Dielectric Properties of Ovine Heart at Microwave Frequencies
Abstract
1. Introduction
2. Background
3. Materials and Methods
3.1. Measurement Method
3.2. Calibration and Validation
3.3. Heart Samples and Measurements
3.4. Fitting Model
3.5. Statistics
4. Results
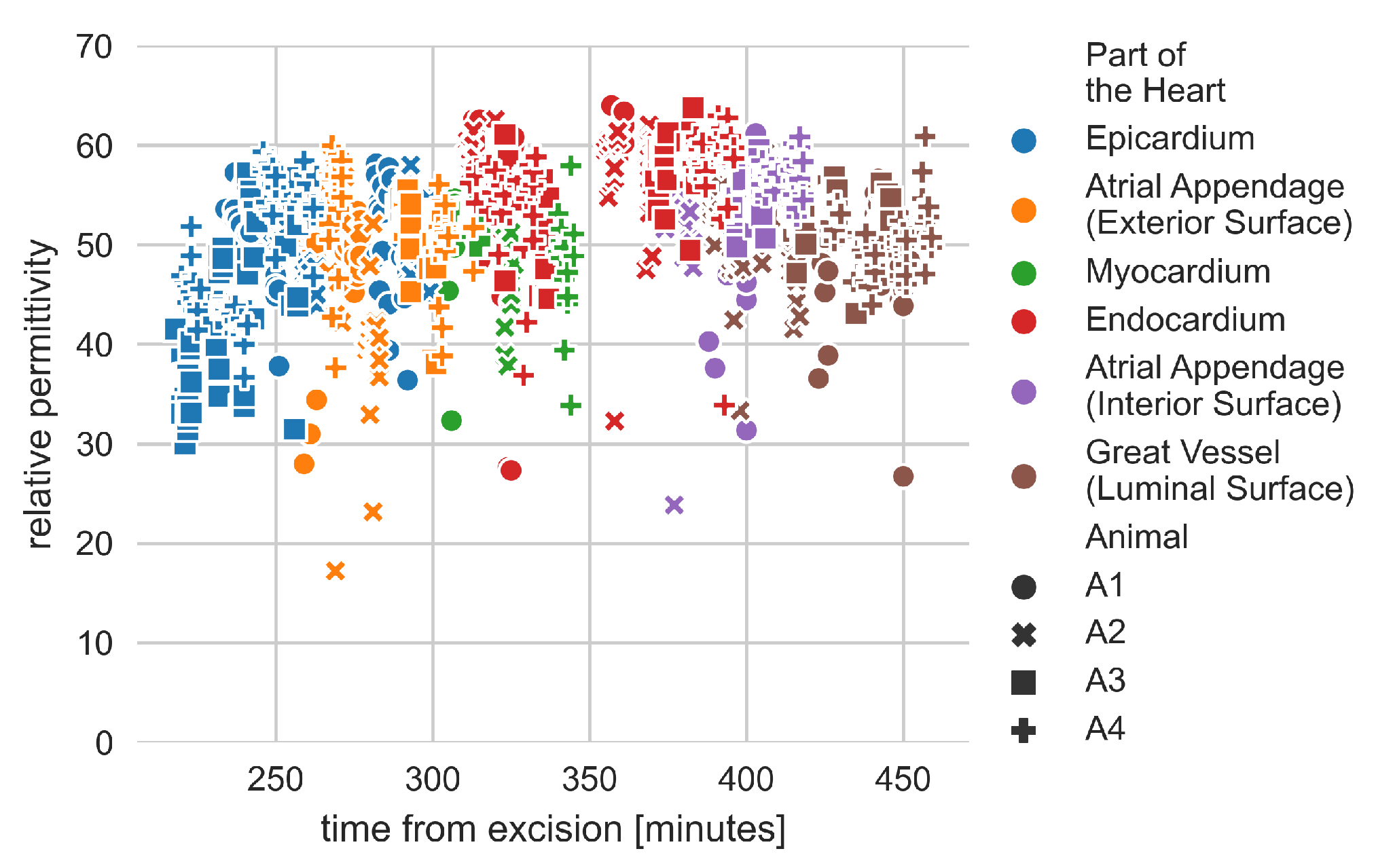
4.1. Dielectric Properties of the Heart
4.2. Debye Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| radiofrequency | RF |
| microwave | MW |
| electromagnetic | EM |
| atrial fibrillation | AF |
| vector network analyzer | VNA |
| E8362B PNA | VNA1 |
| 5063A ENA | VNA2 |
| deionized water | DIW |
| sodium chloride | NaCl |
| animal 1–animal 4 | A1–A4 |
| standard deviation | SD |
References
- La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics 2018, 8, 40. [Google Scholar] [CrossRef]
- Klauenberg, B.J.; Miklavčič, D. (Eds.) Radio Frequency Radiation Dosimetry and Its Relationship to the Biological Effects of Electromagnetic Fields; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Chiang, J.; Hynes, K.; Brace, C.L. Flow-Dependent Vascular Heat Transfer during Microwave Thermal Ablation. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 5582–5585. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Chua, T.C.; Shehata, M.; Alzahrani, N.; Al-Alem, I.; Morris, D.L. Heat Sink Effect on Tumor Ablation Characteristics as Observed in Monopolar Radiofrequency, Bipolar Radiofrequency, and Microwave, Using Ex Vivo Calf Liver Model. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Chaichanyut, M.; Tungjitkusolmun, S. Microwave Ablation Using Four-Tine Antenna: Effects of Blood Flow Velocity, Vessel Location, and Total Displacement on Porous Hepatic Cancer Tissue; Hindawi: London, UK, 2016; volume 2016, p. E4846738. ISSN 1748-670X. [Google Scholar] [CrossRef]
- Brace, C.L. Microwave Tissue Ablation: Biophysics, Technology and Applications. Crit. Rev. Biomed. Eng. 2010, 38, 65–78. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical models of microwave thermal ablation procedures. In Proceedings of the 2014 44th European Microwave Conference, Rome, Italy, 6–9 October 2014; pp. 480–483. [Google Scholar] [CrossRef]
- Brace, C.L. Radiofrequency and Microwave Ablation of the Liver, Lung, Kidney, and Bone: What Are the Differences? Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S. Acquisition of Dielectric Properties of Tissues in the Microwave Range. Ph.D. Thesis, National University of Ireland Galway, Galway, Ireland, 2019. [Google Scholar]
- Dielectric Properties of Body Tissues. Available online: http://niremf.ifac.cnr.it/tissprop/ (accessed on 14 January 2021).
- Dielectric Properties » IT’IS Foundation. Available online: https://itis.swiss/virtual-population/tissue-properties/database/dielectric-properties/ (accessed on 14 January 2021).
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Mealing, G.A.R. Dielectric properties of frog tissues in vivo and in vitro. Phys. Med. Biol. 1985, 30, 117–124. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; Jiang, Z. Measurement of the Dielectric Properties of Biological Substances Using an Improved Open-Ended Coaxial Line Resonator Method. IEEE Trans. Microw. Theory Tech. 1987, 35, 1424–1428. [Google Scholar] [CrossRef]
- Fornes-Leal, A.; Cardona, N.; Frasson, M.; Castelló-Palacios, S.; Nevárez, A.; Beltrán, V.P.; Garcia-Pardo, C. Dielectric Characterization of In Vivo Abdominal and Thoracic Tissues in the 0.5–26.5 GHz Frequency Band for Wireless Body Area Networks. IEEE Access 2019, 7, 31854–31864. [Google Scholar] [CrossRef]
- Porter, E.; Salahuddin, S.; Gioia, A.L.; Elahi, M.A.; Shahzad, A.; Kumar, A.; Kilroy, D.; O’Halloran, M. Characterization of the Dielectric Properties of the Bladder Over the Microwave Range. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 208–215. [Google Scholar] [CrossRef]
- Gray, H. Gray’s Anatomy, 41st Edition: Standring; Elsevier: London, UK, 2015. [Google Scholar]
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2006. [Google Scholar]
- Salahuddin, S.; La Gioia, A.; Shahzad, A.; Elahi, M.; Kumar, A.; Kilroy, D.; Porter, E.; O’Halloran, M. Demonstration of dielectric heterogeneity of previously assumed homogeneous tissues: Examination of the Heart. In Proceedings of the 12th European Conference on Antennas and Propagation (EuCAP 2018), London, UK, 9–13 April 2018; Institution of Engineering and Technology: London, UK, 2018. [Google Scholar] [CrossRef]
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global epidemiology of atrial fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef]
- Feinberg, W.M.; Blackshear, J.L.; Laupacis, A.; Kronmal, R.; Hart, R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 1995, 155, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Shen, W.K. Epidemiology of atrial fibrillation: A current perspective. Heart Rhythm 2007, 4, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Khan, S.; Sheikh, M.A.; Khuder, S.; Grubb, B.; Moukarbel, G.V. Catheter Ablation and Antiarrhythmic Drug Therapy as First- or Second-Line Therapy in the Management of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2014, 7, 853–860. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Jones, D.G.; Haldar, S.K.; Hussain, W.; Sharma, R.; Francis, D.P.; Rahman-Haley, S.L.; McDonagh, T.A.; Underwood, S.R.; Markides, V.; Wong, T. A Randomized Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 1894–1903. [Google Scholar] [CrossRef]
- Arbelo, E. The Atrial Fibrillation Ablation Pilot Study: A European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur. Heart J. 2014, 35, 1466–1478. [Google Scholar] [CrossRef]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar]
- Falk, R.H. Atrial Fibrillation. N. Eng. J. Med. 2001, 344, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Wonnell, T.; Stauffer, P.; Langberg, J. Evaluation of microwave and radio frequency catheter ablation in a myocardium-equivalent phantom model. IEEE Trans. Biomed. Eng. 1992, 39, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.E.; Pacifico, A.; Drago, J.L.; Kilicaslan, F.; Natale, A. Alternative Energy Sources for the Ablation of Arrhythmias. Pacing Clin. Electrophysiol. 2005, 28, 434–443. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection. Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Stauffer, P.R.; Neufeld, E.; Maccarini, P.F.; Kyriakou, A.; Canters, R.A.M.; Diederich, C.J.; Bakker, J.F.; Rhoon, G.C.V. Simulation techniques in hyperthermia treatment planning. Int. J. Hyperth. 2013, 29, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S. Interventional Cardiac Electrophysiology: A Multidisciplinary Approach, 1st ed.; Cardiotext Publishing: Minneapolis, MN, USA, 2015. [Google Scholar]
- Nath, S.; DiMarco, J.P.; Haines, D.E. Basic aspects of radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 1994, 5, 863–876. [Google Scholar] [CrossRef]
- Keane, D. New Catheter Ablation Techniques for the Treatment of Cardiac Arrhythmias. Card. Electrophys. Rev. 2002, 6, 341–348. [Google Scholar] [CrossRef]
- Keysight 85070E Dielectric Probe Kit 200 MHz to 50 GHz; Technical Overview; Keysight Technologies: Santa Rosa, CA, USA, 2017.
- Gabriel, C.; Grant, E.H.; Young, I.R. Use of time domain spectroscopy for measuring dielectric properties with a coaxial probe. J. Phys. E Sci. Instrum. 1986, 19, 843–846. [Google Scholar] [CrossRef]
- Porter, E.; Gioia, A.L.; Salahuddin, S.; Decker, S.; Shahzad, A.; Elahi, M.A.; O’Halloran, M.; Beyan, O. Minimum information for dielectric measurements of biological tissues (MINDER): A framework for repeatable and reusable data. Int. J. RF Microw. Comput. Aided Eng. 2018, 28, e21201. [Google Scholar] [CrossRef]
- European Commission. H2020 Programme Guidelines on FAIR Data Management in Horizon; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Istuk, N.; McDermott, B.; Porter, E.; Santorelli, A.; Abedi, S.; O’Halloran, M.; Joachimowicz, N.; Roussel, H. Dielectric Measurements of Ovine Heart. 2020. Available online: https://zenodo.org/record/3755616#.YFGjo9wRXIU (accessed on 14 January 2021).
- Salahuddin, S.; Porter, E.; Meaney, P.M.; O’Halloran, M. Effect of logarithmic and linear frequency scales on parametric modelling of tissue dielectric data. Biomed. Phys. Eng. Express 2017, 3, 015020. [Google Scholar] [CrossRef]
- Farrugia, L.; Wismayer, P.S.; Mangion, L.Z.; Sammut, C.V. Accurate in vivo dielectric properties of liver from 500 MHz to 40 GHz and their correlation to ex vivo measurements. Electromagn. Biol. Med. 2016, 35, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Gioia, A.L.; Santorelli, A.; O’Halloran, M. Modeling of the dielectric properties of biological tissues within the histology region. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3290–3301. [Google Scholar] [CrossRef]
- Teppati, V.; Ferrero, A.; Sayed, M. Modern RF and Microwave Measurement Techniques; Cambridge University Press: Cambridge, UK, 2013; p. 476. [Google Scholar]
- Peyman, A.; Gabriel, C.; Grant, E.H. Complex permittivity of sodium chloride solutions at microwave frequencies. Bioelectromagnetics 2007, 28, 264–274. [Google Scholar] [CrossRef]
- Vieira de Castro, W.C. The 3Rs and Good Scientific Practice. In Animal Ethics in Animal Research; Röcklinsberg, H., Olsson, I.A.S., Gjerris, M., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 15–40. [Google Scholar] [CrossRef]
- Salahuddin, S.; Porter, E.; Krewer, F.; O’ Halloran, M. Optimised analytical models of the dielectric properties of biological tissue. Med. Eng. Phys. 2017, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; Technical Report; King’S Coll London: London, UK, 1996. [Google Scholar]
- Hurt, W.D. Multiterm Debye Dispersion Relations for Permittivity of Muscle. IEEE Trans. Biomed. Eng. 1985, BME-32, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, C.; Haemmerich, D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L. Temperature-dependent dielectric properties of liver tissue measured during thermal ablation: Toward an improved numerical model. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 230–233. [Google Scholar] [CrossRef]
- Lazebnik, M.; Converse, M.C.; Booske, J.H.; Hagness, S.C. Ultrawideband temperature-dependent dielectric properties of animal liver tissue in the microwave frequency range. Phys. Med. Biol. 2006, 51, 1941–1955. [Google Scholar] [CrossRef]
- Lopresto, V.; Pinto, R.; Lovisolo, G.A.; Cavagnaro, M. Changes in the dielectric properties of Ex Vivo Bov. Liver Microw. Therm. Ablation 2.45 GHz. Phys. Med. Biol. 2012, 57, 2309–2327. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, S.; Jones, M.; Dwyer, R.; O’Halloran, M. Investigation of the effect of dehydration on tissue dielectric properties in ex-vivo measurements. Biomed. Phys. Eng. Express 2017, 3. [Google Scholar] [CrossRef]



| Mean Error (%) | Maximum Error (%) | |
|---|---|---|
| Relative permittivity | 1.36 | 4.92 |
| Conductivity | 1.78 | 3.64 |
| Animal | Part of the Heart | SD () | (S/m) | SD () (S/m) | |
|---|---|---|---|---|---|
| A1 | 53.23 | 6.80 | 1.94 | 0.28 | |
| A2 | Atrial Appendage | 51.51 | 5.94 | 1.87 | 0.22 |
| A3 | (Interior Surface) | 54.65 | 2.54 | 1.98 | 0.10 |
| A4 | 56.68 | 1.98 | 2.03 | 0.09 | |
| A1 | 48.84 | 6.50 | 1.86 | 0.27 | |
| A2 | Atrial Appendage | 44.56 | 9.40 | 1.64 | 0.35 |
| A3 | (Exterior Surface) | 51.11 | 4.11 | 1.93 | 0.15 |
| A4 | 51.19 | 5.74 | 1.91 | 0.24 | |
| A1 | 57.83 | 6.73 | 2.10 | 0.28 | |
| A2 | Endocardium | 57.03 | 4.42 | 2.02 | 0.18 |
| A3 | 56.01 | 4.38 | 2.04 | 0.18 | |
| A4 | 56.14 | 5.31 | 1.99 | 0.23 | |
| A1 | 50.41 | 4.39 | 1.90 | 0.17 | |
| A2 | Epicardium | 50.69 | 3.91 | 1.80 | 0.12 |
| A3 | 44.18 | 8.22 | 1.68 | 0.29 | |
| A4 | 49.31 | 6.28 | 1.83 | 0.17 | |
| A1 | 51.38 | 5.60 | 1.87 | 0.23 | |
| A2 | Myocardium | 46.40 | 4.22 | 1.67 | 0.17 |
| A3 | 52.89 | 1.13 | 1.90 | 0.04 | |
| A4 | 47.76 | 5.54 | 1.81 | 0.19 | |
| A1 | 50.79 | 5.29 | 1.88 | 0.20 | |
| A2 | Great Vessels | 51.10 | 4.55 | 1.85 | 0.17 |
| A3 | (Luminal Surface) | 52.00 | 2.77 | 1.93 | 0.09 |
| A4 | 50.73 | 3.08 | 1.83 | 0.10 |
| Animal | Part of the Heart | (S/m) | (ns) | (ns) | (ns) | ||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | 6.86 | 0.741 | 7.23 | 0.34 | 4.79 | 43.27 | |||
| A2 | Atrial Appendage | 13.02 | 0.263 | 137.91 | 2.15 | 4.32 | 37.74 | ||
| A3 | (Interior Surface) | 7.12 | 0.426 | 88.09 | 1.77 | 4.84 | 46.33 | ||
| A4 | 12.71 | 0.210 | 244.73 | 3.03 | 4.59 | 42.98 | |||
| A1 | 7.00 | 0.373 | 72.86 | 1.39 | 4.95 | 39.72 | |||
| A2 | Atrial Appendage | 11.60 | 0.323 | 96.24 | 2.02 | 4.30 | 31.89 | ||
| A3 | (Exterior Surface) | 7.48 | 0.690 | 11.34 | 0.42 | 4.78 | 40.64 | ||
| A4 | 14.40 | 0.704 | 19.93 | 0.97 | 3.97 | 36.31 | |||
| A1 | 7.53 | 0.124 | 311.85 | 3.41 | 4.70 | 48.61 | |||
| A2 | Endocardium | 13.38 | 0.626 | 44.30 | 1.31 | 3.51 | 43.37 | ||
| A3 | 7.36 | 0.734 | 17.80 | 0.74 | 4.36 | 47.12 | |||
| A4 | 14.22 | 0.433 | 170.62 | 3.31 | 3.97 | 41.88 | |||
| A1 | 7.70 | 0.439 | 63.45 | 1.40 | 5.13 | 40.64 | |||
| A2 | Epicardium | 12.71 | 0.154 | 254.62 | 3.34 | 4.40 | 36.84 | ||
| A3 | 6.74 | 0.550 | 14.43 | 0.54 | 4.49 | 34.95 | |||
| A4 | 14.75 | 0.141 | 207.25 | 2.64 | 4.27 | 33.65 | |||
| A1 | 8.34 | 0.538 | 25.33 | 0.86 | 4.73 | 41.52 | |||
| A2 | Myocardium | 10.49 | 0.035 | 396.00 | 5.37 | 4.81 | 35.23 | ||
| A3 | 8.12 | 0.533 | 26.58 | 0.92 | 5.21 | 43.11 | |||
| A4 | 12.75 | 0.286 | 112.78 | 2.15 | 5.02 | 34.14 | |||
| A1 | 8.76 | 0.369 | 88.96 | 1.65 | 5.00 | 40.53 | |||
| A2 | Great Vessels | 12.75 | 0.012 | 376.15 | 3.97 | 4.68 | 37.08 | ||
| A3 | (Luminal Surface) | 8.02 | 0.674 | 14.78 | 0.60 | 5.28 | 41.48 | ||
| A4 | 14.70 | 0.319 | 152.86 | 2.50 | 4.75 | 34.59 |
| Mean Error (%) | Maximum Error (%) | |
|---|---|---|
| Relative permittivity | 0.35 | 0.51 |
| Conductivity | 0.61 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ištuk, N.; Porter, E.; O’Loughlin, D.; McDermott, B.; Santorelli, A.; Abedi, S.; Joachimowicz, N.; Roussel, H.; O’Halloran, M. Dielectric Properties of Ovine Heart at Microwave Frequencies. Diagnostics 2021, 11, 531. https://doi.org/10.3390/diagnostics11030531
Ištuk N, Porter E, O’Loughlin D, McDermott B, Santorelli A, Abedi S, Joachimowicz N, Roussel H, O’Halloran M. Dielectric Properties of Ovine Heart at Microwave Frequencies. Diagnostics. 2021; 11(3):531. https://doi.org/10.3390/diagnostics11030531
Chicago/Turabian StyleIštuk, Niko, Emily Porter, Declan O’Loughlin, Barry McDermott, Adam Santorelli, Soroush Abedi, Nadine Joachimowicz, Hélène Roussel, and Martin O’Halloran. 2021. "Dielectric Properties of Ovine Heart at Microwave Frequencies" Diagnostics 11, no. 3: 531. https://doi.org/10.3390/diagnostics11030531
APA StyleIštuk, N., Porter, E., O’Loughlin, D., McDermott, B., Santorelli, A., Abedi, S., Joachimowicz, N., Roussel, H., & O’Halloran, M. (2021). Dielectric Properties of Ovine Heart at Microwave Frequencies. Diagnostics, 11(3), 531. https://doi.org/10.3390/diagnostics11030531









