C.; Maleta, K.; Laitinen, K.; Ashorn, P.; Isolauri, E.; Salminen, S. Distinct gut microbiota in southeastern African and northern European infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Reiter, R.J.; Antonuccio, P.; Centorrino, A.; Romeo, C.; Impellizzeri, P.; Gitto, E. Oxidative Stress-Mediated Damage in Newborns with Necrotizing Enterocolitis: A Possible Role of Melatonin. Am. J. Perinatol. 2015, 32, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Swank, G.M.; Deitch, E.A. Role of the gut in multiple organ failure: Bacterial translocation and permeability changes. World J. Surg. 1996, 20, 411–417. [Google Scholar] [CrossRef]
- Berg, R.D.; Garlington, A.W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 1979, 23, 403–411. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Evans, N.J. Assessing the practical differences between model selection methods in inferences about choice response time tasks. Psychon. Bull. Rev. 2019, 26, 1070–1098. [Google Scholar] [CrossRef] [PubMed]
- International Liaison Committee on Resuscitation. 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 7: Neonatal resuscitation. Resuscitation 2005, 67, 293–303. [Google Scholar] [CrossRef]
- Fattuoni, C.; Palmas, F.; Noto, A.; Fanos, V.; Barberini, L. Perinatal asphyxia: A review from a metabolomics perspective. Molecules 2015, 20, 7000–7016. [Google Scholar] [CrossRef]
- Aslam, H.M.; Saleem, S.; Afzal, R.; Iqbal, U.; Saleem, S.M.; Shaikh, M.W.; Shahid, N. Risk factors of birth asphyxia. Ital. J. Pediatr. 2014, 40, 94. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Antonucci, R.; Porcella, A.; Pilloni, M.D. Perinatal asphyxia in the term newborn. J. Pediatric Neonatal Individ. Med. (JPNIM) 2014, 3, e030269. [Google Scholar] [CrossRef]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Torres, M.; Sanchez-Alcoholado, L.; Cardona, F.; Almendros, I.; Gozal, D.; Montserrat, J.M.; Queipo-Ortuno, M.I.; Farre, R. Normoxic Recovery Mimicking Treatment of Sleep Apnea Does Not Reverse Intermittent Hypoxia-Induced Bacterial Dysbiosis and Low-Grade Endotoxemia in Mice. Sleep 2016, 39, 1891–1897. [Google Scholar] [CrossRef]
- Zhdanov, A.V.; Okkelman, I.A.; Golubeva, A.V.; Doerr, B.; Hyland, N.P.; Melgar, S.; Shanahan, F.; Cryan, J.F.; Papkovsky, D.B. Quantitative analysis of mucosal oxygenation using ex vivo imaging of healthy and inflamed mammalian colon tissue. Cell. Mol. Life Sci. CMLS 2017, 74, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Küçükaydin, M.; Kocaoğlu, C.; Köksal, F.; Kontaş, O. Detection of intestinal bacterial translocation in subclinical ischemia-reperfusion using the polymerase chain reaction technique. J. Pediatric Surg. 2000, 35, 41–43. [Google Scholar] [CrossRef]
- Xu, D.Z.; Lu, Q.; Kubicka, R.; Deitch, E.A. The effect of hypoxia/reoxygenation on the cellular function of intestinal epithelial cells. J. Trauma 1999, 46, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X.; Deng, X.; Borjesson, A.; Wallen, R.; Hallberg, E.; Andersson, R. Phagocytic and intestinal endothelial and epithelial barrier function during the early stage of small intestinal ischemia and reperfusion injury. Shock 2000, 13, 209–216. [Google Scholar] [CrossRef]
- Gkiza, E.; Giamarellos-Bourboulis, E.; Tsaganos, T.; Xanthos, T.; Korou, L.M.; Carrer, D.P.; Stergiopoulos, S.; Kouskouni, E.; Perrea, D.N.; Dontas, I.A. Isolation of Aerobic Bacteria in Internal Specimens from Domesticated Pigs Used in Biomedical Research and the Association with Bacterial Translocation. J. Anim. Vet. Adv. 2012, 11, 539–546. [Google Scholar] [CrossRef][Green Version]
- Nayci, A.; Atis, S.; Ersoz, G.; Polat, A.; Talas, D. Oxygen supplementation during airway instrumentation improves intestinal barrier dysfunction. J. Pediatric Surg. 2006, 41, 1386–1391. [Google Scholar] [CrossRef]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef]
- Jesse, N.; Neu, J. Necrotizing Enterocolitis: Relationship to Innate Immunity, Clinical Features, and Strategies for Prevention. Neoreviews 2006, 7, e143–e150. [Google Scholar] [CrossRef]
- Berman, L.; Moss, R.L. Necrotizing enterocolitis: An update. Semin. Fetal Neonatal Med. 2011, 16, 145–150. [Google Scholar] [CrossRef]
- Ostlie, D.J.; Spilde, T.L.; St Peter, S.D.; Sexton, N.; Miller, K.A.; Sharp, R.J.; Gittes, G.K.; Snyder, C.L. Necrotizing enterocolitis in full-term infants. J. Pediatric Surg. 2003, 38, 1039–1042. [Google Scholar] [CrossRef]
- Short, S.S.; Papillon, S.; Berel, D.; Ford, H.R.; Frykman, P.K.; Kawaguchi, A. Late onset of necrotizing enterocolitis in the full-term infant is associated with increased mortality: Results from a two-center analysis. J. Pediatric Surg. 2014, 49, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Iben, S.; Rodriguez, R.J. Neonatal Necrotizing Enterocolitis. In Pediatric Gastrointestinal and Liver Disease, 4th ed.; Wyllie, R., Hyams, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; p. 1104. [Google Scholar]
- Patole, S. Microbiota and Necrotizing Enterocolitis. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Grishin, A.; Bowling, J.; Bell, B.; Wang, J.; Ford, H.R. Roles of nitric oxide and intestinal microbiota in the pathogenesis of necrotizing enterocolitis. J. Pediatric Surg. 2016, 51, 13–17. [Google Scholar] [CrossRef]
- Stevenson, D.K.; Blakely, M.L. Historical Perspectives Necrotizing Enterocolitis: An Inherited Or Acquired Condition? Neoreviews 2006, 7, e125–e134. [Google Scholar] [CrossRef]
- Keely, S.; Glover, L.E.; Weissmueller, T.; MacManus, C.F.; Fillon, S.; Fennimore, B.; Colgan, S.P. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol. Biol. Cell 2010, 21, 538–546. [Google Scholar] [CrossRef]
- Glover, L.E.; Lee, J.S.; Colgan, S.P. Oxygen metabolism and barrier regulation in the intestinal mucosa. J. Clin. Investig. 2016, 126, 3680–3688. [Google Scholar] [CrossRef]
- Tugtekin, I.F.; Radermacher, P.; Theisen, M.; Matejovic, M.; Stehr, A.; Ploner, F.; Matura, K.; Ince, C.; Georgieff, M.; Träger, K. Increased ileal-mucosal-arterial PCO2 gap is associated with impaired villus microcirculation in endotoxic pigs. Intensive Care Med. 2001, 27, 757–766. [Google Scholar] [CrossRef]
- Varvarousis, D.; Xanthos, T.; Ferino, G.; Noto, A.; Iacovidou, N.; Mura, M.; Scano, P.; Chalkias, A.; Papalois, A.; De-Giorgio, F.; et al. Metabolomics profiling reveals different patterns in an animal model of asphyxial and dysrhythmic cardiac arrest. Sci. Rep. 2017, 7, 16575. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Golden, J.M.; Ford, H.R. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 2015, 31, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.J.; Good, M.; Sodhi, C.P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin. Pediatr. Surg. 2013, 22, 76–82. [Google Scholar] [CrossRef]
- Patel, R.M.; Denning, P.W. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatric Res. 2015, 78, 232–238. [Google Scholar] [CrossRef]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Bahrami, S.; Leichtfried, G.; Redl, H.; Ohlinger, W.; Schlag, G. Kinetics of endotoxin and tumor necrosis factor appearance in portal and systemic circulation after hemorrhagic shock in rats. Ann. Surg. 1995, 221, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.M.; Bahrami, S.; Leichtfried, G.; Redl, H.; Schlag, G. Pathogenesis of hemorrhage-induced bacteria/endotoxin translocation in rats. Effects of recombinant bactericidal/permeability-increasing protein. Ann. Surg. 1995, 221, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.J.; Leaphart, C.L.; Mollen, K.P.; Hackam, D.J. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 2007, 27, 124–133. [Google Scholar] [CrossRef]
- Wolfs, T.G.; Derikx, J.P.; Hodin, C.M.; Vanderlocht, J.; Driessen, A.; de Bruine, A.P.; Bevins, C.L.; Lasitschka, F.; Gassler, N.; van Gemert, W.G.; et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm. Bowel Dis. 2010, 16, 68–75. [Google Scholar] [CrossRef]
- Hackam, D.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 49–57. [Google Scholar] [CrossRef]
- Cetin, S.; Dunklebarger, J.; Li, J.; Boyle, P.; Ergun, O.; Qureshi, F.; Ford, H.; Upperman, J.; Watkins, S.; Hackam, D.J. Endotoxin differentially modulates the basolateral and apical sodium/proton exchangers (NHE) in enterocytes. Surgery 2004, 136, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.G.; Leaphart, C.; Cetin, S.; Li, J.; Grishin, A.; Watkins, S.; Ford, H.R.; Hackam, D.J. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology 2005, 128, 1012–1022. [Google Scholar] [CrossRef]
- Corcoran, S.E.; O’Neill, L.A. HIF1alpha and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef]
- Ares, G.J.; McElroy, S.J.; Hunter, C.J. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 29–33. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Foster, D.M.; Cadenas, M.B.; Stone, M.R.; Jacobi, S.K.; Stauffer, S.H.; Pease, A.; Gookin, J.L. Acute necrotizing enterocolitis of preterm piglets is characterized by dysbiosis of ileal mucosa-associated bacteria. Gut Microbes 2011, 2, 234–243. [Google Scholar] [CrossRef]
- Oosterloo, B.C.; Premkumar, M.; Stoll, B.; Olutoye, O.; Thymann, T.; Sangild, P.T.; Burrin, D.G. Dual purpose use of preterm piglets as a model of pediatric GI disease. Vet. Immunol. Immunopathol. 2014, 159, 156–165. [Google Scholar] [CrossRef]
- Aroni, F.; Xanthos, T.; Varsami, M.; Argyri, I.; Alexaki, A.; Stroumpoulis, K.; Lelovas, P.; Papalois, A.; Faa, G.; Fanos, V.; et al. An experimental model of neonatal normocapnic hypoxia and resuscitation in Landrace/Large White piglets. J. Matern. Fetal Neonatal Med. 2012, 25, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef]
- Barre-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).