Abstract
Analysis of circulating cell-free tumor DNA (cftDNA) has emerged as a specific and sensitive blood-based approach to detect epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) patients. Still, there is some debate on what should be the preferential clinical method for plasma-derived cftDNA analysis. We tested 31 NSCLC patients treated with anti-EGFR tyrosine kinase inhibitors (TKIs), at baseline and serially during therapy, by comparing three methodologies in detecting EGFR mutations (L858R, exon 19 deletion, and T790M) from plasma: scorpions-amplification refractory mutation system (ARMS) methodology by using EGFR Plasma RGQ PCR Kit-QIAGEN, peptide nucleic acid (PNA) clamp and PANA RealTyper integration by using PNAClamp EGFR-PANAGENE, and digital real time PCR by using QuantStudio 3D Digital PCR System-Thermo Fisher Scientific. Specificity was 100% for all three mutations, independently from the platform used. The sensitivity for L858R (42.86%) and T790M (100%) did not change based on the method, while the sensitivity for Del 19 differed markedly (Scorpion-ARMS 45%, PNAClamp 75%, and Digital PCR 85%). The detection rate was also higher (94.23%) as measured by Digital PCR, and when we monitored the evolution of EGFR mutations over time, it evidenced the extreme inter-patient heterogeneity in terms of levels of circulating mutated copies. In our study, Digital PCR showed the best correlation with tissue biopsy and the highest sensitivity to attain the potential clinical utility of monitoring plasma levels of EGFR mutations.
1. Introduction
The identification of activating epidermal growth factor receptor (EGFR) mutations plays an important role in determining the treatment response to EGFR tyrosine kinase inhibitors (TKIs), including gefitinib, erlotinib, afatinib and, more recently, osimertinib in advanced non-small cell lung cancer (NSCLC) patients. The most common activating mutations of the EGFR gene are the in-frame deletions of exon 19 and the missense mutations of exon 21 (i.e., p.Leu858Arg), constituting more than 90% of known EGFR activating mutations [1,2]. Unfortunately, almost all patients with NSCLC who respond to EGFR-TKIs therapy soon develop acquired resistance and experience disease progression within 10 to 16 months. The T790M mutation in EGFR exon 20 is a recurrent mechanism of resistance to first-line EGFR-TKIs, detectable in nearly 50% of tissue specimens at progression [3,4,5]. The frequency of T790M mutation in EGFR-TKI-naive patients and its dynamic changes during therapy remains unclear [6,7,8]. The third-generation EGFR-TKI osimertinib, which specifically targets EGFR T790M mutation, was approved for use in some countries, including the United of States (US) and the European Union (EU), in patients who have developed T790M after first and second generation TKI treatment and, more recently, in EGFR mutated treatment naïve patients. Hence, it is of increasing importance to collect information from serial biopsies on T790M to determine the appropriateness of osimertinib treatment [9,10]. However, repeated tissue biopsies in patients with advanced disease is not always feasible, due to the invasiveness of the intervention, and when it is possible it may be difficult to obtain enough tumor DNA for the EGFR mutation test. Moreover, tissue specimens may not be reflective of the patient’s complete disease burden due to spatial and temporal tumor heterogeneity [11,12]. In recent years, new methods to detect disease relevant mutations from liquid biopsy as alternative sources are being developed. In particular, circulating cell-free tumor DNA (cftDNA) in plasma has emerged as a specific and sensitive blood-based biomarker for the detection of EGFR mutations. Genotyping cftDNA in a fresh blood sample represents a noninvasive and feasible method for real-time monitoring of the treatment response to EGFR-TKIs and to predict drug resistance [13,14,15,16,17,18,19]. Moreover, EGFR-activating mutation analysis on cftDNA has been approved as a companion diagnosis to select NSCLC patients for treatment with gefitinib and osimertinib in the EU. However, some technical limitations in detecting EGFR mutations with cftDNA have been reported. For example, the quantity and quality of circulating tumor-derived DNA varies widely between patients [20]. Specifically, the abundance of cftDNA varies from 0.01% to 67% for patients with different kinds of cancers or progression stages [21,22]. Several studies have also evaluated the concordance between mutations detected in tumor tissues and those observed in plasma cftDNA with different sensitivity results depending on the type of technology used [14,23,24,25,26]. DNA from normal cells is always present in plasma together with tumor-released cell free DNA, which often represents only a small fraction of the total circulating DNA, so it is important to use high sensitive technologies to detect tumor-specific somatic mutations. Among several methodologies, such as amplification refractory mutation system (ARMS), Digital PCR and next-generation sequencing (NGS) [27,28,29], there is not a widely accepted and approved method for EGFR mutation analysis from cftDNA. In this study, we reported a performance comparison between three technologies (Scorpion-ARMS EGFR Plasma RGQ PCR Kit-QIAGEN, QuantStudio 3D Digital PCR System-Thermo Fisher Scientific and PNAClamp EGFR-PANAGENE) in detecting clinically-relevant EGFR mutations in tumor tissue and plasma collected from NSCLC patients. We monitored EGFR mutations in plasma samples at baseline and serially during treatment with EGFR-TKIs to predict early development of resistance to treatment.
2. Materials and Methods
2.1. Patient Selection
This was a prospective, multi-institution clinical study that included NSCLC patients treated at the Medical Oncology Division in S. Maria della Misericordia Hospital of Perugia, and at Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS of Meldola (FC), Italy, from November 2014 to July 2017. Patients were considered for inclusion if they met the following criteria: (1) pathologically confirmed diagnoses with advanced or recurrent primary lung cancer according to the seventh Edition of TNM in Lung Cancer [30], with sufficient tissue samples for the study harboring activating EGFR mutations and sufficient peripheral blood; (2) the possibility to make serial blood samples during the TKIs treatment (baseline, 8 days and 20 days after the start of treatment, clinical evaluation and progression of the disease, if occurred during the sampling); (3) complete information obtained, including age, gender, smoking history, and staging; (4) complete medical documentation including follow-up records followed by an eventual systemic objective progression according to response evaluation criteria in solid tumors or World Health Organization criteria (RECIST). Exclusion criteria included: (1) aged below 18 years old; (2) pregnant patients. The study was conducted in accordance with Declaration of Helsinki principles, and approved by the Institutional Review Board of the S. Maria della Misericordia Hospital of Perugia, (Number: 2576/15, approved April 28, 2015 and IRST of Meldola (FC) (Number: 1297/15; approved March 18, 2015. Written informed consent was obtained from each patient prior to study entry.
2.2. Tissue and Blood Sample Collection and cftDNA Extraction
Tissue and blood samples were obtained from advanced NSCLC patients treated with EGFR-TKIs. Tumor tissue genomic DNA was extracted from 10 formalin fixed paraffin embedded (FFPE) slides using the QIAamp DNA FFPE Tissue Kit on the QIAcube Instrument (Qiagen S.p.A. Milan, Italy). Peripheral blood was collected into 2 tubes containing EDTA-K2 anticoagulant (5 mL) (BD Diagnostics, Buccinasco-Milan, Italy) and processed within 30 min. Whole blood was first centrifuged at 1100 g for 15 min to separate the plasma from the peripheral blood cells. The supernatant was collected and transferred into a 2 mL Eppendorf tube (EP tube), followed by centrifugation at 1500 g for 10 min to pellet any remaining cells. Plasma (supernatant) was collected, transferred into a new 2 mL EP tube and stored at −80 °C. CftDNA were extracted from the plasma samples (at least 2 mL) using QIAamp Circulating Nucleic Acid Kit on the QIAvac instrument 24 Plus (Qiagen S.p.A. Milan, Italy), according to the manufacturer’s instruction. The cftDNA was recovered in 55 μL elution buffers (TE Buffer) and immediately stored at −20 °C until use.
2.3. Detection of EGFR Mutations in Tumor Tissue
EGFR mutation testing on tissue samples of patients was performed at the diagnosis using two standardized methodologies, Scorpion-ARMS PCR Kit on Rotor-Gene Q MDx instrument, (Qiagen, S.p.A. Milan, Italy) or MYRIAPOD® Lung status (Diatech Pharmacogenetics, Jesi, Italy) using the MASSArray Sequenom system (Diatech Pharmacogenetics). Assays were performed according to each manufacturer’s protocols.
2.4. Detection of EGFR Mutations in Plasma
Extracted cftDNA of patients harboring activating mutations (exon 19 deletions, L858R and T790M) in tumor tissue, were tested for the same EGFR mutations using three different technologies: Scorpion-ARMS EGFR Plasma RGQ PCR Kit-QIAGEN, PNAClamp R EGFR-PANAGENE and QuantStudio 3D Digital PCR System-Thermo Fisher Scientific. Assays were performed according to each manufacturer’s protocols.
2.5. Scorpion-ARMS EGFR Plasma RGQ
The Scorpion-ARMS EGFR Plasma RGQ PCR Kit is an in vitro diagnostic test for the detection of the 21 EGFR mutations (Del 19, L858R and T790M) on cftDNA extracted from plasma using a real-time polymerase chain reaction (Q-PCR) on the Rotor-Gene Q MDx instrument (Qiagen S.p.A. Milan, Italy). The kit utilizes two technologies, amplification refractory mutation system (ARMS) that ensures distinguishing between a match and a mismatch at the 3’ end of a PCR primer, combined with Scorpions, bifunctional molecules containing a PCR primer covalently linked to a probe to cause increased fluorescence from the reaction tube. The assays were carried out according to manufacturer’s instructions. Each run contains a positive and a negative control and each sample is analyzed for the mutations and for a control assay that amplifies a region of exon 2 of the EGFR gene and is used as a reference to calculate the ∆Ct. The assay provides a qualitative assessment of the mutation status.
2.6. PNAClamp EGFR
The PNAClamp EGFR kit (PANAGENE, Daejeon, Korea), a technology based on peptide nucleic acid (PNA)-mediated real time PCR clamping and melting peak analysis, was used for mutation analysis of the cftDNA. This technology integrates PNAClamp™ and PANA RealTyper™ (PNA probe-based fluorescence melting curve analysis). PNAClamp™ is a kind of PCR technology and uses peptide nucleic acid (PNA) probes which complementarily bind to the wild-type DNA. PANA RealTyper™ uses multiplex melting curve analysis with fluorescence labelled PNA probes. PNAClamp™ takes advantage of both technologies. It is not only able to detect small amounts of mutation with high sensitivity, but it is also able to genotype multiple mutations, simultaneously analyzing their own melting temperature (Tm) value for the sequence changes of the target gene.
2.7. QuantStudio 3D Digital PCR
Digital PCR (dPCR) was performed using the QuantStudio 3D Digital PCR platform (Thermo Fisher Scientific, Monza, Italy). Mutations analysis of cftDNA was performed by an allele specific TaqMan® probe targeting known EGFR mutations (exon 19 deletions, L858R and T790M mutation). Wild Type (WT) EGFR alleles were represented by a VIC fluorescent probe while mutant EGFR alleles were represented by a FAM fluorescent probe. We purchased all 3D Digital PCR reagents from Thermo Fisher Scientific, the custom ordered primers were: T790M (Assay ID: AHRSROS), L858R (Assay ID:AHRSRSV) and Del 19 (Assay ID:Hs00000228_mu). The final 15 μL of TaqMan PCR reaction mixture was made up according to the following: 7.5 μL 2× QuantStudio™ 3D Digital PCR Master Mix, 0.75 μL 20× TaqManAssay (primer/probe mix), 6.75 μL diluted DNA (25 ng), and then loaded into the QuantStudio™ 3D Digital PCR Chip, which has 20,000 mini-chambers. To perform the PCR using the ProFlex™ 2× Flat PCR System, the thermal cycling profile was 10 min of incubation at 96 °C, followed by 39 cycles of 60 °C for 2 min, 98 °C for 30 s, 60 °C for 2 min, and then 4 °C hold. We used the QuantStudio™ 3D Digital PCR instrument to read the chip. The subsequent analysis was performed with the QuantStudio 3D Analysis Suite Software. The QuantStudio™ 3D Digital PCR System provides qualitative detection of target nucleic acid sequences (targets) and relative (% target/total) or absolute quantification (copies/µl) using allele specific post-PCR (endpoint) analysis.
2.8. Statistical Analysis
The limit of detection (LOD) of each assay was defined as the lowest target concentration that could be specifically detected and was determined for each of the three technologies using the Horizon Multiplex I cfDNA Reference Standard Set (HD780) (Horizon Discovery, Cambridge, UK). The reference standard DNAs that were used included Del 19, L858R, and T790M mutations. Each reference mutant DNA contained the mutant sequence at a frequency of 5%, 1%, and 0.1%, respectively. Reference mutant DNA was also diluted with the corresponding WT EGFR reference DNA to obtain 0.5% and 0.01% allele frequencies DNA. Overall survival (OS) and progression free survival (PFS) were modeled via the Cox proportional hazard regression model. Baseline continuous T790M levels were separately considered as covariates. Results are summarized as hazard ratio (HR) with their 95% confidence intervals and the corresponding p-value. Statistical analyses were achieved using the statistical language R version 3.6.3, and its packages Survminer Version 0.4.8 and survival version 3.2.3.
3. Results
3.1. Patient Characteristics
A total of thirty-one NSCLC patients were enrolled, the median age was 68 years old (range, 38–88 years). Patient characteristics at baseline are shown in Table 1. Most patients were female (74.2%), 64.5% were never-smoker, 90.4% had adenocarcinoma and 93.6% were diagnosed at stage IV. According to the results of the tumor tissue EGFR analysis, twenty patients (64.5%) had Del 19 mutation, six patients (19.4%) had L858R mutation, one (3.2%) had both L858R and T790M mutations and four (12.9%) had other mutations (G719S+L833V, L861Q, L858M, G719C, and S768I). All patients received an EGFR-TKI (35.5% gefitinib, 12.9% erlotinib and 51.6% afatinib) as a first-line therapy and 26 (83.8%) developed disease progression and 21 of them died.

Table 1.
Patient characteristics.
3.2. Sensibility, Specificity and Coincidence Rate of the Three Methods for the Three EGFR Mutations
EGFR mutation analysis of cftDNA extracted from baseline plasma was performed with the three methodologies (Scorpion-ARMS EGFR Plasma, PNAClamp R EGFR and QuantStudio 3D Digital PCR) and compared with the EGFR mutation analysis of the corresponding tumor tissue sample. We measured sensitivity, and the coincidence rate of each of these three cftDNA assays, by using the tissue EGFR mutational profiles for the three driver mutations (Del 19, L858R, T790M) as a reference. Specificity was calculated using DNA extracted from the plasma of 12 healthy blood donors. The results showed that specificity was 100% (12/12) for all the three mutations measured by each of the three methods (Figure 1). Digital PCR showed the highest sensitivity in detecting the Del 19, compared to the other methods. The coincidence rate for Del 19 was also different, based on the method used. Sensitivity for Del 19: Scorpion-ARMS 45% (9/20), Digital PCR 85% (17/20), and PNAClamp 75% (15/20); coincidence rate for Del 19: Scorpion-ARMS 65.62% (21/32), Digital PCR 90.62% (29/32), and PNAClamp 84.37% (27/32). For both L858R and T790M sensitivity and coincidence rates were the same no matter what type of method was used. Sensitivity for L858R was 42.86% (3/7) and 100% (1/1) for T790M. The coincidence rate for L858R was 78.95% (15/19) and 100% (13/13) for T790M.
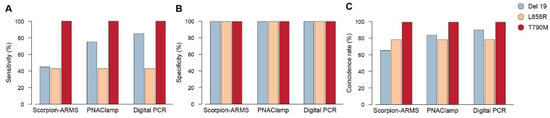
Figure 1.
Levels of sensibility, specificity and coincidence rate of the three methods in detecting epidermal growth factor receptor (EGFR) mutations in plasma samples. EGFR mutations as detected in liquid biopsy at baseline through cell-free tumor DNA (cftDNA) analysis by the Scorpion-ARMS EGFR Plasma RGQ PCR Kit-QIAGEN, PNAClamp EGFR-PANAGENE, and the QuantStudio 3D Digital PCR System-Thermo Fisher Scientific. (A) Sensitivity, (B) specificity, and (C) coincidence rate of each of these three assays, were calculated on the basis of tissue analysis.
3.3. Comparison of the Three Methodologies in Detecting EGFR Mutations in Plasma versus Tissue
Figure 2 represents the results (Table S1) for EGFR mutational analysis in tissue biopsies compared to plasma samples, along with the type of TKI administration and relative best response for each patient (n = 31). The three platforms in liquid biopsy detected at a baseline of a total of three patients with the L858R mutation, seventeen with the Del 19 mutations, and seven with the T790M mutations, compared to seven patients with L858R, twenty with Del 19, and one patient with T790M mutations present in the tumor tissue. Six patients were found positive for the T790M mutation as detected in plasma by Digital PCR while testing negative in tissue analysis (Pt ID.1, 2, 5, 9, 23, 24). In four of these patients the T790M was co-present with either Del 19 (Pt ID. 2, 9) or L858R (Pt ID. 24, 28). In nine patients, the Del 19 mutation observed in tumor tissue was also detected with all three methods in liquid biopsy. None of these mutations was found in plasma by any of the three methods used in the four patients (Pt ID. 8, 19, 22, 27) for which tissue analysis resulted positive for either of the three activating mutations.
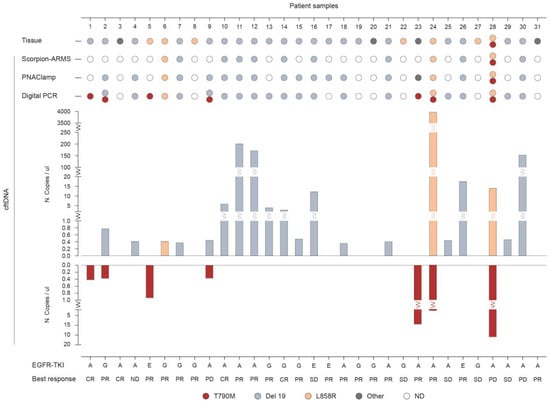
Figure 2.
Comparison of the three methodologies in detecting EGFR mutations in plasma versus tissue analysis. EGFR mutations as detected in liquid biopsy at baseline through cell-free tumor DNA (cftDNA) analysis by Scorpion-ARMS EGFR Plasma RGQ PCR Kit-QIAGEN, PNAClamp EGFR-PANAGENE, and QuantStudio 3D Digital PCR System-Thermo Fisher Scientific. Mutational analysis of EGFR in plasma samples is compared to tissue biopsies (n = 31; ND, not determined). The number of mutated EGFR copies obtained by QuantStudio 3D Digital PCR is indicated as copies/ul. Results are presented along with the type of anti-EGFR treatment and clinical response (TKI, Tyrosin Kinase Inhibitor; A, Afatinib; G, Gefitinib; E, Erlotinib; CR, Complete Response; PR, Partial Response; SD, Stable Disease; PD, Progressive Disease).
3.4. Limit of Detection of the Three Methods for Three Mutations
Analysis with the Horizon Multiplex I cfDNA Reference Standard Set (HD780) demonstrated that the limit of detection (LOD) with Digital PCR for the T790M, Del 19 and L858R mutations was 0.1%, while Scorpion-ARMS and PNAClamp could reveal Del 19 and L858R mutations at a minimal frequency of 0.5%, and T790M mutations at a frequency of 1%. (Table S3)
3.5. EGFR Mutation Detection Rate in Plasma
We found a total of 104 EGFR mutations in the cftDNA of 31 NSCLC patients considering the three platforms together. The overall detection rate of Scorpion-ARMS EGFR Plasma, PNAClamp R EGFR, and QuantStudio 3D Digital PCR was 33.65% (35/104), 62.5% (65/104), and 94.23% (98/104) respectively (Figure 3A). The 104 EGFR mutations were divided into two subgroups (low copy number: < 1 copy/µL; high copy number: ≥ 1 copy/µL). The subdivision was based on the quantitative results obtained by Digital PCR (total 98 mutations).The low copy number group included 52 mutations while the high copy number group includes 46 mutations. We then compared the results obtained with either Scorpion-ARMS and PNAClamp for these subgroups. In the low copy/µL subgroup, the detection rate of Scorpion-ARMS was 11.53% (6/52) and 40.38% (21/52) for Scorpion-ARMS and PNAClamp respectively (Figure 3B). In the high copy/µL subgroup the detection rate of Scorpion-ARMS and PNAClamp was 63.04% (29/46) and 82.6% (38/46) respectively (Figure 3C). We then made the same type of comparison looking at the three main EGFR mutations individually (T790M, Del 19, and L858R). In the low copy/µL subgroup, the detection rate of Scorpion-ARMS and PNAClamp were respectively 4.54% (1/22) and 13.63% (3/22) for T790M, 13.04% (3/23) and 56.52% (13/23) for Del 19, and 28.57% (2/7) and 71.42% (5/7) for L858R (Figure 3D). In the high copy/µL subgroup, the detection rate of Scorpion-ARMS and PNAClamp was, respectively, 33.33% (4/12) and 66.66% (8/12) for T790M, 66.66% (16/24) and 83.33% (20/24) for Del 19, and 90% (9/10) and 100% (10/10) for L858R (Figure 3E).
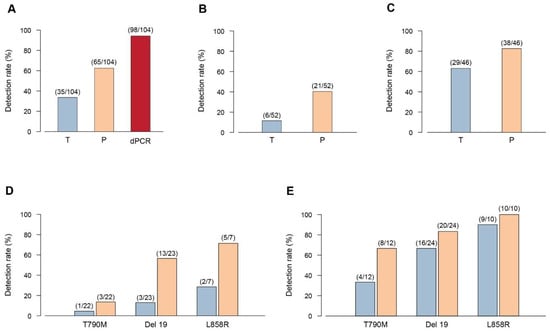
Figure 3.
Detection rate of three platforms in plasma EGFR mutations detection. The detection rate is shown as the frequency of sample that resulted positive when tested in liquid biopsy with the three different methods: Scorpion-ARMS EGFR Plasma RGQ PCR Kit-QIAGEN (T), PNAClamp EGFR-PANAGENE (P), and QuantStudio 3D Digital PCR System-Thermo Fisher Scientific (dPCR). (A) Overall detection rate of the three platforms. (B) Overall detection rate of the Scorpion-ARMS compared to the PNAClamp for the low copy (<1 copy/µL) patient group as measured by Digital PCR. (C) Overall detection rate of Scorpion-ARMS compared to PNAClamp for the high copy (>1 copy/µL) patient group as measured by Digital PCR. (D) Comparison of Scorpion-ARMS (blue) with PNAClamp (orange) for the low copy (<1 copy/µL) patient group as measured by Digital PCR, considered separately for each EGFR main mutation (Del 19, L858R, and T790M). (E) Comparison of Scorpion-ARMS (blue) with PNAClamp (orange) for the high copy (>1 copy/µL) patient group, measured by Digital PCR, considered separately for each EGFR main mutation (T790M, Del 19, and L858R). Numbers in parenthesis indicate the relative amount of mutations detected with each specific method.
3.6. Monitoring the Plasma Levels of T790M, Del 19, and L858R during Therapy
To assess the potential clinical utility of monitoring plasma levels of EGFR mutations (T790M, Del 19, and L858R) in the course of TKI treatment, we analyzed cftDNA at baseline and serially at 8 and 20 days post-treatment, at the first clinical evaluation, and at disease progression (Figure 4; Table S2). For four of the 31 patients monitored (Pt no. 3, 20, 23, 31) was possible to measure only the T790M mutation. Seven patients (Pt no. 1, 5, 8, 17, 19, 22, 27) were negative for either the Del 19 or L858R activating mutations at baseline. Disease progression occurred in 18 patients, ten of which (Pt n. 2, 6, 9, 10, 11, 15, 16, 18, 24, 28) showed concomitant presence in liquid biopsy of the T790M resistance mutation with one of the two activating mutations (either the Del 19 or L858R) at progression. At progression, one patient (Pt no. 5) was positive only for T790M, and 3 patients (Pt no. 8, 14, 26) were positive only for the activating mutations. Interestingly, in Pt n.8 and n.26, theT790M was absent at baseline, appeared at some point during treatment, to disappear again at the time of progression. Finally, one patient (Pt no. 7) was negative at progression for either the T790M or the activating mutation, while another one (Pt no. 31) tested completely negative for the T790M at any time point. In any case, presence of T790M at baseline was significantly associated with a shorter PFS, (HR = 1.246 (1.051, 1.477); p = 0.011) but not OS, (HR = 1.042 (0.926, 1.173); p = 0.491).
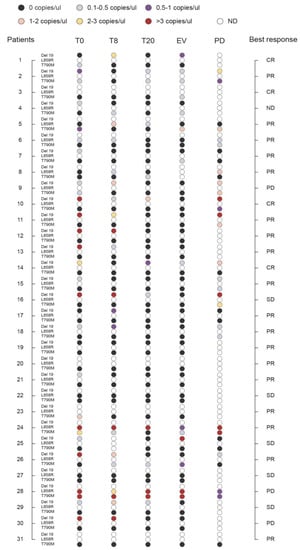
Figure 4.
Monitoring the levels of Del 19, L858R, and T790M during therapy. Plasma levels of EGFR mutations (Del 19, L858R, and T790M) in the course of tyrosine kinase inhibitor (TKI) treatment were monitored by analyzing cell-free tumor DNA (cftDNA) by QuantStudio 3D Digital PCR System-Thermo Fisher Scientific at baseline (T0) and serially at 8 days (T8) and 20 days (T20) post-treatment, at the first clinical evaluation (EV), and at disease progression (PD). Quantitative measures are represented as thresholds of numbers of EGFR mutated copies/ul as reported in Table S1 (n = 31; ND, not determined). Results are presented along with the best clinical response (CR, Complete Response; PR, Partial Response; SD, Stable Disease; PD, Progressive Disease).
4. Discussion
With the approval of liquid biopsy for molecular testing of patients with NSCLC [31], typically in those cases with insufficient tumor tissue or in cases where specimens are not obtainable, and with the advent of third-generation EGFR-TKI, concerns have been raised to establish which platform would be the best to accurately evaluate EGFR mutations. As re-biopsy has several limitations, plasma cftDNA has emerged as a new and promising approach for non-invasive genotyping, facilitating dynamic monitoring of gene mutation in the course of treatment. Several studies have been conducted comparing different platforms [32,33,34,35,36,37,38], but to date there is no standardized procedure or a reference method. The aim of this study was to compare three methodologies that are based on targeted approaches: the QuantStudio 3D Digital PCR, the Scorpion-ARMS EGFR Plasma RGQ PCR Kit, and the PNAClamp EGFR. We applied these three platforms to detect cftDNA EGFR mutations in the plasma of EGFR-TKI treated patients with advanced NSCLC, at baseline and serially during the treatment. Our analysis revealed that the best plasma-tissue correlation was reached by Digital PCR. As well, Digital PCR showed the highest sensitivity in detecting the Del 19 mutation, while there was no difference between the three platforms as for the L858R and T790M mutations, although the latter mutation was observed in only one case. Moreover, analysis with reference standards demonstrated that Digital PCR was the methodology that allowed detection of the three mutations at the lowest allelic frequency. Finally, Digital PCR reached the highest detection rate among the three platforms used, followed by the PNAClamp, in both the low copy/µL and the high copy/µL subgroup.
Many studies have been conducted to find the most accurate method for determining EGFR mutations in the cftDNA of lung cancer patients, including technologies such as ARMS [39,40], PNA-clamp [41], DHPLC [42], and NGS [29]. However, sensitivity and specificity varied significantly among different studies. Droplet Digital (ddPCR) has been reported as the technique with the highest sensitivity and specificity in detecting EGFR mutations in plasma: on average 81.82% and 98.44%, respectively [43].
This is one of the few studies comparing the QuantStudio 3D Digital PCR with other technologies, and demonstrating the high diagnostic accuracy of this method. These results are in accordance with those reported by Feng et al. [44], where the superiority of QuantStudio 3D Digital PCR was demonstrated, in comparison to ARMS-PCR, in detecting T790M EGFR mutations. There is an increasing necessity in clinical practice to characterize tumors for a panel of gene alterations, rather than to a single mutation, and this could be feasible only using NGS methodologies; digital PCR could represent a valid method for the monitoring of specific resistance mutations, such as T790M or others. Digital PCR is a highly sensitive, quick and low in cost method that could be very useful in the clinical practice for the monitoring of selected mutations. In view of the recent approval of the third generation TKI osimertinib in the first line treatment of EGFR mutated patients, other resistance mutations will emerge in future studies, and monitoring will become important during treatment, using methodologies like digital PCR.
Moreover, in view of recent results of the ADAURA study [45] demonstrating the efficacy of osimertinib in EGFR mutated resected NSCLC it will become essential to detect EGFR mutation using highly sensitive methodologies, as the releasing of cftDNA in early stage tumor could be very low, leading to the necessity to use methodologies with a very low limit of detection.
5. Conclusions
Our study investigated three different methodologies for detecting EGFR mutations in plasma samples of NSCLC patients. Results indicated that best correlation data with tissue and highest sensitivity was reached with Digital PCR. Furthermore, Digital PCR on cftDNA provides a promising and non-invasive assay to test EGFR mutations, also providing quantitative data. Compared to other methods Digital PCR would be a robust method for absolute quantification of samples in a longitudinal way, allowing better monitoring of the evolution of mutations over time. Moreover, our results highlight the potential application of liquid biopsy using Digital PCR as a routine assay in clinical practice for both detection and quantification of actionable mutation landscape in NSCLC patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/12/1062/s1, Table S1: Number of EGFR mutated copies as quantified by QuantStudio 3D Digital PCR assay at baseline. The amount of the three main EGFR mutations in cell-free DNA is reported as copied/ul of plasma (ND, not determined; WT, wild type for the specific mutation). Results in the table are referred to in Figure 2. Table S2: Number of EGFR mutated copies as quantified by QuantStudio 3D Digital PCR assay in longitudinal samples. The amount of the three main EGFR mutations in cell-free DNA is reported as copied/ul of plasma (ND, not determined). Results in the table are referred to in Figure 4. Table S3. Results of limit of detection of the three methods for three mutations using Horizon Multiplex I cfDNA Reference Standard Set (HD780).
Author Contributions
Conceived and designed the study: A.S., V.L. and P.U.; methodology, A.S., V.L., L.P. and P.U.; performed the molecular analysis: M.S.R., F.R.T., A.S. and S.B.; involved in the development of the study M.S.R., E.C., F.R.T., S.B. and G.M.; data interpretation and statistical analysis: A.S. and V.L.; wrote the original draft: A.S., V.L., L.P. and P.U. supervision: V.M., L.C., A.D. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by AUCC (Associazione Umbra Contro il Cancro). The study sponsor did not provide writing support for the report. All authors had full access to all the data in the study. The corresponding author had the final responsibility to submit for publication.
Acknowledgments
We thank all individuals who participated at the study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| EGFR | epidermal growth factor receptor |
| TKIs | tyrosine kinase inhibitors |
| cftDNA | cell-free tumor DNA |
| NSCLC | non-small cell lung cancer |
References
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Wang, J.; Ramakrishnan, R.; Tang, Z.; Fan, W.; Kluge, A.; Dowlati, A.; Jones, R.C.; Ma, P.C. Quantifying EGFR Alterations in the Lung Cancer Genome with Nanofluidic Digital PCR Arrays. Clin. Chem. 2010, 56, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLoS Med. 2005, 2, e73. [Google Scholar] [CrossRef] [PubMed]
- Arcila, M.E.; Oxnard, G.R.; Nafa, K.; Riely, G.J.; Solomon, S.B.; Zakowski, M.F.; Kris, M.G.; Pao, W.; Miller, V.A.; Ladanyi, M. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin. Cancer Res. 2011, 17, 1169–1180. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Molina, M.A.; Costa, C.; Simonetti, S.; Gimenez-Capitan, A.; Bertran-Alamillo, J.; Mayo, C.; Moran, T.; Mendez, P.; Cardenal, F.; et al. Pretreatment EGFR T790M Mutation and BRCA1 mRNA Expression in Erlotinib-Treated Advanced Non-Small-Cell Lung Cancer Patients with EGFR Mutations. Clin. Cancer Res. 2011, 17, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-Y.; Chen, H.-Y.; Li, K.-C.; Kuo, M.-L.; Yang, J.C.-H.; Chan, W.-K.; Ho, B.-C.; Chang, G.-C.; Shih, J.-Y.; Yu, S.-L.; et al. Pretreatment Epidermal Growth Factor Receptor (EGFR) T790M Mutation Predicts Shorter EGFR Tyrosine Kinase Inhibitor Response Duration in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 433–440. [Google Scholar] [CrossRef]
- Maheswaran, S.; Sequist, L.V.; Nagrath, S.; Ulkus, L.; Brannigan, B.; Collura, C.V.; Inserra, E.; Diederichs, S.; Iafrate, A.J.; Bell, D.W.; et al. Detection of Mutations inEGFRin Circulating Lung-Cancer Cells. N. Engl. J. Med. 2008, 359, 366–377. [Google Scholar] [CrossRef]
- Jänne, P.A.; Yang, J.C.-H.; Kim, D.-W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.-J.; Kim, S.-W.; Su, W.-C.; Horn, L.; et al. AZD9291 in EGFR Inhibitor–Resistant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef]
- He, Y. Rociletinib in EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 578–579. [Google Scholar] [CrossRef]
- Bintanja, R.; Selten, F.M. Future increases in Arctic precipitation linked to local evaporation and sea-ice retreat. Nat. Cell Biol. 2014, 509, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Meldgaard, P.; Hager, H.; Wu, L.; Wei, W.; Tsai, J.; Khalil, A.A.; Nexo, E.; Sorensen, B.S. Detection of EGFR mutations in plasma and biopsies from non-small cell lung cancer patients by allele-specific PCR assays. BMC Cancer 2014, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, L.; Zheng, D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: A systematic review and meta-analysis. Sci. Rep. 2015, 4, srep06269. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Ostoros, G.; Cobo, M.; Ciuleanu, T.; Cole, R.; McWalter, G.; Walker, J.; Dearden, S.; Webster, A.; Milenkova, T.; et al. Gefitinib Treatment in EGFR Mutated Caucasian NSCLC: Circulating-Free Tumor DNA as a Surrogate for Determination of EGFR Status. J. Thorac. Oncol. 2014, 9, 1345–1353. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Bai, H.; Wu, M.; An, T.; Zhao, J.; Yang, L.; Duan, J.; Zhuo, M.; Wang, Y.; et al. The detection of EGFR mutation status in plasma is reproducible and can dynamically predict the efficacy of EGFR-TKI. Thorac. Cancer 2012, 3, 334–340. [Google Scholar] [CrossRef]
- Vallee, A.; Marcq, M.; Bizieux, A.; El Kouri, C.; Lacroix, H.; Bennouna, J.; Douillard, J.-Y.; Denis, M.G. Plasma is a better source of tumor-derived circulating cell-free DNA than serum for the detection of EGFR alterations in lung tumor patients. Lung Cancer 2013, 82, 373–374. [Google Scholar] [CrossRef]
- Minari, R.; Mazzaschi, G.; Bordi, P.; Gnetti, L.; Alberti, G.; Altimari, A.; Gruppioni, E.; Sperandi, F.; Parisi, C.; Guaitoli, G.; et al. Detection of EGFR-Activating and T790M Mutations Using Liquid Biopsy in Patients With EGFR-Mutated Non–Small-Cell Lung Cancer Whose Disease Has Progressed During Treatment With First- and Second-Generation Tyrosine Kinase Inhibitors: A Multicenter Real-Life Retrospective Study. Clin. Lung Cancer 2020, 21, e464–e473. [Google Scholar] [CrossRef]
- Kim, J.-O.; Shin, J.-Y.; Kim, S.R.; Shin, K.S.; Kim, J.; Kim, M.-Y.; Lee, M.-R.; Kim, Y.; Kim, M.; Hong, S.-H.; et al. Evaluation of Two EGFR Mutation Tests on Tumor and Plasma from Patients with Non-Small Cell Lung Cancer. Cancers 2020, 12, 785. [Google Scholar] [CrossRef]
- Li, B.T.; Janku, F.; Jung, B.; Hou, C.; Madwani, K.; Alden, R.; Razavi, P.; Reis-Filho, J.; Shen, R.; Isbell, J.; et al. Ultra-deep next-generation sequencing of plasma cell-free DNA in patients with advanced lung cancers: Results from the Actionable Genome Consortium. Ann. Oncol. 2019, 30, 597–603. [Google Scholar] [CrossRef]
- Crowley, E.H.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef] [PubMed]
- Holdhoff, M.; Schmidt, K.; Donehower, R.; Diaz, L.A. Analysis of Circulating Tumor DNA to Confirm Somatic KRAS Mutations. J. Natl. Cancer Inst. 2009, 101, 1284–1285. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Ostoros, G.; Cobo, M.; Ciuleanu, T.; McCormack, R.; Webster, A.R.; Milenkova, T. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: A phase-IV, open-label, single-arm study. Br. J. Cancer 2014, 110, 55–62. [Google Scholar] [CrossRef] [PubMed]
- A Punnoose, E.; Atwal, S.; Liu, W.; Raja, R.; Fine, B.M.; Hughes, B.G.M.; Hicks, R.J.; Hampton, G.M.; Amler, L.C.; Pirzkall, A.; et al. Evaluation of Circulating Tumor Cells and Circulating Tumor DNA in Non-Small Cell Lung Cancer: Association with Clinical Endpoints in a Phase II Clinical Trial of Pertuzumab and Erlotinib. Clin. Cancer Res. 2012, 18, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Soria-Comes, T.; Palomar-Abril, V.; Ureste, M.M.; Guerola, M.T.; Maiques, I.C.M. Real-World Data of the Correlation between EGFR Determination by Liquid Biopsy in Non-squamous Non-small Cell Lung Cancer (NSCLC) and the EGFR Profile in Tumor Biopsy. Pathol. Oncol. Res. 2019, 26, 845–851. [Google Scholar] [CrossRef]
- Liu, H.E.; Vuppalapaty, M.; Wilkerson, C.; Renier, C.; Chiu, M.; Lemaire, C.; Che, J.; Matsumoto, M.; Carroll, J.; Crouse, S.; et al. Detection of EGFR Mutations in cfDNA and CTCs, and Comparison to Tumor Tissue in Non-Small-Cell-Lung-Cancer (NSCLC) Patients. Front. Oncol. 2020, 10, 572895. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, F.; Wu, J.; Xue, C.; Zhao, Y.; Jiang, W.; Lin, L.; Wu, X.; Lu, Y.; Bai, H.; et al. Comparison of different methods for detecting epidermal growth factor receptor mutations in peripheral blood and tumor tissue of non-small cell lung cancer as a predictor of response to gefitinib. OncoTargets Ther. 2012, 5, 439–447. [Google Scholar] [CrossRef]
- Taniguchi, K.; Uchida, J.; Nishino, K.; Kumagai, T.; Okuyama, T.; Okami, J.; Higashiyama, M.; Kodama, K.; Imamura, F.; Kato, K. Quantitative Detection of EGFR Mutations in Circulating Tumor DNA Derived from Lung Adenocarcinomas. Clin. Cancer Res. 2011, 17, 7808–7815. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Kang, X.; You, X.; Dai, L.; Tian, D.; Yan, W.; Yang, Y.; Xiong, H.; Liang, Z.; Zhao, G.Q.; et al. Cross-Platform Comparison of Four Leading Technologies for Detecting EGFR Mutations in Circulating Tumor DNA from Non-Small Cell Lung Carcinoma Patient Plasma. Theranostics 2017, 7, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, C. Comparison of cross-platform technologies for EGFR T790M testing in patients with non-small cell lung cancer. Oncotarget 2017, 8, 100801–100818. [Google Scholar] [CrossRef]
- Thress, K.S.; Brant, R.; Carr, T.H.; Dearden, S.; Jenkins, S.; Brown, H.; Hammett, T.; Cantarini, M.; Barrett, J.C. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015, 90, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-N.; Gu, W.-Q.; Zhao, N.; Pan, Y.-M.; Luo, W.; Zhang, H.; Liang, J.-M.; Yang, J.; Deng, Y. Comparison of the SuperARMS and Droplet Digital PCR for Detecting EGFR Mutation in ctDNA From NSCLC Patients. Transl. Oncol. 2018, 11, 542–545. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, N.; Yang, G.; Zhang, Y.; Ye, M.; Cao, J.; Xiong, J.; Han, Z.; Wu, S.; Shang, L.; et al. A comparison of ARMS-Plus and droplet digital PCR for detecting EGFR activating mutations in plasma. Oncotarget 2017, 8, 112014–112023. [Google Scholar] [CrossRef]
- Wang, W.; Song, Z.; Zhang, Y. A Comparison of ddPCR and ARMS for detecting EGFR T790M status in ctDNA from advanced NSCLC patients with acquired EGFR-TKI resistance. Cancer Med. 2016, 6, 154–162. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Q.; Yu, W.; Qiao, L.; Zhao, M.; Zhang, C.; Hu, X.; Yang, G.; Xiong, L.; Lou, J. Quantification of plasma EGFR mutations in patients with lung cancers: Comparison of the performance of ARMS-Plus and droplet digital PCR. Lung Cancer 2017, 114, 31–37. [Google Scholar] [CrossRef]
- Duan, H.; Lu, J.; Lu, T.; Gao, J.; Zhang, J.; Xu, Y.; Wang, M.; Wu, H.; Liang, Z.; Liu, T. Comparison of EGFR mutation status between plasma and tumor tissue in non-small cell lung cancer using the Scorpion ARMS method and the possible prognostic significance of plasma EGFR mutation status. Int. J. Clin. Exp. Pathol. 2015, 8, 13136–13145. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26722512 (accessed on 22 January 2019).
- Goto, K.; Ichinose, Y.; Ohe, Y.; Yamamoto, N.; Negoro, S.; Nishio, K.; Itoh, Y.; Jiang, H.; Duffield, E.; McCormack, R.; et al. Epidermal Growth Factor Receptor Mutation Status in Circulating Free DNA in Serum: From IPASS, a Phase III Study of Gefitinib or Carboplatin/Paclitaxel in Non-small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 115–121. [Google Scholar] [CrossRef]
- Pasquale, R.; Fenizia, F.; Abate, R.E.; Sacco, A.; Esposito, C.; Forgione, L.; Rachiglio, A.M.; Bevilacqua, S.; Montanino, A.; Franco, R.; et al. Assessment of high-sensitive methods for the detection of EGFR mutations in circulating free tumor DNA from NSCLC patients. Pharmacogenomics 2015, 16, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Mao, L.; Wang, H.S.; Zhao, J.; Yang, L.; An, T.T.; Wang, X.; Duan, C.J.; Wu, N.M.; Guo, Z.Q.; et al. Epidermal Growth Factor Receptor Mutations in Plasma DNA Samples Predict Tumor Response in Chinese Patients With Stages IIIB to IV Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2009, 27, 2653–2659. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, X.; Dong, Z.; Lu, Y.C.; Sun, Y.; Liu, Y.; McCormack, R.; Guanshan, Z.; Liu, X. Highly Sensitive Droplet Digital PCR Method for Detection of EGFR-Activating Mutations in Plasma Cell–Free DNA from Patients with Advanced Non–Small Cell Lung Cancer. J. Mol. Diagn. 2015, 17, 265–272. [Google Scholar] [CrossRef]
- Feng, Q.; Gai, F.; Sang, Y.; Zhang, J.; Wang, P.; Wang, Y.; Liu, B.; Lin, N.; Yu, Y.; Fang, J. A comparison of QuantStudio™ 3D Digital PCR and ARMS-PCR for measuring plasma EGFR T790M mutations of NSCLC patients. Cancer Manag. Res. 2018, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).