Abstract
Sarcopenia, characterized by progressive loss of muscle mass and strength, significantly increases health risks in healthy older adults. Resistance training (RT) is believed to counteract sarcopenia through a variety of physiological mechanisms, many of which remain underexplored by public health and physiotherapy professionals. This scoping review aims to consolidate studies that have explored RT programs in mitigating sarcopenia among healthy older adults. A systematic search in four knowledge databases (Web of Science, Scopus, Embase, Cumulative Index for Nursing and Allied Health Sciences Complete) was conducted on 30 April 2024 to consolidate the evidence of RT programs to mitigate sarcopenia risk among healthy older adults. Two reviewers independently screened, consolidated, and synthesized the results based on the Arksey and O’Malley framework. We included 36 studies supporting the RT program for reducing sarcopenia risk among healthy older people. Current evidence, predominantly derived from studies with high selection bias and non-randomized designs, indicates that RT programs may enhance muscle strength in healthy older adults. However, their impact on muscle morphology and mobility appears less pronounced. The dosage and intensity of RT are critical factors influencing these health outcomes. To substantiate the health benefits of RT in healthy older adults and facilitate the translation of research findings into policy-level recommendations, further high-quality, randomized controlled trials are warranted.
Keywords:
resistance training; older; sarcopenia; frailty; physiology; sedentary; sustainable cities 1. Introduction
Sarcopenia is an age-related muscular disorder characterized by loss of muscle mass and strength, eventually creating difficulty in performing basic and instrumental activities of daily living, such as cooking, climbing stairs, and carrying groceries [1]. Furthermore, older adults with sarcopenia are found to have an increased risk of falls, reduced mobility, and osteoporotic fractures, leading to increased dependency and reduced quality of life [2]. The skeletal muscle loss was found to be 3–8% every decade after 40 years, with accelerated deterioration in muscle strength and mass after 65 years. Further, the cross-sectional area of knee extensors reduced by 16.1% in 12 years from middle working age to the older retirement stage [3]. Maintaining muscle mass, strength, and balance is vital to preserving mobility, preventing falls and cognitive deterioration, and maintaining social engagement and quality of life in healthy older people [4]. Hence, advocating for strategies, including resistance training (RT), to delay progressive senescence-related muscle loss early is crucial in alleviating adverse musculoskeletal events in later life.
Senescence-related muscle atrophy, a classical feature of sarcopenia, is postulated to be shaped by several adverse phenomena, including reduction in satellite cell count and activity, increased heat shock proteins and apoptosis, altered muscle architecture and protein kinetics, reduced muscle fiber and size, altered hormones (insulin and thyroid), dysregulation of cytokine (interleukins, tumor necrosis, and tissue growth factors), and increased oxidative stress and mitochondrial dysfunction, which is further compounded by highly sedentary behaviors [5]. Accumulating evidence now claims that RT can be an effective intervention in mitigating, or at least delaying, these putative mechanisms that underpin sarcopenia in middle and older age [6]. The potential mechanisms through which RT programs prevent sarcopenia may include, but are not limited to, optimized neuromuscular metabolism, regulation of oxidative stress and inflammation, and hormones, such as growth hormone, thyroid hormones, adiponectin, and insulin-like growth factors [1,7,8,9,10]. Along with physiological effects, RT programs are also claimed to improve mental health in the elderly population with or without sarcopenia [11,12]. Both the physiological and psychological benefits of RT ultimately lead to enhanced functional capacity and quality of life, a finding now irrefutably supported by most contemporary empirical studies [13].
Although compelling evidence exists to support the RT program as a countermeasure against sarcopenia, the uptake of RT programs among healthy older adults remains low. A significant barrier to implementation is the lack of awareness about the protective effects of RT against sarcopenia, compounded by challenges in translating research into practice and limited knowledge of RT implementation in low-resource settings, such as homes with less access to gyms. This scoping review aimed to examine the evidence demonstrating the potential physiological effects of RT programs and hypothetical inter-linkages in mitigating sarcopenia and the practical implementation of RT in healthy older individuals. The findings from this review may help public health experts design and implement effective RT programs to combat sarcopenia and improve the quality of life of healthy older adults.
2. Materials and Methods
The present scoping review aimed to consolidate the existing evidence that investigates the physiological effects of RT on sarcopenia risks among healthy older adults. The manuscript was reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR). The checklist of PRISMA-ScR is provided as Supplementary File S1. We administered the search and included the studies until 30 April 2024.
The research problem examined the evidence and the physiological mechanisms underpinning RT programs in mitigating sarcopenia risk in healthy older adults. First, we collated the evidence that explored RT programs as countermeasures to sarcopenia risk in healthy older adults. Second, we extracted the putative physiological mechanisms underlying the RT programs against sarcopenia risk in community-dwelling or institutionalized healthy older adults.
2.1. Information Sources and Search
After consulting with our university librarian, we built a search strategy using the following keywords: RT or strength training, healthy older adults, and sarcopenia risk. We administered the following search strategy: “resistance exercise” OR “resistance exercise training” OR dumbbell OR “barbell training” OR “kettlebell” OR “weight training” OR “calisthenics” OR “resistance bands” AND Sarcopenia OR “muscle loss” OR dynapenia OR frailty”. We administered the search strategy in four electronic databases of peer-reviewed journals, including Embase, CINAHL complete, Scopus, and Web of Science. The search was administered from 27 to 30 April 2024. The search strategy is provided in Supplementary File S2. The retrieved citations were imported to EndNote online (https://www.myendnoteweb.com/EndNoteWeb.html), and duplicates were removed. After de-duplication, two authors shared the folder with the citations and started sorting the studies based on the eligibility criteria mentioned below.
2.2. Eligibility Criteria and Source Selection
The eligibility criteria were determined using the PICOS framework (provided in Table 1).

Table 1.
Eligibility criteria of the studies included.
Furthermore, the studies to be included should be published in English, regardless of the year of publication. We excluded studies that administered RT in children, were published in languages other than English, and included RT supplemented by nutrition changes or other concurrent interventions, and protocols and conference proceedings that could not provide contextual information. Two authors (KG and BC) independently screened the studies and met with mutual agreement on the inclusion of the studies.
2.3. Data Charting Process and Data Items
A bespoke data charting Excel sheet was prepared to extract succinct content from the studies included for the review. We used a narrative review or descriptive analytical approach to systematically gather contextual and process-oriented data. The charting elements filled into the Excel sheets were the author, year, study design, participant characteristics, context, intervention details (supervised or unsupervised, mode, frequency, duration, intensity, volume, and progression of RT), outcome measures (with a specific focus on markers of sarcopenia risk, such as muscle metabolism, mitochondrial oxidation, oxidative stress, inflammation, hormones, such as growth hormone, thyroid hormones, adiponectin, and insulin-like growth factors, and physical markers, such as functional capacity, muscle strength, mass, and architecture), and critical findings or implications.
2.4. Synthesis of Results
We adopted a narrative synthesis of the potential findings of the studies that explored the effects of RT in the prevention of sarcopenia risk among healthy older adults. Further charting of data using tables was administered. Following the narrative discussion, a thematic framework was employed to understand the potential physiological mechanisms through which RT programs may mitigate the risk of sarcopenia and to determine the optimal dose required to combat this risk.
3. Results
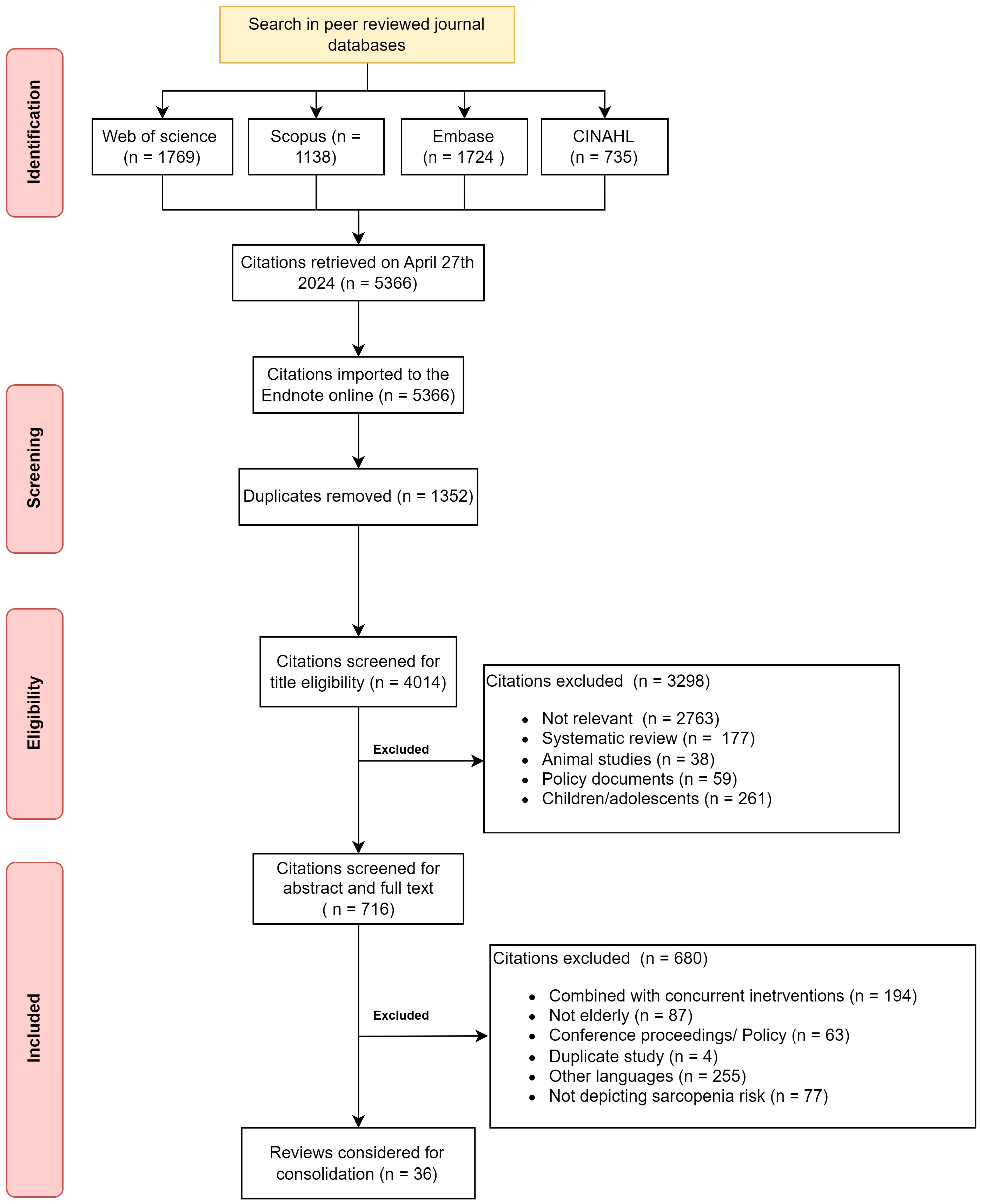
The initial search yielded 5366 citations from the four databases. After duplicates, 4014 citations were available for screening. The common reasons for exclusion were lack of relevance (69%) and focus on children (6%). After the abstract and full-text screening, the final citations that remained for consolidation were 36 citations available to support the RT program to mitigate sarcopenia in healthy older adults. Figure 1 depicts the screening and inclusion of the citations for the scoping review.
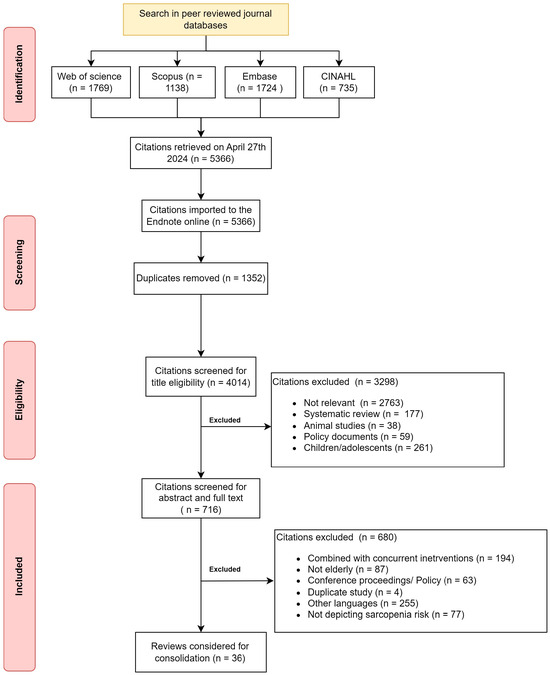
Figure 1.
Flowchart depicting citations searched and included in the review.
3.1. Characteristics of the Included Studies
The characteristics of the included studies are presented in Table 2. Data from each included study were extracted according to the population, interventions, comparator, outcomes, and study design. Most studies were conducted in high-income countries [1,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], with only one study from lower- to middle-income countries (Iran) [47]. The majority of the included studies were randomized controlled trials (n = 22/36, 61%).

Table 2.
Characteristics of the studies and their key findings.
3.2. Population
A total of 2146 older participants were studied. The majority of the studies recruited both genders, while few specifically recruited sedentary men and women with or without sarcopenia or who were at risk of developing sarcopenia or frailty. Standard criteria for diagnosis of sarcopenia were the appendicular skeletal mass index (males < 7.29, females < 5.93), Short Physical Performance Battery (SPPB) ≤ 8 points score, gait speed in 0 m walk test ≤ 1 m/s, and skeletal mass index ≤ 28% or ≤7.76 kg/m2 [15,47]. Few studies involved participants who had sarcopenic obesity with a body mass index of more than 30 kg/m2 [22,26,27,29,33,46,47], while few involved institutionalized individuals [25].
3.3. Intervention
All of the studies included supervised RT programs. The majority of the studies administered gym-based structured RT programs involving weight plates, hydraulic machines, and barbells in well-equipped gyms [1,9,10,16,20,21,22,23,24,25,28,29,30,31,33,34,35,37,39,40,41,42,43,45,46], while few studies administered body supported exercises, TheraBands, and free weights in the community [14,17,18,26,27,36,38,44,47]. The dose commonly seen among studies is as follows: duration of total intervention—12 weeks (28 days–24 months); intensity and volume: eight exercises for large muscle groups in upper and lower limbs, 1st weeks, 1 set 50% one repetition maximum (1-RM), 2nd week two sets of 60% of 1 RM, 3rd to 12th weeks 75–80% of 1 RM three sets, inter-set rest—60–90 s; and frequency (thrice per week for 12 weeks) using the linear periodization model [30,32,33]. The typical exercise protocol was as follows: Monday and Friday: squats, chest press, lateral pulldown, abdominal crunches, and back extensions; Wednesday: leg extensions, leg curls, chest butterflies, upper back rowing, and calf raises [9,10,39,41]. Few trials were compared based on intensity (low vs. high) [32], speed [42], or periodization (linear vs. undulating) [28]. Community-oriented exercises routinely administered squats, marching on the spot, crunches with body weight, or TheraBands. Only five studies (n = 5/36, 14%) mentioned the standardization of the tasks (passive stretching, meditation, routine care) in the control group [10,25,32,35,36]. Only a few trials successfully progressed the intervention (50–80% 1 RM) for the trial period [22,23,29,31,33].
3.4. Outcomes
Almost half of the studies (n = 17/36, 47%) measured the body composition and physical fitness, i.e., handgrip, time-up and test, stair climbing (physical fitness battery), as the primary outcome [1,17,18,22,24,25,27,32,35,36,37,38,40,42,45,46]. Similarly, the subsequent significantly studied outcome was body composition, including lean body mass, fat mass, and appendicular mass indices, through DEXA or bioelectric impedance analysis [10,22,23,24,25,26,27,33,34,39]. A considerable number of studies examined the effects of RT on 1-RM [22,23,29,30], maximum dynamic strength through an isokinetic dynamometer [16,20,23,27,28,30,33,39,43], and peak muscle activation through electromyography [21,28]. Few studies explored the effects on sarcopenic status [32,33,37], Troponin [18], muscle cross-sectional area [28], cognition [29], serum lipids [30], immune–inflammatory markers [insulin-like growth factor IGF-α, T cells and antibodies, C-reactive proteins, CRP, tumor necrosis factor, TNF, interleukins (IL-6, IL-10)] [9,10,14,30,31], reactive oxygen species [1], sleep [30], muscle cross-sectional area through ultrasonogram [1,24], visceral adipose tissue through MRI [23,34], muscle volumes through computer tomography [10,16], bone mineral and fracture risk [47], respiratory functions [32], energy expenditure through accelerometers [36], and behavior change [37,42].
3.5. Key Findings
3.5.1. Positive Findings
Almost all of the studies demonstrated favorable effects on physical performance (handgrip strength, time-up and test speed, chair stand time, stair climb ability, wall push) [1,16,17,18,22,27,40,45,46] and muscle strength (1-RM) and power [20,35,36,39]. Furthermore, maximal voluntary force production, peak torque, power, and 1-RM improved in most studies that employed moderate- to high-intensity traditional progressive RT programs [16,22,23,33,37,39,41,45]. Furthermore, the waist–hip ratio [43], lean body mass, and skeletal muscle index improved with a reduction in fat-free mass, which was evident in many studies [9,15,16,24,27,33,34,39]. Muscle growth factors, such as follistatin and myostatin, were found to be influenced by progressive RT programs among healthy older adults [43,44]. Rufino et al., 2023 significantly improved respiratory muscle strength and dynamic lung volumes [32]. Schulte et al. demonstrated a significant improvement in muscle protein synthesis and myostatin–immunoreactive proteins after a 10-week progressive RT program among physically frail older adults [43]. Stair stepping (forward or sides) was found to elicit muscle (vastus lateralis, gluteus maximus, and biceps femoris) activation similar to traditional RT at 60% and 80% 1-RM test [21]. Functional RT (chair standing, stair climbing, cleaning high places) was found adequate to improve executive functions in sarcopenic obese older adults [29]. Traditional progressive RT of 12 weeks improved sleep onset and reduced sleep latency, apneic episodes, and insomnia severity [30]. Further, a few studies demonstrated favorable effects on immune–inflammatory and immune–senescence markers [10,30,31,39]. Vezzoli et al., 2019 demonstrated a significant reduction in reactive oxygen species production after 12 weeks of progressive RT among healthy older adults over 65 years [1]. All of the favorable changes occurred only in the studies that advocated for moderate- to high-intensity progressive RT programs with moderate to high loads, longer durations, and larger volumes [9,10,36,37]. Any additional intervention added to the traditional RT program improved the outcome measures of the intervention added. For example, when added to traditional RT programs, physical activity advice improved activity levels in addition to the regular benefits of RT programs [37].
3.5.2. Null Findings
Few studies found no significant changes in physiological parameters, such as body composition [18], visceral or subcutaneous fat percentages [23], bone mineral density, telopeptides and fracture risk [47], strength or peak force development [42], muscle mass [45], gait speed, physical performance [1,15,25], muscle mass index, quality of life [26], and accelerometer-based physical activity levels [42]. A single-group pre–post designed trial by Perreault did not find any changes in the inflammatory markers after 16 weeks of the RT program [9]. Conversely, Yuenyongchaiwat et al., 2022 found a difference in IL-6 and TNF-α within 12 weeks of RT with elastic bands and pedometer-based aerobic training [14]. Although any systematically organized RT programs brought significant changes in the muscle cross-sectional area, force development, and muscle activation, differences in periodization strategies did not produce any significant differences among the groups [28].
4. Discussion
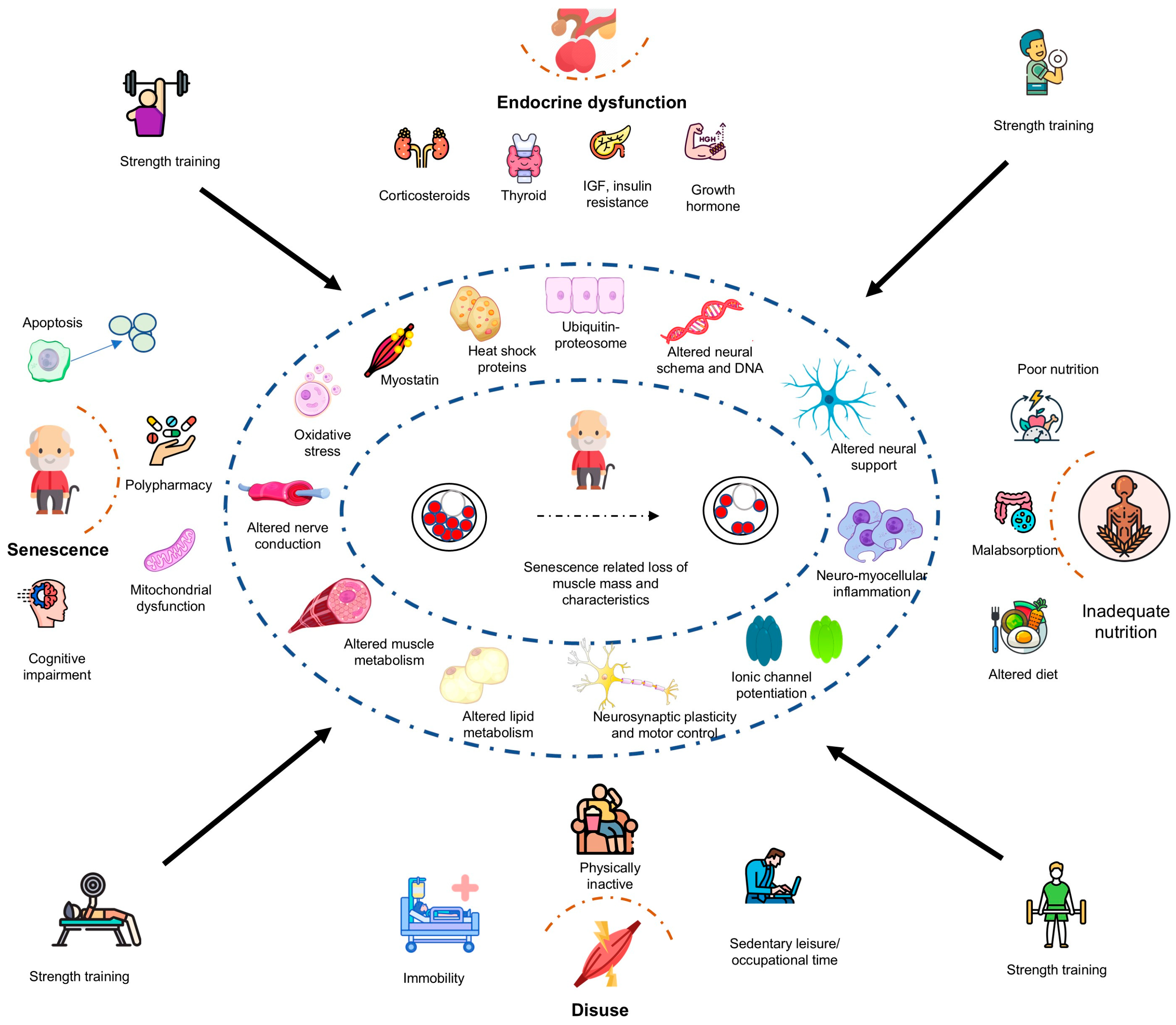
The present scoping review explored the physiological effects of RT programs to mitigate the sarcopenia risk in healthy older adults. While substantial evidence suggests that RT programs improve muscle strength, preserve mass, and enhance functional capacity in healthy older adults, their impact on the molecular mechanisms of protein synthesis, muscle breakdown, inflammaging, sleep quality, mental health, and cognitive functions remains inconclusive (Figure 2). Furthermore, the translation of these physiological changes into improved functional capacity, reduced fall risk, and prevention of senescence-related osteoporosis is still being investigated.
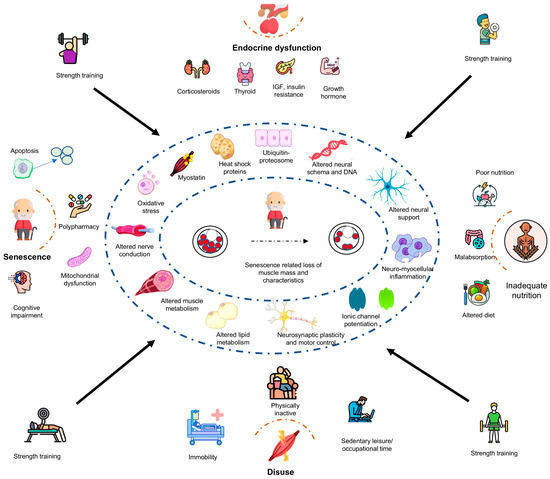
Figure 2.
Potential physiological mechanisms underpinning resistance exercise training in the mitigation of sarcopenia risk among healthy older adults. ACE—angiotensin-converting enzyme, APOE—apolipoprotein E, IGF—insulin-like growth factor, IL—interleukin.
While substantial evidence indicates that RT programs can effectively reduce the risk of sarcopenia in healthy older adults [9,10,16,17,20,21,22,24,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,43,44,46,47,48,49], the majority of trials (n = 13 of 17) did not observe similar favorable effects on muscle mass and biomarkers associated with sarcopenic obesity [1,15,18,23,25,42,45]. This inconsistency may be attributed to variations in intervention characteristics, such as volume, intensity, mode, and frequency, as well as differences in the quantification of outcome measures. A recent systematic review aligns with our findings, indicating that RT has modest effects on both relative and absolute muscle mass but significantly enhances muscle strength in healthy older adults with sarcopenia [50]. The outcomes are influenced by factors like the training period, number of sets, contraction speed, and intensity. As life expectancy increases with the advent of medical advances, healthy older adults in modern society are expected to fulfill several responsibilities, including self-care and being functionally independent in basic and instrumental daily living activities, achieving unrestricted mobility to complete their social roles (taking care of grandchildren, getting groceries), and preventing senescence-related complications (falls and fractures) [51]. RT programs ranging from low- to high-intensity are observed to maintain muscle mass and strength [17,20], thereby offering protection against fall risk and improving physical and social well-being among older adults [21,22].
The majority of trials identified in this scoping review indicated that RT programs have the potential to significantly increase muscle strength and, to a lesser extent, muscle mass in healthy older adults [9,10,25]. Findings from this review are consistent with the meta-analysis provided by Borde et al. (2015), which showed that RT significantly improved muscle strength but had only minor effects on muscle morphology [52]. The meta-regression revealed that the programming parameters of training period, intensity, and total time under tension significantly affected muscle strength. The current literature analysis revealed that RT effects did not or only to a minor extent translate into fall risk reduction, a result that concurs with existing reviews [53]. This finding is also supported by Beijersbergen et al. (2013), who showed only small associations between RT-related improvements in measures of muscle strength, power, and gait speed [53]. Similarly, our review included some studies that investigated RT’s effects on gait speed, which is an important marker of mobility in healthy older adults, and the drawn conclusions remain equivocal [22,25,27,37,46]. More research is needed on the effects of RT on mobility outcomes and fall rates and risk in healthy older adults. Currently, the literature is uniform with regards to RT’s effects on muscle strength, power, and mass. Yet, less is known about the most effective RT methods to improve mobility and reduce fall risks in older adults.
Moreover, RT has been associated with anti-inflammatory effects, which may have implications for cardiovascular disease (CVD) prevention. Sarcopenia and CVD share common inflammatory pathways, including elevated cytokine levels, such as IL-6 and TNF-α, which contribute to vascular dysfunction and metabolic syndrome [54]. Studies indicate that RT reduces systemic inflammation [10,30,55], potentially mitigating CVD risk. By lowering chronic inflammation, RT may serve as a non-pharmacological “polypill” to improve cardiovascular health in sarcopenic populations. A recent systematic review by Momma et al. (2022) demonstrated that RT programs were associated with a 10–20% lower risk of all-cause mortality, cardiovascular disease, total cancer, diabetes, and lung cancer, with the maximum risk reduction observed at approximately 30–60 min per week of performing muscle-strengthening activities [56].
Beyond musculoskeletal benefits, RT plays a role in delaying the onset of chronic dis-eases associated with sarcopenia. Increased muscle mass and strength correlate with both improved insulin sensitivity and upregulation of GLUT-4 transporters, reducing the risk of type 2 diabetes [57]. Furthermore, RT has been shown to influence cancer prognosis by enhancing immune function and reducing systemic inflammation, which is implicated in cancer progression [58]. In this context, Momma et al. (2022) demonstrated in their meta-analysis that RT programs were associated with a reduced risk of total cancer mortality, although dose–response relationships varied across the different cancer types (e.g., colon, kidney, pancreatic, bladder, and lung cancer) [56]. The metabolic improvements associated with RT, including enhanced glucose metabolism and lipid profile regulation, suggest a protective role against metabolic disorders, such as obesity and metabolic syndrome [59].
While increased muscle strength is a direct outcome of RT, its impact on quality of life (QoL) requires further exploration. Ramirez et al. (2018) reported significant QoL improvements in elderly populations following RT programs [40]. However, other studies present mixed results, indicating that the relationship between RT and QoL is not yet fully understood [26,37]. To better understand the impact of RT on QoL, longitudinal studies implementing RT programs in free-living settings are warranted. Although RT is beneficial, its potential risks, particularly for individuals with pre-existing joint conditions, such as osteoarthritis (OA), must be acknowledged. High-intensity RT can exacerbate joint stress, potentially leading to discomfort or injury if not appropriately managed [60]. It is essential to tailor RT programs to accommodate joint limitations by incorporating lower-impact modalities, controlled loading, and progressive overload principles. Studies suggest that supervised RT programs designed with joint health considerations, such as using resistance bands or machines instead of free weights, can mitigate these risks while still providing musculoskeletal benefits [61]. Additionally, proper warm-up, cool-down, and technique correction play a crucial role in preventing joint-related complications in aging individuals.
The evident improvement in physical performance (handgrip strength, time-up and go speed, chair stand time and stair climb ability) may improve daily living and enjoy their social lives [1,17,18,22,45]. Further, sarcopenia prevalence was found to reduce with RT programs, while the control group remained the same [1,32]. Interestingly, few studies reaped success in sleep onset [30], inflammaging status [1], muscle growth factors (follistatin, actinin) [44], anxiety and depression [26] and executive functions [30] among healthy older adults who have undergone classical RT programs. Besides gait speed, an essential determinant of negating roads safely and stair climbing ability, a determinant of negating stairs without inherent fall risks, are found to improve in most of the included studies [22]. Meanwhile, few studies have been conducted to contradict the positive effects of RT programs, primarily on body composition, gait speed, bone density, and muscle mass [18,25,47]. It appears that studies reporting null findings may have implemented low-intensity RT programs, utilizing elastic bands and body weight exercises at suboptimal doses, which may not effectively counteract sarcopenia risk. This could also explain why the majority of these trials did not report adverse events such as falls or cardiovascular incidents during the intervention period. Moreover, the inclusion of studies with small sample sizes and non-randomized designs in this review introduces a significant risk of selection bias, potentially compromising the validity of the findings. Such methodological limitations hinder the ability to draw definitive conclusions regarding the efficacy of RT interventions in healthy older adults.
4.1. Dose of Resistance Exercise Program
Based on the available literature, the design and dosage of progressive resistance exercise training, both in the workplace and during off-hours, can be implemented as outlined in Table 3. This guidance may assist exercise professionals and public health experts in developing and implementing appropriate RT programs for healthy older adults to maximize the physiological and health benefits previously discussed.

Table 3.
Dose of RT programs employed in studies to counter sarcopenia in healthy older adults.
4.2. Caution with Resistance Exercise Training
Resistance testing and training are not without risk among healthy older adults who are healthy with no known disease risk. Exercise-induced muscle damage after acute high-intensity RT may adversely affect the ability to do daily activities and fall risk [62]. Further transient increases in inflammatory markers protein damage may cause transient fatigue and traumatic arthropathies. Hence, appropriate dosing (intensity, duration and frequency) is crucial in preventing muscle injuries, fatigue, and fall risk after RT programs. However, none of the included studies in our review reported adverse events associated with RT programs among healthy older adults.
4.3. Limitations
A few limitations of the present review are the following. (1) We administered the search criteria based on our three team members’ knowledge and the librarian’s suggestions. Furthermore, the search was limited to four databases and included studies only published in English. A search of the gray literature and other languages might have provided us with more results. (2) Only a few studies administered RT programs in low-resource community settings. The findings of this scoping review may not be generalizable to low-resource settings. (3) The majority of the studies involved small sample sizes and used heterogeneous methodologies for administering RT (varying in frequency, intensity, and duration), making it difficult to draw conclusive evidence on the effects of RT programs on sarcopenia risk. (4) Despite conducting a systematic search, our scoping review was subject to selection bias. Notably, only three studies with substantial sample sizes (approximately 150 participants each) were identified [33,39,55], while the remaining studies involved smaller cohorts. This raises concerns about small-study bias, as studies with limited sample sizes are more susceptible to overestimating effect sizes and may lack the statistical power necessary for reliable conclusions. Such biases can compromise the validity and generalizability of the findings. This limitation underscores the pressing need for high-quality randomized controlled trials to enable comprehensive systematic reviews that can clarify the effects of RT on sarcopenia risk among healthy older adults. (5) Furthermore, the safety precautions implemented during these trials remain ambiguous, particularly as many employed low-intensity RT programs. While low-intensity RT has been shown to benefit healthy older adults with sarcopenia, the specific safety measures adopted in these studies are often not clearly reported [63].
5. Conclusions
RT shows promise as a countermeasure against sarcopenia in healthy older adults by modulating protein catabolism, enhancing muscle growth factors, and mitigating immuno-senescence and inflammaging. Evidence suggests that RT can improve both peripheral and respiratory muscle strength, potentially leading to modest gains in gait speed. However, these findings are constrained by methodological limitations, including small sample sizes, non-randomized study designs, and potential selection biases, which hinder the ability to draw definitive causal inferences. Moreover, the long-term health benefits of RT are influenced by broader factors, such as national policies, peer support, and institutional commitment to implementing RT programs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/life15050688/s1, Supplementary File S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist; Supplementary File S2: MeSH term combination and search strategy.
Author Contributions
Conceptualization, K.G., B.C. and U.G.; methodology, K.G., B.C. and U.G.; software, K.G., C.R.R., K.P. and U.G.; validation, K.G., K.P., B.C. and U.G.; formal analysis, K.G., C.R.R., B.C. and U.G.; investigation, K.G. and U.G.; resources, K.G., C.R.R., B.C., K.P. and U.G.; data curation, K.G., B.C. and U.G.; writing—original draft, K.G., B.C. and U.G.; writing—review and editing, C.R.R., K.P., B.C. and U.G.; supervision, B.C. and U.G. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support from the Open Access Publication Fund of the University of Freiburg, Germany.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the Health Sciences Library, the Manipal Academy of Higher Education and Symbiosis International (Deemed University) for the database support and the search strategy for the present scoping review.
Conflicts of Interest
The authors report that there are no competing interests to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| BMC | bone mineral content |
| BMI | body mass index |
| BP | blood pressure |
| CG | control group |
| COP | center of pressure |
| CRP | C-reactive protein |
| DEXA | dual energy X-ray absorptiometry |
| EMG | electromyography |
| FEV | force expiratory volume |
| FFA | free fatty acid |
| HIIRT | high-intensity interval resistance training |
| HSP | heat shock proteins |
| IGF | insulin like growth factor |
| IL | interleukin |
| IPAQ | international physical activity questionnaire |
| LIPA | light-intensity physical activity |
| MEP | maximal expiratory pressure |
| MET | metabolic equivalent |
| MIP | maximal expiratory pressure |
| MVPA | moderate to vigorous physical activity |
| MVV | maximal voluntary ventilation |
| MWD | minute walk distance |
| PA | physical activity |
| PRT | progressive resistance training |
| RM | repetition maximum |
| ROS | reactive oxygen species |
| RPE | rate of perceived exertion |
| RT | resistance training |
| SBP | systolic blood pressure |
| SMI | skeletal muscle index |
| SPPB | short physical performance battery |
| STS | sit to stand |
| TG | triglycerides |
| TMT | trail making test |
| TNF | tissue necrosis factor |
| TUG | Time-Up-Go test |
| VAT | visceral adipose tissue |
| VO2 | oxygen consumed for the workload |
References
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Heidari, D.; Shirvani, H.; Bazgir, B.; Shamsoddini, A. The Resistance Training Effects on Skeletal Muscle Stem Cells in Older Adult: A Systematic Review and Meta-Analysis. Cell J. 2023, 25, 513–523. [Google Scholar] [CrossRef]
- Lidegaard, M.; Jensen, R.B.; Andersen, C.H.; Zebis, M.K.; Colado, J.C.; Wang, Y.; Heilskov-Hansen, T.; Andersen, L.L. Effect of brief daily resistance training on occupational neck/shoulder muscle activity in office workers with chronic pain: Randomized controlled trial. BioMed Res. Int. 2013, 2013, 262386. [Google Scholar] [CrossRef]
- Ho, S.Y.; Chung, Y.C.; Wu, H.J.; Ho, C.C.; Chen, H.T. Effect of high intensity circuit training on muscle mass, muscular strength, and blood parameters in sedentary workers. PeerJ 2024, 12, e17140. [Google Scholar] [CrossRef]
- Perreault, K.; Courchesne-Loyer, A.; Fortier, M.; Maltais, M.; Barsalani, R.; Riesco, E.; Dionne, I.J. Sixteen weeks of resistance training decrease plasma heat shock protein 72 (eHSP72) and increase muscle mass without affecting high sensitivity inflammatory markers’ levels in sarcopenic men. Aging Clin. Exp. Res. 2016, 28, 207–214. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jee, Y.-S. Intensity-effects of strengthening exercise on thigh muscle volume, pro- or anti-inflammatory cytokines, and immunocytes in the older adults: A randomized controlled trial. Arch. Gerontol. Geriatr. 2024, 116, 105136. [Google Scholar] [CrossRef]
- Kong, L.N.; Lyu, Q.; Liu, D.X.; Hu, P. Effects of exercise interventions on physical, psychological and social outcomes in frail older adults: An overview of systematic reviews. J. Clin. Nurs. 2024. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. [Google Scholar] [CrossRef]
- Khodadad Kashi, S.; Mirzazadeh, Z.S.; Saatchian, V. A Systematic Review and Meta-Analysis of Resistance Training on Quality of Life, Depression, Muscle Strength, and Functional Exercise Capacity in Older Adults Aged 60 Years or More. Biol. Res. Nurs. 2023, 25, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Yuenyongchaiwat, K.; Akekawatchai, C.; Khattiya, J. Effects of a Pedometer-Based Walking Home Program Plus Resistance Training on Inflammatory Cytokines and Depression in Thai Older People with Sarcopenia: A Three-Arm Randomized Controlled Trial. Clin. Gerontol. 2023, 46, 717–728. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals With Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Van Roie, E.; Delecluse, C.; Coudyzer, W.; Boonen, S.; Bautmans, I. Strength training at high versus low external resistance in older adults: Effects on muscle volume, muscle strength, and force-velocity characteristics. Exp. Gerontol. 2013, 48, 1351–1361. [Google Scholar] [CrossRef]
- Abreu, E.L.; An-Lin, C.; Kelly, P.J.; Chertoff, K.; Brotto, L.; Griffith, E.; Kinder, G.; Uridge, T.; Zachow, R.; Brotto, M. Skeletal Muscle Troponin as a Novel Biomarker to Enhance Assessment of the Impact of Strength Training on Fall Prevention in the Older Adults. Nurs. Res. 2014, 63, 75–82. [Google Scholar] [CrossRef]
- Adnan, R.; Din, H.M.; Ashari, A.; Minhat, H.S. Effectiveness of a Community-Based Muscle Strengthening Exercise Program to Increase Muscle Strength Among Pre-frail Older Persons in Malaysia: A Pilot Study. Front. Public Health 2021, 9, 610184. [Google Scholar] [CrossRef]
- Akatsu, H.; Manabe, T.; Kawade, Y.; Masaki, Y.; Hoshino, S.; Jo, T.; Kobayashi, S.; Hayakawa, T.; Ohara, H. Effect of Ankle Weights as a Frailty Prevention Strategy in the Community-Dwelling Elderly: A Preliminary Report. Int. J. Environ. Res. Public Health 2022, 19, 7350. [Google Scholar] [CrossRef]
- Aragao-Santos, J.C.; De Resende-Neto, A.G.; Nogueira, A.C.; Feitosa-Neta, M.D.; Brandao, L.H.; Chaves, L.M.; Da Silva-Grigoletto, M.E. The effects of functional and traditional strength training on different strength parameters of elderly women: A randomized and controlled trial. J. Sports Med. Phys. Fit. 2019, 59, 380–386. [Google Scholar] [CrossRef]
- Baggen, R.J.; Van Roie, E.; van Dieën, J.H.; Verschueren, S.M.; Delecluse, C. Weight bearing exercise can elicit similar peak muscle activation as medium-high intensity resistance exercise in elderly women. Eur. J. Appl. Physiol. 2018, 118, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Krawczyk, S.N.; Potiaumpai, M.; Signorile, J.F. High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014, 60, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.F.; Yarasheski, K.E.; Steger-May, K.; Sinacore, D.R.; Brown, M.; Schechtman, K.B.; Holloszy, J.O. Effects of progressive resistance training on body composition in frail older adults: Results of a randomized, controlled trial. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2005, 60, 1425–1431. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A. Short-term heavy resistance training eliminates age-related deficits in muscle mass and strength in healthy older males. J. Strength Cond. Res. 2011, 25, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Cebrià I Iranzo, M.À.; Balasch-Bernat, M.; Tortosa-Chuliá, M.Á.; Balasch-Parisi, S. Effects of Resistance Training of Peripheral Muscles Versus Respiratory Muscles in Older Adults With Sarcopenia Who are Institutionalized: A Randomized Controlled Trial. J. Aging Phys. Act. 2018, 26, 637–646. [Google Scholar] [CrossRef]
- Chang, S.F.; Chiu, S.C. Effect of resistance training on quality of life in older people with sarcopenic obesity living in long-term care institutions: A quasi-experimental study. J. Clin. Nurs. 2020, 29, 2544–2556. [Google Scholar] [CrossRef]
- Chun-De, L.; Jau-Yih, T.; Li-Fong, L.; Shih-Wei, H.; Jan-Wen, K.; Lin-Chuan, C.; Tsan-Hon, L.; Liao, C.-D.; Tsauo, J.-Y.; Lin, L.-F.; et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef]
- Conlon, J.; Newton, R.; Tufano, J.; Peñailillo, L.; Banyard, H.; Hopper, A.; Ridge, A.; Haff, G.; Conlon, J.A.; Newton, R.U.; et al. The efficacy of periodised resistance training on neuromuscular adaptation in older adults. Eur. J. Appl. Physiol. 2017, 117, 1181–1194. [Google Scholar] [CrossRef]
- de Almeida, S.S.; Teixeira, E.L.; Merege, C.A.A.; Brucki, S.M.D.; Painelli, V.D. Acute Effects of Resistance and Functional-Task Exercises on Executive Function of Obese Older Adults: Two Counterbalanced, Crossover, Randomized Exploratory Studies. Sport Exerc. Perform. Psychol. 2021, 10, 102–113. [Google Scholar] [CrossRef]
- de Sá Souza, H.; de Melo, C.M.; Piovezan, R.D.; Miranda, R.E.E.P.C.; Carneiro-Junior, M.A.; Silva, B.M.; Thomatieli-Santos, R.V.; Tufik, S.; Poyares, D.; D’Almeida, V. Resistance Training Improves Sleep and Anti-Inflammatory Parameters in Sarcopenic Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16322. [Google Scholar] [CrossRef]
- Dinh, H.C.; Bautmans, I.; Beyer, I.; Onyema, O.O.; Liberman, K.; De Dobbeleer, L.; Renmans, W.; Vander Meeren, S.; Jochmans, K.; Delaere, A.; et al. Six weeks of strength endurance training decreases circulating senescence-prone T-lymphocytes in cytomegalovirus seropositive but not seronegative older women. Immun. Ageing 2019, 16, 17. [Google Scholar] [CrossRef]
- Flor-Rufino, C.; Barrachina-Igual, J.; Pérez-Ros, P.; Pablos-Monzó, A.; Martínez-Arnau, F.M. Resistance training of peripheral muscles benefits respiratory parameters in older women with sarcopenia: Randomized controlled trial. Arch. Gerontol. Geriatr. 2023, 104, 104799. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.B.; Paiva, F.M.L.; Gauche, R.; de Oliveira, R.J.; Lima, R.M. Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ghasemikaram, M.; Chaudry, O.; Nagel, A.M.; Uder, M.; Jakob, F.; Kemmler, W.; Kohl, M.; Engelke, K. Effects of 16 months of high intensity resistance training on thigh muscle fat infiltration in elderly men with osteosarcopenia. GeroScience 2021, 43, 607–617. [Google Scholar] [CrossRef]
- Kalapotharakos, V.I.; Diamantopoulos, K.; Tokmakidis, S.P. Effects of resistance training and detraining on muscle strength and functional performance of older adults aged 80 to 88 years. Aging Clin. Exp. Res. 2010, 22, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.X.; Bo, L.; Zhu, H.W.; Chen, B.Y.; Wu, Z.; Du, H.D.; Huo, X.P. Effects of lower limb resistance exercise on muscle strength, physical fitness, and metabolism in pre-frail elderly patients: A randomized controlled trial. BMC Geriatr. 2021, 21, 447. [Google Scholar] [CrossRef]
- Nagai, K.; Miyamato, T.; Okamae, A.; Tamaki, A.; Fujioka, H.; Wada, Y.; Uchiyama, Y.; Shinmura, K.; Domen, K. Physical activity combined with resistance training reduces symptoms of frailty in older adults: A randomized controlled trial. Arch. Gerontol. Geriatr. 2018, 76, 41–47. [Google Scholar] [CrossRef]
- Perkin, O.J.; McGuigan, P.M.; Stokes, K.A. Exercise Snacking to Improve Muscle Function in Healthy Older Adults: A Pilot Study. J. Aging Res. 2019, 2019, 7516939. [Google Scholar] [CrossRef]
- Rabelo, H.T.; Bezerra, L.A.; Terra, D.F.; Lima, R.M.; Silva, M.A.F.; Leite, T.K.; De Oliveira, R.J. Effects of 24 weeks of progressive resistance training on knee extensors peak torque and fat-free mass in older women. J. Strength Cond. Res. 2011, 25, 2298–2303. [Google Scholar] [CrossRef]
- Ramirez-Campillo, R.; Alvarez, C.; Garcìa-Hermoso, A.; Celis-Morales, C.; Ramirez-Velez, R.; Gentil, P.; Izquierdo, M. High-speed resistance training in elderly women: Effects of cluster training sets on functional performance and quality of life. Exp. Gerontol. 2018, 110, 216–222. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Picoloto, A.; Nunes, J.P.; Bezerra, E.S.; Schoenfeld, B.J.; Cyrino, E.S. Effects of Different Resistance Training Loads on the Muscle Quality Index in Older Women. J. Strength Cond. Res. 2022, 36, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Saeterbakken, A.H.; Bårdstu, H.B.; Brudeseth, A.; Andersen, V. Effects of Strength Training on Muscle Properties, Physical Function, and Physical Activity among Frail Older People: A Pilot Study. J. Aging Res. 2018, 2018, 8916274. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.N.; Yarasheski, K.E. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, S111–S118. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.W.; Jung, S.W.; Kim, S.W.; Lee, J.M.; Jung, H.C.; Song, J.K. Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: A randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef]
- Silva, A.C.; Pereira, M.A.; Peixoto, L.M.; Rosse, I.C.; Júnior, J.B.F.; de Oliveira, E.C.; Becker, L.K.; Coelho, D.B. 12 weeks of resistance training with progressive intensity improves the diagnostic parameters of sarcopenia in individuals of advanced age. Geriatr. Nurs. 2023, 54, 60–65. [Google Scholar] [CrossRef]
- Stoever, K.; Heber, A.; Eichberg, S.; Brixius, K. Influences of Resistance Training on Physical Function in Older, Obese Men and Women With Sarcopenia. J. Geriatr. Phys. Ther. 2018, 41, 20–27. [Google Scholar] [CrossRef]
- Banitalebi, E.; Ghahfarrokhi, M.M.; Dehghan, M. Effect of 12-weeks elastic band resistance training on MyomiRs and osteoporosis markers in elderly women with Osteosarcopenic obesity: A randomized controlled trial. BMC Geriatr. 2021, 21, 433. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, A.Y.; Kwak, S.; Kwak, S.G. Effect of Resistance Exercise on Depression in Mild Alzheimer Disease Patients With Sarcopenia. Am. J. Geriatr. Psychiatry 2020, 28, 587–589. [Google Scholar] [CrossRef]
- Yuenyongchaiwat, K.; Akekawatchai, C. Beneficial effects of walking-based home program for improving cardio-respiratory performance and physical activity in sarcopenic older people: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2022, 58, 838–844. [Google Scholar] [CrossRef]
- Sun, R.; Wan, J.; Tang, J.; Deng, Y.; Zhang, M.; Liu, C.; Li, J.; Zhang, Q. Effectiveness of resistance training on body composition, muscle strength, and biomarker in sarcopenic older adults: A meta-analysis of randomized controlled trials. Arch. Gerontol. Geriatr. 2025, 128, 105595. [Google Scholar] [CrossRef]
- Xavier, L.N.; do Nascimento, V.B. Professional Narratives about Older Adults and Health Services Responsive to Fall-Inducing Frailty. Int. J. Environ. Res. Public Health 2023, 20, 6975. [Google Scholar] [CrossRef]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Beijersbergen, C.M.; Granacher, U.; Vandervoort, A.A.; DeVita, P.; Hortobágyi, T. The biomechanical mechanism of how strength and power training improves walking speed in old adults remains unknown. Ageing Res. Rev. 2013, 12, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.B.; Escolano, B.T.; Nifas, F.M.; Choi, S.; Carado, G.P.; Lerma, E.; Vijayaraghavan, K.; Yu, M.G. Interrelationship of Sarcopenia and Cardiovascular Diseases: A Review of Potential Mechanisms and Management. J. ASEAN Fed. Endocr. Soc. 2024, 39, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mancus, G.C.; Yuen, H.K.; Watson, J.H.; Lake, M.L.; Jenkins, G.R. Changes in cortisol and dehydroepiandrosterone levels immediately after urban park visits. Int. J. Environ. Health Res. 2023, 33, 206–218. [Google Scholar] [CrossRef]
- Momma, H.; Kawakami, R.; Honda, T.; Sawada, S.S. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: A systematic review and meta-analysis of cohort studies. Br. J. Sports Med. 2022, 56, 755–763. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Salimans, L.; Liberman, K.; Njemini, R.; Kortekaas Krohn, I.; Gutermuth, J.; Bautmans, I. The effect of resistance exercise on the immune cell function in humans: A systematic review. Exp. Gerontol. 2022, 164, 111822. [Google Scholar] [CrossRef]
- Strasser, B.; Siebert, U.; Schobersberger, W. Resistance Training in the Treatment of the Metabolic Syndrome. Sports Med. 2010, 40, 397–415. [Google Scholar] [CrossRef]
- Kristensen, J.; Franklyn-Miller, A. Resistance training in musculoskeletal rehabilitation: A systematic review. Br. J. Sports Med. 2012, 46, 719–726. [Google Scholar] [CrossRef]
- McMaster, D.; Cronin, J.; McGuigan, M. Forms of Variable Resistance Training. Strength Cond. J. 2009, 31, 50–64. [Google Scholar] [CrossRef]
- Hayes, E.J.; Stevenson, E.; Sayer, A.A.; Granic, A.; Hurst, C. Recovery from Resistance Exercise in Older Adults: A Systematic Scoping Review. Sports Med. Open 2023, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T. Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia. Cells 2022, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).