Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Peptide Synthesis and Analysis
2.1.2. In Vitro Experiments
2.1.3. Bacterial Strains and Cells
2.1.4. Animals
2.1.5. In Vivo Experiments
2.2. Methods
2.2.1. Design of the Synthesized Peptides
2.2.2. Molecular Dynamics Simulations
Modeling the L-Form Peptides
Modeling of the D-Form Peptides
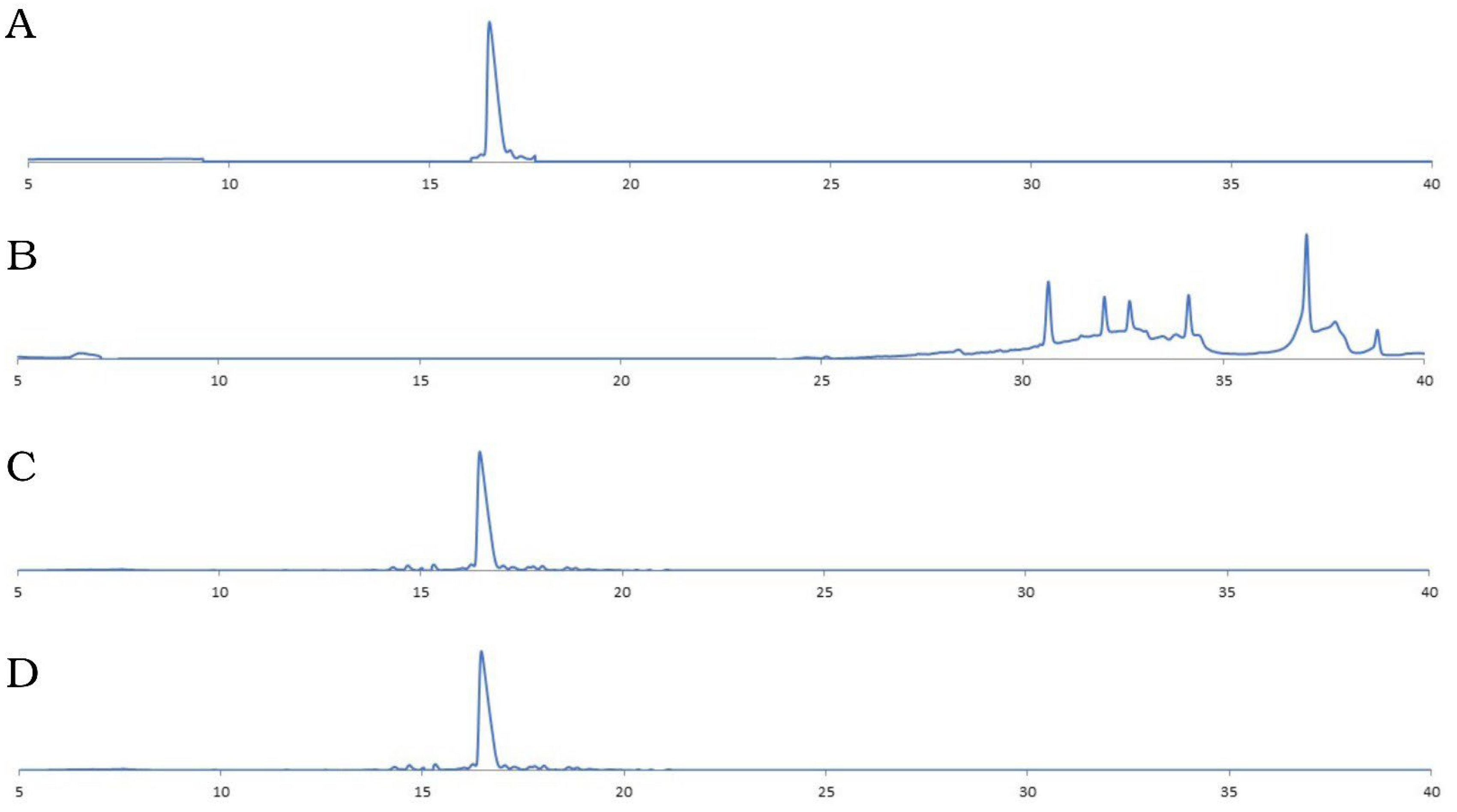
2.2.3. Peptide Synthesis and Characterization
2.2.4. Minimum Inhibitory Concentration
2.2.5. Cell Cytotoxicity
2.2.6. Hemolytic Activity
2.2.7. Stability Toward Enzymatic Degradation
2.2.8. Resistance Development Studies
2.2.9. Electrochemical Measurements
2.2.10. Scanning Electron Microscopy (SEM)
2.2.11. Transmission Electron Microscopy (TEM)
2.2.12. Animal Experiment Design
Full-Thickness Excision Wound Model
Preparation of the Bacteria
Surgical Site Infection Model
2.2.13. Light Microscopy Tissue Processing
2.2.14. Statistical Analysis
3. Results
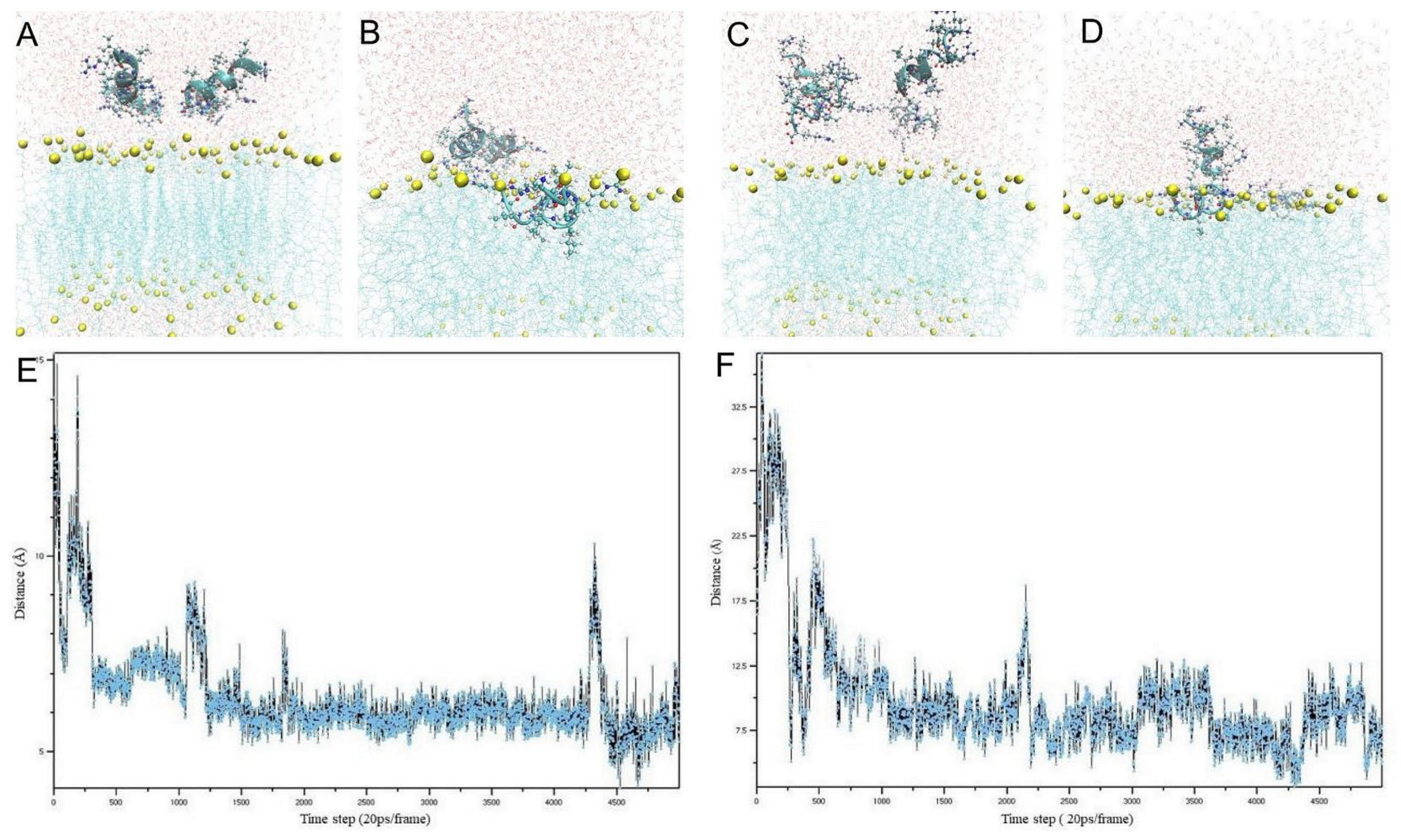
3.1. Molecular Dynamics Simulation
3.2. Antimicrobial Activity of the Peptides
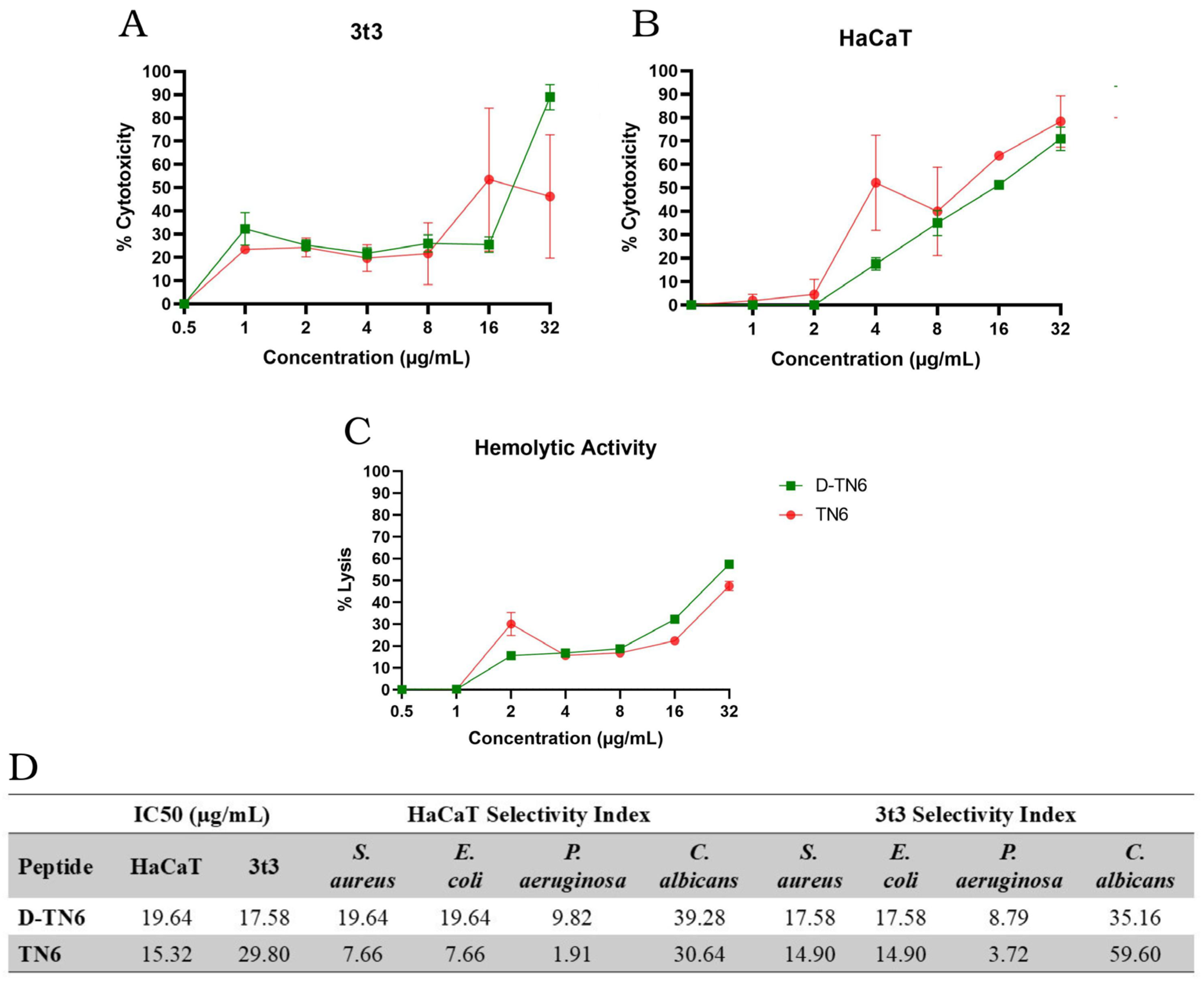
3.3. Evaluation of the Toxicity and Hemolytic Activity of AMPs
3.4. Protease Resistance Assay
3.5. Resistance Development Studies
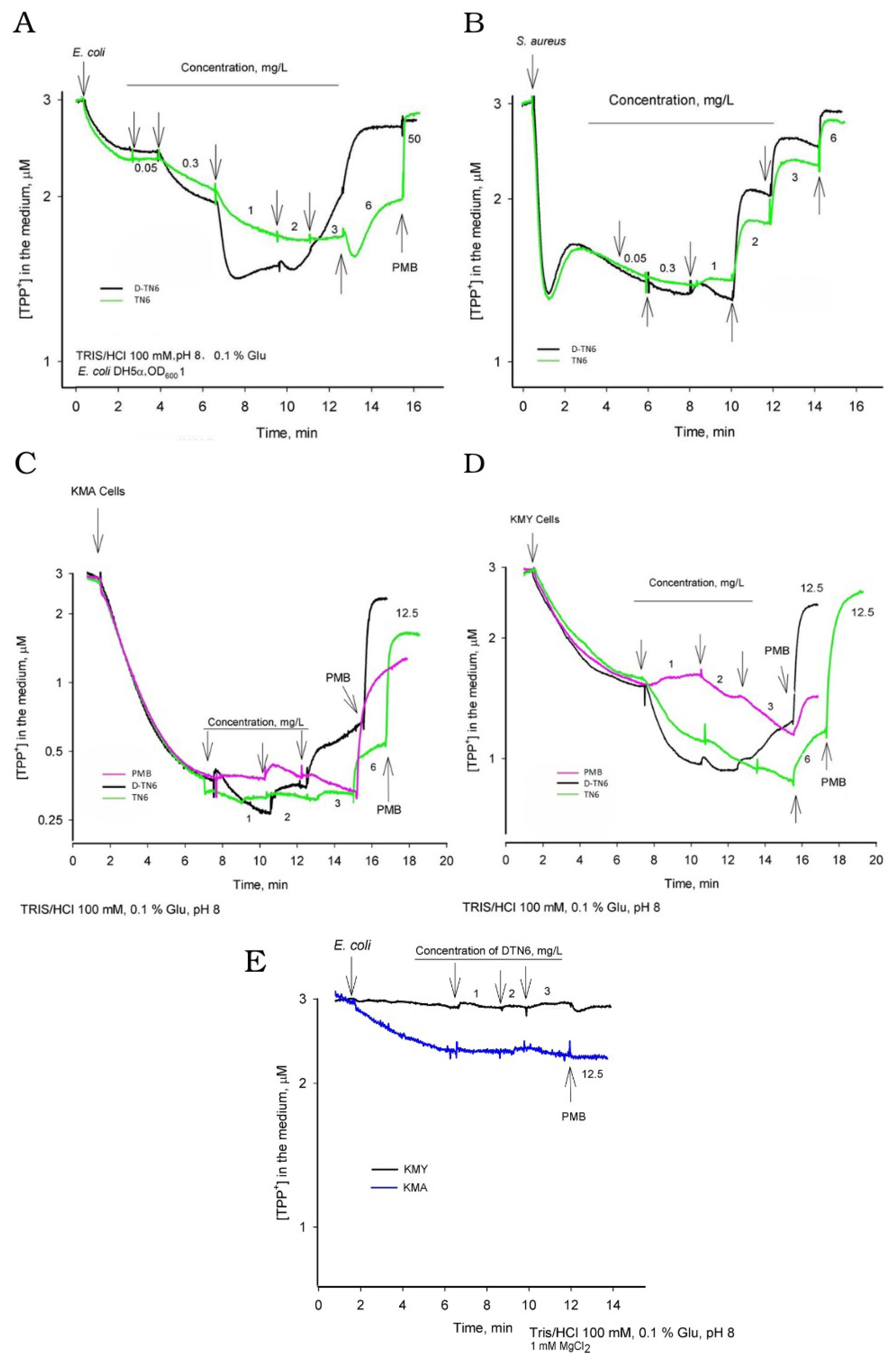
3.6. Electrochemical Measurements
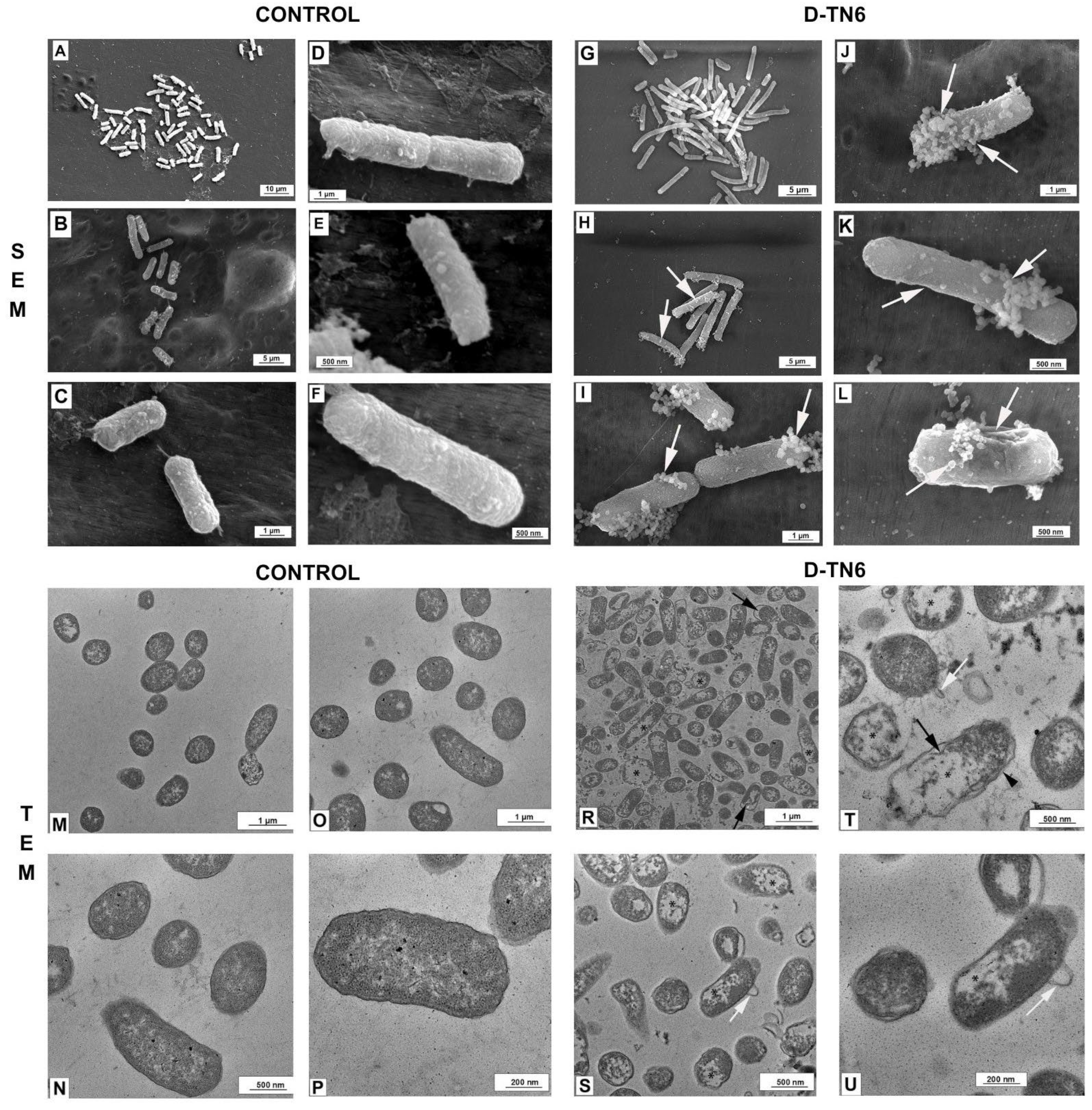
3.7. Morphological Evaluation of E. coli
3.8. Ultrastructural Evaluation of E. coli
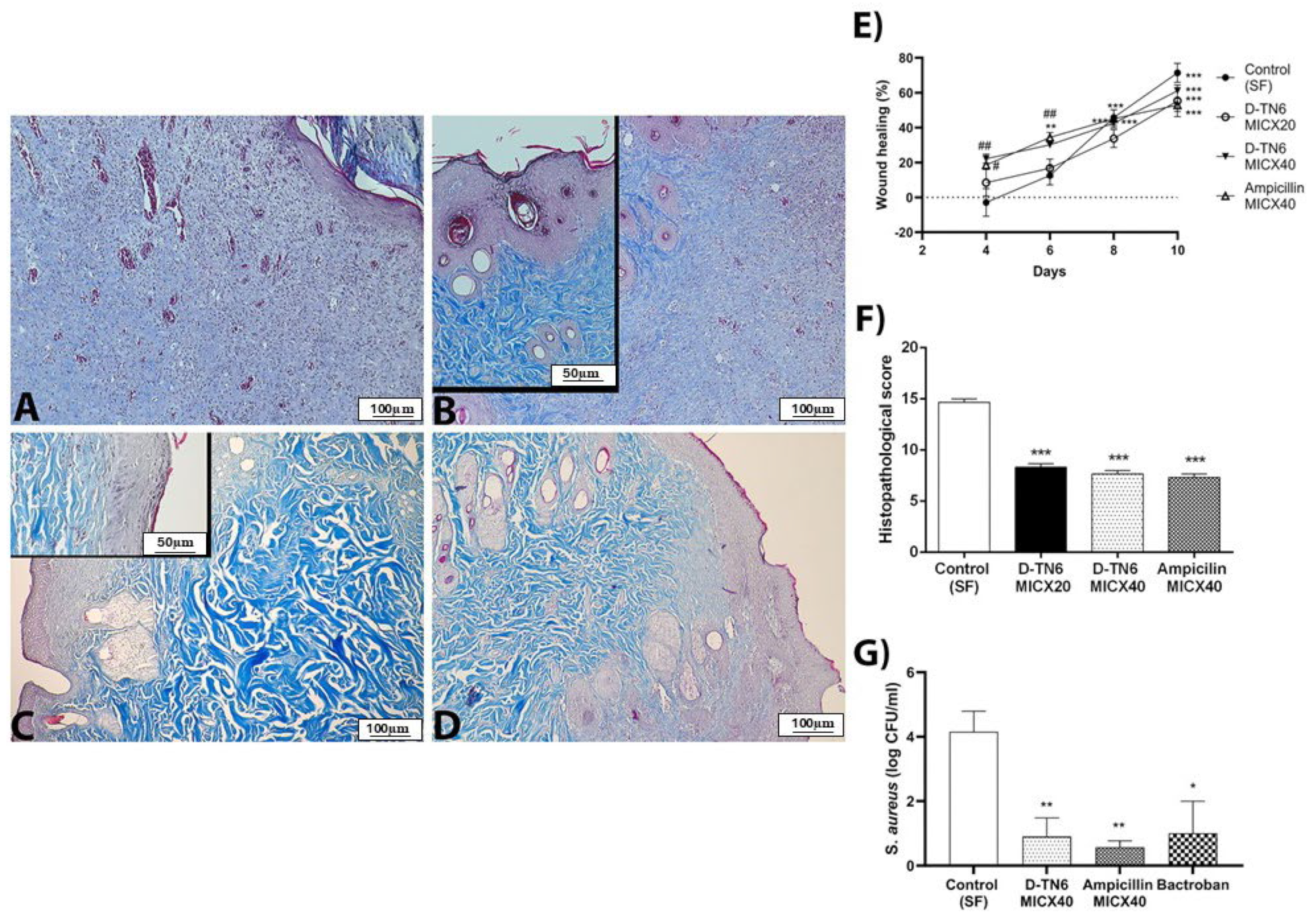
3.9. Animal Experiments
3.10. Histopathological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Zhang, Y.; Wang, J.; Peng, J.; Zhao, P.; Zhang, L.; Wang, K. The effect of halogenation on the antimicrobial activity antibiofilm activity cytotoxicity proteolytic stability of the antimicrobial peptide Jelleine-I. Peptides 2019, 112, 56–66. [Google Scholar] [CrossRef]
- Nayab, S.; Aslam, M.A.; Rahman, S.U.; Sindhu ZU, D.; Sajid, S.; Zafar, N.; Amanullah. A Review of Antimicrobial Peptides: Its Function, Mode of Action and Therapeutic Potential. Int. J. Pept. Res. Ther. 2022, 28, 46. [Google Scholar] [CrossRef]
- Randall, J.R.; Vieira, L.C.; Wilke, C.O.; Davies, B.W. Deep mutational scanning and machine learning for the analysis of antimicrobial-peptide features driving membrane selectivity. Nat. Biomed. Eng. 2024, 8, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Vol. 20, The Lancet Infectious Diseases. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, W.; Veerman, E.C.; Helmerhorst, E.J.; Amerongen, A.V. Antimicrobial peptides: Properties and applicability. Biol. Chem. 2001, 382, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.; McElhaney, R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 2069–2079. [Google Scholar] [CrossRef]
- Desbois, A.P.; Tschorner, D.; Coote, P.J. Survey of Small Antifungal Peptides with Chemotherapeutic Potential. Curr. Pharm. Biotechnol. 2011, 12, 1263–1291. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Manabe, T.; Kawasaki, K. D-form KLKLLLLLKLK-NH2 peptide exerts higher antimicrobial properties than its L-form counterpart via an association with bacterial cell wall components. Sci. Rep. 2017, 7, srep43384. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, K.; Kida, Y.; Zhang, Y.; Shimizu, T.; Kuwano, K. Antimicrobial activity and stability to proteolysis of small linear cationic peptides with D-amino acid substitutions. Microbiol. Immunol. 2002, 46, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Dinu, V.; Lu, Y.; Weston, N.; Lithgo, R.; Coupe, H.; Channell, G.; Adams, G.G.; Gómez, A.T.; Sabater, C.; Mackie, A.; et al. The antibiotic vancomycin induces complexation and aggregation of gastrointestinal and submaxillary mucins. Sci. Rep. 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Würbel, H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Cambridge, MA, USA, 2011; 220p. [Google Scholar]
- Thévenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tufféry, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.; Isgro, T.; Wang, Y.; Mayne, C.; Wen, P.C. Topology File Tutorial. 2012. Available online: www.ks.uiuc.edu/Training/Tutorials/ (accessed on 24 August 2019).
- Bulut, A.; Temur, B.Z.; Kirimli, C.E.; Gok, O.; Balcioglu, B.K.; Ozturk, H.U.; Can, O. A Novel Peptide-Based Detection of SARS-CoV-2 Antibodies. Biomimetics 2023, 8, 89. [Google Scholar] [CrossRef]
- Hasselmann, C. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
- Eren, T.; Som, A.; Rennie, J.R.; Nelson, C.F.; Urgina, Y.; Nüsslein, K.; Tew, G.N. Antibacterial and hemolytic activities of quaternary pyridinium functionalized polynorbornenes. Macromol. Chem. Phys. 2008, 209, 516–524. [Google Scholar] [CrossRef]
- El Shazely, B.; Yu, G.; Johnston, P.R.; Rolff, J. Resistance Evolution Against Antimicrobial Peptides in Staphylococcus aureus Alters Pharmacodynamics Beyond the MIC. Front. Microbiol. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V.; Ghannoum, M.A.; Espinel-Ingroff, A.; Knapp, C.C.; Ostrosky-Zeichner, L.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 22, 25p. [Google Scholar]
- De Lucio, H.; Gamo, A.M.; Ruiz-Santaquiteria, M.; de Castro, S.; Sánchez-Murcia, P.A.; Toro, M.A.; Velázquez, S. Improved proteolytic stability and potent activity against Leishmania infantum trypanothione reductase of α/β-peptide foldamers conjugated to cell-penetrating peptides. Eur. J. Med. Chem. 2017, 140, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Dong, M.; Hang, B.; Sun, Y.; Wang, L.; Wang, Y.; Hu, J.; Zhang, W. HJH-1, a broad-spectrum antimicrobial activity and low cytotoxicity antimicrobial peptide. Molecules 2018, 23, 2026. [Google Scholar] [CrossRef]
- Taştan, C.; Yurtsever, B.; Sir Karakuş, G.; Dilek Kançaği, D.; Demir, S.; Abanuz, S.; Kocagöz, A.S. SARS-CoV-2 isolation and propagation from turkish COVID-19 patients. Turk. J. Biol. 2020, 44, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, R.; Tong, A.; Yang, M.; Deng, J.; Zhou, L.; Zhang, X.; Guo, G. In situ gel-forming AP-57 peptide delivery system for cutaneous wound healing. Int. J. Pharm. 2015, 495, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campillo, M.; Gabaldon, J.; Castillo, J.; Benavente-García, O.; Del Baño, M.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Yumru, M.; Savas, H.A.; Kalenderoglu, A.; Bulut, M.; Celik, H.; Erel, O. Oxidative imbalance in bipolar disorder subtypes: A comparative study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, J.; Björn, C.; Lindgren, K.; Sjöström, E.; Sjöstrand, V.; Mahlapuu, M. Efficacy of the novel topical antimicrobial agent PXL150 in a mouse model of surgical site infections. Antimicrob. Agents Chemother. 2014, 58, 2982–2984. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.; Acikel Elmas, M.; Bingol Ozakpinar, O.; Arbak, S. The Effects of Apocynin on Monosodium Glutamate Induced Liver Damage of Rats. Heliyon 2023, 9, e17327. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Şener, G.; Jahovic, N.; Uslu, B.; Arbak, S.; Yeğen, B.C. β-glucan protects against burn-induced oxidative organ damage in rats. Int. Immunopharmacol. 2006, 6, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Cushnie, B.; Echeverría, J.; Fowsantear, W.; Thammawat, S.; Dodgson, J.L.; Law, S.; Clow, S.M. Bioprospecting for Antibacterial Drugs: A Multidisciplinary Perspective on Natural Product Source Material, Bioassay Selection and Avoidable Pitfalls. Pharm. Res. 2020, 37, 1–24. [Google Scholar] [CrossRef]
- Campoccia, D.; Bottau, G.; De Donno, A.; Bua, G.; Ravaioli, S.; Capponi, E.; Sotgiu, G.; Bellotti, C.; Costantini, S.; Arciola, C.R. Assessing Cytotoxicity, Proteolytic Stability, and Selectivity of Antimicrobial Peptides: Implications for Orthopedic Applications. Int. J. Mol. Sci. 2024, 25, 13241. [Google Scholar] [CrossRef]
- Mora-Navarro, C.; Méndez-Vega, J.; Caraballo-León, J.; Lee, M.R.; Palecek, S.; Torres-Lugo, M.; Ortiz-Bermúdez, P. Hydrophobicity of antifungal ß-peptides is associated with their cytotoxic effect on in vitro human colon Caco-2 and liver HepG2 cells. PLoS ONE 2016, 11, e0149271. [Google Scholar]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides with Enhanced Activities and Specificity/Therapeutic Index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Unubol, N.; Selim Cinaroglu, S.; Elmas, M.A.; Akcelik, S.; Ozal Ildeniz, A.T.; Arbak, S. Peptide Antibiotics Developed by Mimicking Natural Antimicrobial Peptides. Clin. Microbiol. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Sahsuvar, S.; Kocagoz, T.; Gok, O.; Can, O. In vitro efficacy of different PEGylation designs on cathelicidin-like peptide with high antibacterial and antifungal activity. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Asif, F.; Zaman, S.U.; Arnab, M.K.H.; Rahman, M.M.; Hasan, M. Effect of charge on the antimicrobial activity of alpha-helical amphibian antimicrobial peptide. Curr. Res. Microb. Sci. 2023, 4, 100182. [Google Scholar] [CrossRef]
- Alkotaini, B.; Anuar, N.; Kadhum, A.A.H. Evaluation of morphological changes of Staphylococcus aureus and Escherichia coli induced with the antimicrobial peptide AN5-1. Appl. Biochem. Biotechnol. 2015, 175, 1868–1878. [Google Scholar] [CrossRef]
- Tang, Y.L.; Shi, Y.H.; Zhao, W.; Hao, G.; Le, G.W. Insertion mode of a novel anionic antimicrobial peptide MDpep5 (Val-Glu-Ser-Trp-Val) from Chinese traditional edible larvae of housefly and its effect on surface potential of bacterial membrane. J. Pharm. Biomed. Anal. 2008, 48, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Ishibashi, J.; Yukuhiro, F.; Asaoka, A.; Taylor, D.; Yamakawa, M. Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. Biochim. Biophys. Acta (BBA) Gen. Subj. 2003, 1624, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef]
- Rizzo, M.G.; De Plano, L.M.; Franco, D. Regulation of filamentation by bacteria and its impact on the productivity of compounds in biotechnological processes. Appl. Microbiol. Biotechnol. 2020, 104, 4631–4642. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Parfitt, G.J.; Geyfman, M.; Xie, Y.; Jester, J.V. Characterization of quiescent epithelial cells in mouse meibomian glands and hair follicle/sebaceous glands by immunofluorescence tomography. J. Investig. Dermatol. 2015, 135, 1175–1177. [Google Scholar] [CrossRef]
- Miao, F.; Li, Y.; Tai, Z.; Zhang, Y.; Gao, Y.; Hu, M.; Zhu, Q. Antimicrobial Peptides: The Promising Therapeutics for Cutaneous Wound Healing. Macromol. Biosci. 2021, 21, 2100103. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent And control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Mondal, A.H.; Khare, K.; Saxena, P.; Debnath, P.; Mukhopadhyay, K.; Yadav, D. A Review on Colistin Resistance: An Antibiotic of Last Resort. Microorganisms 2024, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Jariyarattanarach, P.; Klubthawee, N.; Wongchai, M.; Roytrakul, S.; Aunpad, R. Novel D-form of hybrid peptide (D-AP19) rapidly kills Acinetobacter baumannii while tolerating proteolytic enzymes. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ren, X.; Luo, X.; Wang, Z.; Li, Z.; Luo, X.; Shen, J.; Li, Y.; Yuan, D.; Nussinov, R.; et al. A Foundation Model Identifies Broad-Spectrum Antimicrobial Peptides against Drug-Resistant Bacterial Infection. Nat. Commun. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Naeem, D.; Alshamrani, M.A.; Aseeri, M.A.; Khan, M.A. Prescribing Empiric Antibiotics for Febrile Neutropenia: Compliance with Institutional Febrile Neutropenia Guidelines. Pharmacy 2018, 6, 83. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Sequence | S. aureus | E. coli | P. aeruginosa | C. albicans |
|---|---|---|---|---|---|
| TN1 | RLLRLLLLRLLR | 4 | 2 | 32 | 0.5 |
| D-TN1 | RLLRLLLLRLLR | 4 | 8 | 4 | 1 |
| TN3-amide | RLLRLLRLLL | 8 | 8 | 4 | 2 |
| TN3-carboxy | RLLRLLRLLL | 16 | 4 | 32 | 1 |
| D-TN3 | RLLRLLRLLL | 8 | 2 | 4 | 0.25 |
| TN3V1 | RVLRVLRVLL | 8 | 16 | 32 | 4 |
| TN3V9 | RVVRVVRVVV | 16 | 64 | 256 | 128 |
| TN6 | RLLRLLLRLLR | 2 | 2 | 8 | 0.5 |
| D-TN6 | RLLRLLLRLLR | 1 | 1 | 2 | 0.5 |
| TN6I1 | RIIRIIIRIIR | 16 | 32 | 128 | 128 |
| TN6I2 | RILRILIRLIR | 2 | 16 | 32 | 128 |
| TN6A1 | RALRALARALR | 128 | 128 | 256 | 256 |
| TN6A5 | RAARAAARAAR | >1024 | 1024 | >1024 | 128 |
| RTN6 | RRLLRLLLRLLR | 1 | 2 | 8 | 4 |
| D/L-TN6 | RLLRLLLRLLR | 8 | 8 | 16 | 8 |
| TN6(2) | RLLRLLRLLLRLLRLLR | 16 | 16 | 64 | 32 |
| TN8 | RLLRLLRLLLL | 8 | 4 | 256 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocagoz, T.; Temur, B.Z.; Unubol, N.; Acikel Elmas, M.; Kanlidere, Z.; Cilingir, S.; Acar, D.; Boskan, G.; Akcelik Deveci, S.; Aybakan, E.; et al. Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity. Life 2025, 15, 242. https://doi.org/10.3390/life15020242
Kocagoz T, Temur BZ, Unubol N, Acikel Elmas M, Kanlidere Z, Cilingir S, Acar D, Boskan G, Akcelik Deveci S, Aybakan E, et al. Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity. Life. 2025; 15(2):242. https://doi.org/10.3390/life15020242
Chicago/Turabian StyleKocagoz, Tanil, Betul Zehra Temur, Nihan Unubol, Merve Acikel Elmas, Zeynep Kanlidere, Sumeyye Cilingir, Dilan Acar, Gizem Boskan, Sumeyye Akcelik Deveci, Esma Aybakan, and et al. 2025. "Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity" Life 15, no. 2: 242. https://doi.org/10.3390/life15020242
APA StyleKocagoz, T., Temur, B. Z., Unubol, N., Acikel Elmas, M., Kanlidere, Z., Cilingir, S., Acar, D., Boskan, G., Akcelik Deveci, S., Aybakan, E., Ozcan Yoner, A., Yurttutan Uyar, N., Serteser, M., Sahsuvar, S., Erdemgil, Y., Yildirim Keles, Z. Z., Demirhan, D., Sakalauskaite, S., Daugelavicius, R., ... Can, O. (2025). Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity. Life, 15(2), 242. https://doi.org/10.3390/life15020242






