Current Infections of the Orofacial Region: Treatment, Diagnosis, and Epidemiology
Abstract
1. Introduction
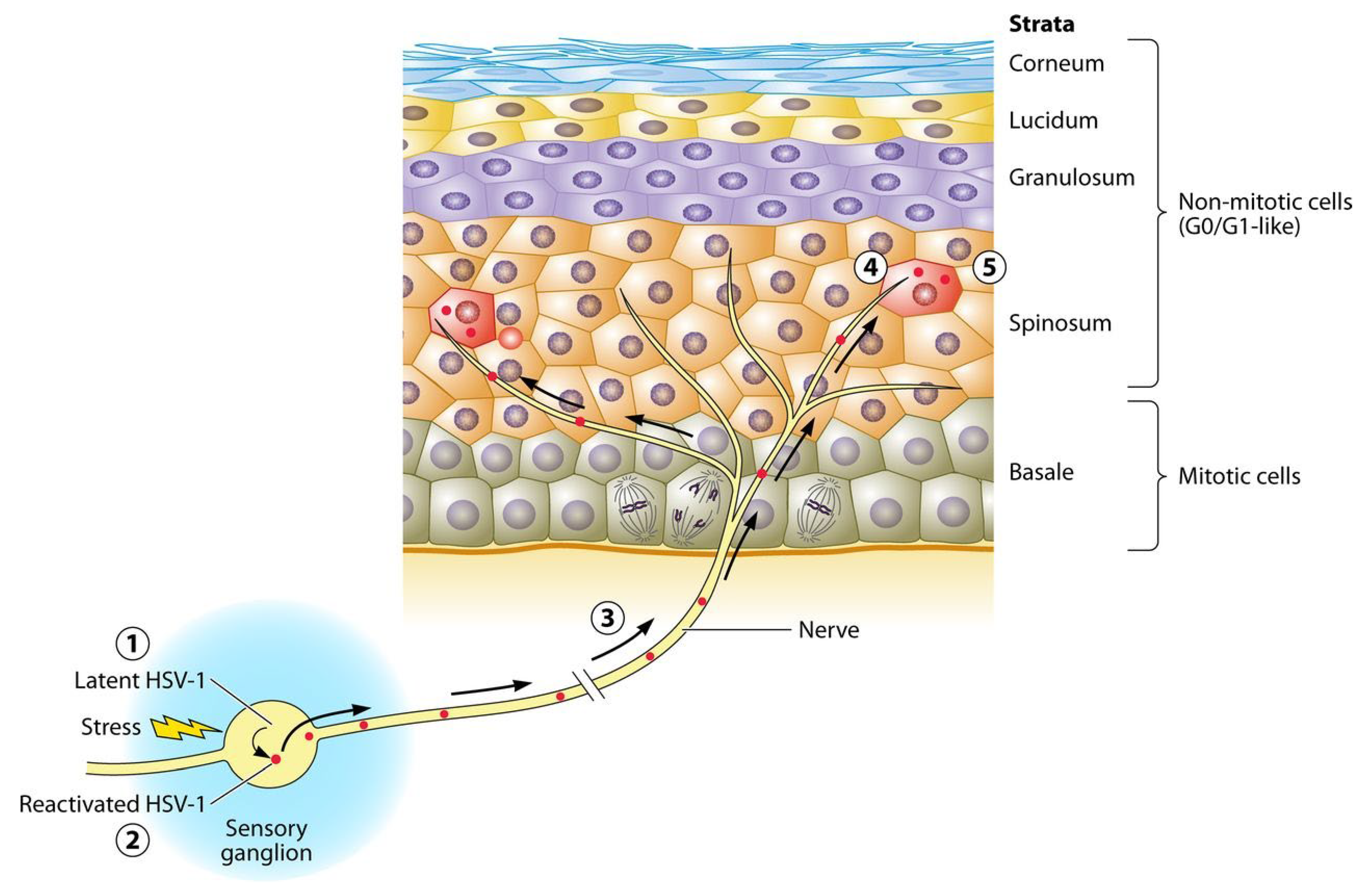
2. Herpes Simplex Virus
2.1. Epidemiology
2.2. Clinical Presentation
2.3. Diagnosis
2.4. Treatment
3. Human Papilloma Viruses
3.1. Epidemiology
3.2. Clinical Presentation
3.3. Risk Factors
3.4. Diagnosis
3.5. Treatment
4. Candida albicans
4.1. Epidemiology
4.2. Clinical Presentation
4.3. Risk Factors
4.4. Diagnosis
4.5. Treatment
5. Aspergillus
5.1. Epidemiology
5.2. Clinical Presentation
5.3. Diagnosis
5.4. Treatment
6. Actinomyces
6.1. Epidemiology
6.2. Risk Factors
6.3. Clinical Presentation
6.4. Diagnosis
6.5. Treatment
7. Streptococcus mutans
7.1. Epidemiology
7.2. Clinical Presentation
7.3. Diagnosis
7.4. Treatment
8. Streptococcus sanguinis
8.1. Epidemiology
8.2. Mechanism of Action
8.3. Clinical Importance
8.4. Diagnosis
9. Conclusions
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santosh, A.B.; Reddy, B.V. Oral Mucosal Infections: Insights into Specimen Collection and Medication Management. Dent. Clin. N. Am. 2017, 61, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Coll, P.P.; Lindsay, A.; Meng, J.; Gopalakrishna, A.; Raghavendra, S.; Bysani, P.; O’Brien, D. The Prevention of Infections in Older Adults: Oral Health. J. Am. Geriatr. Soc. 2020, 68, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, U.; Murthy, R.T.; Felicita, A.S.; Alshehri, A.; Awadh, W.; Almalki, A.; Vinothkumar, T.S.; Baeshen, H.A.; Bhandi, S.; Kathir, A.; et al. Sulfate-Reducing Bacteria in Patients Undergoing Fixed Orthodontic Treatment. Int. Dent. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Heboyan, A.; Avetisyan, A.; Skallevold, H.E.; Rokaya, D.; Marla, V.; Vardanyan, A. Occurrence of Recurrent Aphthous Stomatitis (RAS) as a Rare Oral Manifestation in a Patient with Gilbert’s Syndrome. Case Rep. Dent. 2021, 2021, 6648729. [Google Scholar] [CrossRef]
- Heboyan, A.; Karobari, M.I.; Marya, A. Possible oral manifestation after vaccination against COVID-19: A case report. Oxf. Med. Case Rep. 2022, 2022, omac136. [Google Scholar] [CrossRef]
- Heboyan, A.; Manrikyan, M.; Zafar, M.S.; Rokaya, D.; Nushikyan, R.; Vardanyan, I.; Vardanyan, A.; Khurshid, Z. Bacteriological Evaluation of Gingival Crevicular Fluid in Teeth Restored Using Fixed Dental Prostheses: An In Vivo Study. Int. J. Mol. Sci. 2021, 22, 5463. [Google Scholar] [CrossRef]
- Abbasi, K.; Tavakolizadeh, S.; Hadi, A.; Hosseini, M.; Soufdoost, R.S.; Heboyan, A.; Alam, M.; Fani-Hanifeh, S. The wound healing effect of collagen/adipose-derived stem cells (ADSCs) hydrogel: In vivo study. Vet. Med. Sci. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Dahlén, G. Bacterial infections of the oral mucosa. Periodontol. 2000 2009, 49, 13–38. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rahmani, A.; Tahmasebi, E.; Tebyanian, H.; Yazdanian, A.; Mosaddad, S.A. Current and advanced nanomaterials in dentistry as regeneration agents: An update. Mini-Rev. Med. Chem. 2021, 21, 899–918. [Google Scholar] [CrossRef]
- Ogle, O.E. Odontogenic Infections. Dent. Clin. N. Am. 2017, 61, 235–252. [Google Scholar] [CrossRef]
- Karobari, M.I.; Siddharthan, S.; Adil, A.H.; Khan, M.M.; Venugopal, A.; Rokaya, D.; Heboyan, A.; Marya, C.M.; Marya, A. Modifiable and non-modifiable risk factors affecting oral and periodontal health and quality of life in south asia. Open Dent. J. 2022, 16, e187421062209270. [Google Scholar] [CrossRef]
- Marya, A.; Rokaya, D.; Heboyan, A.; Fernandes, G.V. Biomolecular and Biochemical Aspects of the Oral Cavity. Molecules 2022, 27, 8676. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Karobari, M.I.; Heboyan, A.; Mohamed, R.N.; Mustafa, M.; Basheer, S.N.; Desai, V.; Batool, S.; Ahmed, N.; Zeshan, B. Synthesis of Silver Nanoparticles from Extracts of Wild Ginger (Zingiber zerumbet) with Antibacterial Activity against Selective Multidrug Resistant Oral Bacteria. Molecules 2022, 27, 2007. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Heboyan, A.; Zafar, M.S.; Khurshid, Z.; Marya, A.; Fernandes, G.V.O.; Rokaya, D. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J. Funct. Biomater. 2022, 13, 15. [Google Scholar] [CrossRef]
- Aurelius, E.; Franzen-Röhl, E.; Glimåker, M.; Akre, O.; Grillner, L.; Jorup-Rönström, C.; Studahl, M.; Group, H.-M.S. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: A double-blind, randomized controlled trial. Clin. Infect. Dis. 2012, 54, 1304–1313. [Google Scholar] [CrossRef]
- Schang, L.M. Timing Is Everything. mBio 2018, 9, e02140-17. [Google Scholar] [CrossRef]
- Whitley, R.J. Herpes Simplex Virus Infections of the Central Nervous System. Continuum 2015, 21, 1704–1713. [Google Scholar] [CrossRef]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochem. Biokhimiia 2014, 79, 1635–1652. [Google Scholar] [CrossRef]
- Pires de Mello, C.P.; Bloom, D.C.; Paixão, I.C. Herpes simplex virus type-1: Replication, latency, reactivation and its antiviral targets. Antivir. Ther. 2016, 21, 277–286. [Google Scholar] [CrossRef]
- Levin, M.J.; Weinberg, A.; Schmid, D.S. Herpes Simplex Virus and Varicella-Zoster Virus. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Khadr, L.; Harfouche, M.; Omori, R.; Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J. The Epidemiology of Herpes Simplex Virus Type 1 in Asia: Systematic Review, Meta-analyses, and Meta-regressions. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Betz, D.; Fane, K. Herpetic Whitlow. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Damour, A.; Garcia, M.; Seneschal, J.; Lévêque, N.; Bodet, C. Eczema Herpeticum: Clinical and Pathophysiological Aspects. Clin. Rev. Allergy Immunol. 2020, 59, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sánchez, M. Genital ulcers caused by herpes simplex virus. Enferm. Infecc. Y Microbiol. Clin. 2019, 37, 260–264. [Google Scholar] [CrossRef]
- Corstjens, P.L.; Abrams, W.R.; Malamud, D. Saliva and viral infections. Periodontol. 2000 2016, 70, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, R.; Kuperstein, A.S.; Stoopler, E.T. Update on oral herpes virus infections. Dent. Clin. N. Am. 2014, 58, 265–280. [Google Scholar] [CrossRef]
- Samies, N.L.; James, S.H. Prevention and treatment of neonatal herpes simplex virus infection. Antivir. Res. 2020, 176, 104721. [Google Scholar] [CrossRef]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef]
- Perti, T.; Saracino, M.; Baeten, J.M.; Johnston, C.; Diem, K.; Ocbamichael, N.; Huang, M.L.; Selke, S.; Magaret, A.; Corey, L.; et al. High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: A randomized crossover trial. J. Acquir. Immune Defic. Syndr. 2013, 63, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, N.; Geisler, W.M.; Bachmann, L.H.; Hook, E.W. The effect of valacyclovir on HIV and HSV-2 in HIV-infected persons on antiretroviral therapy with previously unrecognised HSV-2. Int. J. STD AIDS 2015, 26, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Le Cleach, L.; Trinquart, L.; Do, G.; Maruani, A.; Lebrun-Vignes, B.; Ravaud, P.; Chosidow, O. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database Syst. Rev. 2014, 8, Cd009036. [Google Scholar] [CrossRef]
- Donatini, B. Control of oral human papillomavirus (HPV) by medicinal mushrooms, Trametes versicolor and Ganoderma lucidum: A preliminary clinical trial. Int. J. Med. Mushrooms 2014, 16, 497–498. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Timmler, B.; Magaret, A.; Warren, T.; Tyring, S.; Johnston, C.; Fife, K.; Selke, S.; Huang, M.L.; Stobernack, H.P.; et al. Effect of Pritelivir Compared With Valacyclovir on Genital HSV-2 Shedding in Patients With Frequent Recurrences: A Randomized Clinical Trial. JAMA 2016, 316, 2495–2503. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, L.; Wang, Y.; Wang, Y.; Wang, Q.; Song, L.; Li, Y.; Huang, K.; Du, X.; Fan, W.; et al. A randomized open-label clinical trial of an anti-HPV biological dressing (JB01-BD) administered intravaginally to treat high-risk HPV infection. Microbes Infect. 2016, 18, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Tyring, S.K.; Lee, P.; Hill, G.T., Jr.; Silverfield, J.C.; Moore, A.Y.; Matkovits, T.; Sullivan-Bolyai, J. FV-100 versus valacyclovir for the prevention of post-herpetic neuralgia and the treatment of acute herpes zoster-associated pain: A randomized-controlled trial. J. Med. Virol. 2017, 89, 1255–1264. [Google Scholar] [CrossRef]
- Van Wagoner, N.; Fife, K.; Leone, P.A.; Bernstein, D.I.; Warren, T.; Panther, L.; Novak, R.M.; Beigi, R.; Kriesel, J.; Tyring, S.; et al. Effects of Different Doses of GEN-003, a Therapeutic Vaccine for Genital Herpes Simplex Virus-2, on Viral Shedding and Lesions: Results of a Randomized Placebo-Controlled Trial. J. Infect. Dis. 2018, 218, 1890–1899. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad, S.S.; Keyvani, H.; Ghobadi, N. Comparison of Acyclovir and Multistrain Lactobacillus brevis in Women with Recurrent Genital Herpes Infections: A Double-Blind, Randomized, Controlled Study. Probiotics Antimicrob. Proteins 2018, 10, 740–747. [Google Scholar] [CrossRef]
- Nofal, A.; Marei, A.; Ibrahim, A.M.; Nofal, E.; Nabil, M. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J. Am. Acad. Dermatol. 2020, 82, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Nayebzadeh, M.; Peyman, M.; Afshari, N.A.; Pourazizi, M. Topical cyclosporine-A versus prednisolone for herpetic stromal keratitis: A randomized controlled trial. Acta Ophthalmol. 2019, 97, e194–e198. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, N.; Giampaolino, P.; Lavitola, G.; Morra, I.; Formisano, C.; Nappi, C.; Bifulco, G. Effect of Immunomodulatory Supplements Based on Echinacea Angustifolia and Echinacea Purpurea on the Posttreatment Relapse Incidence of Genital Condylomatosis: A Prospective Randomized Study. BioMed. Res. Int. 2019, 2019, 3548396. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, Y.L.; Duan, S.M.; Zhan, S.B.; Guan, R.L.; Yue, T.F.; Kong, L.H.; Zhou, L.; Deng, L.H.; Huang, C.; et al. REBACIN® as a noninvasive clinical intervention for high-risk human papillomavirus persistent infection. Int. J. Cancer 2019, 145, 2712–2719. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Raboud, J.M.; Szadkowski, L.; Grinsztejn, B.; Madruga, J.V.; Figueroa, M.I.; Cahn, P.; Barton, S.E.; Clarke, A.; Fox, J.; et al. Effect of valaciclovir on CD4 count decline in untreated HIV: An international randomized controlled trial. J. Antimicrob. Chemother. 2019, 74, 480–488. [Google Scholar] [CrossRef]
- Allen, C.T.; Lee, S.; Norberg, S.M.; Kovalovsky, D.; Ye, H.; Clavijo, P.E.; Hu-Lieskovan, S.; Schlegel, R.; Schlom, J.; Strauss, J.; et al. Safety and clinical activity of PD-L1 blockade in patients with aggressive recurrent respiratory papillomatosis. J. Immunother. Cancer 2019, 7, 119. [Google Scholar] [CrossRef]
- Breier, A.; Buchanan, R.W.; D’Souza, D.; Nuechterlein, K.; Marder, S.; Dunn, W.; Preskorn, S.; Macaluso, M.; Wurfel, B.; Maguire, G.; et al. Herpes simplex virus 1 infection and valacyclovir treatment in schizophrenia: Results from the VISTA study. Schizophr. Res. 2019, 206, 291–299. [Google Scholar] [CrossRef]
- Luyt, C.E.; Forel, J.M.; Hajage, D.; Jaber, S.; Cayot-Constantin, S.; Rimmelé, T.; Coupez, E.; Lu, Q.; Diallo, M.H.; Penot-Ragon, C.; et al. Acyclovir for Mechanically Ventilated Patients With Herpes Simplex Virus Oropharyngeal Reactivation: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 263–272. [Google Scholar] [CrossRef]
- Murray, M.L.; Meadows, J.; Doré, C.J.; Copas, A.J.; Haddow, L.J.; Lacey, C.; Jit, M.; Soldan, K.; Bennett, K.; Tetlow, M.; et al. Human papillomavirus infection: Protocol for a randomised controlled trial of imiquimod cream (5%) versus podophyllotoxin cream (0.15%), in combination with quadrivalent human papillomavirus or control vaccination in the treatment and prevention of recurrence of anogenital warts (HIPvac trial). BMC Med. Res. Methodol. 2018, 18, 125. [Google Scholar] [CrossRef]
- Syrjänen, S. Oral manifestations of human papillomavirus infections. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 49–66. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, K.L.; Vidhya, M. Human papilloma virus and oral infections: An update. J. Cancer Res. Ther. 2011, 7, 120–127. [Google Scholar] [CrossRef]
- Rettig, E.M.; D’Souza, G. Epidemiology of head and neck cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Lerman, M.A.; Almazrooa, S.; Lindeman, N.; Hall, D.; Villa, A.; Woo, S.B. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2017, 30, 1646–1654. [Google Scholar] [CrossRef]
- Antonsson, A.; Cornford, M.; Perry, S.; Davis, M.; Dunne, M.P.; Whiteman, D.C. Prevalence and risk factors for oral HPV infection in young Australians. PLoS ONE 2014, 9, e91761. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wong, J.; D’Souza, G.; Riemer, A.B.; Lorch, J.; Haddad, R.; Pai, S.I.; Longtine, J.; McClean, M.; LaBaer, J.; et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br. J. Cancer 2011, 104, 1896–1905. [Google Scholar] [CrossRef]
- Tezal, M.; Scannapieco, F.A.; Wactawski-Wende, J.; Hyland, A.; Marshall, J.R.; Rigual, N.R.; Stoler, D.L. Local inflammation and human papillomavirus status of head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 669–675. [Google Scholar] [CrossRef]
- Shipilova, A.; Dayakar, M.M.; Gupta, D. High risk human papillomavirus in the periodontium: A case control study. J. Indian Soc. Periodontol. 2017, 21, 380–385. [Google Scholar] [CrossRef]
- Chai, R.C.; Lambie, D.; Verma, M.; Punyadeera, C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015, 4, 596–607. [Google Scholar] [CrossRef]
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Seifi Kafshgari, H.; Yazdanian, M.; Ranjbar, R.; Tahmasebi, E.; Mirsaeed, S.; Tebyanian, H.; Ebrahimzadeh, M.A.; Goli, H.R. The effect of Citrullus colocynthis extracts on Streptococcus mutans, Candida albicans, normal gingival fibroblast and breast cancer cells. J. Biol. Res. 2019, 92, 8201. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and white manifestations in the oral cavity. Head Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef]
- Hood, S.; Denning, D. Treatment of fungal infection in AIDS. J. Antimicrob. Chemother. 1996, 37, 71–85. [Google Scholar] [CrossRef]
- Reinhardt, L.; Nascente, P.; Ribeiro, J.; Guimarães, V.; Etges, A.; Lund, R. Sensitivity to antifungals by Candida spp samples isolated from cases of chronic atrophic candidiasis (CAC). Braz. J. Biol. 2020, 80, 266–272. [Google Scholar] [CrossRef]
- Benito-Cruz, B.; Aranda-Romo, S.; López-Esqueda, F.J.; de la Rosa-García, E.; Rosas-Hernández, R.; Sánchez-Vargas, L.O. Oral Candida isolates and fluconazole susceptibility patterns in older Mexican women. Arch. Gerontol. Geriatr. 2016, 65, 204–210. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Watthanasaen, S.; Merchant, A.T.; Luengpailin, S.; Chansamak, N.; Pisek, A.; Pitiphat, W. Xylitol-containing Chewing Gum for Caries Prevention in Students with Disabilities: A Randomised Trial. Oral Health Prev. Dent. 2017, 15, 519–527. [Google Scholar] [CrossRef]
- Cabras, M.; Gambino, A.; Broccoletti, R.; Lodi, G.; Arduino, P.G. Treatment of angular cheilitis: A narrative review and authors’ clinical experience. Oral Dis. 2020, 26, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O′Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Awotiwon, A.A.; Johnson, S.; Rutherford, G.W.; Meintjes, G.; Eshun-Wilson, I. Primary antifungal prophylaxis for cryptococcal disease in HIV-positive people. Cochrane Database Syst. Rev. 2018, 8, Cd004773. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Shackleton, J.-A.; Coogan, M.M.; Galpin, J. Antifungal effect of mouth rinses on oral Candida counts and salivary flow in treatment-naïve HIV-infected patients. AIDS Patient Care STDs 2008, 22, 613–618. [Google Scholar] [CrossRef]
- Arya, N.R.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Custodio, W.; Silva, W.J.; Paes Leme, A.F.; Cury, J.A.; Del Bel Cury, A.A. Plasma proteins in the acquired denture pellicle enhance substrate surface free energy and Candida albicans phospholipase and proteinase activities. J. Investig. Clin. Dent. 2015, 6, 273–281. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Gacon, I.; Loster, J.E.; Wieczorek, A. Relationship between oral hygiene and fungal growth in patients: Users of an acrylic denture without signs of inflammatory process. Clin. Interv. Aging 2019, 14, 1297–1302. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Mayer, M.P.; Miyazima, T.Y.; Matsubara, V.H.; Silva, E.G.; Paula, C.R.; Campos, T.T.; Nakamae, A.E. A multispecies probiotic reduces oral Candida colonization in denture wearers. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2015, 24, 194–199. [Google Scholar] [CrossRef]
- Vento-Zahra, E.; De Wever, B.; Decelis, S.; Mallia, K.; Camilleri, S. Randomized, double-blind, placebo-controlled trial to test the efficacy of nitradine tablets in maxillary removable orthodontic appliance patients. Quintessence Int. 2011, 42, 37–43. [Google Scholar]
- Voskamp, A.L.; Gillman, A.; Symons, K.; Sandrini, A.; Rolland, J.M.; O’Hehir, R.E.; Douglass, J.A. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. Pract. 2015, 3, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kraft-Bodi, E.; Jørgensen, M.R.; Keller, M.K.; Kragelund, C.; Twetman, S. Effect of Probiotic Bacteria on Oral Candida in Frail Elderly. J. Dent. Res. 2015, 94, 181s–186s. [Google Scholar] [CrossRef] [PubMed]
- Colombari, B.; Tagliazucchi, D.; Odorici, A.; Pericolini, E.; Foltran, I.; Pinetti, D.; Meto, A.; Peppoloni, S.; Blasi, E. Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release. Int. J. Environ. Res. Public Health 2022, 19, 4146. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A.; Weerakoon, H.S.; de Silva, S.H. Randomized, double-blind, comparative study on efficacy and safety of itraconazole pulse therapy and terbinafine pulse therapy on nondermatophyte mold onychomycosis: A study with 90 patients. J. Dermatol. Treat. 2016, 27, 364–372. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Sushma, R.; Sathe, T.T.; Farias, A.; Sanyal, P.K.; Kiran, S. “Nature cures:” An alternative herbal formulation as a denture cleanser. Ann. Afr. Med. 2017, 16, 6–12. [Google Scholar] [CrossRef]
- Atai, Z.; Atai, M.; Amini, J.; Salehi, N. In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. J. Oral Sci. 2017, 59, 425–430. [Google Scholar] [CrossRef]
- Miyazima, T.Y.; Ishikawa, K.H.; Mayer, M.; Saad, S.; Nakamae, A. Cheese supplemented with probiotics reduced the Candida levels in denture wearers-RCT. Oral. Dis. 2017, 23, 919–925. [Google Scholar] [CrossRef]
- Sandison, T.; Ong, V.; Lee, J.; Thye, D. Safety and Pharmacokinetics of CD101 IV, a Novel Echinocandin, in Healthy Adults. Antimicrob. Agents Chemother. 2017, 61, e01627-16. [Google Scholar] [CrossRef]
- Keller, M.K.; Kragelund, C. Randomized pilot study on probiotic effects on recurrent candidiasis in oral lichen planus patients. Oral. Dis. 2018, 24, 1107–1114. [Google Scholar] [CrossRef]
- Mofatteh, M.R.; Naseripour Yazdi, Z.; Yousefi, M.; Namaei, M.H. Comparison of the recovery rate of otomycosis using betadine and clotrimazole topical treatment. Braz. J. Otorhinolaryngol. 2018, 84, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Dhooria, S.; Singh Sehgal, I.; Aggarwal, A.N.; Garg, M.; Saikia, B.; Behera, D.; Chakrabarti, A. A Randomized Trial of Itraconazole vs Prednisolone in Acute-Stage Allergic Bronchopulmonary Aspergillosis Complicating Asthma. Chest 2018, 153, 656–664. [Google Scholar] [CrossRef]
- de Senna, A.M.; Vieira, M.M.F.; Machado-de-Sena, R.M.; Bertolin, A.O.; Núñez, S.C.; Ribeiro, M.S. Photodynamic inactivation of Candida ssp. on denture stomatitis. A clinical trial involving palatal mucosa and prosthesis disinfection. Photodiagnosis Photodyn. Ther. 2018, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Viscoli, C.; Pappas, P.G.; Vazquez, J.; Ostrosky-Zeichner, L.; Rotstein, C.; Sobel, J.D.; Herbrecht, R.; Rahav, G.; Jaruratanasirikul, S.; et al. Isavuconazole Versus Caspofungin in the Treatment of Candidemia and Other Invasive Candida Infections: The ACTIVE Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 1981–1989. [Google Scholar] [CrossRef]
- Saeed, A.; Haider, A.; Zahid, S.; Khan, S.A.; Faryal, R.; Kaleem, M. In-vitro antifungal efficacy of tissue conditioner-chitosan composites as potential treatment therapy for denture stomatitis. Int. J. Biol. Macromol. 2019, 125, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Afroozi, B.; Zomorodian, K.; Lavaee, F.; Zare Shahrabadi, Z.; Mardani, M. Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagnosis Photodyn. Ther. 2019, 27, 193–197. [Google Scholar] [CrossRef]
- de Souza, R.F.; Silva-Lovato, C.H.; de Arruda, C.N.; Regis, R.R.; Zanini, A.P.; Longo, D.L.; Peracini, A.; de Andrade, I.M.; Watanabe, E.; Paranhos, H.F. Efficacy of a propolis solution for cleaning complete dentures. Am. J. Dent. 2019, 32, 306–310. [Google Scholar] [PubMed]
- Mustafa, M.W.; Ungphaiboon, S.; Phadoongsombut, N.; Pangsomboon, K.; Chelae, S.; Mahattanadul, S. Effectiveness of an Alcohol-Free Chitosan-Curcuminoid Mouthwash Compared with Chlorhexidine Mouthwash in Denture Stomatitis Treatment: A Randomized Trial. J. Altern. Complement. Med. 2019, 25, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, H.; Kaneda, T.; Kida, Y.; Kaneko, M.; Fujii, H.; Hayashi, M.; Tomii, K.; Tada, K.; Suzuki, Y.; Karino, T. [An open, noncomparative multicenter study of the efficacy and safety of itraconazole injections and high dose capsules in chronic pulmonary aspergillosis]. Kansenshogaku Zasshi J. Jpn. Assoc. Infect. Dis. 2011, 85, 644–651. [Google Scholar] [CrossRef][Green Version]
- Mammen, M.P.; Armas, D.; Hughes, F.H.; Hopkins, A.M.; Fisher, C.L.; Resch, P.A.; Rusalov, D.; Sullivan, S.M.; Smith, L.R. First-in-Human Phase 1 Study To Assess Safety, Tolerability, and Pharmacokinetics of a Novel Antifungal Drug, VL-2397, in Healthy Adults. Antimicrob. Agents Chemother. 2019, 63, e00969-19. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagnosis Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef]
- Tasso, C.O.; de Oliveira Zoccolotti, J.; Ferrisse, T.M.; Malavolta, I.F.; Jorge, J.H. Effectiveness of Disinfectant Liquid Soaps in the Reduction of Candida spp Present in Complete Dentures: A Crossover Randomized Clinical Trial. Int. J. Prosthodont. 2020, 33, 620–628. [Google Scholar] [CrossRef]
- Doppalapudi, R.; Vundavalli, S.; Prabhat, M.P. Effect of probiotic bacteria on oral Candida in head- and neck-radiotherapy patients: A randomized clinical trial. J. Cancer Res. Ther. 2020, 16, 470–477. [Google Scholar] [CrossRef]
- Jimenez-Garcia, L.; Celis-Aguilar, E.; Díaz-Pavón, G.; Muñoz Estrada, V.; Castro-Urquizo, Á.; Hernández-Castillo, N.; Amaro-Flores, E. Efficacy of topical clotrimazole vs. topical tolnaftate in the treatment of otomycosis. A randomized controlled clinical trial. Braz. J. Otorhinolaryngol. 2020, 86, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Obar, J.J. Sensing the threat posed by Aspergillus infection. Curr. Opin. Microbiol. 2020, 58, 47–55. [Google Scholar] [CrossRef]
- Van De Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latge, J.-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661. [Google Scholar] [CrossRef]
- Bongomin, F.; Batac, C.; Richardson, M.D.; Denning, D.W. A review of onychomycosis due to Aspergillus species. Mycopathologia 2018, 183, 485–493. [Google Scholar] [CrossRef]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. In Proceedings of Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 141–152. [Google Scholar]
- Williams, C.; Rajendran, R.; Ramage, G. Aspergillus biofilms in human disease. Fungal Biofilms Relat. Infect. 2016, 931, 1–11. [Google Scholar]
- Rowe-Jones, J.M.; Moore-Gillon, V. Destructive noninvasive paranasal sinus aspergillosis: Component of a spectrum of disease. J. Otolaryngol. 1994, 23, 92–96. [Google Scholar] [PubMed]
- Chakrabarti, A.; Kaur, H. Allergic aspergillus rhinosinusitis. J. Fungi 2016, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Telles, D.R.; Karki, N.; Marshall, M.W. Oral fungal infections: Diagnosis and management. Dent. Clin. 2017, 61, 319–349. [Google Scholar]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [PubMed]
- Page, I.D.; Richardson, M.D.; Denning, D.W. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J. Infect. 2016, 72, 240–249. [Google Scholar] [CrossRef]
- Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Misset, B.; Meersseman, W.; Cardoso, T.; Paiva, J.-A.; Blasco-Navalpotro, M.; De Laere, E.; Dimopoulos, G. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit. Care 2015, 19, 7. [Google Scholar] [CrossRef]
- Kawashima, J.; Nakajo, K.; Washio, J.; Mayanagi, G.; Shimauchi, H.; Takahashi, N. Fluoride-sensitivity of growth and acid production of oral Actinomyces: Comparison with oral Streptococcus. Microbiol. Immunol. 2013, 57, 797–804. [Google Scholar] [CrossRef]
- Valour, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 2014, 7, 183. [Google Scholar]
- Boyanova, L.; Kolarov, R.; Mateva, L.; Markovska, R.; Mitov, I. Actinomycosis: A frequently forgotten disease. Future Microbiol. 2015, 10, 613–628. [Google Scholar] [CrossRef]
- Wong, V.K.; Turmezei, T.; Weston, V. Actinomycosis. BMJ 2011, 343, d6099. [Google Scholar] [CrossRef]
- Moghimi, M.; Salentijn, E.; Debets-Ossenkop, Y.; Karagozoglu, K.H.; Forouzanfar, T. Treatment of cervicofacial actinomycosis: A report of 19 cases and review of literature. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e627. [Google Scholar] [CrossRef] [PubMed]
- Attaway, A.; Flynn, T. Actinomyces meyeri: From “lumpy jaw” to empyema. Infection 2013, 41, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Stájer, A.; Ibrahim, B.; Gajdács, M.; Urbán, E.; Baráth, Z. Diagnosis and management of cervicofacial actinomycosis: Lessons from two distinct clinical cases. Antibiotics 2020, 9, 139. [Google Scholar] [CrossRef]
- Moturi, K.; Kaila, V. Cervicofacial actinomycosis and its management. Ann. Maxillofac. Surg. 2018, 8, 361. [Google Scholar] [PubMed]
- Balbinot, K.M.; Sousa, N.W.A.; Pinheiro, J.d.J.V.; Ribeiro, A.L.R. Surgical debridement as a treatment strategy for cervicofacial actinomycosis—Literature review and case report. Int. J. Surg. Case Rep. 2020, 73, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.D.; Shetty, N.L.; Kanuri, M. Comparative evaluation of garlic extract mouthwash and chlorhexidine mouthwash on salivary Streptococcus mutans count—An in vitro study. Oral Health Prev. Dent. 2010, 8, 369–374. [Google Scholar]
- Haas, A.N.; Silva-Boghossian, C.M.; Colombo, A.P.; Susin, C.; Albandar, J.M.; Oppermann, R.V.; Rösing, C.K. Adjunctive azithromycin in the treatment of aggressive periodontitis: Microbiological findings of a 12-month randomized clinical trial. J. Dent. 2012, 40, 556–563. [Google Scholar] [CrossRef]
- Faveri, M.; Rebello, A.; de Oliveira Dias, R.; Borges-Junior, I.; Duarte, P.M.; Figueiredo, L.C.; Feres, M. Clinical and microbiologic effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized chronic periodontitis: Smokers versus non-smokers. J. Periodontol. 2014, 85, 581–591. [Google Scholar] [CrossRef]
- Tulsani, S.G.; Chikkanarasaiah, N.; Siddaiah, S.B.; Krishnamurthy, N.H. The effect of Propolis and Xylitol chewing gums on salivary Streptococcus mutans count: A clinical trial. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2014, 25, 737–741. [Google Scholar] [CrossRef]
- Parkar, S.G.; Eady, S.; Cabecinha, M.; Skinner, M.A. Consumption of apple-boysenberry beverage decreases salivary Actinomyces naeslundii and their adhesion in a multi-species biofilm model. Benef. Microbes 2017, 8, 299–307. [Google Scholar] [CrossRef]
- Marya, C.M.; Taneja, P.; Nagpal, R.; Marya, V.; Oberoi, S.S.; Arora, D. Efficacy of Chlorhexidine, Xylitol, and Chlorhexidine + Xylitol against Dental Plaque, Gingivitis, and Salivary Streptococcus mutans Load: A Randomised Controlled Trial. Oral Health Prev. Dent. 2017, 15, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.M.; Abell, G.C.J.; Thomson, R.; Morgan, L.; Waterer, G.; Gordon, D.L.; Taylor, S.L.; Leong, L.E.X.; Wesselingh, S.L.; Burr, L.D.; et al. Impact of Long-Term Erythromycin Therapy on the Oropharyngeal Microbiome and Resistance Gene Reservoir in Non-Cystic Fibrosis Bronchiectasis. mSphere 2018, 3, e00103-18. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Jung, H.I.; Kim, B.I. Susceptibility of oral bacteria to antibacterial photodynamic therapy. J. Oral Microbiol. 2019, 11, 1644111. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Peng, X.; Wang, S.; Han, Q.; Li, B.; Zhou, X.; Ren, B.; Xu, H.H.K.; Weir, M.D.; Li, M.; et al. A novel antibacterial resin-based root canal sealer modified by Dimethylaminododecyl Methacrylate. Sci. Rep. 2019, 9, 10632. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Esteban-Fernández, A.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement. Altern. Med. 2019, 19, 145. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef]
- Rafiee, A.; Memarpour, M.; Najibi, Y.; Khalvati, B.; Kianpour, S.; Morowvat, M.H. Antimicrobial Efficacy of a Novel Antibiotic-Eluting Injectable Platelet-Rich Fibrin Scaffold against a Dual-Species Biofilm in an Infected Immature Root Canal Model. BioMed. Res. Int. 2020, 2020, 6623830. [Google Scholar] [CrossRef]
- Vinothkumar, T.S.; Apathsakayan, R.; El-Shamy, F.M.M.; Homeida, H.E.; Hommedi, A.I.M.; Safhi, M.Y.A.; Alsalhi, H.A.M. Antibacterial effect of diode laser on different cariogenic bacteria: An In-vitro study. Niger. J. Clin. Pract. 2020, 23, 1578–1582. [Google Scholar] [CrossRef]
- Bueno, J.; Sánchez, M.C.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antimicrobial effect of nanostructured membranes for guided tissue regeneration: An in vitro study. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 1566–1577. [Google Scholar] [CrossRef]
- Fei, X.; Li, Y.; Weir, M.D.; Baras, B.H.; Wang, H.; Wang, S.; Sun, J.; Melo, M.A.S.; Ruan, J.; Xu, H.H.K. Novel pit and fissure sealant containing nano-CaF(2) and dimethylaminohexadecyl methacrylate with double benefits of fluoride release and antibacterial function. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 1241–1253. [Google Scholar] [CrossRef]
- Marcoux, E.; Lagha, A.B.; Gauthier, P.; Grenier, D. Antimicrobial activities of natural plant compounds against endodontic pathogens and biocompatibility with human gingival fibroblasts. Arch. Oral Biol. 2020, 116, 104734. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, K.; Miyaji, H.; Miyata, S.; Nishida, E.; Furihata, T.; Kanemoto, Y.; Sugaya, T.; Shitomi, K.; Akasaka, T. Antibacterial coating of tooth surface with ion-releasing pre-reacted glass-ionomer (S-PRG) nanofillers. Heliyon 2021, 7, e06147. [Google Scholar] [CrossRef] [PubMed]
- Sumioka, R.; Nakata, M.; Okahashi, N.; Li, Y.; Wada, S.; Yamaguchi, M.; Sumitomo, T.; Hayashi, M.; Kawabata, S. Streptococcus sanguinis induces neutrophil cell death by production of hydrogen peroxide. PLoS ONE 2017, 12, e0172223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Zhang, H.; Zheng, X.; Wang, J.; Jia, X.; Peng, X.; Xie, Q.; Zou, J.; Zheng, L.; et al. Probiotic Streptococcus salivarius K12 Alleviates Radiation-Induced Oral Mucositis in Mice. Front. Immunol. 2021, 12, 684824. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.W.; Chanyi, R.M.; Macklaim, J.M.; Cadieux, P.A.; Reid, G.; Burton, J.P. Streptococcus salivarius inhibits immune activation by periodontal disease pathogens. BMC Oral Health 2021, 21, 245. [Google Scholar] [CrossRef]
- Puccio, T.; An, S.S.; Schultz, A.C.; Lizarraga, C.A.; Bryant, A.S.; Culp, D.J.; Burne, R.A.; Kitten, T. Manganese transport by Streptococcus sanguinis in acidic conditions and its impact on growth in vitro and in vivo. Mol. Microbiol. 2022, 117, 375–393. [Google Scholar] [CrossRef]
- Odorici, A.; Colombari, B.; Bellini, P.; Meto, A.; Venturelli, I.; Blasi, E. Novel Options to Counteract Oral Biofilm Formation: In Vitro Evidence. Int. J. Environ. Res. Public Health 2022, 19, 8056. [Google Scholar] [CrossRef]
- Lemos, J.A.; Quivey, R.G., Jr.; Koo, H.; Abranches, J. Streptococcus mutans: A new Gram-positive paradigm? Microbiology 2013, 159, 436. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Moghaddam, M.M.; Kahnamoei, M.B. Evaluation of antimicrobial and cytotoxic effects of Echinacea and Arctium extracts and Zataria essential oil. AMB Express 2022, 12, 75. [Google Scholar] [CrossRef]
- Strużycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis–a comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.M.; Mendes, F.M.; Ekstrand, K.R. Detection activity assessment and diagnosis of dental caries lesions. Dent. Clin. 2010, 54, 479–493. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Macey, R.; Walsh, T.; Riley, P.; Hogan, R.; Glenny, A.-M.; Worthington, H.V.; Clarkson, J.E.; Ricketts, D. Transillumination and optical coherence tomography for the detection and diagnosis of enamel caries. Cochrane Database Syst. Rev. 2021, 1, CD013855. [Google Scholar]
- Cochrane, N.; Walker, G.; Manton, D.; Reynolds, E. Comparison of quantitative light-induced fluorescence, digital photography and transverse microradiography for quantification of enamel remineralization. Aust. Dent. J. 2012, 57, 271–276. [Google Scholar] [CrossRef]
- Gomez, J. Detection and diagnosis of the early caries lesion. BMC Oral Health 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.; Innes, N.; Schwendicke, F. Managing Carious Lesions: Why Do We Need Consensus on Terminology and Clinical Recommendations on Carious Tissue Removal? SAGE Publications Sage CA: Los Angeles, CA, USA, 2016. [Google Scholar]
- Philip, N.; Suneja, B.; Walsh, L. Beyond Streptococcus mutans: Clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br. Dent. J. 2018, 224, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Stingu, C.S.; Eschrich, K.; Rodloff, A.C.; Schaumann, R.; Jentsch, H. Periodontitis is associated with a loss of colonization by Streptococcus sanguinis. J. Med. Microbiol. 2008, 57, 495–499. [Google Scholar] [CrossRef]
- Zeng, L.; Walker, A.R.; Lee, K.; Taylor, Z.A.; Burne, R.A. Spontaneous Mutants of Streptococcus sanguinis with Defects in the Glucose-Phosphotransferase System Show Enhanced Post-Exponential-Phase Fitness. J. Bacteriol. 2021, 203, e0037521. [Google Scholar] [CrossRef]
- Dye, B.A.; Thornton-Evans, G.; Li, X.; Iafolla, T.J. Dental caries and sealant prevalence in children and adolescents in the United States, 2011–2012. NCHS Data Brief 2015, 191, 1–8. [Google Scholar]
- Alpsoy, E.; Bozca, B.C.; Bilgic, A. Behçet disease: An update for dermatologists. Am. J. Clin. Dermatol. 2021, 22, 477–502. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Qi, F.; Caufield, P.W. Identification of Streptococcus sanguinis with a PCR-generated species-specific DNA probe. J. Clin. Microbiol. 2003, 41, 3481–3486. [Google Scholar] [CrossRef]
| Species | Samples | Intervention | Outcomes | Ref. Year |
|---|---|---|---|---|
| HPV | Patients with progressed oropharyngeal cancer | Accelerated fractionation radiotherapy and standard-fractionation radiotherapy | Over 6o percent of patients were HPV-positive and showed better overall survival in 3 years compared to HPV-negative tumors. | [31] 2010 |
| HSV-2 | Patients with acute primary or recurrent HSV-2 | 1 g valacyclovir 3 times daily for 1 week, followed by 0.5 g valaciclovir twice daily for 1 year. | The following-up prescription showed insufficiency in prohibiting recurrent meningitis. | [16] 2012 |
| HPV | Patients under cervical surgery | Three doses of quadrivalent HPV vaccine or placebo at days 1, 60, and 180 | Quadrivalent HPV vaccination after surgical treatment significantly reduced recurrent HPV-related diseases. | [32] 2012 |
| HSV-1 and 2 | Patients with HIV-1 and HSV-2 | Valacyclovir 1000 mg or acyclovir 400 mg twice a week for ~3 months | High-dose valacyclovir was more successful in reducing the plasma HIV-1 RNA levels compared to the standard dose. | [33] 2013 |
| HSV-2 | Patients with HIV and HSV-2 in co-infection | Patients randomly received valacyclovir or placebo (N = 35) | The CD4+ T-lymphocyte count or HIV viral load did not change, but asymptomatic HSV-2 shedding reduced slightly. | [34] 2014 |
| HSV-1 or HSV-2 | RCTs | Effectiveness of oral antiviral drugs (acyclovir, famciclovir, and valacyclovir) | Researchers found there was a significantly lower number of patients with at least one genital herpes recurrence when acyclovir, valacyclovir, or famciclovir was used to treat patients with at least four recurrences per year as compared with placebo in patients with at least four recurrences. | [35] 2014 |
| HPV | Patients examined for gingivitis | Three medicinal mushrooms: Laetiporus sulphureus, Ganoderma lucidum, and Trametes Versicolor | Laetiporus sulphureus exerted 5% oral HPV clearance, while Trametes Versicolor plus Ganoderma lucidum showed a clearance of 88%. | [36] 2014 |
| HPV-16 and HPV-18 | Patients from 36 gynecology practices in seven countries | 6 mg VGX-3100 or placebo | This vaccine is the first to show effectiveness against CIN2/3 associated with HPV-16 and HPV-18 and provides a new treatment outlook. | [37] 2015 |
| HSV-2 | Healthy adults with recurrent genital HSV-2 | 100 mg oral pritelivir with 500 mg valaciclovir once a day | People with frequently recurrent genital HSV-2 using pritelivir experienced a lower percentage of HSV+ swabs. | [38] 2016 |
| HPV | Patients with high-risk cervical HPV infection | An anti-HPV biological dressing (JB01-BD) | JB01-BD could effectively decrease the viral load. | [39] 2016 |
| HSV-1 and 2 | Patients with post-herpetic neuralgia | 200 mg or 400 mg valnivudine hydrochloride (FV-100) once daily, or 1000 mg valacyclovir three times daily | Treatment with FV-100 decreased the neuralgia in a dose-dependent manner better than valacyclovir. | [40] 2017 |
| HSV-2 | Adults with symptomatic HSV-2 | 30 or 60 µg antigen against glycoprotein D2 and viral transcription factor ICP4.2 | The GEN-003 vaccine combinations with higher amounts of antigen and adjuvant showed more efficacy. | [41] 2018 |
| HSV-2 | Patients with recurrent genital HSV-2 | A vaginal capsule of multistrain Lactobacillus brevis or oral acyclovir | Probiotic therapy with multi-strain L. brevis was a promising low-cost treatment for recurrent genital herpes simplex virus infection compared with acyclovir. | [42] 2018 |
| HPV | Patients with multiple common warts | Intramuscular and intralesional bivalent HPV vaccine | Both HPV vaccination roots showed potential for treating warts. | [43] 2019 |
| HSV-1 | Patients with herpetic stromal keratitis | Topical cyclosporine-A 2% eye drop with prednisolone acetate 1% eye drop | Both treatments could similarly improve the cornea’s optical density to a significant extent. | [44] 2019 |
| HPV | Patients with genital condylomatosis | Dry extracts of Echinacea purpurea and Elaeagnus angustifolia (HPVADL18®) | HPVADL18® was suggested as a potential adjuvant therapy for reducing recurrent lesions after treating genital condylomatosis. | [45] 2019 |
| HPV | Patients with high-risk HPV | A proprietary combination of antiviral biologics (REBACIN®) | The antiviral agent could significantly repress the expression of E7 and E6 oncogenes in HPV and clear persistent HPV infections. | [46] 2019 |
| HSV-1 and 2 | HIV-1-positive adults | 500 mg valaciclovir twice daily | Valaciclovir modestly lowered the HIV viral load but did not slow the CD4 count decline. | [47] 2019 |
| HPV | Patients with recurrent respiratory papillomatosis | 10 mg/kg avelumab every 2 weeks for three doses | Avelumab treatment led to fewer surgical interventions and reduced HPV viral load. | [48] 2019 |
| HSV-1 | Schizophrenic patients with or without HSV-1 | 1.5 g valacyclovir or placebo for 16 weeks | Valacyclovir showed no effect on the viral infection. HSV-1 infection co-occurred with a more severe form of schizophrenia. | [49] 2019 |
| HSV-1 and 2 | Patients receiving mechanical ventilation for over 4 days | Intravenous acyclovir 5 mg/kg | Acyclovir did not decrease the duration of mechanical ventilation and did not increase the number of ventilator-free days in patients with HSV oropharyngeal reactivation. | [50] 2019 |
| HPV | Patients with anogenital warts | Podophyllotoxin cream 0.15% or imiquimod cream 5% with vaccination | Imiquimod and podophyllotoxin creams could similarly clear the wart, but the vaccine benefit was not observed. | [51] 2020 |
| Species | Sample | Intervention | Outcomes | Ref. Year |
|---|---|---|---|---|
| C. albicans | Children under treatment with a removable maxillary appliance | NitrAdine tablets | The treatment had no significant effect on the salivary Candida load. | [83] 2011 |
| Aspergillus | Patients with allergic bronchopulmonary Aspergillosis | Omalizumab | The treatment was successful in preventing the exacerbation of the infection. | [84] 2014 |
| C. albicans | Adults living in nursing homes | Probiotics, including Lactobacillus reuteri (strains DSM 17938 and ATCC PTA 5289) | The probiotics significantly reduced the oral Candida counts. | [85] 2015 |
| C. albicans | In vitro study | Pomegranate peel extract (PomeGr) | The PomeGr treatment altered biofilm formation, fungal growth, and AI release. Moreover, fungal cells substantially reduced PomeGr’s phenolic content | [86] 2022 |
| Aspergillus | Patients with non-dermatophyte mold onychomycosis | Traconazole or terbinafine | Both treatment efficacy was non-statistically significant (clinical cure of 54–65%). | [87] 2016 |
| Aspergillus | Patients with suspected invasive mold infection | Intravenous injection of isavuconazonium sulfate or voriconazole followed by further oral administration | Isavuconazole efficacy was not worse than voriconazole. It was well tolerated with fewer adverse events. | [88] 2016 |
| C. albicans | Patients wearing dentures | Triphala churna and chlorhexidine gluconate | Triphala showed a more antifungal effect than conventional chlorhexidine. | [89] 2017 |
| C. albicans | Patients with denture stomatitis | Low-molecular-weight chitosan and nystatin | Chitosan solution showed a significant antifungal effect. | [90] 2017 |
| C. albicans | Patients wearing dentures | Two probiotics (Lactobacillus acidophilus or Lactobacillus rhamnosus) enriched into cheese | The enriched cheese with probiotics reduced oral Candida colonization. | [91] 2017 |
| Candida and Aspergillus | Patients with candidemia and invasive candidiasis | A member of echinocandins (CD101 IV) | The dosing of CD101 IV was safe, minimally accumulative, plasma-persistent, and well-tolerated with negligible renal excretion. | [92] 2017 |
| C. albicans | Patients with symptomatic oral lichen planus | Probiotics, including Lactobacilli reuteri | The probiotic used did not affect the Candida load. | [93] 2018 |
| Aspergillus and C. albicans | Patients with otomycosis | Topical betadine and clotrimazole | The agents showed similar antifungal potential for treating otomycosis. | [94] 2018 |
| Aspergillus | Patients in the acute stage of allergic bronchopulmonary Aspergillosis | Oral administration of itraconazole or prednisolone | Prednisolone induced a better immunologic response but more side effects. | [95] 2018 |
| C. albicans | Patients with denture stomatitis | Photodynamic inactivation using a diode laser and methylene blue | The inactivation operation reduced the fungal and inflammation levels. | [96] 2018 |
| C. albicans | Patients with candidemia or invasive candidiasis | Intravenous and oral isavuconazole comparedto caspofungin and voriconazole | Isavuconazole showed a lower minimal inhibitory concentration than caspofungin. | [97] 2019 |
| C. albicans | In vitro study | Tissue conditioner modified by chitosan or chitosan-oligosaccharide | Both formulations reduced the C. albicans density. | [98] 2019 |
| C. albicans | Patients wearing complete dentures with stomatitis | Photodynamic therapy using indocyanine green was added to the routine antifungal therapy with nystatin mouthwash alone | The combined therapy helped to improve the denture stomatitis showing no adverse effects. | [99] 2019 |
| S. mutans, C. albicans, C. glabrata, and C. parapsilosis | In vitro study | Propolis, saline, or alkaline peroxide solutions | The propolis solution had an antimicrobial effect against S. mutans and C. albicans, showing no immediate effect on denture biofilm. | [100] 2019 |
| C. albicans | Patients wearing removable dentures | Chitosan-curcuminoid/PEG mouthwash compared to chlorhexidine | The composite alcohol-free mouthwash was a safe topical therapeutic for treating candida-associated denture stomatitis. | [101] 2019 |
| Aspergillus | Patients with chronic pulmonary Aspergillosis | Intravenous followed by oral administration of itraconazole | The treatments with itraconazole were effective on chronic pulmonary Aspergillosis. | [102] 2019 |
| Aspergillus | Patients with invasive Aspergillosis | Single and multiple ascending intravenous doses of an antifungal drug (VL-2397) | The dosing of VL-2397 was safe, non-accumulative, and tolerable in both healthy subjects and patients. | [103] 2019 |
| C. albicans | Patients wearing dentures with stomatitis | Photodynamic inactivation by GaA1As diode laser in comparison with nystatin | Both treatments were equally effective in treating denture stomatitis. | [104] 2019 |
| C. albicans | Patients wearing maxillary dentures | Dettol and Lifebuoy liquid soaps compared to sodium hypochlorite and phosphate-buffered saline solution as positive and negative controls | The liquid soaps efficiently reduced the fungal biofilm. | [105] 2020 |
| C. albicans | Patients after head and neck radiotherapy | Probiotics, including L. acidophilus, B. longum, L. rhamnosus, and S. boulardii | The probiotic bacteria could effectively reduce the oral Candida load. | [106] 2020 |
| Aspergillus | Patients with otomycosis | Clotrimazole cream and tolnaftate solution | Clotrimazole improved otitis better. | [107] 2020 |
| Species | Sample | Intervention | Outcomes | Ref. Year |
|---|---|---|---|---|
| S. mutans | Healthy subjects | Chlorhexidine and garlic extract mouthwash | Garlic extract inhibited S. mutans in both in vitro and in vivo studies. | [130] 2010 |
| A. gerencseriae | Patients with aggressive periodontitis | Systemic azithromycin | Azithromycin could slightly reduce the subgingival periodontal pathogens | [131] 2012 |
| Actinomyces spp. | Smoker and non-smoker subjects | Metronidazole and Amoxicillin | The non-smokers showed the lowest proportions of the orange complex and a meaningful increase in the proportions of Actinomyces species. | [132] 2013 |
| S. mutans | Children with decayed, missing, or filled teeth | Propolis and xylitol chewing gums | Both gums reduced the bacterial saliva load suggesting them an anti-cariogenic agent. | [133] 2014 |
| Lactobacillus spp. Actinomyces naeslundii S. mutans | Healthy adult subjects | Beverages containing apple fiber and polyphenols from boysenberry | The apple-boysenberry beverage exerted the most reduction on the colonization and biofilm adhesion. | [134] 2017 |
| S. mutans | Adolescent subjects with disabilities | Xylitol gum | The xylitol gum significantly reduced the caries rate. | [73] 2017 |
| S. mutans | Young subjects | Mouthwashes containing chlorhexidine, xylitol, and chlorhexidine + xylitol | All mouthwashes effectively reduced plaque, gingivitis, and bacterial saliva level. | [135] 2017 |
| S. pseudopneumoniae A. odontolyticus | Adults with bronchiectasis | Erythromycin | The intervention significantly decreased the oropharyngeal microbiota composition. | [136] 2018 |
| A. actinomycetemcomitans A. naeslundii A. viscosus E. faecalis E. coli L. casei S. oralis S. sanguinis C. albicans | In vitro study | Several flavonoids | Among the eight tested flavonoids, morin was the most effective; however other four flavonoids, including luteolin, naringin, quercetin, and rutin, could also decrease bacterial and fungal growth. | [137] 2019 |
| A. israelii E. faecium F. nucleatum L. gasseri S. mutans V. parvula | In vitro study | Curcumin, protoporphyrin IX, resazurin, riboflavin, and light irradiation | All tested agents decreased the oral bacterial growth. | [138] 2019 |
| E. faecalis S. gordonii A. naeslundii L. acidophilus | In vitro study | Dimethylaminododecyl methacrylate (DMADDM) and EndoREZ | The two tested sealers showed similar cytotoxicity, apical sealing ability, and solubility; however, DMADDM showed different properties at a mass fraction of 5%. | [139] 2019 |
| S. oralis A. naeslundii V. parvula F. nucleatum P. gingivalis A. actinomycetemcomitans | In vitro study | Red wine, dealcoholized red wine, polyphenols-rich extracts from wine, and polyphenols-rich extracts from grape seeds | Conventional and dealcoholized red wine reduced the bacteria load within the biofilm, especially about P. gingivalis and A. actinomycetemcomitans. | [140] 2019 |
| S. mutans A. naeslundii | In vitro study | Surface pre-reacted glass-ionomer filler in a resin-based composite incorporated with 2-methacryloyloxyethyl phosphorylcholine | The polymer added in the filler composite inhibited bacterial attachment and biofilm growth. | [141] 2019 |
| A. naeslundii E. faecalis | Extracted single-rooted human mandibular first premolars | Platelet-rich fibrin scaffold with or without a 3-antibiotic mixture containing minocycline, metronidazole, and ciprofloxacin | The combination of scaffold and antibiotic mixture showed the highest antibacterial activity. | [142] 2020 |
| S. mutans L. casei A. naeslundii | Human mandibular third molars | Diode laser and 2% chlorhexidine gluconate solution | All three cariogenic bacteria were affected by a diode. | [143] 2020 |
| S. oralis A. naeslundii V. parvula F. nucleatum P. gingivalis A. actinomycetemcomitans | In vitro study in a subgingival biofilm model | Doxycycline, zinc, and calcium doped polymeric nanostructured membrane that is non-resorbable | The nanostructured membrane significantly reduced the biofilm growth dynamics and bacterial load. | [144] 2020 |
| S. mutans S. gordonii S. sobrinus A. naeslundii F. nucleatum A. actinomycetemcomitans P. gingivalis E. faecalis | In vitro study in planktonic culture | Fruit juices derived from blackcurrant, redcurrant, cranberry, and raspberry | Blackcurrant, redcurrant, and cranberry juices had the most suppressing effect on bacterial growth, respectively, while raspberry only significantly suppressed the growth of P. gingivalis. | [53] 2020 |
| S. mutans | In vitro study on extracted teeth | A composite of nano-calcium fluoride and dimethylaminohexadecyl methacrylate | The composite showed promising fluoride release features and antibacterial functions. | [145] 2020 |
| E. faecalis S. mutans A. israelii | In vitro study | Three licorice-derived polyphenols and cinnamon oil | The tested natural plant-derived compounds showed promising root canal disinfection properties. | [146] 2020 |
| S. mutans S. sobrinus A. viscosus L. acidophilus | In vitro study | Caffeic acid phenethyl ester | The tested agent exerted high bactericidal and inhibitory activities against biofilms, cariogenic bacteria, and their virulence. | [147] 2020 |
| S. mutans A. naeslundii | Human dentin blocks Periodontitis animal model | Surface pre-reacted glass-ionomer nanofillers | The tested nanofiller coating showed antibacterial effects on the tooth surfaces and improved the clinical parameters of periodontitis. | [148] 2021 |
| S. sanguinis | In vitro study | The concentration of H2O2 | It has been shown that S. sanguinis evades neutrophil killing in vitro and counteracts innate immunity by the action of SpxB in collected blood | [149] 2017 |
| S. salivarius | Induced oral mucositis by experimental radiation in mice | The mouse oral cavity was treated daily with S. salivarius K12 | Cancer patients receiving radiotherapy may benefit from S. salivarius K12 as an adjuvant treatment. | [150] 2021 |
| S. salivarius | Primary human gingival fibroblasts | Pathogen-induced fibroblasts were treated with S. salivarius M18K12, K12, and fractions of its supernatant and whole-cell lysate | Periodontal disease pathogens were prevented from activating the immune system by S.salivarius M18 and K12. After chewing gum was administered with S. salivarius K12, the salivary microbiome and immune system did not change. | [151] 2021 |
| S. sanguinis | In vitro study | Measurement of SsaACB manganese transporter | In acidic conditions, SK36 mutants lacking SsaACB display reduced growth and manganese uptake. S. sanguinis may have a variety of manganese transporters due to the heterogeneity of its oral environment. | [152] 2022 |
| C. albicans, S. aureus and P. aeruginosa | In vitro study | Pomegranate (PomeGr) and microRepair (MicroR) | There were similarities in the effects of MicroR and PomeGr; however, the effectiveness of the two, given separately or in combination, varied based on which microbial agent was being treated. | [153] 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahmasebi, E.; Keshvad, A.; Alam, M.; Abbasi, K.; Rahimi, S.; Nouri, F.; Yazdanian, M.; Tebyaniyan, H.; Heboyan, A.; Fernandes, G.V.O. Current Infections of the Orofacial Region: Treatment, Diagnosis, and Epidemiology. Life 2023, 13, 269. https://doi.org/10.3390/life13020269
Tahmasebi E, Keshvad A, Alam M, Abbasi K, Rahimi S, Nouri F, Yazdanian M, Tebyaniyan H, Heboyan A, Fernandes GVO. Current Infections of the Orofacial Region: Treatment, Diagnosis, and Epidemiology. Life. 2023; 13(2):269. https://doi.org/10.3390/life13020269
Chicago/Turabian StyleTahmasebi, Elahe, Ali Keshvad, Mostafa Alam, Kamyar Abbasi, Saeide Rahimi, Farzad Nouri, Mohsen Yazdanian, Hamid Tebyaniyan, Artak Heboyan, and Gustavo Vicentis Oliveira Fernandes. 2023. "Current Infections of the Orofacial Region: Treatment, Diagnosis, and Epidemiology" Life 13, no. 2: 269. https://doi.org/10.3390/life13020269
APA StyleTahmasebi, E., Keshvad, A., Alam, M., Abbasi, K., Rahimi, S., Nouri, F., Yazdanian, M., Tebyaniyan, H., Heboyan, A., & Fernandes, G. V. O. (2023). Current Infections of the Orofacial Region: Treatment, Diagnosis, and Epidemiology. Life, 13(2), 269. https://doi.org/10.3390/life13020269










