Methods of Non-Invasive In Vivo Optical Diagnostics in the Assessment of Structural Changes in the Skin Induced by Ultraviolet Exposure in an Experimental Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modeling and Assessment of Photoaging In Vivo Using Optical Methods
2.1.1. Laboratory Animals and Experimental Design
2.1.2. Optical Diagnostics
2.1.3. Histological Examination
2.1.4. Statistical Analysis
2.2. In Vivo UV Erythema Modeling and Colorimetric Assay
2.2.1. Laboratory Animals and Experimental Design
2.2.2. Evaluation of the Erythema Index
3. Results and Discussion
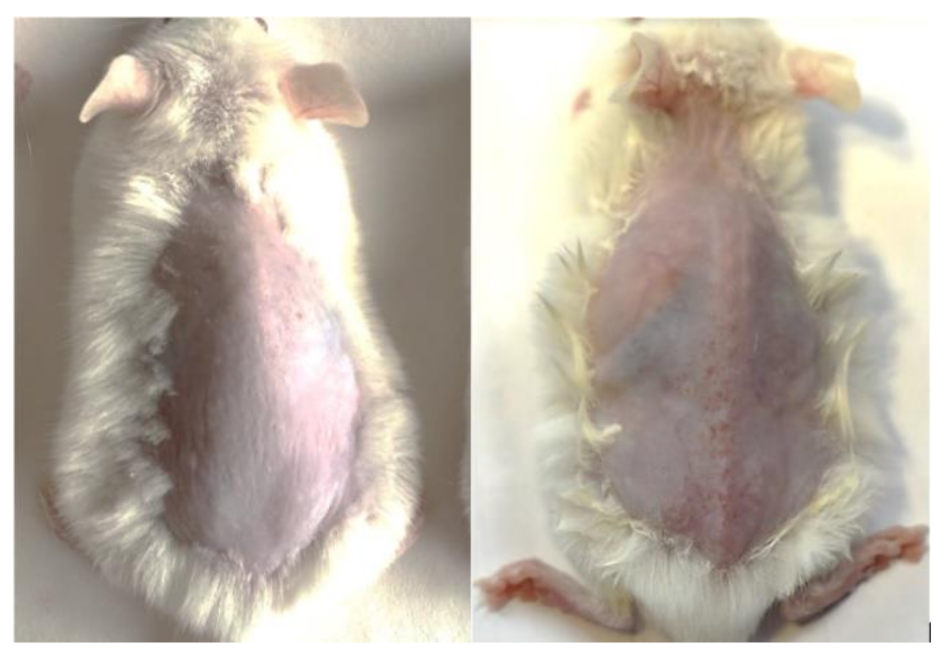
3.1. Photoaging
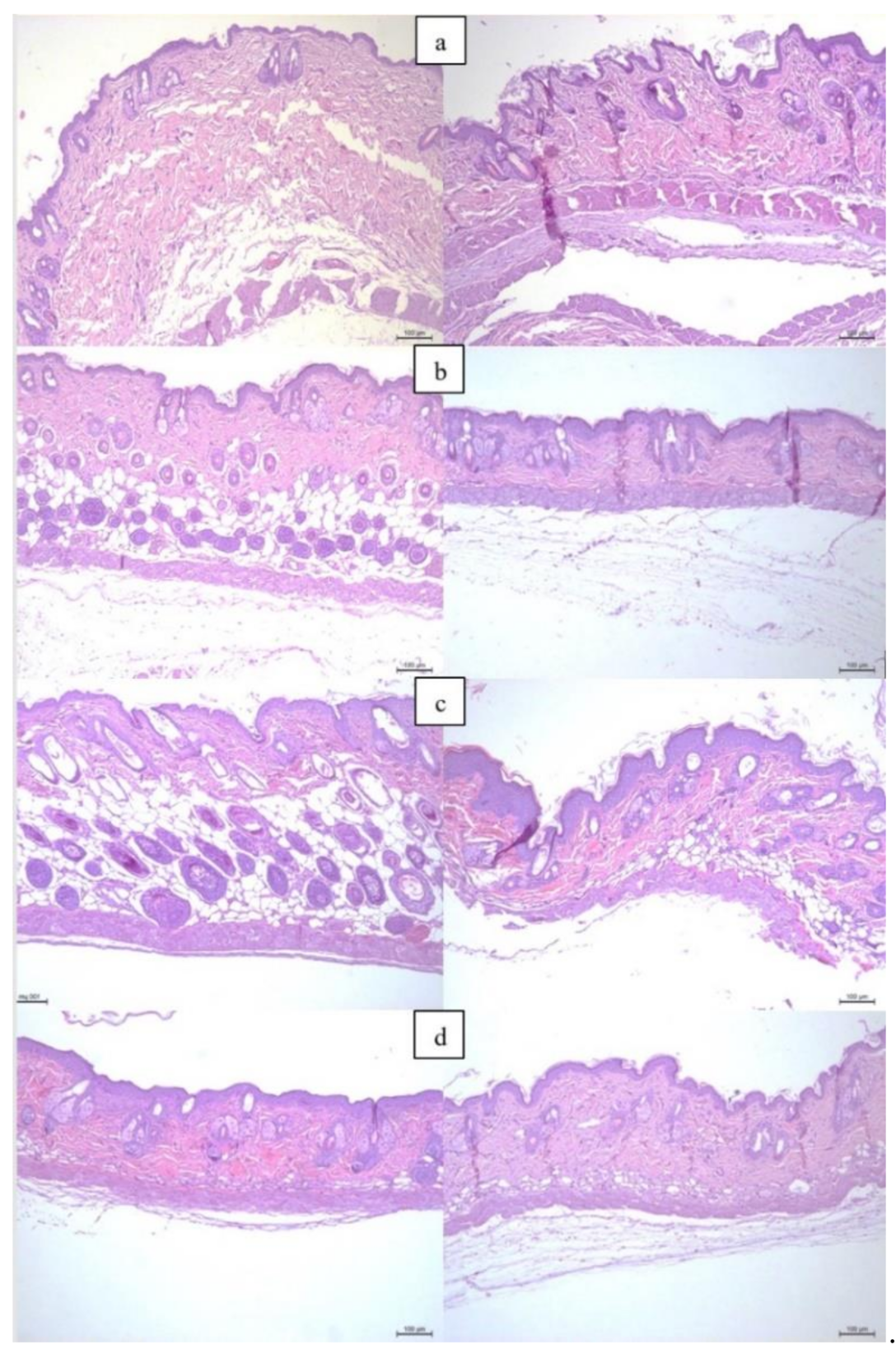
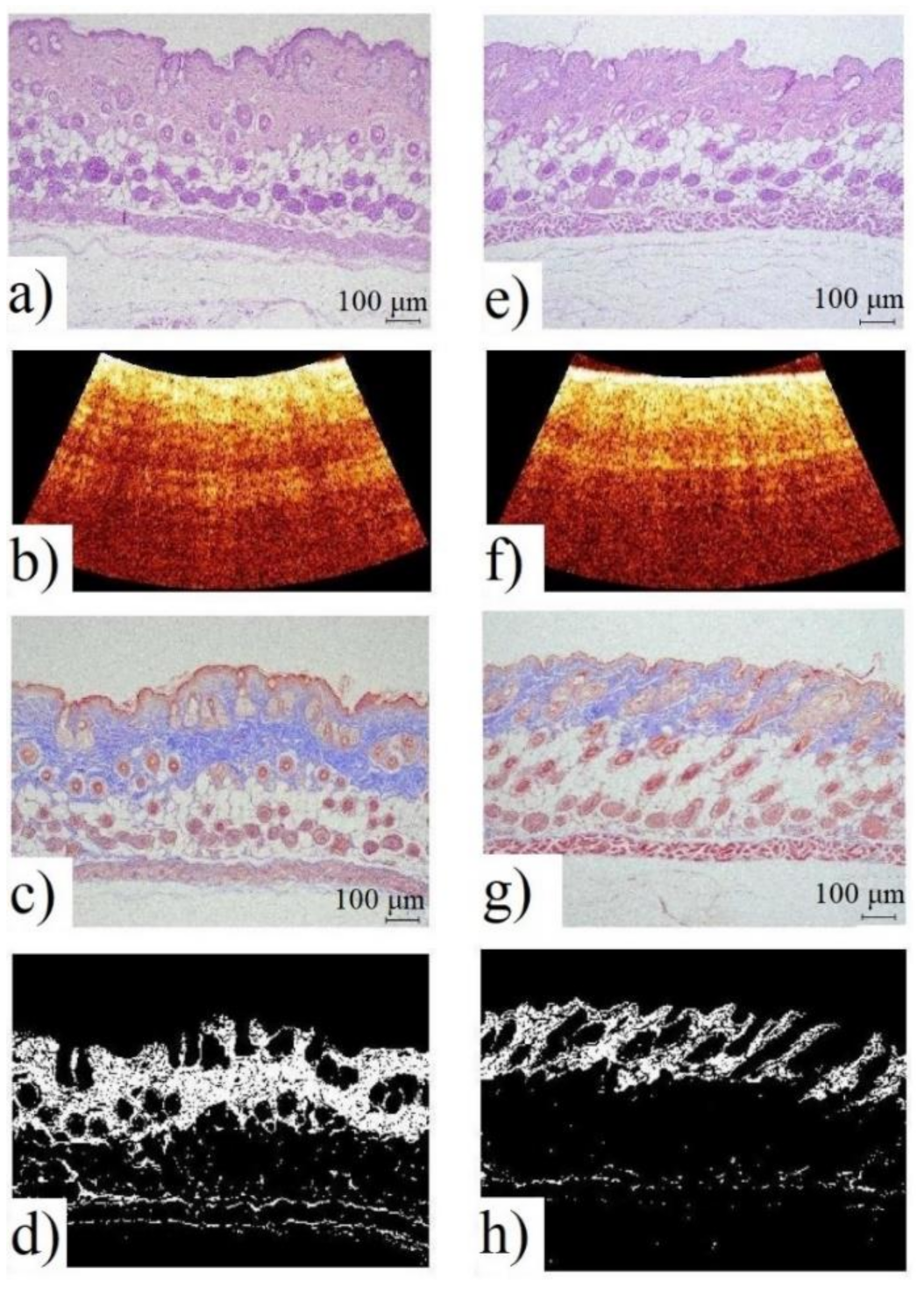
3.1.1. Histological Examination
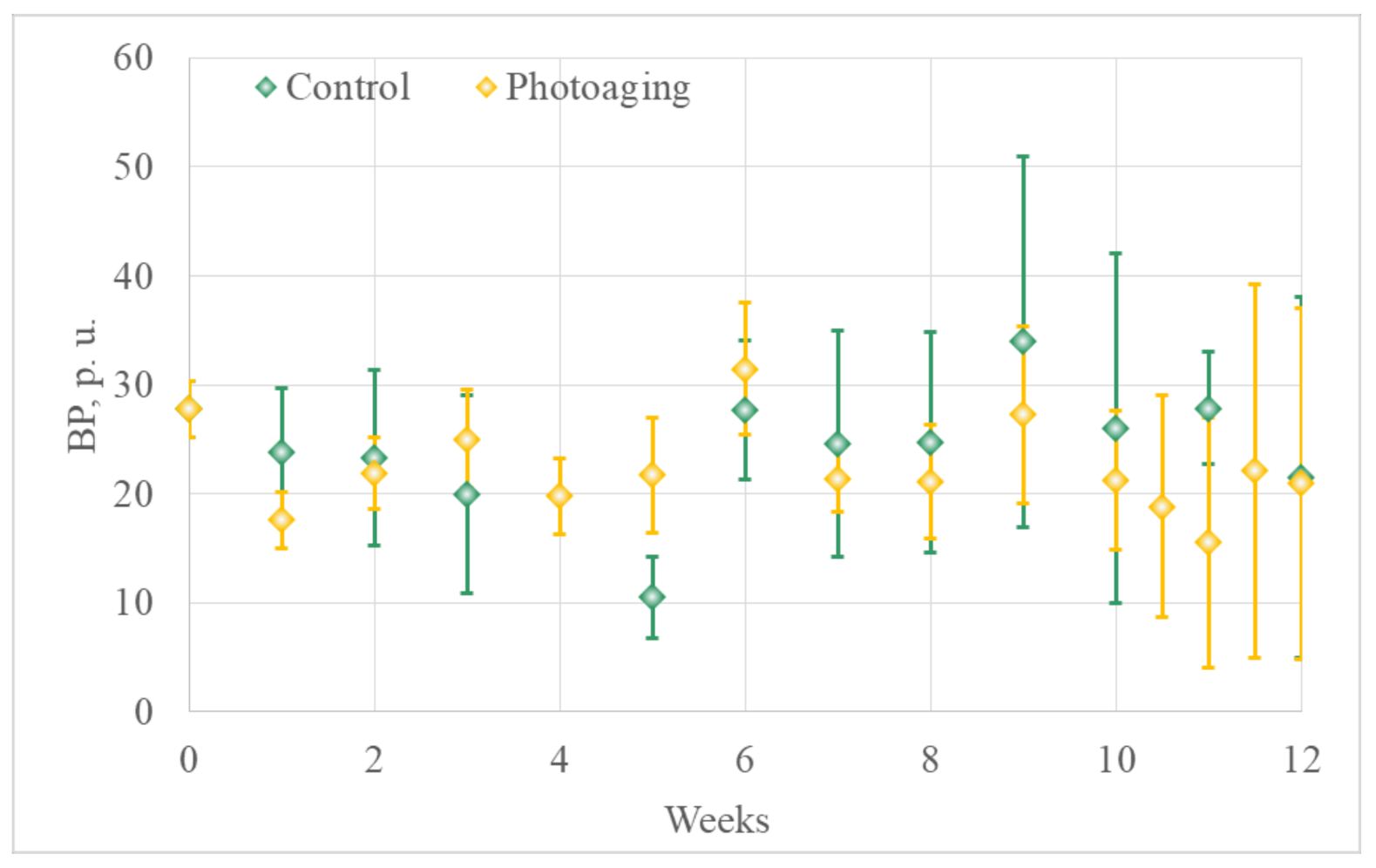
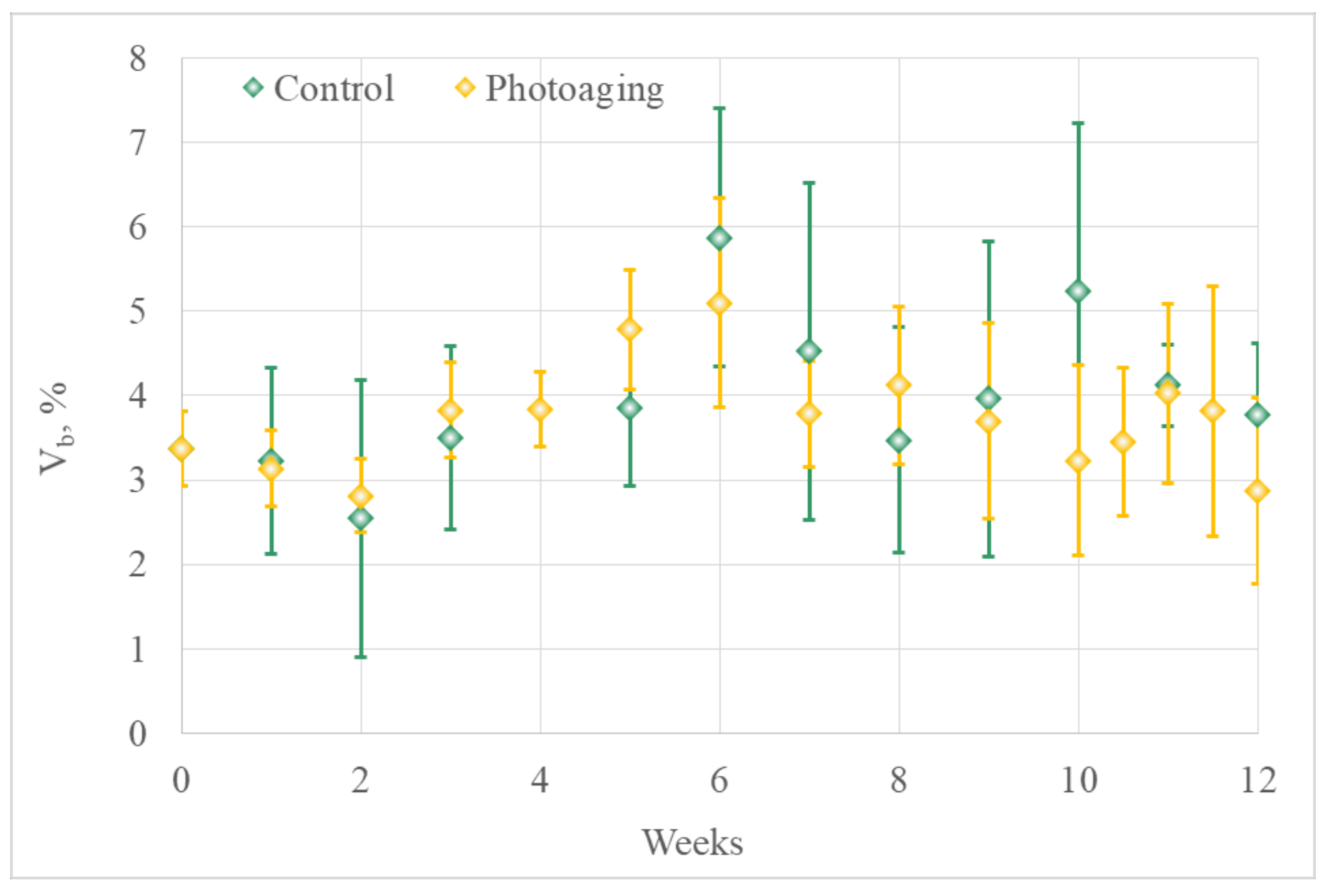
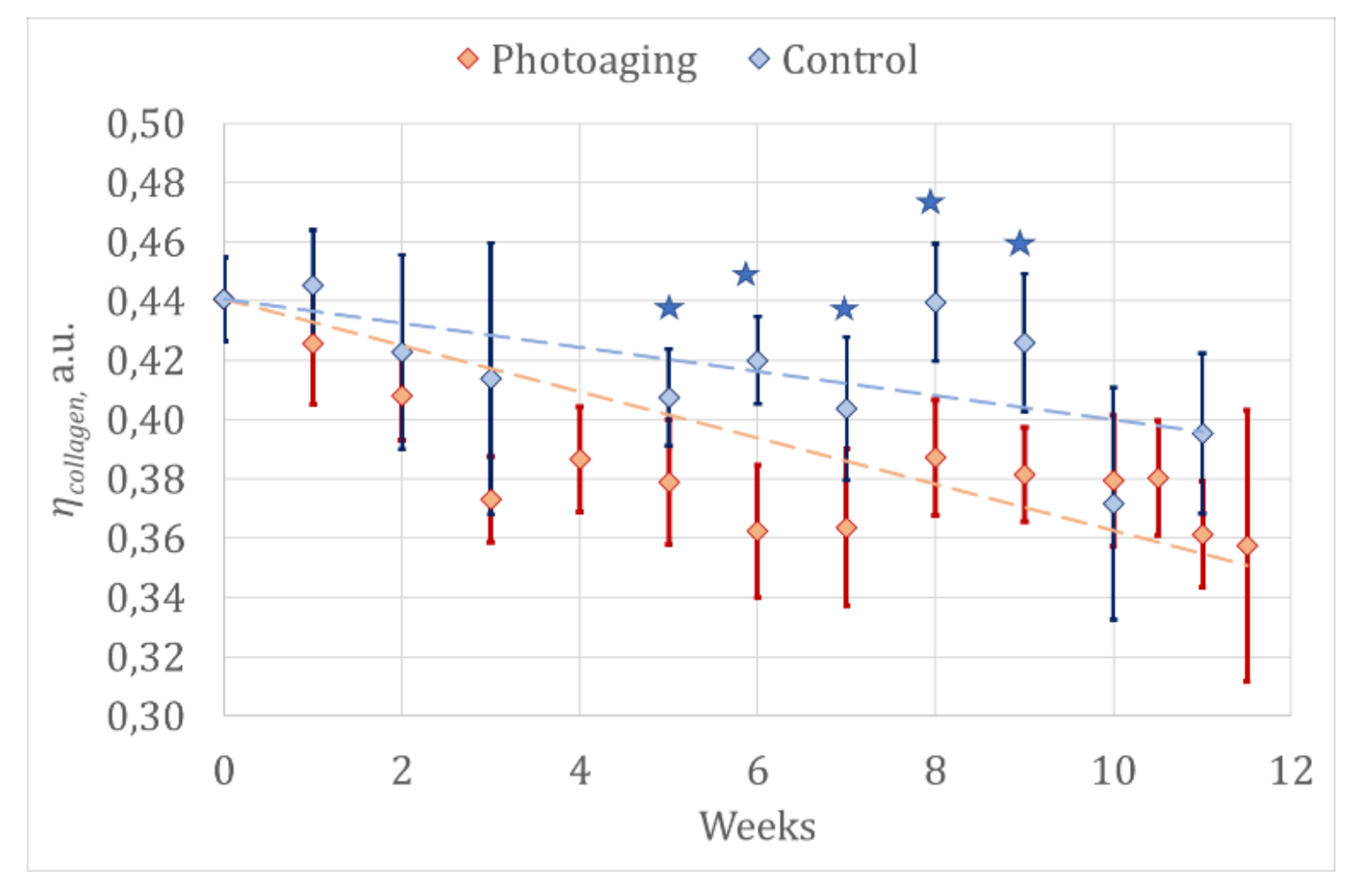
3.1.2. Optical Diagnostics Results
- Laser Doppler flowmetry (LDF) and optical tissue oximetry
- 2.
- The comparison of the results of optical diagnostics and histological examination
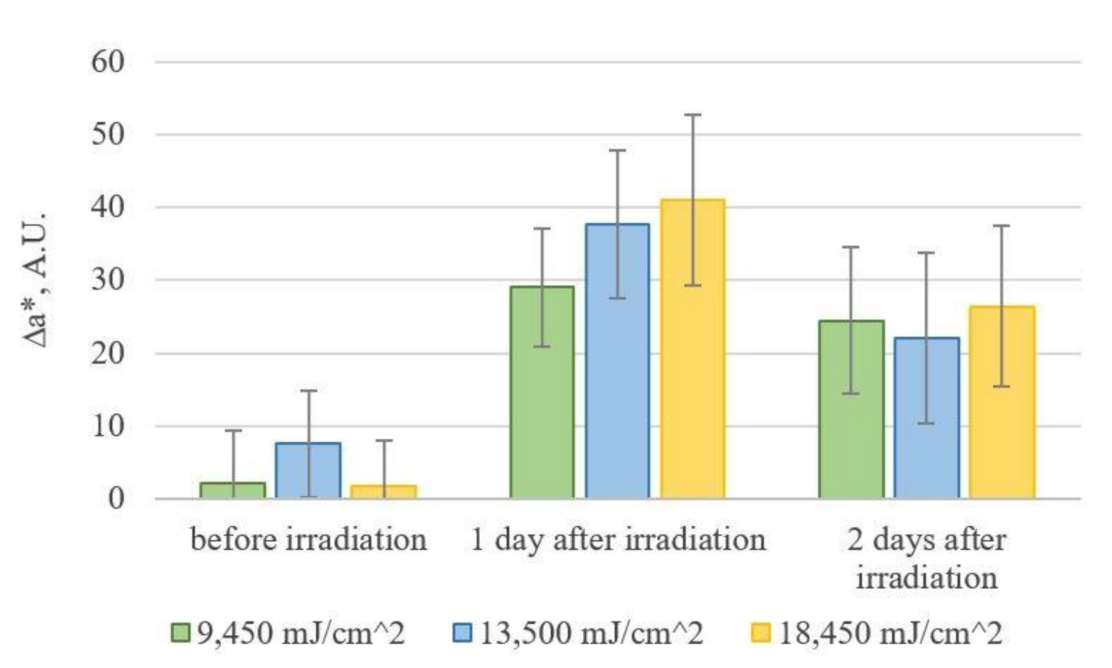
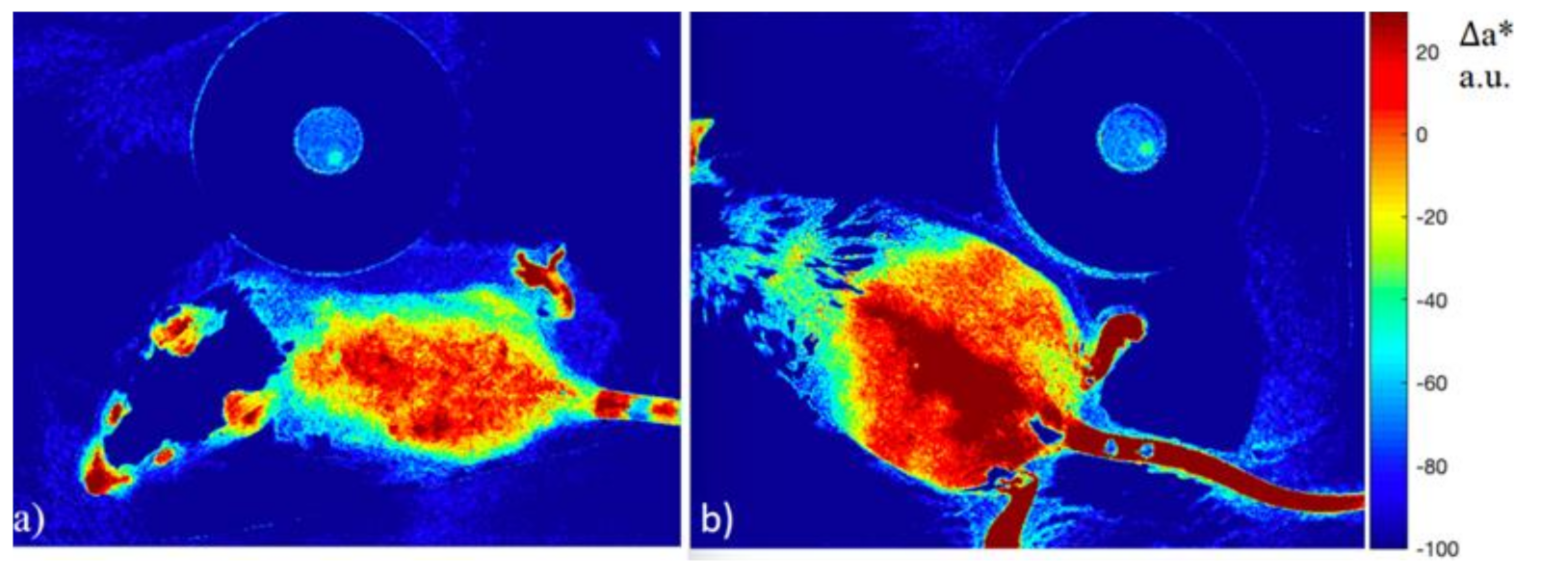
3.2. Colorimetric Assay of Erythema from an Image Using a Digital RGB Camera
3.3. Limitations of Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
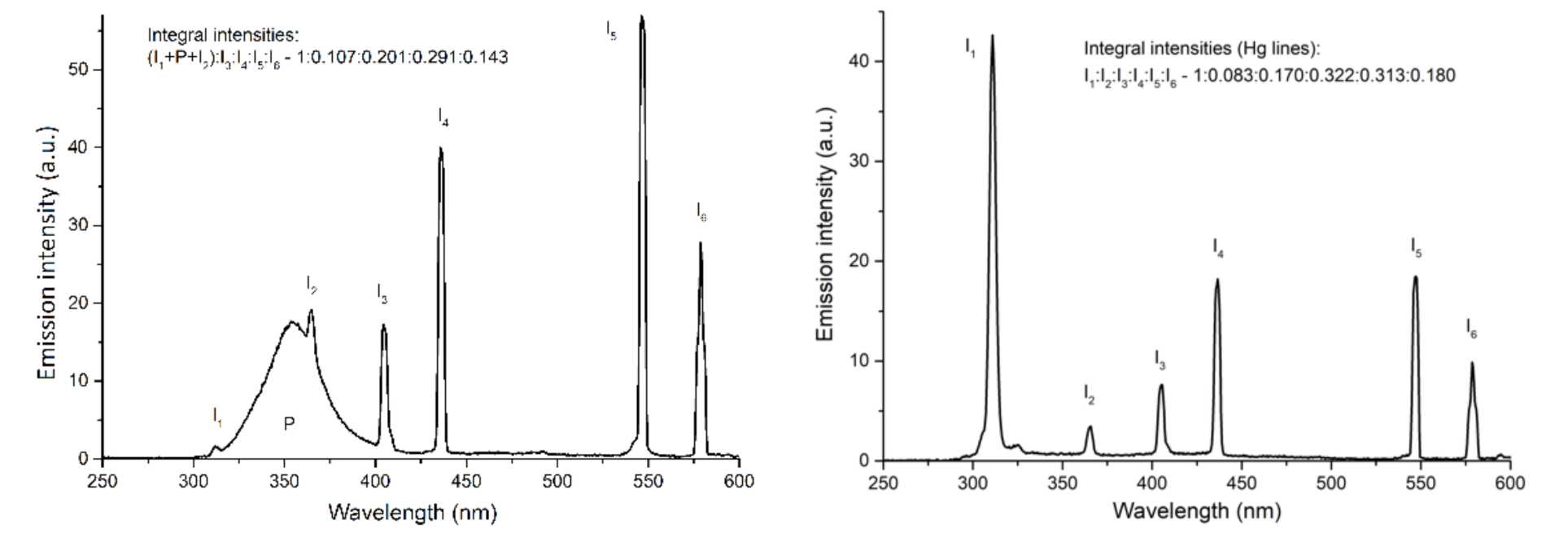
Appendix B
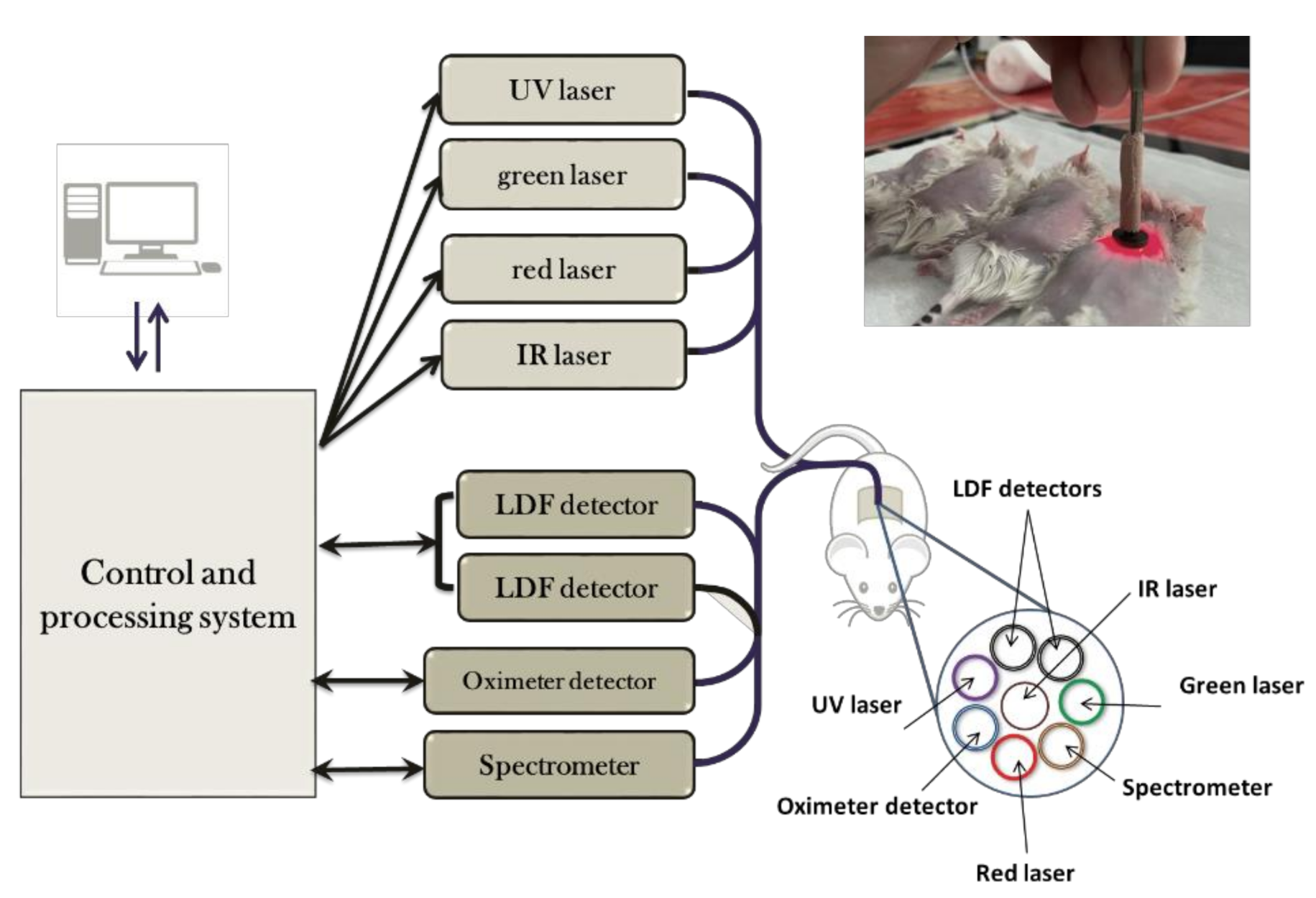
Appendix C
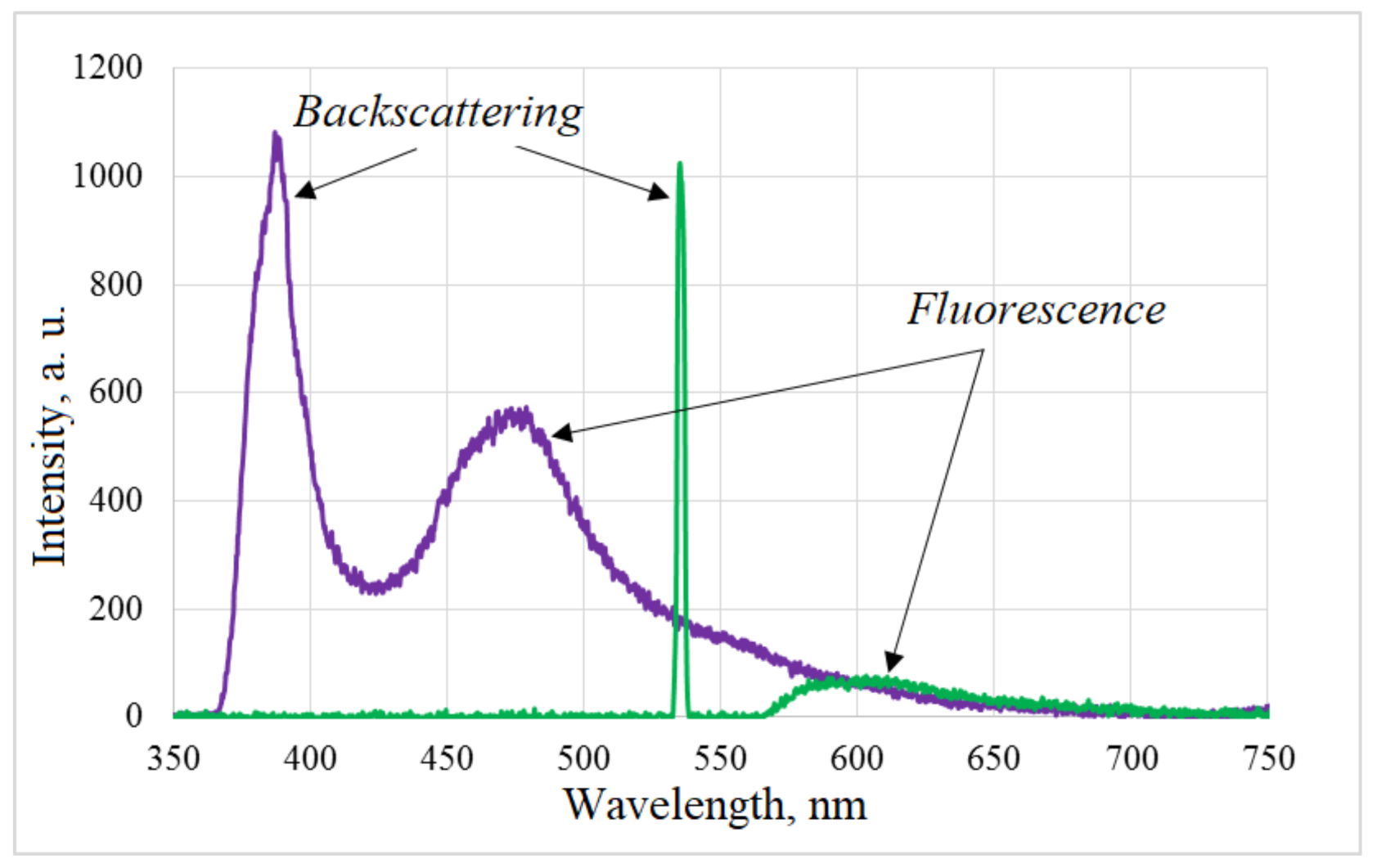
Appendix D
Appendix E
References
- Gallagher, R.P.; Lee, T.K. Adverse effects of ultraviolet radiation: A brief review. Prog. Biophys. Mol. Biol. 2006, 92, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.S.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Molecular mechanisms of photocarcinogenesis. Front. Biosci. 2002, 7, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Queille, S.; Luron, L.; Spatz, A.; Avril, M.F.; Ribrag, V.; Duvillard, P.; Hiesse, C.; Sarasin, A.; Daya-Grosjean, L. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis 2007, 28, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.; Ordóñez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010, 463, 191–196. [Google Scholar] [CrossRef]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef]
- Stein, C.; Hesse. The Action of Uviol Light on the Skin, and its Therapeutic Use in Dermatology. Arch. Roentgen Ray 1907, 12, 70–78. [Google Scholar] [CrossRef]
- Goeckerman, W.H. Treatment of psoriasis: Continued observations on the use of crude coal tar and ultraviolet light. Arch. Dermatol. Syphilol. 1931, 24, 446–450. [Google Scholar] [CrossRef]
- Krenitsky, A.; Ghamrawi, R.I.; Feldman, S.R. Phototherapy: A Review and Update of Treatment Options in Dermatology. Curr. Dermatol. Rep. 2020, 9, 10–21. [Google Scholar] [CrossRef]
- Heckman, C.J.; Chandler, R.; Kloss, J.D.; Benson, A.; Rooney, D.; Munshi, T.; Darlow, S.D.; Perlis, C.; Manne, S.L.; Oslin, D.W. Minimal Erythema Dose (MED) testing. J. Vis. Exp. 2013, 75, e50175. [Google Scholar] [CrossRef]
- Crisan, D.; Lupsor, M.; Boca, A.; Crisan, M.; Badea, R. Ultrasonographic assessment of skin structure according to age. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 519. [Google Scholar] [CrossRef]
- Cinotti, E.; Bovi, C.; Tonini, G.; Labeille, B.; Heusèle, C.; Nizard, C.; Schnebert, S.; Aubailly, S.; Barthélémy, J.C.; Cambazard, F.; et al. Structural skin changes in elderly people investigated by reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Casari, A.; Beretti, F.; Cesinaro, A.M.; Pellacani, G. Skin aging: In Vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J. Am. Acad. Dermatol. 2013, 68, e73–e82. [Google Scholar] [CrossRef]
- de Paula Campos, C.; de Paula D’Almeida, C.; Nogueira, M.S.; Moriyama, L.T.; Pratavieira, S.; Kurachi, C. Fluorescence spectroscopy in the visible range for the assessment of UVB radiation effects in hairless mice skin. Photodiagnosis Photodyn. Ther. 2017, 20, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.S.; Kurachi, C. Assessing the photoaging process at sun exposed and non-exposed skin using fluorescence lifetime spectroscopy. Proc. SPIE 2016, 9703, 97031W. [Google Scholar] [CrossRef]
- Kollias, N.; Gillies, R.M.; Moran, M.; Kochevar, I.E.; Anderson, R.R. Endogenous Skin Fluorescence Includes Bands that may Serve as Quantitative Markers of Aging and Photoaging. J. Investig. Dermatol. 1998, 111, 776–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdlaty, R.; Hayward, J.; Farrell, T.; Fang, Q. Skin erythema and pigmentation: A review of optical assessment techniques. Photodiagnosis Photodyn. Ther. 2021, 33, 102–127. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.H. The ultraviolet-irradiated hairless mouse: A model for photoaging. J. Am. Acad. Dermatol. 1989, 21, 623–631. [Google Scholar] [CrossRef]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D.; Learn, D.B. The hairless mouse model of photoaging: Evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem. Photobiol. 1992, 56, 505–511. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, E.; Sunkari, C.; Neidrauer, M.; Klement, J.F.; Uitto, J. Noninvasive Assessment of UV-induced Skin Damage: Comparison of Optical Measurements to Histology and MMP Expression. Photochem. Photobiol. 2010, 86, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, M.N.; Ranganathaiah, C. Chemical and photochemical degradation of human hair: A free-volume microprobe study. J. Photochem. Photobiol. B 2010, 101, 286–294. [Google Scholar] [CrossRef]
- Johns Hopkins University Animal Care and Use: Policies and Guidelines. Available online: https://web.jhu.edu/animalcare/policies/ (accessed on 1 July 2021).
- Petritskaya, E.N.; Kulikov, D.A.; Rogatkin, D.A.; Guseva, I.A.; Kulikova, P.A. Use of fluorescence spectroscopy for diagnosis of hypoxia and inflammatory processes in tissue. J. Opt. Technol. 2015, 82, 810–814. [Google Scholar] [CrossRef]
- Bachmann, L.; Zezell, D.; Ribeiro, A.; Gomes, L.; Ito, A. Fluorescence Spectroscopy of Biological Tissues—A Review. Appl. Spectrosc. Rev. 2006, 41, 575–590. [Google Scholar] [CrossRef]
- Smirnova, O.D.; Rogatkin, D.A.; Litvinova, K.S. Collagen as in vivo quantitative fluorescent biomarkers of abnormal tissue changes. J. Innov. Opt. Health Sci. 2012, 5, 1250010. [Google Scholar] [CrossRef]
- Rogatkin, D.A.; Lapaeva, L.G.; Petritskaya, E.N.; Sidorov, V.V.; Shumskiy, V.I. Multifunctional Laser Noninvasive Spectroscopic System for Medical Diagnostics and Metrological Provisions for That. In Proceedings of the SPIE-OSA Biomedical Optics (Optical Society of America 2009), Munich, Germany, 14–18 June; 2009. [Google Scholar] [CrossRef]
- Ferrante di Ruffano, L.; Dinnes, J.; Deeks, J.J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013189. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Fotouhi, A.; Turk, D.; Sriranga, P.; Rajabi-Estarabadi, A.; Nouri, K.; Daveluy, S.; Mehregan, D.; Nasiriavanaki, M. OCT image atlas of healthy skin on sun-exposed areas. Skin Res. Technol. 2018, 24, 570–586. [Google Scholar] [CrossRef]
- Kunisada, M.; Kumimoto, H.; Ishizaki, K.; Sakumi, K.; Nakabeppu, Y.; Nishigori, C. Narrow-band UVB induces more carcinogenic skin tumors than broad-band UVB through the formation of cyclobutane pyrimidine dimer. J. Investig. Dermatol. 2007, 127, 2865–2871. [Google Scholar] [CrossRef]
- El-Ghorr, A.A.; Norval, M. Biological effects of narrow-band (311 nm TL01) UVB irradiation: A review. J. Photochem. Photobiol. B 1997, 38, 99–106. [Google Scholar] [CrossRef]
- Jung, B.; Choi, B.; Durkin, A.J.; Kelly, K.M.; Nelson, J.S. Characterization of port wine stain skin erythema and melanin content using cross-polarized diffuse reflectance imaging. Lasers Surg. Med. 2004, 34, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatas, G.N.; Zmudzka, B.Z.; Kollias, N.; Beer, J.Z. Non-invasive measurements of skin pigmentation in situ. Pigment. Cell Res. 2004, 17, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Piérard, G.E. EEMCO guidance for the assessment of skin colour. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 1–11. [Google Scholar] [CrossRef]
- Hunt, R.W.G.; Pointer, M.R. Measuring Colour, 4th ed.; John Wiley & Sons: New York, NY, USA, 2011; ISBN 978-1-119-97537-3. [Google Scholar]
- Kong, S.Z.; Shi, X.G.; Feng, X.X.; Li, W.J.; Liu, W.H.; Chen, Z.W.; Xie, J.H.; Lai, X.P.; Zhang, S.X.; Zhang, X.J.; et al. Inhibitory effect of hydroxysafflor yellow a on mouse skin photoaging induced by ultraviolet irradiation. Rejuvenation Res. 2013, 16, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Porto, B.N.; Alves, L.S.; Fernández, P.L.; Dutra, T.P.; Figueiredo, R.T.; Graça-Souza, A.V.; Bozza, M.T. Heme Induces Neutrophil Migration and Reactive Oxygen Species Generation through Signaling Pathways Characteristic of Chemotactic Receptors. J. Biol. Chem. 2007, 282, 24430–24436. [Google Scholar] [CrossRef] [Green Version]
- Schneckenburger, H.; Lang, M.; Köllner, T.; Rück, A.; Herzog, M.; Hörauf, H.; Steiner, R. Fluorescence spectra and microscopic imaging of porphyrins in single cells and tissues. Laser Med. Sci. 1989, 4, 159–166. [Google Scholar] [CrossRef]
- Makmatov-Rys, M.B.; Raznitsyna, I.A.; Mosalskaya, D.V.; Bobrov, M.A.; Ivleva, A.L.; Sekirin, A.B.; Kaznacheeva, E.V.; Gureeva, M.A.; Molochkov, A.V.; Rogatkin, D.A. Application of laser fluorescence spectroscopy in non-invasive assessment of ultraviolet-induced skin immune response in vivo. J. Phys. Conf. Ser. 2020, 1560, 012075. [Google Scholar] [CrossRef]
- Leffell, D.J.; Stetz, M.L.; Milstone, L.M.; Deckelbaum, L.I. In vivo fluorescence of human skin. A potential marker of photoaging. Arch Dermatol. 1988, 124, 1514–1518. [Google Scholar] [CrossRef]
- Jones, J.D.; Ramser, H.E.; Woessner, A.E.; Veves, A.; Quinn, K.P. Quantifying Age-Related Changes in Skin Wound Metabolism Using In Vivo Multiphoton Microscopy. Adv. Wound Care 2020, 9, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Leithäuser, B.; Landgraf, H.; Jünger, M.; Franzeck, U.; Pries, A.; Sternitzky, R.; Franke, R.P.; Forconi, S.; Ehrly, A.M. Laser Doppler flux measurement for the assessment of cutaneous microcirculation—Critical remarks. Clin. Hemorheol. Microcirc. 2013, 55, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.J.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 15885. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mac-Mary, L.; Sainthillier, J.M.; Nouveau, S.; de Lacharriere, O.; Humbert, P. Age-Related Changes of the Cutaneous Microcirculation in vivo. Gerontology 2006, 52, 142–153. [Google Scholar] [CrossRef]
- Heinrich, U.; Moore, C.E.; De Spirt, S.; Tronnier, H.; Stahl, W. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambichler, T.; Matip, R.; Moussa, G.; Altmeyer, P.; Hoffmann, K. In vivo data of epidermal thickness evaluated by optical coherence tomography: Effects of age, gender, skin type, and anatomic site. J. Dermatol. Sci. 2006 44, 145–152. [CrossRef]
- Gambichler, T.; Huyn, J.; Tomi, N.S.; Moussa, G.; Moll, C.; Sommer, A.; Altmeyer, P.; Hoffmann, K. A comparative pilotstudy on ultraviolet-induced skin changes assessed by noninvasive imaging techniques in vivo. Photochem. Photobiol. 2006, 82, 1103–1107. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhang, X.; Li, Z. Optical features for chronological aging and photoaging skin by optical coherence tomography. Lasers Med. Sci. 2013, 28, 445–450. [Google Scholar] [CrossRef]
- Coelho, S.G.; Miller, S.A.; Zmudzka, B.Z.; Beer, J.Z. Quantification of UV-induced erythema and pigmentation using computer-assisted digital image evaluation. Photochem. Photobiol. 2006, 82, 651–655. [Google Scholar] [CrossRef]
- Hindelang, B.; Aguirre, J.; Berezhnoi, A.; Biedermann, T.; Darsow, U.; Eberlein, B.; Ntziachristos, V. Quantification of skin sensitivity to ultraviolet radiation using ultrawideband optoacoustic mesoscopy. Br. J. Dermatol. 2021, 184, 352–354. [Google Scholar] [CrossRef]
| Week | Control Group | Photoaging Group | p-Value * |
|---|---|---|---|
| 1 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.328 |
| 2 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.401 |
| 3 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.709 |
| 5 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.123 |
| 6 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.180 |
| 7 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.037 * |
| 8 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.256 |
| 9 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.049 * |
| 10 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.776 |
| 11 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.548 |
| 12 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.400 |
| Group | Value | Standard Error | p-Value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Bottom Border | Upper Border | |||||
| Control | b | 0.431 | 0.008 | <0.001 * | 0.415 | 0.446 |
| k | −0.003 | 0.001 | 0.0021 * | −0.0053 | −0.0005 | |
| Photoaging | b | 0.420 | 0.005 | <0.001 * | 0.411 | 0.430 |
| k | −0.006 | 0.001 | <0.001 * | −0.0075 | −0.0041 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikov, D.; Makmatov-Rys, M.; Raznitsyna, I.; Glazkova, P.; Gerzhik, A.; Glazkov, A.; Andreeva, V.; Kassina, D.; Rogatkin, D. Methods of Non-Invasive In Vivo Optical Diagnostics in the Assessment of Structural Changes in the Skin Induced by Ultraviolet Exposure in an Experimental Model. Diagnostics 2021, 11, 1464. https://doi.org/10.3390/diagnostics11081464
Kulikov D, Makmatov-Rys M, Raznitsyna I, Glazkova P, Gerzhik A, Glazkov A, Andreeva V, Kassina D, Rogatkin D. Methods of Non-Invasive In Vivo Optical Diagnostics in the Assessment of Structural Changes in the Skin Induced by Ultraviolet Exposure in an Experimental Model. Diagnostics. 2021; 11(8):1464. https://doi.org/10.3390/diagnostics11081464
Chicago/Turabian StyleKulikov, Dmitry, Mikhail Makmatov-Rys, Irina Raznitsyna, Polina Glazkova, Anastasiia Gerzhik, Alexey Glazkov, Viktoriya Andreeva, Darya Kassina, and Dmitry Rogatkin. 2021. "Methods of Non-Invasive In Vivo Optical Diagnostics in the Assessment of Structural Changes in the Skin Induced by Ultraviolet Exposure in an Experimental Model" Diagnostics 11, no. 8: 1464. https://doi.org/10.3390/diagnostics11081464
APA StyleKulikov, D., Makmatov-Rys, M., Raznitsyna, I., Glazkova, P., Gerzhik, A., Glazkov, A., Andreeva, V., Kassina, D., & Rogatkin, D. (2021). Methods of Non-Invasive In Vivo Optical Diagnostics in the Assessment of Structural Changes in the Skin Induced by Ultraviolet Exposure in an Experimental Model. Diagnostics, 11(8), 1464. https://doi.org/10.3390/diagnostics11081464






