NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic–Metabolic Interplay
Abstract
1. Introduction
2. NAFLD and T2DM Bidirectional Relationship
3. Natural History of NAFLD
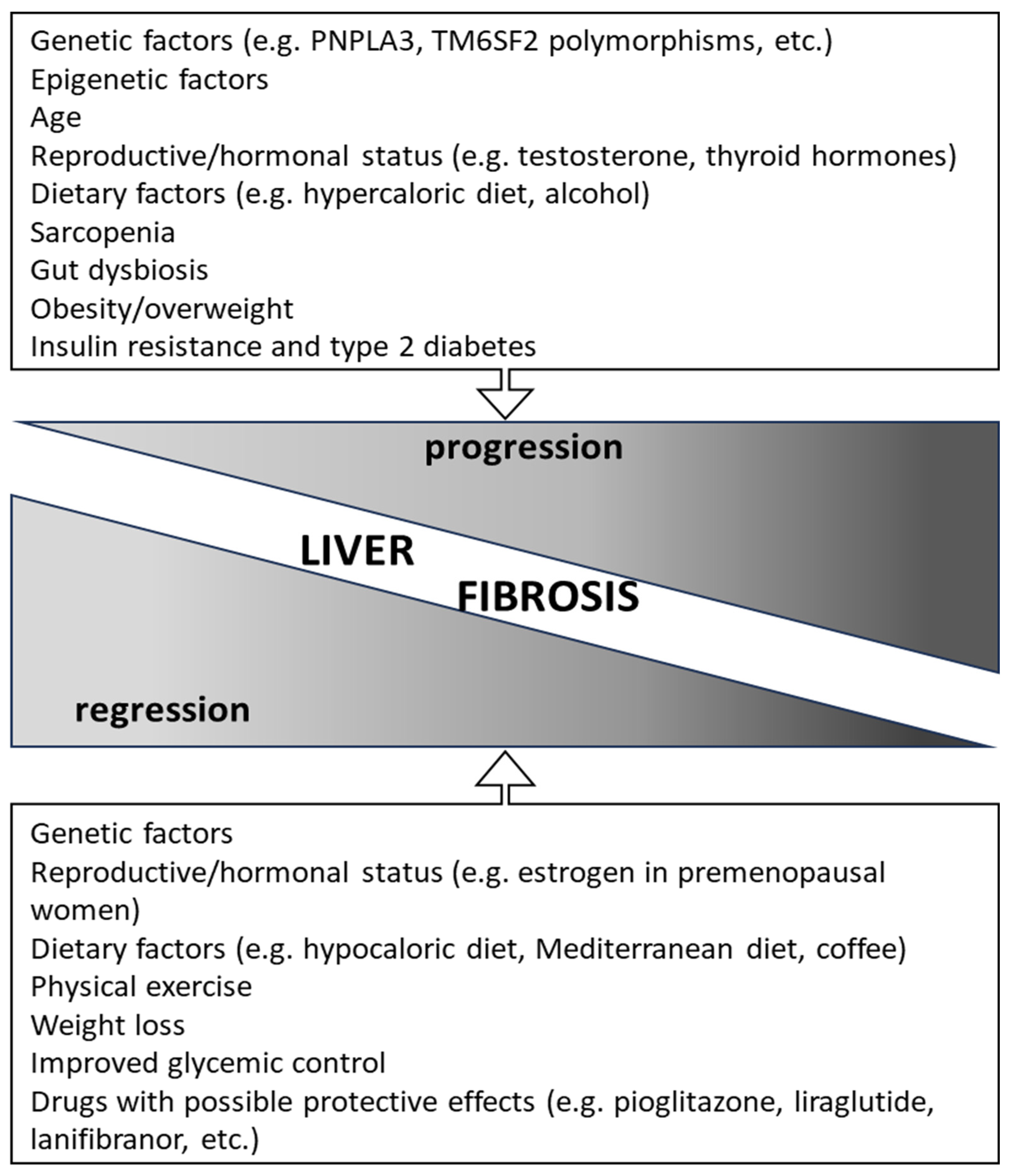
4. Factors Associated with Fibrosis Progression and Regression
5. Liver Fibrogenesis and the Role of Metabolism
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Liebe, R.; Esposito, I.; Bock, H.H.; Vom Dahl, S.; Stindt, J.; Baumann, U.; Luedde, T.; Keitel, V. Diagnosis and management of secondary causes of steatohepatitis. J. Hepatol. 2021, 74, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Raz, I. NAFLD in type 2 diabetes mellitus: Still many challenging questions. Diabetes Metab. Res. Rev. 2021, 37, e3386. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N. Japan Study Group of Nafld Jsg-Nafld. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- New NAFLD Nomenclature. Available online: https://www.aasld.org/new-nafld-nomenclature (accessed on 19 August 2023).
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Henry, L.; Paik, J.; Younossi, Z.M. Review article: The epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment. Pharmacol. Ther. 2022, 56, 942–956. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, K.J.; Han, K.H. Prevalence and Predictors of Significant Fibrosis Among Subjects with Transient Elastography-Defined Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2017, 62, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Makker, J.; Tariq, H.; Kumar, K.; Ravi, M.; Shaikh, D.H.; Leung, V.; Hayat, U.; Hassan, M.T.; Patel, H.; Nayudu, S.; et al. Prevalence of advanced liver fibrosis and steatosis in type-2 diabetics with normal transaminases: A prospective cohort study. World J. Gastroenterol. 2021, 27, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Monti, T.; Perseghin, G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults with Type 2 Diabetes. Diabetes Care 2021, 44, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Onișor, D. Screening and interventions to prevent nonalcoholic fatty liver disease/nonalcoholic steatohepatitis-associated hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 286–309. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kang, D.; Cao, W.; Wang, Y.; Liu, Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2012, 28, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes Is Associated with Increased Risk of Hepatocellular Carcinoma in Patients with Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 907–916. [Google Scholar] [CrossRef]
- Huang, D.Q.; Noureddin, N.; Ajmera, V.; Amangurbanova, M.; Bettencourt, R.; Truong, E.; Gidener, T.; Siddiqi, H.; Majzoub, A.M.; Nayfeh, T.; et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: An individual participant-level data meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 829–836. [Google Scholar] [CrossRef]
- Aller de la Fuente, R.; Mora Cuadrado, N.; Tafur, C.; López Gómez, J.J.; Gómez de la Cuesta, S.; García Sánchez, M.C.; Antolin Melero, B.; de Luis Román, D.A. Histopathological differences in patients with biopsy-proven non-alcoholic fatty liver disease with and without type 2 diabetes. Endocrinol. Diabetes Nutr. 2018, 65, 354–360. [Google Scholar] [CrossRef]
- Puchakayala, B.K.; Verma, S.; Kanwar, P.; Hart, J.; Sanivarapu, R.R.; Mohanty, S.R. Histopathological differences utilizing the nonalcoholic fatty liver disease activity score criteria in diabetic (type 2 diabetes mellitus) and non-diabetic patients with nonalcoholic fatty liver disease. World J. Hepatol. 2015, 7, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Zhu, X.; Xia, M.; Yan, H.; Chang, X.; Hu, X.; Pan, B.; Guo, W.; Li, X.; Gao, X. Impact of TYPE 2 diabetes on nonalcoholic steatohepatitis and advanced fibrosis in patients with nonalcoholic fatty liver disease. Endocr. Pract. 2020, 26, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, H.; Craig, D.; Barker, R.; Spiers, G.; Stow, D.; Anstee, Q.M.; Hanratty, B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020, 17, e1003100. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.H.; Morling, J.R.; McAllister, D.A.; Kerssens, J.; Fischbacher, C.; Parkes, J.; Roderick, P.J.; Sattar, N.; Byrne, C.D.; Scottish and Southampton Diabetes and Liver Disease Group; et al. Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J. Hepatol. 2016, 64, 1358–1364. [Google Scholar] [CrossRef]
- Liu, Z.J.; Yan, Y.J.; Weng, H.L.; Ding, H.G. Type 2 diabetes mellitus increases liver transplant-free mortality in patients with cirrhosis: A systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5514–5525. [Google Scholar] [CrossRef]
- Bertot, L.C.; Jeffrey, G.P.; de Boer, B.; MacQuillan, G.; Garas, G.; Chin, J.; Huang, Y.; Adams, L.A. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018, 38, 1793–1802. [Google Scholar] [CrossRef]
- Tada, T.; Toyoda, H.; Sone, Y.; Yasuda, S.; Miyake, N.; Kumada, T.; Tanaka, J. Type 2 diabetes mellitus: A risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019, 34, 2011–2018. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: Real-world study of 18 million patients in four European cohorts. BMC Med. 2019, 17, 95. [Google Scholar] [CrossRef]
- Colosimo, S.; Tan, G.D.; Petroni, M.L.; Marchesini, G.; Tomlinson, J.W. Improved glycaemic control in patients with type 2 diabetes has a beneficial impact on NAFLD, independent of change in BMI or glucose lowering agent. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, O.P.; Strassburger, K.; Strom, A.; Bönhof, G.J.; Karusheva, Y.; Antoniou, S.; Bódis, K.; Markgraf, D.F.; Burkart, V.; Müssig, K.; et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: A 5-year follow-up study. Lancet Diabetes Endocrinol. 2019, 7, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, O.P.; Strassburger, K.; Knebel, B.; Kupriyanova, Y.; Karusheva, Y.; Wolkersdorfer, M.; Bódis, K.; Markgraf, D.F.; Burkart, V.; Hwang, J.H.; et al. Role of Patatin-Like Phospholipase Domain-Containing 3 Gene for Hepatic Lipid Content and Insulin Resistance in Diabetes. Diabetes Care. 2020, 43, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.E.; Zaccardi, F.; Khunti, K.; Davies, M.J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 2019, 39, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Björkström, K.; Stål, P.; Hultcrantz, R.; Hagström, H. Histologic Scores for Fat and Fibrosis Associate with Development of Type 2 Diabetes in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1461–1468. [Google Scholar] [CrossRef]
- Nasr, P.; Ignatova, S.; Kechagias, S.; Ekstedt, M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatol. Commun. 2017, 2, 199–210. [Google Scholar] [CrossRef]
- Ampuero, J.; Aller, R.; Gallego-Durán, R.; Crespo, J.; Calleja, J.L.; García-Monzón, C.; Gómez-Camarero, J.; Caballería, J.; Lo Iacono, O.; Ibañez, L.; et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J. Hepatol. 2020, 73, 17–25. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tsuboya, T.; Tsuji, K.; Dohke, M.; Maguchi, H. Independent Association between Improvement of Nonalcoholic Fatty Liver Disease and Reduced Incidence of Type 2 Diabetes. Diabetes Care. 2015, 38, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Hwang, S.; Park, J.I.; Yang, M.J.; Hwang, J.C.; Yoo, B.M.; Lee, K.M.; Shin, S.J.; Lee, K.J.; Kim, J.H.; et al. Improvement of Nonalcoholic Fatty Liver Disease Reduces the Risk of Type 2 Diabetes Mellitus. Gut Liver. 2019, 13, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Wang, J.; Tauchi, S.; Dohke, M.; Hanawa, N.; Katanuma, A.; Saisho, Y.; Kamitani, T.; Fukuhara, S.; Yamamoto, Y. Inverse Association between Fatty Liver at Baseline Ultrasonography and Remission of Type 2 Diabetes Over a 2-Year Follow-up Period. Clin. Gastroenterol. Hepatol. 2021, 19, 556–564.e5. [Google Scholar] [CrossRef]
- Xin, Z.; Huang, J.; Cao, Q.; Wang, J.; He, R.; Hou, T.; Ding, Y.; Lu, J.; Xu, M.; Wang, T.; et al. Nonalcoholic fatty liver disease in relation to the remission and progression along the glycemic continuum. J. Diabetes. 2022, 14, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Henry, Z.H.; Argo, C.K. How to Identify the Patient with Nonalcoholic Steatohepatitis Who Will Progress to Cirrhosis. Gastroenterol. Clin. North. Am. 2020, 49, 45–62. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef]
- Pais, R.; Maurel, T. Natural History of NAFLD. J. Clin. Med. 2021, 10, 1161. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, e1–e9. [Google Scholar] [CrossRef]
- Le, P.; Payne, J.Y.; Zhang, L.; Deshpande, A.; Rothberg, M.B.; Alkhouri, N.; Herman, W.; Hernandez, A.V.; Schleicher, M.; Ye, W.; et al. Disease State Transition Probabilities Across the Spectrum of NAFLD: A Systematic Review and Meta-Analysis of Paired Biopsy or Imaging Studies. Clin. Gastroenterol. Hepatol. 2023, 21, 1154–1168. [Google Scholar] [CrossRef]
- Ng, C.H.; Xiao, J.; Lim, W.H.; Chin, Y.H.; Yong, J.N.; Tan, D.J.H.; Tay, P.; Syn, N.; Foo, R.; Chan, M.; et al. Placebo effect on progression and regression in NASH: Evidence from a meta-analysis. Hepatology. 2022, 75, 1647–1661. [Google Scholar] [CrossRef]
- Pennisi, G.; Celsa, C.; Enea, M.; Vaccaro, M.; Di Marco, V.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Vernuccio, F.; et al. Effect of pharmacological interventions and placebo on liver Histology in nonalcoholic steatohepatitis: A network meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2279–2288. [Google Scholar] [CrossRef]
- Ampuero, J.; Gallego-Durán, R.; Maya-Miles, D.; Montero, R.; Gato, S.; Rojas, Á.; Gil, A.; Muñoz, R.; Romero-Gómez, M. Systematic review and meta-analysis: Analysis of variables influencing the interpretation of clinical trial results in NAFLD. J. Gastroenterol. 2022, 57, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Giorda, C.; Forlani, G.; Manti, R.; Mazzella, N.; De Cosmo, S.; Rossi, M.C.; Nicolucci, A.; Russo, G.; Di Bartolo, P.; Ceriello, A.; et al. Occurrence over time and regression of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Metab. Res. Rev. 2017, 33, e2878. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Wilson, L.A.; Behling, C.; Kleiner, D.E.; Kowdley, K.V.; Dasarathy, S.; Amangurbanova, M.; Terrault, N.A.; Diehl, A.M.; Chalasani, N.; et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People with Diabetes Versus People without Diabetes: A Multicenter Study. Gastroenterology. 2023, 165, 463–472.e5. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Hyogo, H.; Yoneda, M.; Sumida, Y.; Eguchi, Y.; Fujii, H.; Ono, M.; Kawaguchi, T.; Imajo, K.; Aikata, H.; et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J. Gastroenterol. 2014, 49, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [PubMed]
- Jonas, W.; Schürmann, A. Genetic and epigenetic factors determining NAFLD risk. Mol. Metab. 2021, 50, 101111. [Google Scholar] [CrossRef]
- Valenti, L.; Al-Serri, A.; Daly, A.K.; Galmozzi, E.; Rametta, R.; Dongiovanni, P.; Nobili, V.; Mozzi, E.; Roviaro, G.; Vanni, E.; et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2010, 51, 1209–1217. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef]
- Singal, A.G.; Manjunath, H.; Yopp, A.C.; Beg, M.S.; Marrero, J.A.; Gopal, P.; Waljee, A.K. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 325–334. [Google Scholar] [CrossRef]
- Pingitore, P.; Dongiovanni, P.; Motta, B.M.; Meroni, M.; Lepore, S.M.; Mancina, R.M.; Pelusi, S.; Russo, C.; Caddeo, A.; Rossi, G.; et al. PNPLA3 overexpression results in reduction of proteins predisposing to fibrosis. Hum. Mol. Genet. 2016, 25, 5212–5222. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Pirola, C.J.; Sookoian, S.; Wilson, L.A.; Belt, P.; Liang, T.; Liu, W.; Chalasani, N. Impact of the Association between PNPLA3 Genetic Variation and Dietary Intake on the Risk of Significant Fibrosis in Patients with NAFLD. Am. J. Gastroenterol. 2021, 116, 994–1006. [Google Scholar] [CrossRef]
- Chen, V.L.; Oliveri, A.; Miller, M.J.; Wijarnpreecha, K.; Du, X.; Chen, Y.; Cushing, K.C.; Lok, A.S.; Speliotes, E.K. PNPLA3 Genotype and Diabetes Identify Patients with Nonalcoholic Fatty Liver Disease at High Risk of Incident Cirrhosis. Gastroenterology 2023, 164, 966–977.e17. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef]
- Liu, Y.L.; Reeves, H.L.; Burt, A.D.; Tiniakos, D.; McPherson, S.; Leathart, J.B.; Allison, M.E.; Alexander, G.J.; Piguet, A.C.; Anty, R.; et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 4309. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Petta, S.; Maglio, C.; Fracanzani, A.L.; Pipitone, R.; Mozzi, E.; Motta, B.M.; Kaminska, D.; Rametta, R.; Grimaudo, S.; et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 2015, 61, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Rau, M.; Schattenberg, J.M.; Bantel, H.; Pathil, A.; Demir, M.; Kluwe, J.; Boettler, T.; Lammert, F.; Geier, A.; et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: A multicenter biopsy-based study. J. Lipid Res. 2017, 58, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.; Abeysekera, K.W.M.; Adams, L.; Aigner, E.; Anstee, Q.M.; Banales, J.M.; Banerjee, R.; Basu, P.; Berg, T.; Bhatnagar, P.; et al. rs641738C>T near MBOAT7 is associated with liver fat, ALT and fibrosis in NAFLD: A meta-analysis. J. Hepatol. 2021, 74, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego-Durán, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef]
- Petta, S.; Miele, L.; Bugianesi, E.; Cammà, C.; Rosso, C.; Boccia, S.; Cabibi, D.; Di Marco, V.; Grimaudo, S.; Grieco, A.; et al. Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLoS ONE 2014, 9, e87523, Erratum in PLoS ONE 2014, 9, e92497. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Govaere, O.; Cockell, S.; Tiniakos, D.; Queen, R.; Younes, R.; Vacca, M.; Alexander, L.; Ravaioli, F.; Palmer, J.; Petta, S.; et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 2020, 12, eaba4448. [Google Scholar] [CrossRef]
- Villani, R.; Magnati, G.P.; De Girolamo, G.; Sangineto, M.; Romano, A.D.; Cassano, T.; Serviddio, G. Genetic Polymorphisms and Clinical Features in Diabetic Patients with Fatty Liver: Results From a Single-Center Experience in Southern Italy. Front. Med. 2021, 8, 737759. [Google Scholar] [CrossRef]
- Lavrado, N.C.; Salles, G.F.; Cardoso, C.R.L.; de França, P.H.C.; Melo, M.F.D.G.G.; Leite, N.C.; Villela-Nogueira, C.A. Impact of PNPLA3 and TM6SF2 polymorphisms on the prognosis of patients with MASLD and type 2 diabetes mellitus. Liver Int. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Gellert-Kristensen, H.; Richardson, T.G.; Davey Smith, G.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Stender, S. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020, 72, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Duseja, A. Genetic and epigenetic disease modifiers: Non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD). Transl. Gastroenterol. Hepatol. 2021, 6, 2. [Google Scholar] [CrossRef]
- Zeybel, M.; Hardy, T.; Robinson, S.M.; Fox, C.; Anstee, Q.M.; Ness, T.; Masson, S.; Mathers, J.C.; French, J.; White, S.; et al. Differential DNA methylation of genes involved in fibrosis progression in non-alcoholic fatty liver disease and alcoholic liver disease. Clin. Epigenetics. 2015, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Zeybel, M.; Day, C.P.; Dipper, C.; Masson, S.; McPherson, S.; Henderson, E.; Tiniakos, D.; White, S.; French, J.; et al. Plasma DNA methylation: A potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut 2017, 66, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.K.; Yang, H.; Moylan, C.A.; Pang, H.; Dellinger, A.; Abdelmalek, M.F.; Garrett, M.E.; Ashley-Koch, A.; Suzuki, A.; Tillmann, H.L.; et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 1076–1087. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Gupta, S.; Maurya, M.R.; Subramaniam, S.; Loomba, R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: A prospective study. Gut 2016, 65, 1546–1554. [Google Scholar] [CrossRef]
- Hanson, A.; Wilhelmsen, D.; DiStefano, J.K. The Role of Long Non-Coding RNAs (lncRNAs) in the Development and Progression of Fibrosis Associated with Nonalcoholic Fatty Liver Disease (NAFLD). Noncoding RNA 2018, 4, 18. [Google Scholar] [CrossRef]
- Riazi, K.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. Race and Ethnicity in Non-Alcoholic Fatty Liver Disease (NAFLD): A Narrative Review. Nutrients 2022, 14, 4556. [Google Scholar] [CrossRef]
- Rich, N.E.; Oji, S.; Mufti, A.R.; Browning, J.D.; Parikh, N.D.; Odewole, M.; Mayo, H.; Singal, A.G. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 198–210.e2. [Google Scholar] [CrossRef] [PubMed]
- Truong, E.; Yeo, Y.H.; Cook-Wiens, G.; Muthiah, M.; Yang, J.D.; Sundaram, V.; Chang, D.; Todo, T.; Kim, I.K.; Lu, S.C.; et al. Nonalcoholic fatty liver disease prevalence and severity in Asian Americans from the national health and nutrition examination surveys 2017–2018. Hepatol Commun. 2022, 6, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Schattenberg, J.M. NAFLD in the Elderly. Clin. Interv. Aging. 2021, 16, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Miyaaki, H.; Ichikawa, T.; Nakao, K.; Yatsuhashi, H.; Furukawa, R.; Ohba, K.; Omagari, K.; Kusumoto, Y.; Yanagi, K.; Inoue, O.; et al. Clinicopathological study of nonalcoholic fatty liver disease in Japan: The risk factors for fibrosis. Liver Int. 2008, 28, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Day, C.P.; Henderson, E.; Burt, A.D.; Newton, J.L. Non-alcoholic fatty liver disease in older people. Gerontology 2009, 55, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Nagral, A.; Bangar, M.; Menezes, S.; Bhatia, S.; Butt, N.; Ghosh, J.; Manchanayake, J.H.; Mahtab, M.A.; Singh, S.P. Gender Differences in Nonalcoholic Fatty Liver Disease. Euroasian J. Hepatogastroenterol. 2022, 12 (Suppl. S1), S19–S25. [Google Scholar] [PubMed]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef]
- Sarkar, M.A.; Suzuki, A.; Abdelmalek, M.F.; Yates, K.P.; Wilson, L.A.; Bass, N.M.; Gill, R.; Cedars, M.; Terrault, N.; NASH Clinical Research Network. Testosterone is Associated with Nonalcoholic Steatohepatitis and Fibrosis in Premenopausal Women with NAFLD. Clin. Gastroenterol. Hepatol. 2021, 19, 1267–1274.e1. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Wang, Y.; Wang, N.; Chen, Y.; Lu, Y.; Xia, F. The associations of total testosterone with probable nonalcoholic steatohepatitis and nonalcoholic fatty liver disease fibrotic progression in men with type 2 diabetes: A cross-sectional study. Eur. J. Med. Res. 2022, 27, 307. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Tomlinson, J.W. Non-alcoholic fatty liver disease in common endocrine disorders. Eur. J. Endocrinol. 2013, 169, R27–R37. [Google Scholar] [CrossRef] [PubMed]
- Arefhosseini, S.; Ebrahimi-Mameghani, M.; Najafipour, F.; Tutunchi, H. Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones. Front. Endocrinol. 2022, 13, 1032361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Liu, H. Correlation between the thyroid hormone levels and nonalcoholic fatty liver disease in type 2 diabetic patients with normal thyroid function. BMC Endocr. Disord. 2022, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Tsompanaki, E.; Thanapirom, K.; Papatheodoridi, M.; Parikh, P.; Chotai de Lima, Y.; Tsochatzis, E.A. Systematic Review and Meta-analysis: The Role of Diet in the Development of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 1462–1474.e24. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Feature of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Miryan, M.; Darbandi, M.; Moradi, M.; Najafi, F.; Soleimani, D.; Pasdar, Y. Relationship between the Mediterranean diet and risk of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A cross-sectional analysis of the RaNCD cohort. Front. Nutr. 2023, 10, 1062008. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz-Del-Campo, N.; Castelnuovo, G.; Rosso, C.; Nicolosi, A.; Guariglia, M.; Dileo, E.; Armandi, A.; Caviglia, G.P.; Bugianesi, E. Impact of Health Related QoL and Mediterranean Diet on Liver Fibrosis in Patients with NAFLD. Nutrients 2023, 15, 3018. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Boutari, C.; Chrysohoou, C.; Fragkopoulou, E.; Antonopoulou, S.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B.; Mantzoros, C.S.; ATTICA study Investigators. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: Results from the ATTICA prospective cohort study. Clin. Nutr. 2021, 40, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Cammisotto, V.; Tozzi, G.; Coronati, M.; Bartimoccia, S.; Castellani, V.; Nocella, C.; D’Amico, A.; Angelico, F.; Carnevale, R.; et al. High Compliance to Mediterranean Diet Associates with Lower Platelet Activation and Liver Collagen Deposition in Patients with Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Curci, R.; Bianco, A.; Franco, I.; Bonfiglio, C.; Campanella, A.; Mirizzi, A.; Giannuzzi, V.; Cozzolongo, R.; Veronese, N.; Osella, A.R. Lifestyle Modification: Evaluation of the Effects of Physical Activity and Low-Glycemic-Index Mediterranean Diet on Fibrosis Score. Nutrients 2023, 15, 3520. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, D.; Ranjbar, G.; Rezvani, R.; Goshayeshi, L.; Razmpour, F.; Nematy, M. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2019, 12, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, V.; Long, M.T.; Singh, S.R.; Kim, Y.; Zhang, X.; Rogers, G.; Jacques, P.F.; Levy, D.; Ma, J. A Healthy Diet is Associated with a Lower Risk of Hepatic Fibrosis. J. Nutr. 2023, 153, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Johari, M.I.; Yusoff, K.; Haron, J.; Nadarajan, C.; Ibrahim, K.N.; Wong, M.S.; Hafidz, M.I.A.; Chua, B.E.; Hamid, N.; Arifin, W.N.; et al. A Randomised Controlled Trial on the Effectiveness and Adherence of Modified Alternate-day Calorie Restriction in Improving Activity of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 11232. [Google Scholar] [CrossRef]
- López-Bautista, F.; Barbero-Becerra, V.J.; Ríos, M.Y.; Ramírez-Cisneros, M.Á.; Sánchez-Pérez, C.A.; Ramos-Ostos, M.H.; Uribe, M.; Chávez-Tapia, N.C.; Juárez-Hernández, E. Dietary consumption and serum pattern of bioactive fatty acids in NAFLD patients. Ann. Hepatol. 2020, 19, 482–488. [Google Scholar] [CrossRef]
- Guerrerio, A.L.; Colvin, R.M.; Schwartz, A.K.; Molleston, J.P.; Murray, K.F.; Diehl, A.; Mohan, P.; Schwimmer, J.B.; Lavine, J.E.; Torbenson, M.S.; et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2012, 95, 892–900. [Google Scholar] [CrossRef]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef]
- Khodami, B.; Hatami, B.; Yari, Z.; Alavian, S.M.; Sadeghi, A.; Varkaneh, H.K.; Santos, H.O.; Hekmatdoost, A. Effects of a low free sugar diet on the management of nonalcoholic fatty liver disease: A randomized clinical trial. Eur. J. Clin. Nutr. 2022, 76, 987–994. [Google Scholar] [CrossRef]
- Protopapas, A.A.; Cholongitas, E.; Chrysavgis, L.; Tziomalos, K. Alcohol consumption in patients with nonalcoholic fatty liver disease: Yes, or no? Ann. Gastroenterol. 2021, 34, 476–486. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, Y.K.; Kim, Y.; Sung, E.; Ahn, J.; Jung, H.S.; Yun, K.E.; Shin, H.; Ryu, S. Nonheavy Drinking and Worsening of Noninvasive Fibrosis Markers in Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology 2019, 69, 64–75. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Holmqvist, M.; Bendtsen, P.; Mathiesen, U.L.; Bodemar, G.; Kechagias, S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2009, 44, 366–374. [Google Scholar] [CrossRef]
- Åberg, F.; Puukka, P.; Salomaa, V.; Männistö, S.; Lundqvist, A.; Valsta, L.; Perola, M.; Färkkilä, M.; Jula, A. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology 2020, 71, 835–848. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Joo, S.K.; Koo, B.K.; Lin, H.C.; Kim, W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2021, 41, 494–504. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Oshida, N.; Oh, S.; Okada, K.; Shoda, J. Progressive reduction in skeletal muscle mass to visceral fat area ratio is associated with a worsening of the hepatic conditions of non-alcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2019, 12, 495–503. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Joo, S.K.; Koo, B.K.; Lin, H.C.; Lee, D.H.; Chang, M.S.; Park, J.H.; So, Y.H.; Kim, W.; Innovative Target Exploration of NAFLD (ITEN) Consortium. Myosteatosis, but not Sarcopenia, Predisposes NAFLD Subjects to Early Steatohepatitis and Fibrosis Progression. Clin. Gastroenterol. Hepatol. 2023, 21, 388–397.e10. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Nicolosi, A.; Caviglia, G.P.; Abate, M.L.; Olivero, A.; D’Amato, D.; Vernero, M.; Gaggini, M.; Saracco, G.M.; et al. Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 635. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Martínez-Montoro, J.I.; Kaur, P.; Fernández-García, J.C.; Ramos-Molina, B. Non-alcoholic fatty liver disease-related fibrosis and sarcopenia: An altered liver-muscle crosstalk leading to increased mortality risk. Ageing Res. Rev. 2022, 80, 101696. [Google Scholar] [CrossRef]
- Zhu, W.; Sahar, N.E.; Javaid, H.M.A.; Pak, E.S.; Liang, G.; Wang, Y.; Ha, H.; Huh, J.Y. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 2021, 10, 3306. [Google Scholar] [CrossRef]
- Zhao, J.; Qiao, L.; Dong, J.; Wu, R. Antioxidant Effects of Irisin in Liver Diseases: Mechanistic Insights. Oxid. Med. Cell Longev. 2022, 2022, 3563518. [Google Scholar] [CrossRef]
- Oh, S.; So, R.; Shida, T.; Matsuo, T.; Kim, B.; Akiyama, K.; Isobe, T.; Okamoto, Y.; Tanaka, K.; Shoda, J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 43029. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, P.; Naimimohasses, S.; Monaghan, A.; Kennedy, M.; Melo, A.M.; Ní Fhloinn, D.; Doherty, D.G.; Beddy, P.; Finn, S.P.; Moore, J.B.; et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment. Pharmacol. Ther. 2020, 52, 1387–1398. [Google Scholar] [CrossRef]
- Chen, G.; Banini, B.; Do, A.; Lim, J.K. The independent effect of exercise on biopsy-proven non-alcoholic fatty liver disease: A systematic review. Clin. Mol. Hepatol. 2023, 29, 414–416. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Chen, D.; Le, T.H.; Shahidipour, H.; Read, S.A.; Ahlenstiel, G. The Role of Gut-Derived Microbial Antigens on Liver Fibrosis Initiation and Progression. Cells 2019, 8, 1324. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- de Faria Ghetti, F.; Oliveira, D.G.; de Oliveira, J.M.; de Castro Ferreira, L.E.V.V.; Cesar, D.E.; Moreira, A.P.B. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur. J. Nutr. 2018, 57, 861–876. [Google Scholar] [CrossRef]
- Bastian, W.P.; Hasan, I.; Lesmana, C.R.A.; Rinaldi, I.; Gani, R.A. Gut Microbiota Profiles in Nonalcoholic Fatty Liver Disease and Its Possible Impact on Disease Progression Evaluated with Transient Elastography: Lesson Learnt from 60 Cases. Case Rep. Gastroenterol. 2019, 13, 125–133. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, A.M.; Ruiz-Limón, P.; Salas-Salvadó, J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Atzeni, A.; Torres-Collado, L.; Álvarez-Sala, A.; et al. Gut microbiota in nonalcoholic fatty liver disease: A PREDIMED-Plus trial sub analysis. Gut Microbes. 2023, 15, 2223339. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Lv, J.; Yu, C.; Deng, Y.; Yin, J.; Wang, B.; Li, L.; Liu, H. Association between metabolically healthy obesity and non-alcoholic fatty liver disease. Hepatol. Int. 2022, 16, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Sabatini, S.; Gaggini, M.; Carli, F.; Rosso, C.; Positano, V.; Armandi, A.; Caviglia, G.P.; Faletti, R.; Bugianesi, E.; et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 2022, 42, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Conde, M.; Llop, E.; Carrillo, C.F.; Tormo, B.; Abad, J.; Rodriguez, L.; Perelló, C.; Gomez, M.L.; Martínez-Porras, J.L.; Puga, N.F.; et al. Estimation of visceral fat is useful for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 6658–6668. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chang, Y.; Cho, Y.K.; Ahn, J.; Shin, H.; Ryu, S. Metabolically healthy versus unhealthy obesity and risk of fibrosis progression in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chang, Y.; Cho, Y.K.; Ahn, J.; Shin, H.; Ryu, S. Obesity and Weight Gain Are Associated with Progression of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 543–550.e2. [Google Scholar] [CrossRef] [PubMed]
- Julián, M.T.; Ballesta, S.; Pera, G.; Pérez-Montes de Oca, A.; Soldevila, B.; Caballería, L.; Morillas, R.; Expósito, C.; Martínez-Escudé, A.; Puig-Domingo, M.; et al. Abdominal obesity and dsyglycemia are risk factors for liver fibrosis progression in NAFLD subjects: A population-based study. Front. Endocrinol. 2023, 13, 1051958. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Jebb, S.A.; Tomlinson, J.W.; Cobbold, J.F.; Aveyard, P. Association of Weight Changes with Changes in Histological Features and Blood Markers in Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2022, 20, e538–e547. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V.; LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Adams, L.A.; Sanderson, S.; Lindor, K.D.; Angulo, P. The histological course of nonalcoholic fatty liver disease: A longitudinal study of 103 patients with sequential liver biopsies. J. Hepatol. 2005, 42, 132–138. [Google Scholar] [CrossRef]
- Wajsbrot, N.B.; Leite, N.C.; Franca, P.H.C.; Cardoso, C.R.L.; Salles, G.F.; Villela-Nogueira, C.A. Parental History of Type 2 Diabetes Mellitus and PNPLA3 Polymorphism Increase the Risk of Severe Stages of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2023, 69, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; van der Schouw, Y.T.; Stolk, R.P.; Spijkerman, A.; van der, A.D.L.; Navis, G.; Bakker, S.J.; Beulens, J.W. Parental history of type 2 diabetes and cardiometabolic biomarkers in offspring. Eur. J. Clin. Investig. 2012, 42, 974–982. [Google Scholar] [CrossRef]
- De Pergola, G.; Castellana, F.; Zupo, R.; De Nucci, S.; Panza, F.; Castellana, M.; Lampignano, L.; Di Chito, M.; Triggiani, V.; Sardone, R.; et al. A family history of type 2 diabetes as a predictor of fatty liver disease in diabetes-free individuals with excessive body weight. Sci. Rep. 2021, 11, 24084. [Google Scholar] [CrossRef]
- Schiaffini, R.; Liccardo, D.; Alisi, A.; Benevento, D.; Cappa, M.; Cianfarani, S.; Nobili, V. Early Glucose Derangement Detected by Continuous Glucose Monitoring and Progression of Liver Fibrosis in Nonalcoholic Fatty Liver Disease: An Independent Predictive Factor? Horm. Res. Paediatr. 2016, 85, 29–34. [Google Scholar] [CrossRef]
- Hashiba, M.; Ono, M.; Hyogo, H.; Ikeda, Y.; Masuda, K.; Yoshioka, R.; Ishikawa, Y.; Nagata, Y.; Munekage, K.; Ochi, T.; et al. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PLoS ONE 2013, 8, e76161. [Google Scholar] [CrossRef]
- Chang, X.; Bian, H.; Xia, M.; Zhu, X.; Sun, X.; Yang, X.; Gao, J.; Lin, H.; Yan, H.; Gao, X. Postprandial glucose is correlated with an increasing risk of liver fibrosis in Chinese patients with nonalcoholic fatty liver disease. Diabetes Metab. 2022, 48, 101377. [Google Scholar] [CrossRef] [PubMed]
- Sako, S.; Takeshita, Y.; Takayama, H.; Goto, H.; Nakano, Y.; Ando, H.; Tsujiguchi, H.; Yamashita, T.; Arai, K.; Kaneko, S.; et al. Trajectories of Liver Fibrosis and Gene Expression Profiles in Nonalcoholic Fatty Liver Disease Associated with Diabetes. Diabetes 2023, 72, 1297–1306. [Google Scholar] [CrossRef]
- Khairnar, R.; Islam, M.A.; Fleishman, J.; Kumar, S. Shedding light on non-alcoholic fatty liver disease: Pathogenesis, molecular mechanisms, models, and emerging therapeutics. Life Sci. 2023, 312, 121185. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef]
- Masuda, K.; Noguchi, S.; Ono, M.; Ochi, T.; Munekage, K.; Okamoto, N.; Suganuma, N.; Saibara, T. High fasting insulin concentrations may be a pivotal predictor for the severity of hepatic fibrosis beyond the glycemic status in non-alcoholic fatty liver disease patients before development of diabetes mellitus. Hepatol. Res. 2017, 47, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, N.; Noureddin, M.; Singh, A.; Alkhouri, N. Progression of Nonalcoholic Fatty Liver Disease-Associated Fibrosis in a Large Cohort of Patients with Type 2 Diabetes. Dig. Dis. Sci. 2022, 67, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Perlemuter, G.; Bonvoust, F.; Dargere, D.; Parfait, B.; Vidaud, M.; Conti, M.; Huet, S.; Ba, N.; Buffet, C.; et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: A potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001, 34 Pt 1, 738–744. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Ridolfi, F.; Di Sario, A.; Casini, A.; Marucci, L.; Gaggiotti, G.; Orlandoni, P.; Macarri, G.; Perego, L.; Benedetti, A.; et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: Differential effects on signal transduction pathways. Hepatology 1999, 29, 1743–1751. [Google Scholar] [CrossRef]
- Carbone, L.J.; Angus, P.W.; Yeomans, N.D. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 23–31. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic Steatohepatitis: A metaanalysis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Mikami, S.; Haruki, U.; Yoshikata, K.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; et al. Antifibrotic effect and long-term outcome of SGLT2 inhibitors in patients with NAFLD complicated by diabetes mellitus. Hepatol. Commun. 2022, 6, 3073–3082. [Google Scholar] [CrossRef]
- Thong, V.D.; Quynh, B.T.H. Correlation of Serum Transaminase Levels with Liver Fibrosis Assessed by Transient Elastography in Vietnamese Patients with Nonalcoholic Fatty Liver Disease. Int. J. Gen. Med. 2021, 14, 1349–1355. [Google Scholar] [CrossRef]
- Pais, R.; Rusu, E.; Ratziu, V. The impact of obesity and metabolic syndrome on chronic hepatitis B and drug-induced liver disease. Clin. Liver Dis. 2014, 18, 165–178. [Google Scholar] [CrossRef]
- Bessone, F.; Dirchwolf, M.; Rodil, M.A.; Razori, M.V.; Roma, M.G. Review article: Drug-induced liver injury in the context of nonalcoholic fatty liver disease—A physiopathological and clinical integrated view. Aliment. Pharmacol. Ther. 2018, 48, 892–913. [Google Scholar] [CrossRef]
- Allard, J.; Le Guillou, D.; Begriche, K.; Fromenty, B. Drug-induced liver injury in obesity and nonalcoholic fatty liver disease. Adv. Pharmacol. 2019, 85, 75–107. [Google Scholar]
- Massart, J.; Begriche, K.; Moreau, C.; Fromenty, B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J. Clin. Transl. Res. 2017, 3 (Suppl. S1), 212–232. [Google Scholar]
- Cernea, S.; Cahn, A.; Raz, I. Pharmacological management of nonalcoholic fatty liver disease in type 2 diabetes. Expert. Rev. Clin. Pharmacol. 2017, 10, 535–547. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of non-alcoholic fatty liver disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef]
- Prikhodko, V.A.; Bezborodkina, N.N.; Okovityi, S.V. Pharmacotherapy for Non-Alcoholic Fatty Liver Disease: Emerging Targets and Drug Candidates. Biomedicines 2022, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Liu, S.; Yang, M. Treatment of liver fibrosis: Past, current, and future. World J. Hepatol. 2023, 15, 755–774. [Google Scholar] [CrossRef]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients with Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- A Study of Tirzepatide (LY3298176) in Participants with Nonalcoholic Steatohepatitis (NASH) (SYNERGY-NASH). Available online: https://clinicaltrials.gov/study/NCT04166773?term=NCT04166773&rank=1 (accessed on 10 February 2024).
- Dapagliflozin Efficacy and Action in NASH (DEAN). Available online: https://clinicaltrials.gov/study/NCT03723252?term=NCT03723252&rank=1 (accessed on 10 February 2024).
- A Phase 3 Study to Evaluate the Efficacy and Safety of MGL-3196 (Resmetirom) in Patients with NASH and Fibrosis (MAESTRO-NASH). Available online: https://clinicaltrials.gov/study/NCT03900429?term=NCT03900429&rank=1 (accessed on 10 February 2024).
- A Clinical Study to Evaluate the Efficacy and Safety of Aramchol in Subjects with NASH (ARMOR) (ARMOR). Available online: https://clinicaltrials.gov/study/NCT04104321?term=NCT04104321&rank=1 (accessed on 10 February 2024).
- Ratziu, V.; Yilmaz, Y.; Lazas, D.; Friedman, S.L.; Hayardeny, L.; Kadosh, S.; Gorfine, T.; Sanya, A.J. New Data on Aramchol™: Efficacy of a Higher Daily Exposure to Aramchol on Fibrosis Improvement in the ARMOR Study Open-Label Part. EMJ Hepatol. 2022, 10 (Suppl. S1), 2–8. [Google Scholar]
- Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of BFKB8488A Compared with Placebo in Participants with Non-Alcoholic Steatohepatitis (BANFF). Available online: https://clinicaltrials.gov/study/NCT04171765?term=NCT04171765&rank=1 (accessed on 10 February 2024).
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Dasatinib and Quercetin to Treat Fibrotic Non-alcoholic Fatty Liver Disease. Available online: https://clinicaltrials.gov/study/NCT05506488?term=NCT05506488&rank=1 (accessed on 11 February 2024).
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar] [CrossRef]
- Tacke, F.; Weiskirchen, R. Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: Mechanisms, treatment and prevention. Ann. Transl. Med. 2021, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Gilgenkrantz, H.; Mallat, A.; Moreau, R.; Lotersztajn, S. Targeting cell-intrinsic metabolism for antifibrotic therapy. J. Hepatol. 2021, 74, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, G.; Afarin, R.; Bavarsad, S.S.; Aslani, F.; Zadeh, S.A.; Shakerian, E. Comparison of the effects of cholesterol, palmitic acid, and glucose on activation of human hepatic stellate cells to induce liver fibrosis. J. Diabetes Metab. Disord. 2022, 21, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Enjoji, M.; Kohjima, M.; Tsuruta, S.; Fukushima, M.; Iwao, M.; Sonta, T.; Kotoh, K.; Inoguchi, T.; Nakamuta, M. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int. 2005, 25, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.K.; Liu, Y.C.; Ma, G.; Shi, L.L.; He, X.M. High levels of glucose promote the activation of hepatic stellate cells via the p38-mitogen-activated protein kinase signal pathway. Genet. Mol. Res. 2016, 15, gmr.15038419. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Wang, S.; Friedman, S.L. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021, 33, 242–257. [Google Scholar] [CrossRef]
- Hernández-Gea, V.; Friedman, S.L. Autophagy fuels tissue fibrogenesis. Autophagy 2012, 8, 849–850. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Subramanian, S.; Chait, A.; Haigh, W.G.; Yeh, M.M.; Farrell, G.C.; Lee, S.P.; Savard, C. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 2017, 58, 1067–1079. [Google Scholar] [CrossRef]
- Leroux, A.; Ferrere, G.; Godie, V.; Cailleux, F.; Renoud, M.L.; Gaudin, F.; Naveau, S.; Prévot, S.; Makhzami, S.; Perlemuter, G.; et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J. Hepatol. 2012, 57, 141–149. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
| Drug Name/ Mechanism of Action | Study/Clinical Trial Ref. | Study Population/Number | Primary Objective | Main Results (If Available) |
|---|---|---|---|---|
| Monotherapy | ||||
| Pioglitazone/PPAR-γ agonist; insulin sensitizer | RCT (pioglitazone 45 mg/day vs. pbo), NCT00994682 [177] | T2DM/prediabetes and biopsy-proven NASH (n = 101) | Reduction of ≥2 NAS points in two histologic categories without worsening of fibrosis. | Primary outcome: TD: 41 [23; 59] percentage points; p < 0.001. Additional outcome: mean change in fibrosis score: TD: −0.5 [−0.9; 0.0]; p = 0.039. |
| Lanifibranor/pan-PPAR agonist | RCT (lanifibranor 1200 mg/800 mg per day or pbo), NCT03008070 [178] | Non-cirrhotic, highly active NASH (n = 247) | Decrease of ≥2 points in SAF-A score without worsening of fibrosis. | Primary outcome: RR: 1.7 [1.2; 2.3]; p = 0.007 (1200 mg dose vs. pbo); RR: 1.5 [1.0; 2.1]; p = 0.07 (800 mg dose vs. pbo). Additional outcome: Improvement in fibrosis stage of ≥1 without worsening of NASH: RR: 1.7 [1.2; 2.5] (1200 mg dose vs. pbo); RR: 1.2 [0.7; 1.9] (800 mg dose vs. pbo). |
| Liraglutide/ GLP-1 RA | RCT (liraglutide 1.8 mg/day vs. pbo), NCT01237119 [158] | Biopsy-proven NASH (n = 52; 17 with T2DM) | Resolution of definite NASH with no worsening of fibrosis. | Primary outcome: RR: 4.3 [1.0; 17.7]; p = 0.019. Additional results: patients with worsening of fibrosis: mean change from baseline vs. pbo: 0.2 [0.1; 1.0]; p = 0.04. |
| Tirzepatide/ Dual GLP-1 and GIP RA | RCT (tirzepatide 5 mg/10 mg/15 mg per wk vs. pbo), NCT04166773 [179] | Biopsy-proven NASH, and stage F2/3 fibrosis, or without T2DM (n = 196 estimated) | Percentage of participants with absence of NASH with no worsening of fibrosis. Secondary outcomes: percentage of participants with ≥1 point decrease in fibrosis stage with no worsening of NASH; percentage of participants with ≥1 point increase in fibrosis stage with no worsening of NASH. | N/A; ongoing; phase 2 |
| Dapagliflozin/ SGLT 2 inhibitors | RCT (dapagliflozin 10 mg/day vs. pbo), NCT03723252 [180] | Biopsy-proven NASH (n = 148 estimated) | Improvement in scored liver histology over 12 months. Secondary outcome: change in fibrosis score. | N/A; ongoing; phase 3 |
| Resmetirom/ THR β-selective agonist | RCT (resmetirom (MGL-3196) 80 mg/100 mg per day vs. pbo), NCT03900429 [181] | NASH fibrosis (n = 1759 enrolled) | Proportion with resolution of NASH associated with ≥2-point reduction in NAS without worsening of fibrosis stage OR proportion with ≥1-point improvement in fibrosis stage with no worsening of NAS. | N/A; ongoing; phase 3 |
| Aramchol/ partial inhibitor of hepatic SCD1 | RCT (aramchol 300 mg BID or pbo), double-blind and open-label; NCT04104321 [182,183] | Biopsy-proven NASH with fibrosis stage F2/3, overweight/obesity, and prediabetes, or T2DM (for double-blind) (150 for open-label; n = 2000 estimated for double-blind) | Proportion of subjects with improvement in liver fibrosis ≥1 and no worsening of NASH. Proportion of subjects with resolution of NASH and no worsening of liver fibrosis. | Open-label interim analysis: Primary outcome: 60.0% had fibrosis improvement of ≥1 stage (of first 20 patients). N/A; phase 3 |
| BFKB8488A/ FGF receptor 1/Klothoβ agonist | RCT (individualized or fixed doses of BFKB8488A vs. pbo), NCT04171765 [184] | Biopsy-proven NASH with stage F2/3 fibrosis, and liver fat ≥8% | Proportion of participants with NASH resolution without worsening of fibrosis. Secondary outcome: proportion of participants with improvement in liver fibrosis of ≥1 stage and no worsening of NASH. | N/A; phase 2 |
| Efruxifermin/ Fc-FGF21 fusion protein; FGF receptor agonist | RCT (efruxifermin 28 mg/50 mg/70 mg per wk vs. pbo), NCT03976401 [185] | Biopsy-proven NASH and ≥10% liver fat content (n = 80) | Absolute change from baseline in HFF measured by magnetic resonance imaging. Secondary outcomes: change from baseline in liver stiffness; change from baseline in non-invasive biomarkers including liver fibrosis. | Primary outcome: absolute changes in HFF: −12.3%, −13.4%, and −14.1% (28, 50, and 70 mg) vs. 0.3% (pbo); p < 0.0001. Additional outcomes: reduction in ELF scores (p = 0.0008 (28 mg), p = 0.0005 (50 mg), and p = 0.03 (70 mg) vs. pbo); 55% (across all efruxifermin arms with liver biopsies) had a fibrosis improvement of ≥1 stage (no statistical analysis vs. pbo). |
| Combination therapy | ||||
| Dasatinib and Quercetin/tyrosine kinase inhibitor and flavonoid | RCT (dasatinib (100 mg/day) plus quercetin (1000 mg/day) on three consecutive days for three consecutive wks), NCT05506488 [186] | Biopsy-proven NAFLD with stage >F2 fibrosis, but no cirrhosis (n = 30 estimated) | Improvement of fibrosis with at least 1-point without worsening of fibrosis and NAFLD score based on histology. | N/A; ongoing; phase 1, 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernea, S. NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic–Metabolic Interplay. Life 2024, 14, 272. https://doi.org/10.3390/life14020272
Cernea S. NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic–Metabolic Interplay. Life. 2024; 14(2):272. https://doi.org/10.3390/life14020272
Chicago/Turabian StyleCernea, Simona. 2024. "NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic–Metabolic Interplay" Life 14, no. 2: 272. https://doi.org/10.3390/life14020272
APA StyleCernea, S. (2024). NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic–Metabolic Interplay. Life, 14(2), 272. https://doi.org/10.3390/life14020272






