Abstract
Many studies have suggested a prognostic value of one or several positron emission tomography (PET) parameters in patients with small cell lung cancer (SCLC). However, studies are often small, and there is a considerable interstudy disagreement about which PET parameters have a prognostic value. The objective of this study was to perform a review and meta-analysis to identify the most promising PET parameter for prognostication. PubMed®, Cochrane, and Embase® were searched for papers addressing the prognostic value of any PET parameter at any treatment phase with any endpoint in patients with SCLC. Pooled hazard ratios (HRs) were calculated by a random effects model for the prognostic value of the baseline maximum standardized uptake value (SUVmax) and metabolic tumor volume (MTV). The qualitative analysis included 38 studies, of these, 19 studies were included in the meta-analyses. The pooled results showed that high baseline MTV was prognostic for overall survival (OS) (HR: 2.83 (95% confidence interval [CI]: 2.00–4.01) and progression-free survival (PFS) (HR: 3.11 (95% CI: 1.99–4.90)). The prognostic value of SUVmax was less pronounced (OS: HR: 1.50 (95% CI: 1.17–1.91); PFS: HR: 1.24 (95% CI: 0.94–1.63)). Baseline MTV is a strong prognosticator for OS and PFS in patients with SCLC. MTV has a prognostic value superior to those of other PET parameters, but whether MTV is superior to other prognosticators of tumor burden needs further investigation.
1. Introduction
Small cell lung cancer (SCLC) is an aggressive cancer, and most patients present at an advanced stage [1]. Treatment options are limited. Patients with limited disease (LD) are treated with concomitant thoracic radiotherapy and platin-based chemoradiotherapy. Patients presenting at an advanced stage (extensive disease; ED) are treated with palliative platin-based chemotherapy. Up to 40% of patients do not achieve objective response to first-line therapy [2], but even when objective response is achieved, it is often followed by a quick and fatal relapse, and overall survival (OS) is poor [2]. The introduction of immunotherapy for first-line treatment and for treatment of relapse gives hope for an improved clinical outcome [3,4,5].
2-Deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography (PET)/computed tomography (CT) has an established role in the staging of SCLC with a sensitivity approximating 100% and a specificity exceeding 90% [6,7]. Compared with CT, FDG–PET/CT causes stage migration in up to 40% of patients, thus having a great impact on treatment choice [8]. FDG–PET/CT for early or final response evaluation seems feasible [9]; however, the role of FDG–PET/CT after therapy has not been proven to be superior to that of CT [10]. Several studies have shown a prognostic value of FDG–PET/CT, but studies are inconsistent in regard to which parameters have a prognostic value and cutoff values differ [9]. Better prognostication in order to personalize the aggressiveness of the treatment course and surveillance after the end of treatment is warranted.
In this study, we present an overview of all published studies of the prognostic value of FDG–PET parameters before, during, and after treatment in patients with SCLC, including quantification by a meta-analysis of baseline PET parameters, in order to identify the most promising PET parameter(s) for prognostication.
2. Materials and Methods
2.1. Eligibility Criteria
Studies concerning the prognostic evaluation of any FDG–PET parameter in patients with SCLC were eligible. Studies were not selected based on the stage of SCLC, treatment, or other clinical characteristics.
FDG–PET performed at any phase of the disease was accepted: before treatment, during treatment, after the end of treatment, and during follow-up.
Any PET parameter was accepted (uptake values, metabolic tumor volumes, and their combinations).
PET parameters within any anatomical region were accepted (within primary tumor, lymph nodes, metastases, and their combinations).
Any prognostic endpoint was accepted (progression-free survival (PFS), distant failure, time to progression, OS, and so forth).
2.1.1. Search Strategy
A search was performed in PubMed®, Cochrane Library, and Embase® on 24 September 2020. MeSH® terms were used in PubMed® and Cochrane Library, and Emtree® terms in Embase®, in combination with the search of keywords.
The search in PubMed® and Cochrane Library was constructed as follows: ((carcinoma, small cell lung [MeSH terms]), OR (SCLC)) AND ((positron emission tomography [MeSH Terms]) OR (positron emission tomography) OR (PET)) AND ((18f fluorodeoxyglucose [MeSH Terms]) OR (fluorodeoxyglucose) OR (FDG)) AND ((prognosis) OR (prognosis [MeSH Terms])).
The search in Embase® was constructed as follows: ((small cell lung cancer/) OR (SCLC.mp)) AND ((positron emission tomography/) OR (PET.mp) OR (positron emission tomography.mp)) AND ((fluorodeoxyglucose f 18/) OR (fluorodeoxyglucose/) OR (fluorodeoxyglucose.mp) OR (FDG.mp)) AND ((prognosis/) OR (prognosis.mp)).
2.1.2. Study Selection
The papers identified by the database search were screened for inclusion. Reviews, cases, meta-analyses, letters, preclinical studies, trial notes, and studies in languages other than English were excluded. Reference lists from the included studies were screened for additional records.
Studies with overlapping cohorts were included if different PET parameters or endpoints were addressed; otherwise, the study with the largest cohort was included.
Studies of baseline FDG–PET providing hazard ratio (HR) and 95% confidence intervals (CI) for PFS or OS or sufficient data to extract HR and 95% CI were included in the meta-analysis.
2.2. Data
Clinical data, PET parameters, and prognostic data were extracted from the identified records.
The prognostic value of PET parameters at variant time periods in regard to treatment was qualitatively described. The independent prognostic value of PET parameters was compared with that of clinical parameters in studies providing multivariate analysis.
Risk of bias in the studies was assessed by six domains using the Quality in Prognostic Studies (QUIPS) tool [11]. In the “study confounding” domain, inclusion of the covariates stage, age, and sex was assessed.
2.3. Statistics
The meta-analysis was performed for the baseline maximum standardized uptake value (SUVmax) and baseline metabolic tumor volume (MTV) measured within the primary tumor (tSUVmax, tMTV) or in the whole body (wbSUVmax, wbMTV). Separate analyses were performed for the most common endpoints: OS and PFS.
HR and 95% CI from univariate analysis were collected. In studies not providing HR and 95% CI, data were extracted from Kaplan–Meier curves either with readable data points or combined with the available p-value and recalculated into the Cox model. In studies providing HR for continuous values of SUVmax or MTV, data points for individual patients were extracted from Kaplan–Meier curves when available, or a Cox model was reconstructed for the dichotomized SUVmax or MTV. If individual data points were not available, the difference of the median value in the high group and the low group was applied, and HR was estimated for the dichotomized PET parameters. See Supplementary Materials File S2 for further details on the reconstruction of data.
Meta-analyses were performed using the functions “metagen,” “forest,” and “funnel” in the R package “meta” version 4.9-1 (R Foundation for Statistical Computing, Vianna, Austria). Due to the inherent heterogeneity of the studies owing to differences of study designs and definitions of PET parameters, random effects models were used. Forest plots and pooled HR and 95% CI were generated. HR greater than one implies worse survival for patients with larger PET parameters. Heterogeneity between the studies was evaluated by I2 and tau2 statistics. Funnel plots were constructed to identify the presence of publication bias.
3. Results
The search on PubMed®, Cochrane, and Embase® resulted in 181 individual records. After excluding 144 records, 37 studies were included in the qualitative review. One additional study was identified through screening the references of the included studies. Nineteen studies were included in the quantitative meta-analysis. The identification process and reasons for exclusion are illustrated in Figure 1. Four studies had a partial overlap of patient cohorts with one other study each [12,13,14,15]. They were all included in the qualitative review as their designs differed. The smallest study of Oh et al. [13] was excluded from the meta-analysis in favor of a larger study [12]. The study of Kim et al. [14] was excluded from the meta-analysis due to insufficient data.
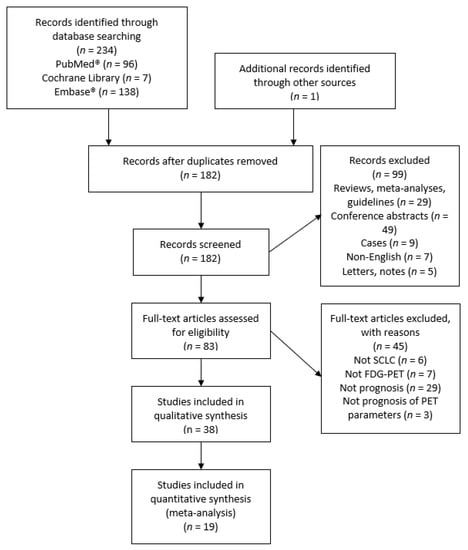
Figure 1.
Prisma flowchart of included and excluded studies. SCLC: small cell lung cancer; FDG–PET: 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography.
From the 38 included studies, 30 studies addressed the prognostic value of baseline PET parameters. Post-treatment PET parameters were evaluated in 7 studies, the prognostic value of changes in PET parameters was evaluated in four studies, and further three studies evaluated the prognostic value of PET parameters in different timings, because the patient cohorts consisted of patients who had performed PET before or after treatment or before and during therapy.
The 38 studies present 73 different approaches of measuring PET parameters. Table 1 defines the 73 different PET parameters.

Table 1.
PET parameters. Definitions of PET parameters used in the included studies.
3.1. Quality of the Studies
Figure 2 presents the risk of bias in the included studies evaluated using the QUIPS tool. There was a high risk of bias in “study participation,” reflecting a retrospective design of 35 of the included studies. Available PET and medical records were inclusion criteria in most studies, causing inclusion of as little as 13% of all SCLC patients from the recruiting period [16].
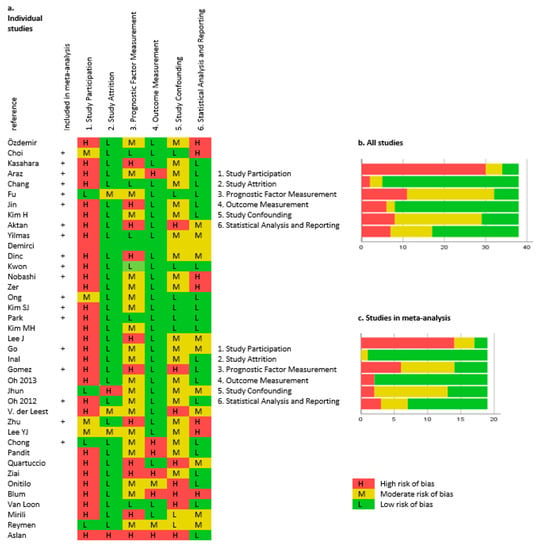
Figure 2.
Risk of bias according to the Quality in Prognostic Studies (QUIPS) tools. Individual studies are shown in (a), results from all studies in (b), and results from studies included in the meta-analysis in (c).
“Prognostic factor measurement” had moderate or high risk of bias in 32 studies, including 14 studies in the meta-analysis. The risk of bias for the prognostic factor measurement was often caused by the use of optimal cutoff (n = 6), median cutoff (n = 17), or no available information of which cutoff was used (n = 4). PET acquisition and definition of PET parameters rarely contributed to bias. Few studies did not provide sufficient data, and in one study, baseline PET performed up to 4 months prior to the start of treatment was assessed [17].
3.2. Qualitative Analysis: Prognostic Value of Baseline PET Parameters
Results from the 30 baseline studies are presented in Table 2. Each study included 8 to 344 patients.

Table 2.
Prognostic value of baseline PET parameters.
3.2.1. Baseline SUV
Baseline SUVmax was addressed in 28 studies, but only seven studies showed a significant prognostic value of SUVmax for OS and/or PFS [18,19,20,21,22,23,24].
Twelve studies included baseline SUVmax in a multivariate analysis. In five studies, SUVmax were independently prognostic for OS [18,19,20,21,25]. No study showed an independent prognostic value for PFS [12,25,32,33]. Compared with other covariates included in the multivariate analysis, an additional independent or superior prognostic value of SUVmax to stage, age, blood lactate dehydrogenase (LDH), sex, and performance status (PS) was sporadic (Figure 3a).
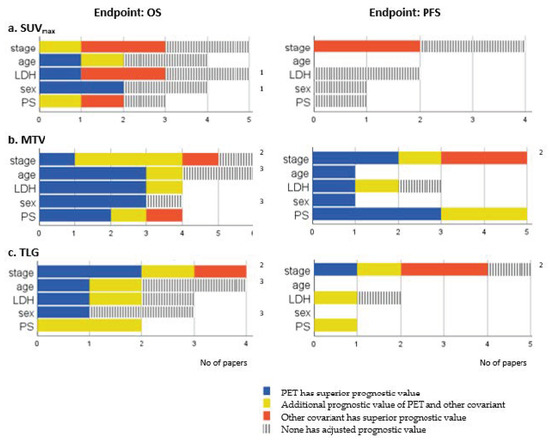
Figure 3.
Comparisons of PET parameters and other covariates included in the multivariate analysis. Number of papers showing either superior (blue), additional (yellow), inferior (red), or no prognostic value (grey) of adjusted SUVmax (a), MTV (b), and TLG (c) compared with the five most frequently used covariates. 1 Özdemir accounted twice due to different results in subgroups, 2 Nobashi accounted twice due to different results in subgroups, 3 Choi accounted twice due to different results in subgroups.
Other uptake parameters than SUVmax have been evaluated for prognostic value. SUVpeak [26,29] and SUVmean [16,26,34,35] did not show a significant prognostic value in any studies. MeanSUVmax (mean of SUVmax from all lesions) was prognostic for OS and PFS in one of four studies [40]. Lesser-used PET parameters were addressed in one study each, all showing a prognostic value: MeanSUVmean (mean of SUVmean from all lesions) [23], SUVmax corrected for blood glucose level (SUVmax(glu)) [27], and SUVmax corrected for liver-FDG uptake (SUVmax(liver)). However, in contrast to other uptake parameters, high SUVmax(liver) was associated with a better prognosis (HR by univariate analysis: 0.31) [36].
Three uptake parameters showed an independent prognostic value for OS and/or PFS in one study each: wb-meanSUVmax (HR for OS: 3.74; HR for PFS: 2.25) [40], t-SUVmax(glu) (HR for PFS: 3.38) [27], and tSUVmax(liver) (HR for OS 0.194) [36].
3.2.2. Baseline MTV
Baseline MTV was addressed in univariate analysis in 13 studies. All studies showed significant prognostic results for OS, PFS, and/or distant failure.
Absolute threshold was the most frequently used delineation method. Large MTV2.5 was prognostic for lower OS in four of five studies [16,21,23,27] and for lower PFS in four of five studies [16,21,23,29]: MTV2.5 measured throughout the whole body (wbMTV2.5) was prognostic for OS and PFS in two of two studies [21,23]. MTV2.5 measured within the primary tumor (tMTV2.5) was prognostic for OS in one of two studies [27], but not for PFS [16,27]. MTV2.5 measured within the primary tumor and lymph node metastases (tnMTV2.5) was prognostic for PFS in two of two studies [16,29], and for OS in one of two studies [16].
MTV3.0 throughout the whole body (wbMTV3.0) had a prognostic value for OS in four of four studies [12,13,18,28], though partial cohort overlap of two of the studies should be noticed [12,13]. wbMTV3.0 had a prognostic value for PFS in two of three studies [13,28]. MTV3.0 measured in the primary tumor (tMTV3.0), measured in all intrathoracic tumors, or in the hottest tumor did not show a significant prognostic value [13,18].
MTV with relative thresholds of 40% or 42% of SUVmax (MTV40; MTV42) showed a prognostic value for OS and PFS in one [32] of two studies. Ong et al. [34] showed a prognostic value of tMTV42 for distant failure, but not for OS or PFS.
Software-delineated MTV (MTVsoftware) was prognostic for OS in two of two studies [26,35]. Both studies used a patient-specific SUV threshold for delineation based on SUV in the liver. The prognostic value of MTVsoftware for PFS has not been investigated.
Results from multivariate analysis of baseline MTV were available from 14 studies, accounting for the above 13 studies and the study of Zer et al. [33] that had only published results from multivariate analysis.
Baseline MTV had an independent prognostic value for OS (HR: 1.001–16.7) and/or PFS (HR: 1.8–6.11) in 12 of 14 studies.
PET parameters and clinical parameters were comparable for OS in 10 studies, and for PFS in 8 studies. Figure 3b gives an overview of the independent prognostic value of PET parameters and the most investigated covariates. MTV had an additional or superior prognostic value to stage [12,23,32,33,35], age [18,26,28,35], LDH [12,18,23,26], sex [18,26,28], and PS [12,13,16,28] in most studies. Only three studies identified a clinical covariate with a superior prognostic value to MTV: stage [32], stage and treatment response [29], and PS, chemotherapy and number of extrathoracic metastases [13].
3.2.3. Baseline PET Parameters Combining SUV with Tumor Volume
Eleven studies addressed total lesion glycolysis (TLG; the product of MTV and SUVmean within MTV). In nine studies, TLG provided similar results as MTV [16,18,21,23,27,29,32,33,35]. However, in the studies of Araz et al. [26] and Ong et al. [34], TLG did not show a prognostic value, whereas MTV did.
TLG had an independent prognostic value for OS (HR: 1.0003–11.19) in six studies [16,18,23,32,33,35] and for PFS (HR: 3.2–12.48) in three [16,23,32]. Stage was the most frequently investigated clinical parameter in addition to TLG. TLG had an additional or superior prognostic value to stage for OS [23,32,33,35], but the results applied only for one subgroup in the study of Nobashi et al. [1]. Other clinical parameters were sporadic included in the multivariate analysis (Figure 3c).
The sum of SUVmax from all lesions (sumSUVmax) was addressed in two studies, both showing a prognostic value for PFS and OS [14,37]. Baseline sumSUVmax had an independent prognostic value (OS: HR: 2.676–3.970; PFS: HR: 2.219–2.296) in both studies. SumSUVmax was a stronger prognosticator for OS than stage and sex.
3.3. Qualitative Analysis: Prognostic Value of Post-Treatment PET Parameters
Table 3 presents results from seven studies addressing the prognostic value of FDG–PET/CT after treatment. The studies included 22–164 patients each. The majority of studies investigated the prognostic value of PET within 4 months after the end of treatment, although Pandit et al. [41] included patients up to 4 years after treatment.

Table 3.
Prognostic value of post-treatment PET parameters.
In five studies, either SUVmax [29,41,43], SUVpeak [29], wbSULpeak [43], presence of PET-positive lesions [41,43,44,45], MTV2.5, or TLG2.5 [29] showed a prognostic value. Two studies, including the largest study, did not find a significant prognostic value of any post-treatment PET parameter [36,42].
Multivariate analysis showed an independent prognostic value of post-treatment PET parameter in two of three studies: tnMTV2.5 was independently prognostic for PFS (HR: 2.8 (95% CI: 1.5–5.2), p = 0.001) in addition to initial stage and response by The Response Evaluation Criteria in Solid Tumors (RECIST) [29]; sum-wbSULpeak and presence of PET-positive lesions were independently prognostic for OS and/or PFS (HR 1.046) [43].
3.4. Qualitative Analysis: Prognostic Value of PET Parameter Change, Early and Final Response Evaluation
Results from four studies evaluating the prognostic value of a PET parameter change from baseline PET to PET during or after the end of treatment are presented in Table 4.

Table 4.
Prognostic value of PET parameter change, early and final response evaluation. All PET parameters were compared with the baseline PET parameter.
Van Loon et al. showed a prognostic value of early response measured as the reduction of MTV after one cycle of chemotherapy, despite a small study size (n = 15) [46]. The PET parameter change from baseline to the end of therapy (i.e., final response evaluation) had a prognostic value in three of three studies; however, different PET parameters were tested: reductions of tSUVmax, tSUVpeak, tn-meanSUVmax(liver), tSULpeak, and tnMTV2.5 were prognostic for PFS and/or OS [29,36,43]. Change of tSUVmax(liver) and tnTLG2.5 did not show a prognostic value [29,36]. Reduction of SUVpeak had an independent prognostic value for OS over stage; however, for PFS, stage had an independent prognostic value over SUVpeak [29]. Reduction of tn-meanSUVmax(liver) had an independent prognostic value for PFS in addition to LDH [36].
3.5. Qualitative Analysis: Prognostic Value of PET Parameters at Mixed Treatment Phases
Three studies investigated the prognostic value of PET parameters at mixed treatment phases (Table 5).

Table 5.
Prognostic value of PET parameters in studies with PET at mixed treatment phases.
Two studies investigated a cohort mixed of patients who had baseline PET or post-treatment PET [47,49]. Both studies investigated SUVmax, SUVmean, MTV, and TLG. Most analyses did not find any prognostic value. Mirili et al. [47] showed a prognostic value of SUVmax and MTV. Arslan et al. [49] found a prognostic value for OS of only TLG.
Gross tumor volume (GTV) used for radiotherapy planning based on pre- and post-chemotherapy PET/CT was prognostic for OS [48].
3.6. Quantitative Analysis: Prognostic Value of Baseline PET Parameters
3.6.1. Baseline SUVmax
Fourteen studies with a total of 1194 patients were included in the meta-analysis of the prognostic value of SUVmax with OS as endpoint. Nine studies with a total of 716 patients were included with PFS as endpoint. SUVmax-cutoff for dichotomizing patients into two groups of high and low SUVmax ranged from 5.1 to 16. The cutoffs in the studies were median SUVmax (n = 7), optimal cutoff (n = 6), and recalculated median SUVmax from HR of a continuously increasing SUVmax (n = 3). Information of cutoff and definitions of SUVmax in the studies are available in Supplementary Materials File S1, Table S1.
Random effects meta-analysis revealed a slightly increased HR for OS with large SUVmax (pooled HR: 1.50 (1.17–1.91), p = 0.001). SUVmax was not significantly prognostic for PFS (pooled HR: 1.24 (0.94–1.63), p = 0.13). Forest plots are presented in Figure 4. The heterogeneity between the studies was moderate (OS as endpoint: I2 = 56%, tau2 = 0.1132; PFS as endpoint: I2 = 49%, tau2 = 0.0902). Funnel plots showed a tendency toward asymmetry (Figure 5), which can be caused by interstudy heterogeneity or publication/reporting bias. The corresponding test for asymmetry was significant with OS as endpoint (p = 0.02), and not significant with PFS as endpoint (p = 0.35).
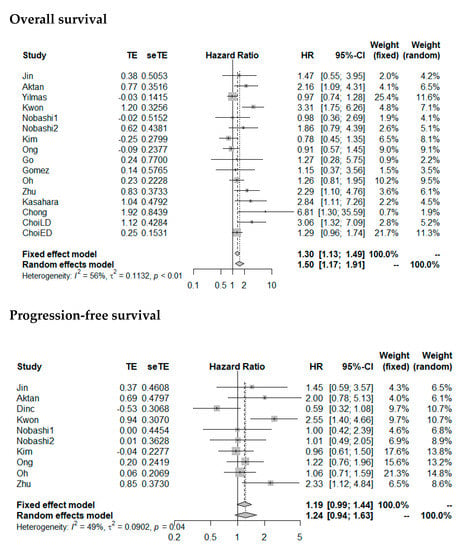
Figure 4.
Forest plots of HRs of SUVmax for overall survival and progression-free survival. Nobashi1 refers to the results of the central type of SCLC. Nobashi2 refers to the results of the peripheral type of SCLC. ChoiLD refers to the result of LD-SCLC. ChoiED refers to the results of SCLC-ED.
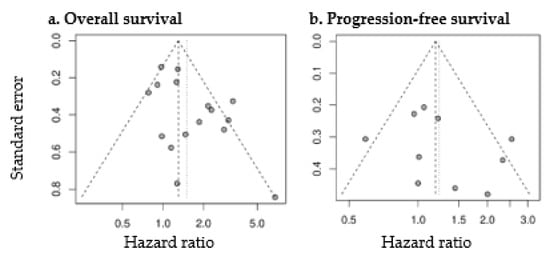
Figure 5.
Funnel plots of studies assessing the prognostic value of SUVmax for OS (a) and PFS (b).
3.6.2. Baseline MTV
Eleven studies with a total of 1015 patients were included in the meta-analysis of the prognostic value of MTV with OS as endpoint. Seven studies with a total of 627 patients were included in the meta-analysis with PFS as endpoint. MTV cutoff for dichotomizing patients in two groups with high and low MTV ranged from 21.45 (tMTV42) to 266.5 (wbMTV3.0). The cutoff in the studies was median MTV (n = 6), 75th percentile MTV (n = 1), or optimal cutoff (n = 3), as well as recalculated median MTV from HR using MTV as a continuous variable (n = 2). MTV was delineated with an absolute threshold in seven studies, with a relative threshold in three studies, and with a software-based method in two studies. Cutoffs and definitions of MTV in the studies included in the meta-analyses are available in Supplementary Materials File S1, Table S1.
HR for OS and PFS was significantly higher with high MTV (pooled HR for OS: 2.83 (2.00–4.01), p < 0.0001; pooled HR for PFS: 3.22 (1.96–5.28), p < 0.0001). Forest plots are presented in Figure 6. The heterogeneity between the studies was high (OS as endpoint: I2 = 77%, tau2 = 0.2745; PFS as endpoint: I2 = 82%, tau2 = 0.3952). Funnel plots were asymmetric with larger HR for studies with lower precision (p = 0.04 for OS; p = 0.08 for PFS) (Figure 7), corresponding to the large interstudy heterogeneity, although publication bias is possible.
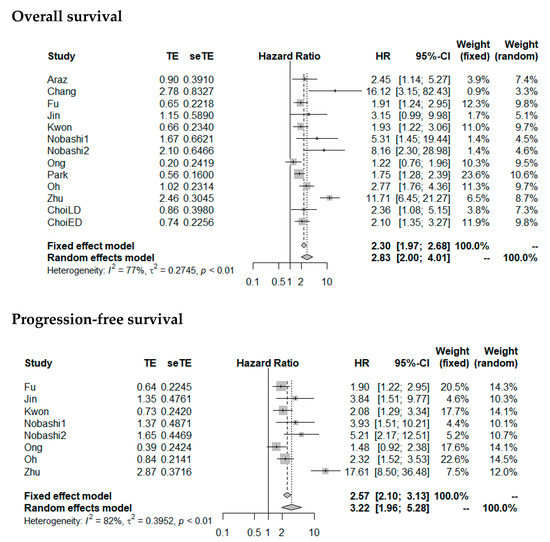
Figure 6.
Forest plots of HRs of MTV for overall survival and progression-free survival. Nobashi1 refers to the results of the central type of SCLC. Nobashi2 refers to the results of the peripheral type of SCLC.
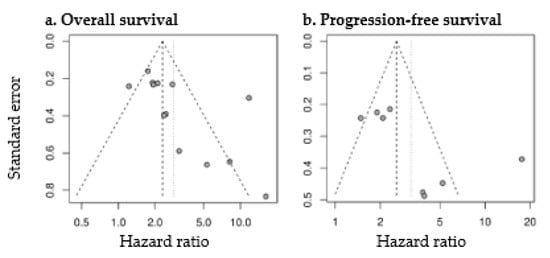
Figure 7.
Funnel plots of studies assessing the prognostic value of MTV for OS (a) and PFS (b).
4. Discussion
This paper provides an overview and meta-analyses of PET parameters for prognostication in SCLC in order to identify the most valuable PET parameter for prognostication. From the available results, baseline MTV, regardless of the delineation method, performed well in individual studies, in the meta-analysis, and in multivariate analysis in the individual studies. MTV measured throughout the whole body performed better than MTV in the primary tumor. MTV was a stronger prognosticator than most clinical parameters and had an equal or additional prognostic value to stage. Baseline SUVmax did not show a convincing prognostic value in the qualitative analysis and showed only a slight prognostic value in the meta-analysis. TLG, combining MTV and SUVmax, did not add a prognostic value to MTV. The compound parameter sumSUVmax showed promise in univariate and multivariate analyses, with either an additional or stronger prognostic value, compared with stage and objective response but was addressed in only two studies [14,37].
The prognostic value of PET parameters after treatment were addressed in seven studies and during treatment only in one study. Results were encouraging; however, due to the large variety of investigated PET parameters, it cannot be justified to appoint a superior PET parameter.
A previous meta-analysis on patients with SCLC established a small prognostic value of SUVmax for PFS (HR: 1.09) and OS (HR: 1.13) [50], similar to our results. However, a limitation to the meta-analyses of Zhu et al. is pooling of HR of high vs. low SUVmax with HR for continuously increasing SUVmax and inclusion of results from univariate and multivariate analyses. HR and 95% CI for a continuous increase is smaller than HR for a dichotomized parameter, affecting the weight of the studies in the pooled analysis. The meta-analysis of Zhu et al. included 1062 patients from 12 studies; however, more than 80% of the weight in the meta-analyses was based on data from one study with 59 patients [21]. Zhu et al. did not perform meta-analysis on MTV. In other cancers, including non-small cell lung cancer NSCLC [51], lymphoma [52], and head and neck squamous cell carcinoma [53], meta-analysis also demonstrated a superiority of MTV over SUVmax. However, SUVmax, but not MTV, was prognostic for event-free survival in a meta-analysis in patients with breast cancer [54]. It has previously been suggested that in advanced cancers, SUVmax may not be representative of tumor metabolism or tumor burden [55]. This may contribute to the different results seen in different cancers and could explain why wbMTV is a better prognosticator than SUVmax in SCLC. SUVmax represents the metabolism in one single voxel, whereas wbMTV reflects the entire tumor burden. In an aggressive cancer such as SCLC with a high metabolic activity in the vast majority of cases, it is likely that a prognosticator to even a higher extent needs to reflect the entire tumor burden to add value compared with that in other cancers.
Numerous PET parameters have been evaluated for prognostic value in patients with SCLC; however, to our knowledge, radiomic features have not yet been addressed in SCLC. Results from the prognostic value of radiomic features in patients with NSCLC have been inconsistent [56]. A validation study did not find an independent prognostic value of PET radiomics in NSCLC [57].
A comparison of the prognostic value of MTV with those of other parameters of tumor burden (i.e., volume measured by other imaging modalities or by the tumor, node, metastasis (TNM) staging system) would be relevant. Except for stage (ED vs. LD), LDH, and metastases, other parameters of tumor burden were not included in the papers. In NSCLC, a large validation study showed an independent prognostic value of MTV and TNM stage, and a combined index of MTV, TNM stage, and age improves the accuracy of OS prognosis [58].
This study has limitations. Meta-analyses often overestimate HR [59], and the possibility of publication bias must be considered. Funnel plots showed tendencies toward asymmetry, particularly for MTV, suggesting the presence of publication bias. However, interpretation of asymmetry tests should be done with caution when the included studies show large interstudy heterogeneity [60] and when the analysis includes censored data [61]. In these instances, which are both relevant for this meta-analysis, the asymmetry can be caused by heterogeneity. Most studies identified at least one PET parameter with a prognostic value, but in addition to their positive results, negative results from other PET parameters were also presented, and therefore, a small study effect does not seem obvious. However, the selection of which PET parameters are presented in each study may be biased. With 73 different approaches used to quantify PET parameters presented in the included 38 studies, and the fact that almost all studies identified at least one significant prognosticator, this calls for a concern for selective analysis reporting, favoring the presentation of PET parameters with positive results and, to a lesser extent, including PET parameters with negative results in the papers.
The risk of bias in the included studies was evaluated using the QUIPS tools. There was a high risk of bias within the domain “study participation” due to the retrospective design of 35 of 38 studies. Patients were included only if a baseline FDG–PET/CT was available, but the reasons for not having an available FDG–PET/CT were not given. The risk of bias in the domain “study confounding” was moderate to high in 29 of 38 studies and in 13 of 19 studies included in the meta-analysis. The prognostic value of adjusted PET parameters is more clinically relevant than an unadjusted prognostic value, and it has been recommended that the adjusted HR is used in meta-analyses [59]. However, different multivariate study designs were used in each study; thus a comparison of adjusted HRs in the meta-analysis would be highly biased. Additionally, the measurement of the PET parameters was associated with risks of bias, often caused by using a study-specific (optimal) cutoff for dichotomizing the patients into groups with high and low PET parameters.
The studies included in our meta-analysis showed a large interstudy heterogeneity. Apart from the different cutoff values for dichotomizing high vs. low SUVmax and MTV, differences in the included study populations, PET protocols, and definitions for PET parameters contributed to the heterogeneity. To accommodate the interstudy heterogeneity, random effects model meta-analyses were applied. We found a significant prognostic value of MTV for OS and PFS, and a lesser pronounced prognostic value of SUVmax. A strong prognosticator should be able to prove its worth under a slightly varying condition, and the prognostic value of MTV may exist regardless of the delineation method, anatomical boundaries, and cutoff value, but it rather represents an increasing risk when MTV increases.
5. Conclusions
From these review and meta-analyses, we have identified baseline MTV as a strong prognosticator for PFS and OS in patients with SCLC. MTV has a prognostic value that is superior to those of other PET parameters, but whether MTV is superior to other prognosticators of tumor burden, such as stage and CT volumetrics, needs further investigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/11/2/174/s1: Supplementary File S1: Table S1: Studies included in meta-analysis. Definitions of PET parameters and cutoff. Supplementary File S2: Techniques used in meta-analysis when estimate and standard error (SE) were not directly available.
Author Contributions
Conceptualization: T.N.C.; methodology: T.N.C., B.M.B.F., and P.K.A.; formal analysis: T.N.C. and P.K.A.; investigation: T.N.C.; data curation: T.N.C.; writing—original draft preparation: T.N.C.; writing—review and editing: T.N.C., P.K.A., S.W.L., and B.M.B.F.; visualization: T.N.C. and P.K.A.; supervision: S.W.L. and B.M.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dayen, C.; Debieuvre, D.; Molinier, O.; Raffy, O.; Paganin, F.; Virally, J.; Larive, S.; Desurmont-Salasc, B.; Perrichon, M.; Martin, F.; et al. New insights into stage and prognosis in small cell lung cancer: An analysis of 968 cases. J. Thorac. Dis. 2017, 9, 5101–5111. [Google Scholar] [CrossRef] [PubMed]
- Lattuca-Truc, M.; Timsit, J.F.; Levra, M.G.; Ruckly, S.; Villa, J.; Dumas, I.; Pinsolle, J.; Ferrer, L.; Guillem, P.; Moro-Sibilot, D.; et al. Trends in response rate and survival in small-cell lung cancer patients between 1997 and 2017. Lung Cancer 2019, 131, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zimmermann, S.; Parikh, K.; Mansfield, A.S.; Adjei, A.A. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin. Proc. 2019, 94, 1599–1622. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Z.; Luo, F.; Zhao, Y.; Hou, X.; Liu, T.; Wang, K.; Zhao, H.; Huang, Y.; Zhang, L. Comparison of First-Line Treatments for Patients with Extensive-Stage Small Cell Lung Cancer: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2020, 3, e2015748. [Google Scholar] [CrossRef]
- Ruben, J.D.; Ball, D.L. The efficacy of PET staging for small-cell lung cancer: A systematic review and cost analysis in the Australian setting. J. Thorac. Oncol. 2012, 7, 1015–1020. [Google Scholar] [CrossRef]
- Fischer, B.M.; Mortensen, J.; Langer, S.W.; Loft, A.; Berthelsen, A.K.; Petersen, B.I.; Daugaard, G.; Lassen, U.; Hansen, H.H. A prospective study of PET/CT in initial staging of small-cell lung cancer: Comparison with CT, bone scintigraphy and bone marrow analysis. Ann. Oncol. 2007, 18, 338–345. [Google Scholar] [CrossRef]
- Van Loon, J.; van Baardwijk, A.; Boersma, L.; Ollers, M.; Lambin, P.; De Ruysscher, D. Therapeutic implications of molecular imaging with PET in the combined modality treatment of lung cancer. Cancer Treat. Rev. 2011, 37, 331–343. [Google Scholar] [CrossRef]
- Langer, N.H.; Christensen, T.N.; Langer, S.W.; Kjaer, A.; Fischer, B.M. PET/CT in therapy evaluation of patients with lung cancer. Expert Rev. Anticancer Ther. 2014, 14, 595–620. [Google Scholar] [CrossRef]
- Fischer, B.M.; Mortensen, J.; Langer, S.W.; Loft, A.; Berthelsen, A.K.; Daugaard, G.; Lassen, U.; Hansen, H.H. PET/CT imaging in response evaluation of patients with small cell lung cancer. Lung Cancer 2006, 54, 41–49. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Cote, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.R.; Seo, J.H.; Chong, A.; Min, J.J.; Song, H.C.; Kim, Y.C.; Bom, H.S. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.R.; Seo, J.H.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Min, J.J.; Song, H.C.; Bom, H.S.; Kim, Y.C.; et al. Extra-thoracic tumor burden but not thoracic tumor burden on (18)F-FDG PET/CT is an independent prognostic biomarker for extensive-disease small cell lung cancer. Lung Cancer 2013, 81, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.S.; Mok, J.H.; Lee, K.; Kim, K.U.; Park, H.K.; Kim, S.J.; Lee, M.K. Metabolic burden measured by (18)f-fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in patients with small cell lung cancer. Cancer Res. Treat. 2014, 46, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chang, S. Limited Prognostic Value of SUV max Measured by F-18 FDG PET/CT in Newly Diagnosed Small Cell Lung Cancer Patients. Oncol. Res. Treat. 2015, 38, 577–585. [Google Scholar] [CrossRef]
- Jin, F.; Qu, B.; Fu, Z.; Zhang, Y.; Han, A.; Kong, L.; Yu, J. Prognostic Value of Metabolic Parameters of Metastatic Lymph Nodes on (18)F-FDG PET/CT in Patients With Limited-stage Small-cell Lung Cancer With Lymph Node Involvement. Clin. Lung Cancer 2018, 19, e101–e108. [Google Scholar] [CrossRef]
- Gomez, D.R.; Gladish, G.W.; Wei, X.; Kotamarti, K.R.; Allen, P.K.; Cox, J.D.; O’Reilly, M.S.; Erasmus, J.J.; Fossella, F.V.; Komaki, R. Prognostic value of positron emission tomography/computed tomography findings in limited-stage small cell lung cancer before chemoradiation therapy. Am. J. Clin. Oncol. 2014, 37, 77–80. [Google Scholar] [CrossRef]
- Choi, E.K.; Park, M.; Im, J.J.; Chung, Y.A.; Oh, J.K. Prognostic value of (18)F-FDG PET/CT metabolic parameters in small cell lung cancer. J. Int. Med. Res. 2019. [Google Scholar] [CrossRef]
- Kasahara, N.; Kaira, K.; Yamaguchi, K.; Masubuchi, H.; Tsurumaki, H.; Hara, K.; Koga, Y.; Sakurai, R.; Higuchi, T.; Handa, T.; et al. Fluorodeoxyglucose uptake is associated with low tumor-infiltrating lymphocyte levels in patients with small cell lung cancer. Lung Cancer 2019, 134, 180–186. [Google Scholar] [CrossRef]
- Aktan, M.; Koc, M.; Kanyilmaz, G.; Yavuz, B.B. Prognostic value of pre-treatment (18)F-FDG-PET uptake in small-cell lung cancer. Ann. Nucl. Med. 2017, 31, 462–468. [Google Scholar] [CrossRef]
- Kwon, S.H.; Hyun, S.H.; Yoon, J.K.; An, Y.S.; Oh, Y.T.; Choi, J.H.; Park, K.J.; Lee, S.J. The Highest Metabolic Activity on FDG PET Is Associated with Overall Survival in Limited-Stage Small-Cell Lung Cancer. Medicine 2016, 95, e2772. [Google Scholar] [CrossRef]
- Van der Leest, C.; Smit, E.F.; Baas, J.; Versteijlen, R.J.; van Walree, N.; Hoogsteden, H.C.; Aerts, J.G. SUVmax during 18FDG-PET scanning in small cell lung cancer: Similar information as in non-small cell lung cancer? Lung Cancer 2012, 76, 67–71. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, T.; Niu, Z.; Zheng, J.; Han, A.; Zhao, S.; Yu, J. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer 2011, 73, 332–337. [Google Scholar] [CrossRef]
- Chong, S.; Lee, K.S.; Kim, B.T.; Choi, J.Y.; Yi, C.A.; Chung, M.J.; Oh, D.K.; Lee, J.Y. Integrated PET/CT of pulmonary neuroendocrine tumors: Diagnostic and prognostic implications. AJR Am. J. Roentgenol. 2007, 188, 1223–1231. [Google Scholar] [CrossRef]
- Ozdemir, O.; Batum, O.; Ermin, S.; Aksel, N.; Komurcuoglu, B.; Mertoglu, A.; Deniz, S.; Balci, G.; Koparal, H.; Ozbilek, E.; et al. Metabolic activity of primary tumour on PET/CT has a relationship with survival in stages I-III small-cell lung carcinoma. Clin. Respir. J. 2020. [Google Scholar] [CrossRef]
- Araz, M.; Soydal, C.; Ozkan, E.; Sen, E.; Nak, D.; Kucuk, O.N.; Gonullu, U.; Kir, K.M. Prognostic value of metabolic parameters on baseline 18F-FDG PET/CT in small cell lung cancer. Q. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef]
- Chang, H.; Lee, S.J.; Lim, J.; Lee, J.S.; Kim, Y.J.; Lee, W.W. Prognostic significance of metabolic parameters measured by (18)F-FDG PET/CT in limited-stage small-cell lung carcinoma. J. Cancer Res. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, Y.; Jing, W.; Guo, D.; Kong, L.; Yu, J. Incorporation of circulating tumor cells and whole-body metabolic tumor volume of (18)F-FDG PET/CT improves prediction of outcome in IIIB stage small-cell lung cancer. Chin. J. Cancer Res. 2018, 30, 596–604. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, I.R.; Boo, S.H.; Park, H.L.; Kim, S.H. Prognostic Value of Pre- and Post-Treatment FDG PET/CT Parameters in Small Cell Lung Cancer Patients. Nucl. Med. Mol. Imaging 2018, 52, 31–38. [Google Scholar] [CrossRef]
- Yilmaz Demirci, N.; Yilmaz, U.; Biner Uslu, I.; Dikmen, A.; Yilmaz, A.; Erdogan, Y. Prognostic significance of standardised uptake value (SUVmax) measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Eur. J. Cancer Care 2017, 26. [Google Scholar] [CrossRef]
- Dinc, N.S.; Aydin, K.; Odabas, H.; Ercelep, O.; Tufan, G.; Seker, M.; Yasar, N.; Aydin, D.; Yuksel, S.; Mert, A.; et al. Pretreatment PET/CT Standardized Uptake Values Play a Role in Predicting Response to Treatment and Survival in Patients with Small Cell Lung Cancer. Oncol. Res. Treat. 2016, 39, 130–134. [Google Scholar] [CrossRef]
- Nobashi, T.; Koyasu, S.; Nakamoto, Y.; Kubo, T.; Ishimori, T.; Kim, Y.H.; Yoshizawa, A.; Togashi, K. Prognostic value of fluorine-18 fludeoxyglucose positron emission tomography parameters differs according to primary tumour location in small-cell lung cancer. Br. J. Radiol. 2016, 89, 20150618. [Google Scholar] [CrossRef]
- Zer, A.; Domachevsky, L.; Rapson, Y.; Nidam, M.; Flex, D.; Allen, A.M.; Stemmer, S.M.; Groshar, D.; Bernstine, H. The Role of 18F-FDG PET/CT on Staging and Prognosis in Patients with Small Cell Lung Cancer. Eur. Radiol. 2016, 26, 3155–3161. [Google Scholar] [CrossRef]
- Ong, L.T.; Dunphy, M.; Foster, A.; Woo, K.M.; Zhang, Z.; Perez, C.A.; Pietanza, C.M.; Rosenzweig, K.E.; Gelblum, D.Y.; Rimner, A.; et al. Prognostic Value of Preradiotherapy (18)F-FDG PET/CT Volumetrics in Limited-Stage Small-Cell Lung Cancer. Clin. Lung Cancer 2016, 17, 184–188. [Google Scholar] [CrossRef]
- Park, S.B.; Choi, J.Y.; Moon, S.H.; Yoo, J.; Kim, H.; Ahn, Y.C.; Ahn, M.J.; Park, K.; Kim, B.T. Prognostic value of volumetric metabolic parameters measured by [18F]fluorodeoxyglucose-positron emission tomography/computed tomography in patients with small cell lung cancer. Cancer Imag. 2014, 14, 2. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.O.; Jung, C.K.; Kim, Y.S.; Yoo, I.R.; Choi, W.H.; Jeon, E.K.; Hong, S.H.; Chun, S.H.; Kim, S.J.; et al. Metabolic activity on [18f]-fluorodeoxyglucose-positron emission tomography/computed tomography and glucose transporter-1 expression might predict clinical outcomes in patients with limited disease small-cell lung cancer who receive concurrent chemoradiation. Clin. Lung Cancer. 2014, 15, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Go, S.I.; Song, H.N.; Kang, J.H.; Kang, M.H.; Kim, M.J.; Jung, J.; Chung, S.I.; Choi, B.H.; Hwang, I.G.; Kim, S.H.; et al. The clinical impact of the sum of the maximum standardized uptake value on the pretreatment with F-FDG-PET/CT in small-cell lung cancer. Oncology 2014, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inal, A.; Kucukoner, M.; Kaplan, M.A.; Urakci, Z.; Nas, N.; Guven, M.; Dostbil, Z.; Altindag, S.; Isikdogan, A. Is (18)F-FDG-PET/CT prognostic factor for survival in patients with small cell lung cancer? Single center experience. Rev. Port. Pneumol. 2013, 19, 260–265. [Google Scholar] [CrossRef]
- Jhun, B.W.; Lee, K.J.; Jeon, K.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Sun, J.M.; Ahn, J.S.; Ahn, M.J.; et al. Clinical applicability of staging small cell lung cancer according to the seventh edition of the TNM staging system. Lung Cancer 2013, 81, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cho, A.; Cho, B.C.; Yun, M.; Kim, S.K.; Chang, J.; Moon, J.W.; Park, I.K.; Choi, H.J.; Kim, J.H. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin. Cancer Res. 2009, 15, 2426–2432. [Google Scholar] [CrossRef]
- Pandit, N.; Gonen, M.; Krug, L.; Larson, S.M. Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 78–84. [Google Scholar] [CrossRef]
- Quartuccio, N.; Evangelista, L.; Alongi, P.; Caobelli, F.; Altini, C.; Cistaro, A.; Lambertini, A.; Schiorlin, I.; Popescu, C.E.; Linguanti, F.; et al. Prognostic and diagnostic value of [18F]FDG-PET/CT in restaging patients with small cell lung carcinoma: An Italian multicenter study. Nucl. Med. Commun. 2019. [Google Scholar] [CrossRef]
- Ziai, D.; Wagner, T.; El Badaoui, A.; Hitzel, A.; Woillard, J.B.; Melloni, B.; Monteil, J. Therapy response evaluation with FDG-PET/CT in small cell lung cancer: A prognostic and comparison study of the PERCIST and EORTC criteria. Cancer Imag. 2013, 13, 73–80. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Engel, J.M.; Demos, J.M.; Mukesh, B. Prognostic significance of 18 F-fluorodeoxyglucose—positron emission tomography after treatment in patients with limited stage small cell lung cancer. Clin. Med. Res. 2008, 6, 72–77. [Google Scholar] [CrossRef][Green Version]
- Blum, R.; MacManus, M.P.; Rischin, D.; Michael, M.; Ball, D.; Hicks, R.J. Impact of positron emission tomography on the management of patients with small-cell lung cancer: Preliminary experience. Am. J. Clin. Oncol. 2004, 27, 164–171. [Google Scholar] [CrossRef]
- Van Loon, J.; Offermann, C.; Ollers, M.; van Elmpt, W.; Vegt, E.; Rahmy, A.; Dingemans, A.M.; Lambin, P.; De Ruysscher, D. Early CT and FDG-metabolic tumour volume changes show a significant correlation with survival in stage I-III small cell lung cancer: A hypothesis generating study. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2011, 99, 172–175. [Google Scholar] [CrossRef]
- Mirili, C.; Guney, I.B.; Paydas, S.; Seydaoglu, G.; Kapukaya, T.K.; Ogul, A.; Gokcay, S.; Buyuksimsek, M.; Yetisir, A.E.; Karaalioglu, B.; et al. Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET-CT metabolic parameters in small cell lung cancer (SCLC). Int. J. Clin. Oncol. 2019, 24, 168–178. [Google Scholar] [CrossRef]
- Reymen, B.; Van Loon, J.; van Baardwijk, A.; Wanders, R.; Borger, J.; Dingemans, A.M.; Bootsma, G.; Pitz, C.; Lunde, R.; Geraedts, W.; et al. Total gross tumor volume is an independent prognostic factor in patients treated with selective nodal irradiation for stage I to III small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1319–1324. [Google Scholar] [CrossRef]
- Arslan, N.; Tuncel, M.; Kuzhan, O.; Alagoz, E.; Budakoglu, B.; Ozet, A.; Ozguven, M.A. Evaluation of outcome prediction and disease extension by quantitative 2-deoxy-2-[18F] fluoro-D-glucose with positron emission tomography in patients with small cell lung cancer. Ann. Nucl. Med. 2011, 25, 406–413. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Wang, L.; Chen, J.; Byanju, S.; Zhang, H.; Liao, M. Prognostic value of the maximum standardized uptake value of pre-treatment primary lesions in small-cell lung cancer on 18F-FDG PET/CT: A meta-analysis. Acta Radiol. 2017, 284185117745907. [Google Scholar] [CrossRef]
- Im, H.J.; Pak, K.; Cheon, G.J.; Kang, K.W.; Kim, S.J.; Kim, I.J.; Chung, J.K.; Kim, E.E.; Lee, D.S. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: A meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 241–251. [Google Scholar] [CrossRef]
- Xie, M.; Wu, K.; Liu, Y.; Jiang, Q.; Xie, Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: A meta-analysis with 702 participants. Med. Oncol. 2015, 32, 446. [Google Scholar] [CrossRef]
- Bonomo, P.; Merlotti, A.; Olmetto, E.; Bianchi, A.; Desideri, I.; Bacigalupo, A.; Franco, P.; Franzese, C.; Orlandi, E.; Livi, L.; et al. What is the prognostic impact of FDG PET in locally advanced head and neck squamous cell carcinoma treated with concomitant chemo-radiotherapy? A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2122–2138. [Google Scholar] [CrossRef]
- Wen, W.; Xuan, D.; Hu, Y.; Li, X.; Liu, L.; Xu, D. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with breast cancer: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0225959. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, H. Prognostic Value of (18)F-Fluorodeoxyglucose PET/Computed Tomography in Non-Small-Cell Lung Cancer. PET Clin. 2018, 13, 59–72. [Google Scholar] [CrossRef]
- Han, S.; Woo, S.; Suh, C.H.; Kim, Y.J.; Oh, J.S.; Lee, J.J. A systematic review of the prognostic value of texture analysis in (18)F-FDG PET in lung cancer. Ann. Nucl. Med. 2018, 32, 602–610. [Google Scholar] [CrossRef]
- Krarup, M.M.K.; Nygard, L.; Vogelius, I.R.; Andersen, F.L.; Cook, G.; Goh, V.; Fischer, B.M. Heterogeneity in tumours: Validating the use of radiomic features on (18)F-FDG PET/CT scans of lung cancer patients as a prognostic tool. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 144, 72–78. [Google Scholar] [CrossRef]
- Finkle, J.H.; Penney, B.C.; Pu, Y. An updated and validated PET/CT volumetric prognostic index for non-small cell lung cancer. Lung Cancer 2018, 123, 136–141. [Google Scholar] [CrossRef]
- Riley, R.D.; Moons, K.G.M.; Snell, K.I.E.; Ensor, J.; Hooft, L.; Altman, D.G.; Hayden, J.; Collins, G.S.; Debray, T.P.A. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019, 364, k4597. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Debray, T.P.A.; Moons, K.G.M.; Riley, R.D. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res. Synth. Methods 2018, 9, 41–50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).