Computed Tomography-Guided Percutaneous Radiofrequency Ablation of the Splanchnic Nerves as a Single Treatment for Pain Reduction in Patients with Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Evaluation
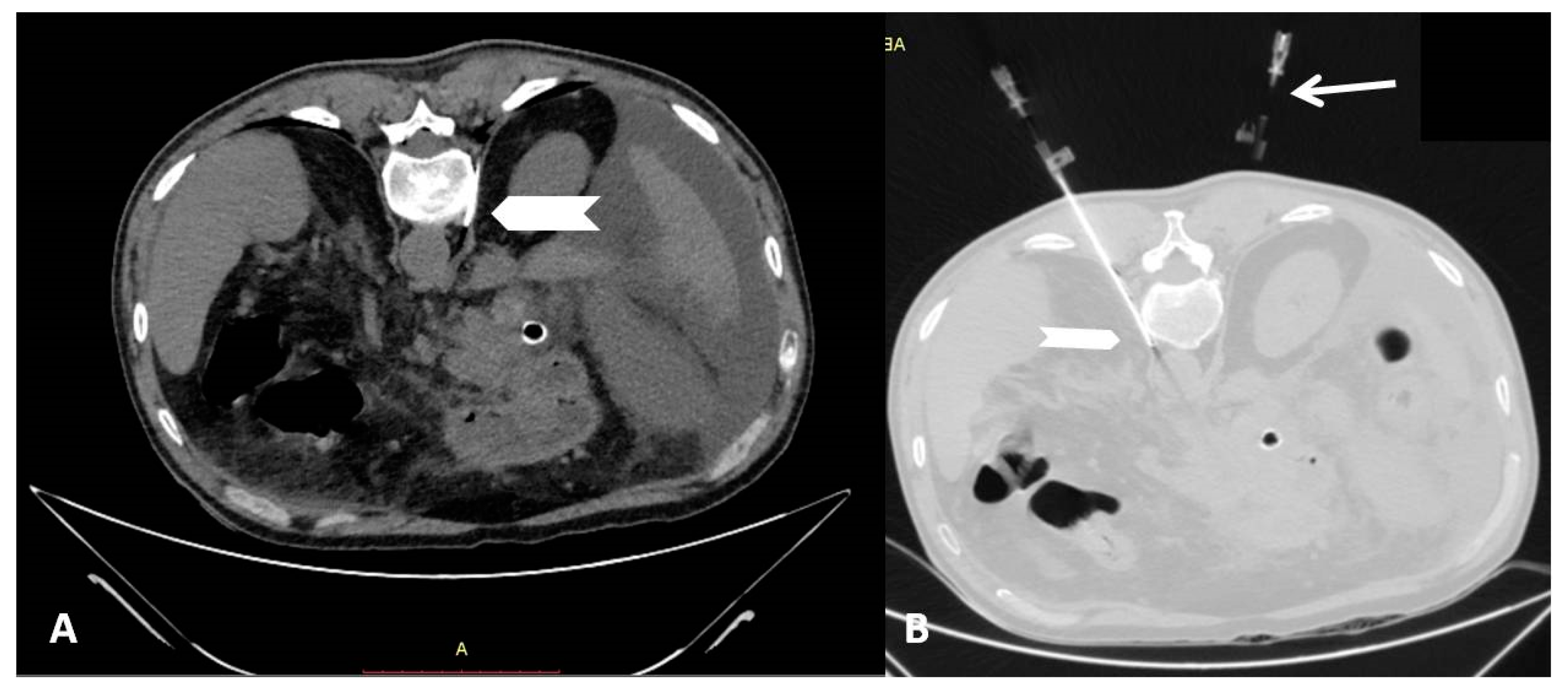
2.2. Percutaneous Radiofrequency Neurolysis
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drewes, A.M.; Campbell, C.M.; Ceyhan, G.O.; Delhaye, M.; Garg, P.K.; van Goor, H.; Laquente, B.; Morlion, B.; Olesen, S.S.; Singh, V.K.; et al. Pain in pancreatic ductal adenocarcinoma: A multidisciplinary, international guideline for optimized management. Pancreatology 2018, 18, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.L.; Loscalzo, M.; Trask, P.C.; Zabora, J.; Philip, E.J. Psychological distress in patients with pancreatic cancer—An understudied group. Psychooncology 2010, 19, 1313.e20. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Peirce-Sandner, S.; Baron, R.; Bellamy, N.; Burke, L.B.; Chappell, A.; Chartier, K.; Cleeland, C.S.; Costello, A.; et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010, 149, 177.e93. [Google Scholar] [CrossRef]
- WHO. Cancer Pain Relief: With a Guide to Opioid Availability, 2nd ed.; World Health Organization: Geneva, Switzerland, 1996; Available online: http://apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf (accessed on 31 January 2018).
- Filippiadis, D.K.; Tselikas, L.; Tsitskari, M.; Kelekis, A.; de Baere, T.; Ryan, A.G. Percutaneous Neurolysis for Pain Management in Oncological Patients. Cardiovasc. Interv. Radiol. 2019, 42, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, K.; Chary, A.; Patel, S.; Mitchell, J.W.; Fleishon, H.; Prologo, J.D. Percutaneous CT-Guided Cryoablation of the Celiac Plexus: A Retrospective Cohort Comparison with Ethanol. J. Vasc. Interv. Radiol. JVIR 2020, 31, 1216–1220. [Google Scholar] [CrossRef]
- Arcidiacono, P.G.; Calori, G.; Carrara, S.; McNicol, E.D.; Testoni, P.A. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst. Rev. 2011, 78, CD007519. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, H.; Nakamoto, D.A.; Azar, N.; Hayek, S.M.; Haaga, J.R. Percutaneous computed tomography guided cryoablation of the celiac plexus as an alternative treatment for intractable pain caused by pancreatic cancer. J. Cancer Res. Ther. 2011, 7, 481–483. [Google Scholar] [CrossRef]
- Cornman-Homonoff, J.; Holzwanger, D.J.; Lee, K.S.; Madoff, D.C.; Li, D. Celiac plexus block and neurolysis inthe management of chronic upper abdominal pain. Semin. Intervent. Radiol. 2017, 34, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Kambadakone, A.; Thabet, A.; Gervais, D.A.; Mueller, P.R.; Arellano, R.S. CT-guided Celiac Plexus Neurolysis: A review of anatomy, indications, technique, and tips for successful treatment. RadioGraphics 2011, 31, 1599–1621. [Google Scholar] [CrossRef] [PubMed]
- Comlek, S. Pain control with splanchnic neurolysis in pancreatic cancer patients unresponsive to celiac plexus neurolysis. J. Pain Res. 2020, 13, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.H.; Monard, E.; Moulin, M.A.; Vignaud, E.; Laveissiere, F.; Ben Ammar, M.; Nouri-Neuville, M.; Barral, M.; Lombart, B. Sedation and analgesia in interventional radiology: Where do we stand, where are we heading and why does it matter? Diagn. Interv. Imaging 2019, 100, 753–762. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Shwita, A.H.; Amr, Y.M.; Okab, M.I. Comparative study of the effects of the retrocrural celiac plexus block versus splanchnic nerve block, c-arm guided, for upper gastrointestinal tract tumors on pain relief and the quality of life at a six-month follow up. Korean J. Pain 2015, 28, 22–31. [Google Scholar] [CrossRef]
- Amr, S.A.; Reyad, R.M.; Othman, A.H.; Mohamad, M.F.; Mostafa, M.M.; Alieldin, N.H.; Hamed, F.A. Comparison between radiofrequency ablation and chemical neurolysis of thoracic splanchnic nerves for the management of abdominal cancer pain, randomized trial. Eur. J. Pain 2018, 22, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumah, R.; Urits, I.; Viswanath, O.; Kaye, A.D.; Hasoon, J. Radiofrequency Ablation and Alcohol Neurolysis of the Splanchnic Nerves for a Patient with Abdominal Pain from Pancreatic Cancer. Cureus 2020, 12, e10758. [Google Scholar] [CrossRef]
- Oh, T.K.; Lee, W.J.; Woo, S.M.; Kim, N.W.; Yim, J.; Kim, D.H. Impact of celiac plexus neurolysis on survival in patients with unresectable pancreatic cancer: A retrospective, propensity score matching analysis. Pain Physician 2017, 20, E357–E365. [Google Scholar] [PubMed]
- Wang, P.J.; Shang, M.Y.; Qian, Z.; Shao, C.W.; Wang, J.H.; Zhao, X.H. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom. Imaging 2006, 31, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Bahn, B.M.; Erdek, M.A. Celiac plexus block and neurolysis for pancreatic cancer. Curr. Pain Headache Rep. 2013, 17, 310. [Google Scholar] [CrossRef]
- Plancarte, R.; Guajardo-Rosas, J.; Reyes-Chiquete, D.; Chejne-Gómez, F.; Plancarte, A.; González-Buendía, N.I.; Cerezo-Camacho, O.; Lee, A.; Medina-Santillan, R. Management of chronic upper abdominal pain in cancer: Transdiscal blockade of the splanchnic nerves. Reg. Anesth. Pain Med. 2010, 35, 500–506. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Biondetti, P.; Coppola, A.; Fumarola, E.M.; Biasina, A.M.; Alessio Angileri, S.; Carrafiello, G. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg. 2018, 7, 59–66. [Google Scholar] [CrossRef]
- Carrafiello, G.; Ierardi, A.M.; Fontana, F.; Petrillo, M.; Floridi, C.; Lucchina, N.; Cuffari, S.; Dionigi, G.; Rotondo, A.; Fugazzola, C. Microwave ablation of pancreatic head cancer: Safety and efficacy. J. Vasc. Interv. Radiol. 2013, 24, 1513–1520. [Google Scholar] [CrossRef]
| N (%) | |
|---|---|
| Gender | |
| Females | 11 (36.7) |
| Males | 19 (63.3) |
| Age, mean (SD) | 65.4 (10.8) |
| Age | |
| <67 | 15 (50.0) |
| >67 | 15 (50.0) |
| Pain | N | Mean (SD) | Mean Change from Previous Measurement (SD) | P+ |
|---|---|---|---|---|
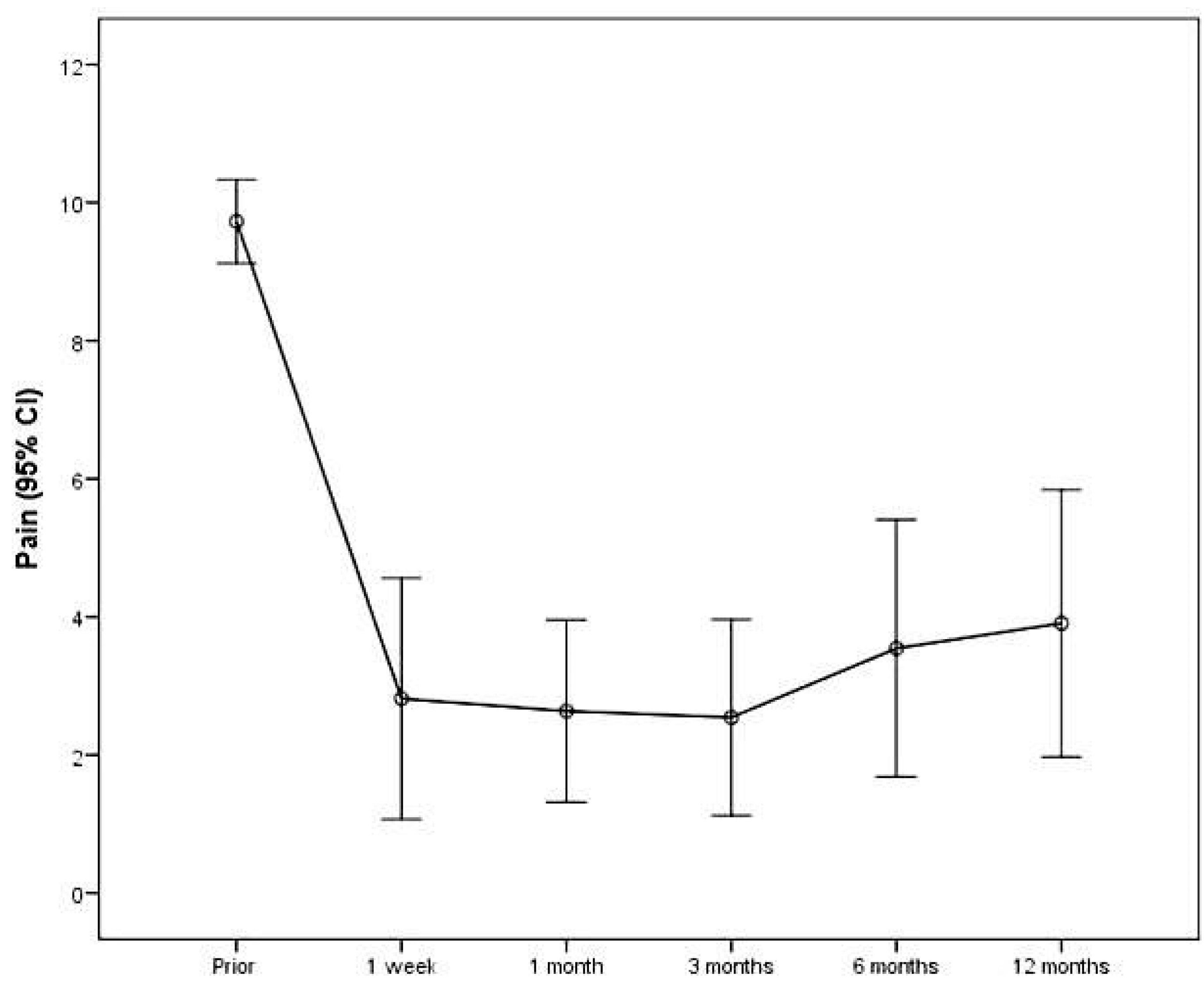
| Prior | 30 | 9.0 (1.4) | - | |
| 1 week | 30 | 2.9 (2.2) | −6.0 (2.7) | <0.001 |
| 1 month | 30 | 3.1 (2.1) | 0.2 (2.3) | 1.000 |
| 3 months | 28 | 3.6 (2.3) | 0.4 (1.4) | 1.000 |
| 6 months | 17 | 3.8 (2.7) | 0.6 (1.4) | 0.869 |
| 12 months | 11 | 3.9 (2.9) | 0.4 (0.9) | 1.000 |
| Mean change from prior to 12 months (SD) | −5.8 (2.8) | <0.001 |
| Pain | Gender | Age | ||
|---|---|---|---|---|
| Females | Males | <67 | >67 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Prior | 9.2 (1.5) | 8.8 (1.5) | 8.9 (1.6) | 9.0 (1.4) |
| 1 week | 2.7 (2.4) | 3.1 (2.2) | 2.9 (2.2) | 3.0 (2.4) |
| 1 month | 3.3 (2.5) | 3.0 (1.9) | 3.5 (2.1) | 2.7 (2.0) |
| 3 months | 3.6 (2.8) | 3.6 (2.0) | 4.2 (2.2) | 3.2 (2.4) |
| 6 months | 4.0 (3.0) | 3.6 (2.7) | 4.8 (3.2) | 2.9 (1.9) |
| 12 months | 2.3 (1.5) | 4.9 (3.1) | 4.9 (3.1) | 2.3 (1.5) |
| Changes: | ||||
| From prior to 1 week | −6.5 (3.2) | −5.8 (2.4) | −6.1 (2.7) | −6.0 (2.8) |
| From 1 week to 1 month | 0.5 (1.9) | −0.1 (2.5) | 0.7 (2.5) | −0.3 (2.0) |
| From 1 month to 3 months | 0.4 (1.5) | 0.5 (1.3) | 0.3 (1.5) | 0.5 (1.2) |
| From 3 to 6 months | 0.3 (1.8) | 0.7 (1.6) | 0.9 (1.7) | 0.3 (1.0) |
| From 6 to 12 months | 0.0 (0.0) | 0.6 (1.1) | 0.6 (1.1) | 0.0 (0.0) |
| From prior to 12 months | −7.8 (1.5) | −4.7 (2.98) | −4.7 (2.9) | −7.8 (1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriadis, S.; Tsitskari, M.; Ioannidi, M.; Zavridis, P.; Kotsantis, I.; Kelekis, A.; Filippiadis, D. Computed Tomography-Guided Percutaneous Radiofrequency Ablation of the Splanchnic Nerves as a Single Treatment for Pain Reduction in Patients with Pancreatic Cancer. Diagnostics 2021, 11, 303. https://doi.org/10.3390/diagnostics11020303
Grigoriadis S, Tsitskari M, Ioannidi M, Zavridis P, Kotsantis I, Kelekis A, Filippiadis D. Computed Tomography-Guided Percutaneous Radiofrequency Ablation of the Splanchnic Nerves as a Single Treatment for Pain Reduction in Patients with Pancreatic Cancer. Diagnostics. 2021; 11(2):303. https://doi.org/10.3390/diagnostics11020303
Chicago/Turabian StyleGrigoriadis, Stavros, Maria Tsitskari, Maria Ioannidi, Periklis Zavridis, Ioannis Kotsantis, Alexis Kelekis, and Dimitrios Filippiadis. 2021. "Computed Tomography-Guided Percutaneous Radiofrequency Ablation of the Splanchnic Nerves as a Single Treatment for Pain Reduction in Patients with Pancreatic Cancer" Diagnostics 11, no. 2: 303. https://doi.org/10.3390/diagnostics11020303
APA StyleGrigoriadis, S., Tsitskari, M., Ioannidi, M., Zavridis, P., Kotsantis, I., Kelekis, A., & Filippiadis, D. (2021). Computed Tomography-Guided Percutaneous Radiofrequency Ablation of the Splanchnic Nerves as a Single Treatment for Pain Reduction in Patients with Pancreatic Cancer. Diagnostics, 11(2), 303. https://doi.org/10.3390/diagnostics11020303








