Abstract
Background: to explore the diagnostic accuracy of 18F-Fluciclovine positron-emission tomography (PET) in prostate cancer (PCa), considering both primary staging prior to radical therapy, biochemical recurrence, and advanced setting. Methods: A systematic web search through Embase and Medline was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Studies performed from 2011 to 2020 were evaluated. The terms used were “PET” or “positron emission tomography” or “positron emission tomography/computed tomography” or “PET/CT” or “positron emission tomography-computed tomography” or “PET-CT” and “Fluciclovine” or “FACBC” and “prostatic neoplasms” or “prostate cancer” or “prostate carcinoma”. Only studies reporting about true positive (TP), true negative (TN), false positive (FP) and false negative (FN) findings of 18F-fluciclovine PET were considered eligible. Results: Fifteen out of 283 studies, and 697 patients, were included in the final analysis. The pooled sensitivity for 18F-Fluciclovine PET/CT for diagnosis of primary PCa was 0.83 (95% CI: 0.80–0.86), the specificity of 0.77 (95% CI: 0.74–0.80). The pooled sensitivity for preoperative LN staging was 0.57 (95% CI: 0.39–0.73) and specificity of 0.99 (95% CI: 0.94–1.00). The pooled sensitivity for the overall detection of recurrence in relapsed patients was 0.68 (95% CI: 0.63–0.73), and specificity of 0.68 (95% CI: 0.60–0.75). Conclusion: This meta-analysis showed promising results in term of sensitivity and specificity for 18F-Fluciclovine PET/CT to stage the primary lesion and in the assessment of nodal metastases, and for the detection of PCa locations in the recurrent setting. However, the limited number of studies and the broad heterogeneity in the selected cohorts and in different investigation protocols are limitation affecting the strength of these results.
1. Introduction
Prostate cancer (PCa) is the second most common cancer in the male population and represents the 15% of all cancers diagnosed [1,2] with a prevalence in elderly age of 59% (48–71%) [3]. Monitoring of the disease after radical prostatectomy (RP) is handled by measuring the Prostate Specific Antigen (PSA) serum levels, as defined by the definition of biochemical recurrence (BCR): PSA values above 0.2 ng/mL in patients treated with RP or PSA greater than 2 ng/mL above the nadir in patients treated with primary radiotherapy. However, the identification of the exact site(s) of the disease (pelvic vs. extra-pelvic recurrence) is crucial, and while PSA evaluation can optimally identify recurrent patients, it cannot provide anatomical functional information. The proper detection of the exact PCa location might significantly influence the therapeutic plan [4]. Recently, the use of positron emission tomography/computed tomography with several radiopharmaceuticals is gaining importance in this setting. In this scenario, 18F-Fluciclovine (18F-FACBC/1-amino-3fluorine 18F-fluorocyclobutane-1-carboxylic acid) has been proposed and has already received the Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval for its use for PET imaging in biochemical recurrence of PCa. 18F-Fluciclovine is a synthetic amino acid transported across mammalian cell membranes by amino acid transporters such as LAT-1 and ASCT2. The upregulation of LAT-1 and ASCT2 activities in PCa is responsible for 18F-Fluciclovine uptake in PCa lesions [5]. After its uptake in the tumor cell, 18F-Fluciclovine is not furtherly metabolized. Thus, the intensity of uptake defined as “pathological” in PET image is highly dependent by the site and size of the lesion [6,7]. The 18F-Fluciclovine uptake in PCa cell lines is higher than that of methionine, glutamine, choline, and acetate [8]. Currently, 18F-flucicovine PET is recommended by the European Association of Urology (EAU) guidelines, in the case of BCR when prostate specific membrane antigen (PSMA) PET is not available, and the PSA serum level is higher than 1 ng/mL. While PSMA PET demonstrated superior diagnostic performance compared to choline PET and 18F-fluciclovine PET [9,10], this diagnostic procedure still holds limited availability in daily clinical practice. Furthermore, a non-negligible percentage of PCa phenotypes do not express PSMA (overall attested up to 5%). Accordingly, 18F-fluciclovine still represents a valid alternative to investigate recurrent PCa patients. Finally, present EAU guidelines do not suggest the use of new generation imaging to stage the disease prior to radical surgery. However, new emerging data strongly support its use in this scenario, namely in high-risk patients [11]. Therefore, the aim of this systematic review is to explore the diagnostic accuracy of 18F-Fluciclovine PET in PCa, both considering primary staging prior to radical therapy, biochemical recurrence, and advanced setting. A dedicated meta-analysis has been performed considering the meta-data derived by the reviewing process.
2. Materials and Methods
A systematic web search through Embase and Medline was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Studies performed from 2011 to 2020 were evaluated. The terms used were “PET” or “positron emission tomography” or “positron emission tomography/computed tomography” or “PET/CT” or “positron emission tomography-computed tomography” or “PET-CT” and “Fluciclovine” or “FACBC” and “prostatic neoplasms” or “prostate cancer” or “prostate carcinoma”. Full-text publications in English were considered. The study population comprised male patients with histologically proven PCa. Studies using 18F-Fluciclovine in pre-surgery setting as well as biochemical recurrence and advanced setting were considered. Two investigators (C.C. and C.R.) independently performed the literature search. The inclusion criteria considered for each study included an assessment of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) results of 18F-Fluciclovine PET or PET / CT for the diagnosis of primary cancer, lymph node staging and the detection of recurrence. Only studies providing these information were finally included in the study. The overall quality of the studies included was independently assessed by two authors, based on the 15 modified items Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2, Table 1) [12]. Discrepancies between researchers were resolved with consensus.

Table 1.
Flow-chart for risk of bias and applicability concerns in Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2).
3. Results
3.1. Patient Population
The whole abstract revision process is displayed in Figure 1. A total of 283 studies were screened. Forty-six were excluded after reviewing their content. A further 217 studies were subsequently excluded as the content was not considered relevant considering the aim of the analysis. Five studies were subsequently excluded due to insufficient data to evaluate sensitivity and specificity. In conclusion, 15 studies matched the inclusion criteria of the systematic review and were considered eligible for the meta-analysis. The number of subjects included in the final analysis were 697 in total. A multicentric study evaluated the diagnostic accuracy of 18F-Fluciclovine PET/CT in the diagnosis of primitive and preoperative lymph node staging [13]. Six studies investigated the diagnostic role of 18F-Fluciclovine PET/CT in detecting primary prostate cancer [14,15,16,17,18,19]. Three studies evaluated the role of lymph node staging [17,20,21] and six investigated the role of 18F-Fluociclovine in recurrence of prostate disease [13,22,23,24,25,26]. A study compared 18F-Fluciclovine PET-CT with bone scan in the evaluation of bone metastases [27].
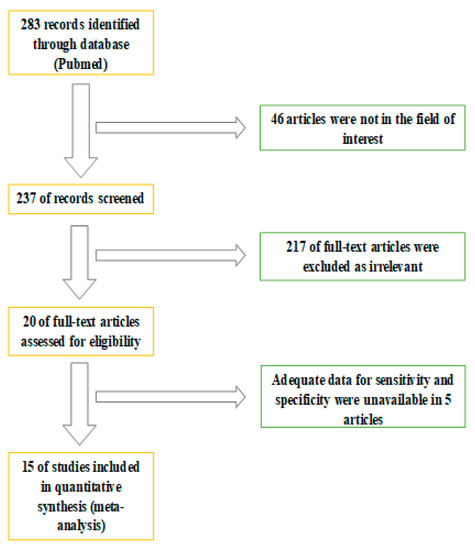
Figure 1.
Flowchart of information through the different phases of the systematic review.
3.2. Diagnostic Performance of 18F-Fluciclovine PET/CT in Different Clinical Setting
The diagnostic performance results of 18F-Fluciclovine PET/CT for the diagnosis of primary cancer, pre-operative LN staging, for prostate disease recovery, and for bone metastasis assessment are shown in Table 2. Pooled sensitivity and specificity were calculated according to a patient-based analysis.

Table 2.
Authors, patients, and study characteristics.
3.2.1. Detection of Primary Prostate Cancer Lesion
The per-patient pooled sensitivity for 18F-Fluciclovine PET/CT for the diagnosis of primary PCa was 0.83 (95% CI: 0.80–0.86), with I-square: 89.6% (Figure 2a) and a pooled of specificity of 0.77 (95% CI: 0.74–0.80) with I –square: 94.8% (Figure 2b). The likelihood ratio (LR) syntheses gave an overall LR+ of 5.22 (95% CI: 2.15–12.67) (Figure 2c) and LR– of 0.17 (95% CI: 0.8–0.38) (Figure 2d). The pooled DOR was 35.43 (95%CI: 6.66–188.55) (Figure 2e). The SROC curve indicates that the area under the cure was: 0.9224 (Figure 2f).
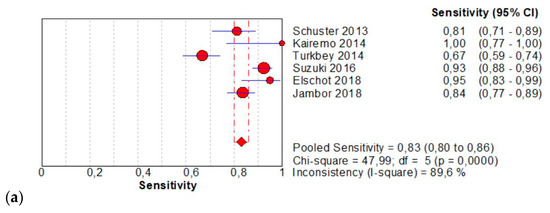
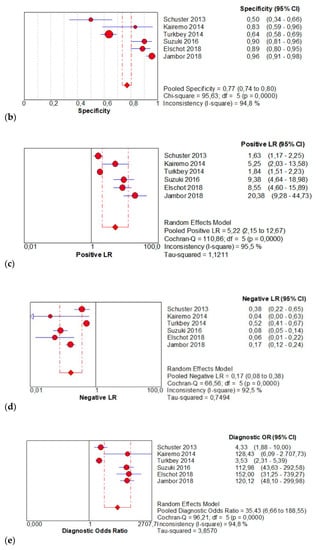
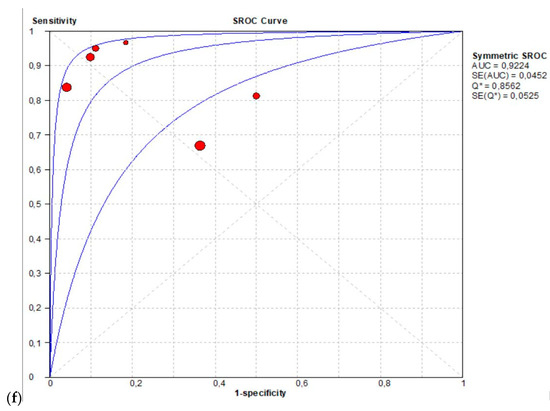
Figure 2.
(a,b) Forest plot of 18F-Fluciclovine PET/CT for the diagnosis of prostate cancer (sensibility and specificity). (c,d) Forest plot of 18F-Fluciclovine PET/CT for the diagnosis of prostate cancer (positive and negative likelihood ratio). (e,f): Pooled DOR and SROC curve of 18F-luciclovine PET/CT for the diagnosis of prostate cancer.
3.2.2. Preoperative LN Staging
The per-patient pooled sensitivity for 18F-Fluciclovine PET/CT for preoperative LN staging was 0.57 (95% CI: 0.39–0.73), with I-square: 0.0% (Figure 3a) and a pooled specificity of 0.99 (95% CI: 0.94–1.00) with I –square: 0.0% (Figure 3b). Likelihood ratio (LR) syntheses gave an overall LR+ of 22.91 (95% CI: 5.67–92.65) (Figure 3c) and LR– of 0.48 (95% CI: 0.34–0.68) (Figure 3d). The pooled DOR was 52.55 (95% CI: 11.19–246.86) (Figure 3e). The SROC curve indicates that the area under the cure was 0.9410 (Figure 3f).
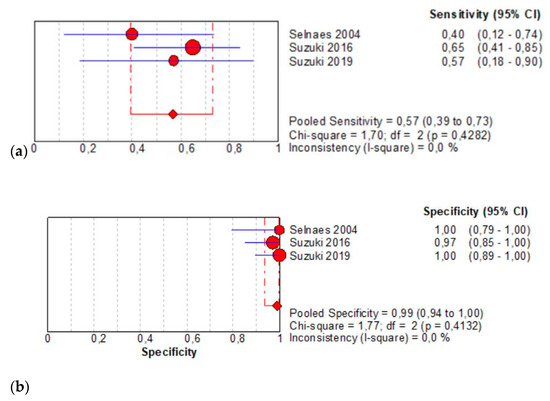
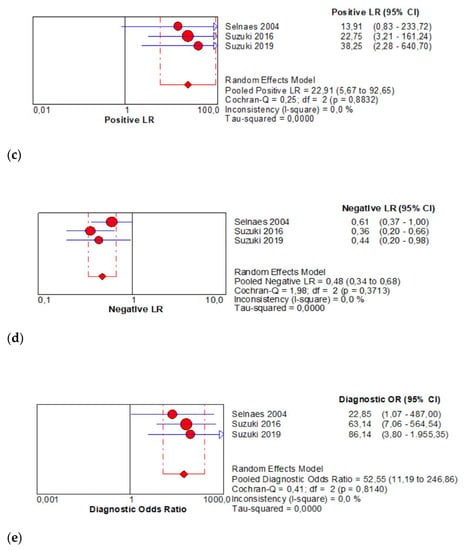
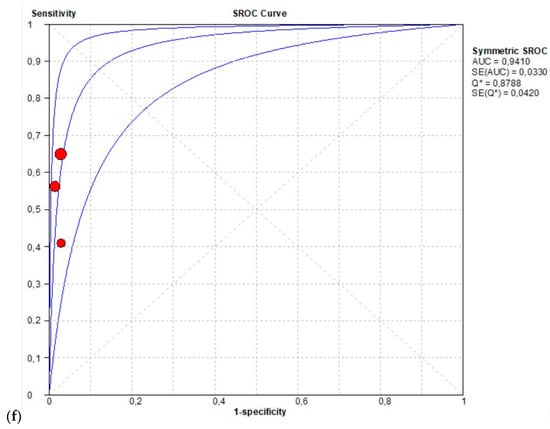
Figure 3.
(a,b) Forest plot of 18F-Fluciclovine PET/CT for preoperative LN staging (sensibility and specificity). (c,d) Forest plot of 18F-Fluciclovine PET/CT for preoperative LN staging (positive and negative likelihood ratio). (e,f) Pooled DOR and SROC curve of 18F-luciclovine PET/CT for preoperative LN staging.
3.2.3. Detection of Recurrent Disease
The per-patient pooled sensitivity for 18F-Fluciclovine PET/CT for the detection of recurrent disease was 0.68 (95% CI: 0.63–0.73), with I-square 91.8% (Figure 4a) and a pooled of specificity of 0.68 (95% CI: 0.60–0.75) with I-square 0.0% (Figure 4b). The likelihood ratio (LR) syntheses gave an overall LR+ of 2.43 (95% CI: 1.93–3.06) (Figure 4c) and LR– of 0.33 (95% CI: 0.20–0.54) (Figure 4d). The pooled DOR was 8.40 (95% CI: 5.13–13.75) (Figure 4e). The SROC curve indicates that the area under the cure was: 0.8086 (Figure 4f).
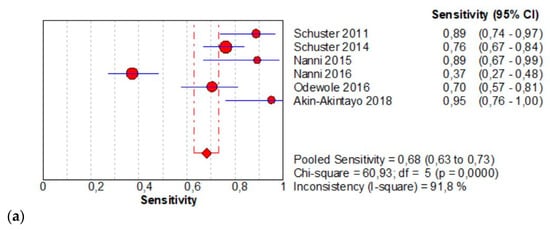
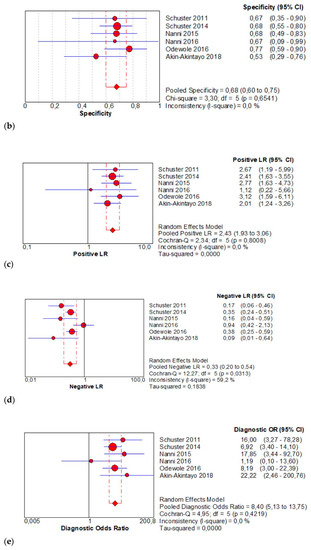
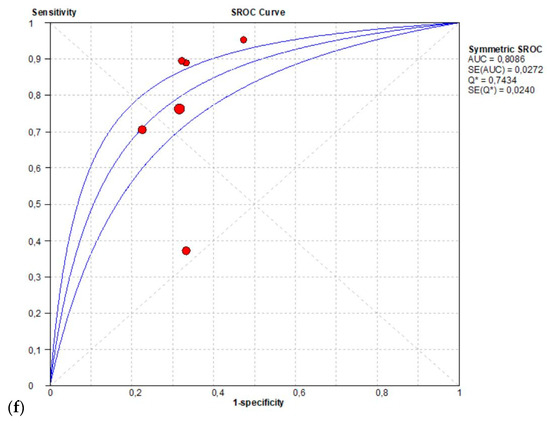
Figure 4.
(a,b) Forest plot of 18F-Fluciclovine PET/CT for the detection of recurrent disease (sensibility and specificity). (c,d) Forest plot of 18F-Fluciclovine PET/CT for the detection of recurrent disease (positive and negative likelihood ratio). (e,f) Pooled DOR and SROC curve of 18F-luciclovine PET/CT for the detection of recurrent disease.
3.2.4. Evaluation of Bone Metastases
It was not possible to perform a meta-analysis on bone metastases as there is only one study.
3.2.5. Evaluation of Heterogeneity in Meta-Regression Analysis
A heterogeneity in sensitivity was found among the studies presented (I-square > 20%). Therefore, the random-effect model was used as the most accurate model for all analyzes. The heterogeneity found is most likely due to the disparity in the number of populations included in the two or three study groups, particularly in preoperative LN staging. This is even more enveloped by the low number of studies reviewed, which was only three, and the detection of recurrent disease with a large number of false negatives reported in one study [20].
4. Discussion
18F-Fluciclovine is a synthetic amino acid used as a PET tracer for PCa. Cell uptake is a system mediated transport of neutral amino acids such as LAT 1 and ASCT2 across mammalian cell membranes, which are overexpressed in many cancer cells, including those from prostate cancer. Once inside the cell, 18F-Fluciclovine is not metabolized and is incorporated into proteins and only a small part is excreted in the urinary system. Similar to other tracers, the PET tracer 18F-Fluciclovine is a non-specific uptake by benign inflammatory prostatic tissue and in a variety of other malignancies. The aim of the study was to evaluate the diagnostic and specific sensitivity of 18F-Fluciclovine for the diagnosis of primary cancer, pre-operative LN staging, for the recovery of prostate disease and for the evaluation of bone metastases. In several studies, 18F-Fluciclovine PET/CT shows a higher uptake in intra-prostatic tumor foci than in normal prostate tissue (Figure 5a,b). However, 18F-Fluciclovine uptake in tumors is similar to that in BPH (benign prostate hyperplastic) nodules [23,26]. The Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved 18F-Fluciclovine in the recurrent setting only [28]. After definitive treatment for prostate cancer, patients are routinely followed up with serum PSA level. 18F-Fluciclovine is highly useful in the detection of recurrent prostate cancer, even in the presence of non-conclusive conventional imaging. In fact, CT or MRI scans may not detect or accurately characterize the biochemical relapse at the earliest stage [29]. However, functional imaging with Choline or Fluciclovine PET/CT associated with multi-parametric MRI (MP-MR) seems to be the most valuable technique in the detection of prostate cancer relapse [23]. These functional images are cost-effectiveness when PSA doubling time is short. 18F-Fluciclovine PET/CT shows detection rates of 72.0%, 83.3%, and 100% at PSA levels <1, 1–2. and >2 ng/mL, respectively [19]. In comparison with MP-MR, many studies concluded that 18F-Fluciclovine imaging for the evaluation of primary PCa was limited. Delayed imaging (20–28 min) could improve diagnostic performance for the characterization of primary cancer and can help guide biopsy in high-risk disease [17,23]. A study compared prospectively 18F-Fluciclovine and PSMA PET/CT scans for localizing recurrence of PCa after prostatectomy in patients with a PSA level <2.0 ng/mL. PSMA PET/CT detection rates for pelvic and extra-pelvic metastasis were higher than those for 18F-Flucicloviune PET/CT [10]. Other results, obtained in a more heterogeneous and at higher risk population, showed a better detection rate for 18F-Fluciclovine compared to PSMA for the detection of prostate bed recurrences in areas close to the bladder (37.9% and 27.6%, respectively) [30]. The lower urinary excretion of 18F-Flucicloviune PET compared to PSMA PET might be the explanation of this finding. However, PET/CT findings validation is not always feasible, especially in the recurrent setting. Nevertheless, it is well established that 18F-Fluciclovine is highly useful in the detection of recurrent prostate cancer, when conventional bone scan and CT and/or MRI imaging are negative [29]. Our meta-analysis study showed promising results in terms of sensitivity and specificity of 18F-Fluciclovine PET/CT, as recently reported in other meta-analysis recently published [31,32,33]. High specificity values have been observed for preoperative LN staging (almost 100%); acceptable (although lower) pooled specificity (68%) was obtained for the detection of PCa recurrence in terms of local recurrence and nodal localization. Discrepancy may be a consequence of a smaller number of studies included in meta-analysis of preoperative LN staging (which may have somehow reduced the statistical power of this sub-analysis) compared to the recurrent setting. The validation of positive findings still represents a challenge for medical imaging in oncology. In pre-surgery setting, a more accurate approach can be designed, and PET results can be validated by histology. Generally, lesion- or region-based validation is preferable and (especially for lymph node metastasis) positive PET lesions are compared with surgery templates. On the contrary, in the recurrent setting, the standard of truth is generally composite. Histological confirmation of metastatic sites is not often feasible due to ethical and practical reasons. Thus, PET findings are generally validated with informative conventional imaging that might have lower diagnostic accuracy compared to new generation imaging. Further validation can be obtained by complete PSA response in subjects treated with image-guided therapy. This heterogeneity might explain the different specificity observed in primary staging vs. recurrent setting.
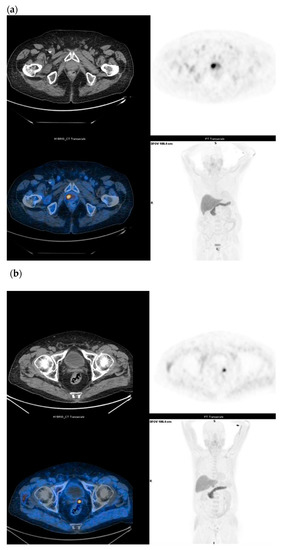
Figure 5.
(a) Staging in un-operated prostate cancer under drug treatment (Gleason Score: 4 + 3) and with a moderate increase in serum PSA (1.67 ng/mL); 18F-Fluciclovine PET/CT shows intense parenchymal uptake (SUVmax 15) in the right paramedian site of the prostate. (b) Biochemical recurrence in prostate cancer (Gleason Score: 4 + 4) subjected to radical HIFU treatment three years ago and with PSA elevation (3.3 ng/mL); 18F-Fluciclovine PET/CT shows a focal uptake in the left prostatic lodge (SUV max 7).
For the detection of bone metastases, further studies will be necessary, as the few studies considered are not able to provide statistically acceptable results. However, in this context, the physiological uptake of 18F-Fluciclovine might represent a limit.
An accurate knowledge of normal and physiologic distribution and variants is important for proper interpretation of 18F-Fluciclovine PET/CT imaging. Uptake may also be present in benign conditions such as inflammation and infection, in other metabolically active benign lesions or in other malignancies. Nowadays, 18F-Fluciclovine is also being investigated for other cancer indications, such as brain metastases, gliomas and breast cancers. Similar to many other statistical techniques, meta-analysis is a powerful tool, but it needs to fulfill several key requirements to ensure the validity of its results. In our study, we defined objectives, including definitions of clinical variables, evaluation of risk of bias in the selection of studies and considering heterogeneity of the population.
5. Conclusions
This systematic review and the related meta-analysis demonstrated promising results in term of sensitivity and specificity for 18F-Fluciclovine PET/CT to stage the primary lesion and in the assessment of nodal metastases, and for the detection of PCa locations in the recurrent setting. 18F-Fluciclovne PET can still be considered a valid new generation imaging procedure for staging PCa patients. However, the limited number of studies and the heterogeneity in the selected cohorts and different investigation protocols are limitations affecting the strength of these results.
Author Contributions
Design, C.R., C.C. (Chiara Cottignoli), G.B. and L.B.; methodology, G.B. and L.B.; software, C.C. (Carmelo Caldarella); validation, A.P. and F.M.F.; formal analysis, C.R., C.C. (Carmelo Caldarella), and G.B.; data curation, C.C. (Carmelo Caldarella); drafting, C.R., C.C. (Chiara Cottignoli), G.B., F.C., and L.B.; review and editing, A.P., F.C., and F.M.F.; visualization, C.C. (Chiara Cottignoli); supervision, C.R. and G.B.; project administration, C.C. (Chiara Cottignoli); funding acquisition, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The publication fee for this article has been supported by Blue Earth Diagnostics.
Acknowledgments
We would like to thank all the nuclear medicine technical and nursing staff for their professional contribution.
Conflicts of Interest
The authors declare no conflict of interest with respect to the authorship, research, and/or publication of this article.
References
- Ferlay, J.; Soerjomataran, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef]
- Haas, G.P.; Delongchamps, N.; Brawley, O.W.; Wang, C.Y.; De la Roza, G. The worldwide epidemiology of prostate cancer: Perspectives from autopsy studies. Can. J. Urol. 2008, 15, 3866–3871. [Google Scholar]
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. EAU Guidelines. In Proceedings of the EAU Annual Congress Amsterdam, 20–24 March 2020; ISBN 978-94-92671-07-3. [Google Scholar]
- Ono, M.; Oka, S.; Okudaira, H.; Nakanishi, T.; Mizolami, A.; Kobayashi, M.; Schuster, D.M.; Goodman, M.M.; Shirakami, Y.; Kawai, K. [(14)C] fluciclovine (alias anti-[(14)C] FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl. Med. Biol. 2015, 42, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. Axumin (fluciclovine F 18) Imaging & Interpretation Manual. v2.0 Final; Blue Earth Diagnostics Ltd.: Oxford, UK, 2019; pp. 1–21. [Google Scholar]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sorensen, J.; Owenius, R.; Chovke, P.; Turkbey, B.; et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: Physiologic uptake patterns, incidental findings, and variants that may simulate disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef]
- Tade, F.I.; Cohen, M.A.; Styblo, T.M.; Odewole, O.A.; Holbrook, A.I.; Newell, M.S.; Savir-Baruch, B.; Li, X.B.; Goodman, M.M.; Nye, J.A.; et al. Anti3-18F-FACBC (fluciclovine) PET/CT of breast cancer: An exploratory study. J. Nucl. Med. 2016, 57, 1357–1363. [Google Scholar] [CrossRef]
- Mapelli, P.; Incerti, E.; Ceci, F.; Castellucci, P.; Fanti, S.; Picchio, M. 11C- or 18F-choline PET/CT for imaging evaluation of biochemical recurrence of prostate cancer. J. Nucl. Med. 2016, 57, 43–48S. [Google Scholar]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. (18)F-fluciclovine PET-CT and (68) Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Odewole, O.A.; Tade, F.I.; Nieh, P.T.; Savir-Baruch, B.; Jani, A.B.; Master, V.A.; Rossi, P.J.; Halkar, R.K.; Osunkoya, A.O.; Akin-Akintayo, O.; et al. Recurrent prostate cancer detection with anti-3-[18F] FACBC PET/CT: Comparison with CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1773–1783. [Google Scholar] [CrossRef]
- Schuster, D.M.; Taleghani, P.A.; Nieh, P.T.; Master, V.A.; Amzat, R.; Savir-Baruch, B.; Halkar, R.K.; Fox, T.; Osunkoya, A.O.; Moreno, C.S.; et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[18F]-fluoro cyclobutane-1-carboxylic acid (anti-3-[18F] FACBC) uptake. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 85–96. [Google Scholar] [PubMed]
- Kairemo, K.; Rasulova, N.; Partanen, K.; Joensuu, T. Preliminary clinical experience of trans-1-amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar]
- Turkbey, B.; Mena, E.; Shih, J.; Pinto, P.A.; Merino, M.J.; Lindenberg, M.L.; Bernardo, M.; McKinney, Y.L.; Adler, S.; Owenius, R.; et al. Localized prostate cancer detection with 18F FACBC PET/CT: Comparison with MR imaging and histopathologic analysis. Radiology 2014, 270, 849–856. [Google Scholar] [CrossRef]
- Suzuki, H.; Inoue, Y.; Fujimoto, H.; Yonese, J.; Tanabe, K.; Fukasawa, S.; Inoue, T.; Saito, S.; Ueno, M.; Otaka, A. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F] fluorocyclobutanecarboxylic acid)—PET/CT in primary prostate cancer: Multicenter Phase IIb clinical trial. Jpn. J. Clin. Oncol. 2016, 46, 152–162. [Google Scholar] [CrossRef]
- Elschot, M.; Selnæs, K.M.; Sandsmark, E.; Krüger-Stokke, B.; Størkersen, Ø.; Giskeødegård, G.F.; Tessem, M.; Moestue, S.A.; Bertilsson, H.; Bathen, T.F. Combined 18F-fluciclovine PET/MRI shows potential for detection and characterization of high-risk prostate cancer. J. Nucl. Med. 2018, 59, 762–768. [Google Scholar] [CrossRef]
- Jambor, I.; Kuisma, A.; Kahkonen, E.; Kemppainen, J.; Merisaari, H.; Eskola, O.; Teuho, J.; Montoya Perez, I.; Pesola, M.; Aronen, H.J.; et al. Prospective evaluation of 18F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate—to high—risk prostate cancer patients (FLUCIPRO trial). Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 355–364. [Google Scholar] [CrossRef]
- Selnæs, K.M.; Kruger-Stokke, B.; Elschot, M.; Willoch, F.; Størkersen, Ø.; Sandsmark, E.; Moestue, S.A.; Tessem, M.; Halvorsen, D.; Kjøbli, E.; et al. 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur. Radiol. 2018, 28, 3151–3159. [Google Scholar] [CrossRef]
- Suzuki, H.; Jinnouchi, S.; Kaji, Y.; Kishida, T.; Kinoshita, H.; Yamaguchi, S.; Tobe, T.; Okamura, T.; Kawakita, M.; Furukawa, J.; et al. Diagnostic performance of 18F-fluciclovine PET/CT for regional lymph node metastases in patients with primary prostate cancer: A multicenter phase II clinical trial. Jpn. J. Clin. Oncol. 2019, 49, 803–811. [Google Scholar]
- Schuster, D.M.; Savir-Baruch, B.; Nieh, P.T.; Master, V.A.; Halkar, R.K.; Rossi, P.J.; Lewis, M.M.; Nye, J.A.; Yu, W.; Bowman, F.D.; et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology 2011, 259, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Nieh, P.T.; Jani, A.B.; Amzat, R.; Bowman, F.D.; Haslkar, R.K.; Master, V.A.; Nye, J.A.; Odewole, O.A.; Osunkoya, A.O.; et al. Anti-3-[18F] FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J. Urol. 2014, 191, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Boschi, S.; Borghesi, M.; Zanoni, L.; Pettinato, C.; Martorana, G.; Fanti, S. 18F-fluciclovine PET/CT for the detection of prostate cancer relapse: A comparison to 11C-choline PET/ CT. Clin. Nucl. Med. 2015, 40, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Zanoni, L.; Pultrone, C.; Schiavina, R.; Brunocilla, E.; Lodi, F.; Malizia, C.; Ferrari, M.; Rigatti, P.; Fonti, C.; et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1601–1610. [Google Scholar] [CrossRef]
- Akin-Akintayo, O.; Tade, F.; Mittal, P.; Moreni, C.; NIeh, P.T.; Rossi, P.; Patil, D.; Halkar, R.; Fei, B.; Master, V.; et al. Prospective evaluation of 18F-Fluciclovine PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur. J. Radiol. 2018, 102, 1–8. [Google Scholar] [PubMed]
- Chen, B.; Wei, P.; Macapinlac, H.A.; Lu, Y. Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nuc. Med. Commun. 2019, 40, 940–946. [Google Scholar]
- Bach-Gansmo, T.; Nanni, C.; Nieh, P.Y.; Zanoni, L.; Bogsrud, T.V.; Sletten, H.; Korsan, K.A.; Kieboom, K.J.; Tade, F.I.; Odewole, O.; et al. Multisite experience of the safety, detection rate and diagnostic performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the staging of biochemically recurrent prostate cancer. J. Urol. 2017, 197, 676–683. [Google Scholar] [CrossRef]
- De Visschere, P.J.L.; Standaert, C.; Fütterer, J.J.; Villeirs, G.M.; Panebianco, V.; Walz, J.; Maurer, T.; Hadaschik, B.A.; Leucovet, F.E.; Giannarini, G.; et al. A Systematic Review on the Role of Imaging in Early Recurrent Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Pernthaler, B.; Kuinik, R.; Gstettner, C.; Salamon, S.; Aigner, R.M.; Kvaternik, H. A perspective head-to-head comparison of 18F-fluciclovine with 68Ga-PSMA-11 in biochemical recurrence of prostate cancer in PET/CT. Clin. Nucl. Med. 2019, 44, 566–573. [Google Scholar] [CrossRef]
- Bin, X.; Yong, S.; Kong, Q.F.; Zhao, S.; Zhang, G.Y.; Wu, J.P.; Chen, S.Q.; Zhu, W.D.; Pan, K.H.; Du, M.L.; et al. Diagnostic performance of PET/CT using 18F-FACBC in prostate cancer: A meta-analysis. Front Oncol. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Laudicella, R.; Albano, D.; Alongi, P.; Argiroffi, G.; Bauckneht, M.; Baldari, S.; Bertagna, F.; Boero, M.; De Vincentis, G.; Del Sole, A.; et al. (18) F-Facbc in Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1348. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, S. The role of (18) F-fluciclovine PET in the management of prostate cancer: A systematic review and meta-analysis. Clin. Radiol. 2019, 74, 886–892. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).