Disease Activity Is More Associated with IL-1 Than with IL-6 in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection, Laboratory Assessments, and Carotid Ultrasound Assessment
2.3. Statistical Analysis
3. Results
3.1. Demographics and Disease-Related Data
3.2. Relationship between Demographics and disease data and IL-6 and IL-1ra
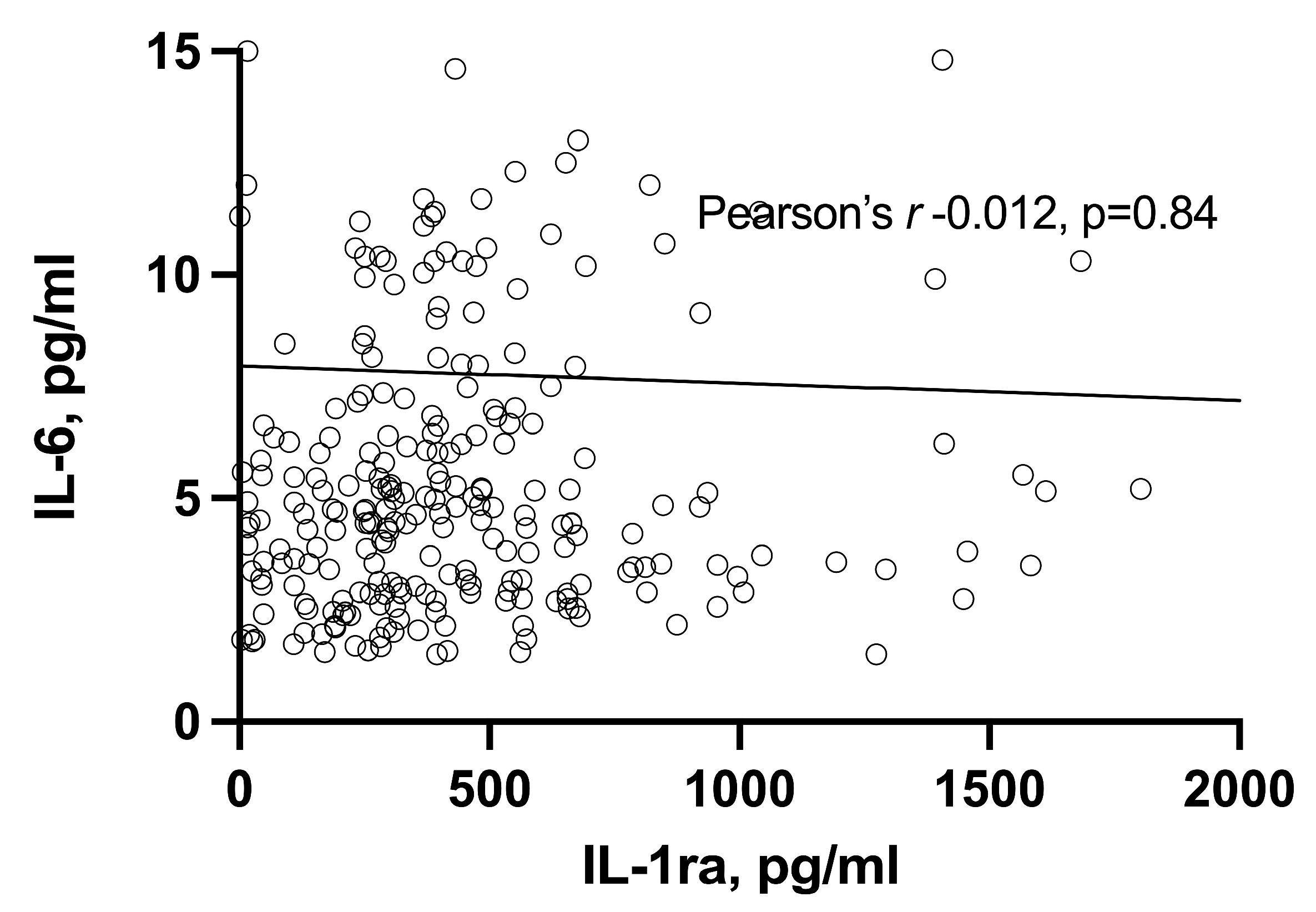
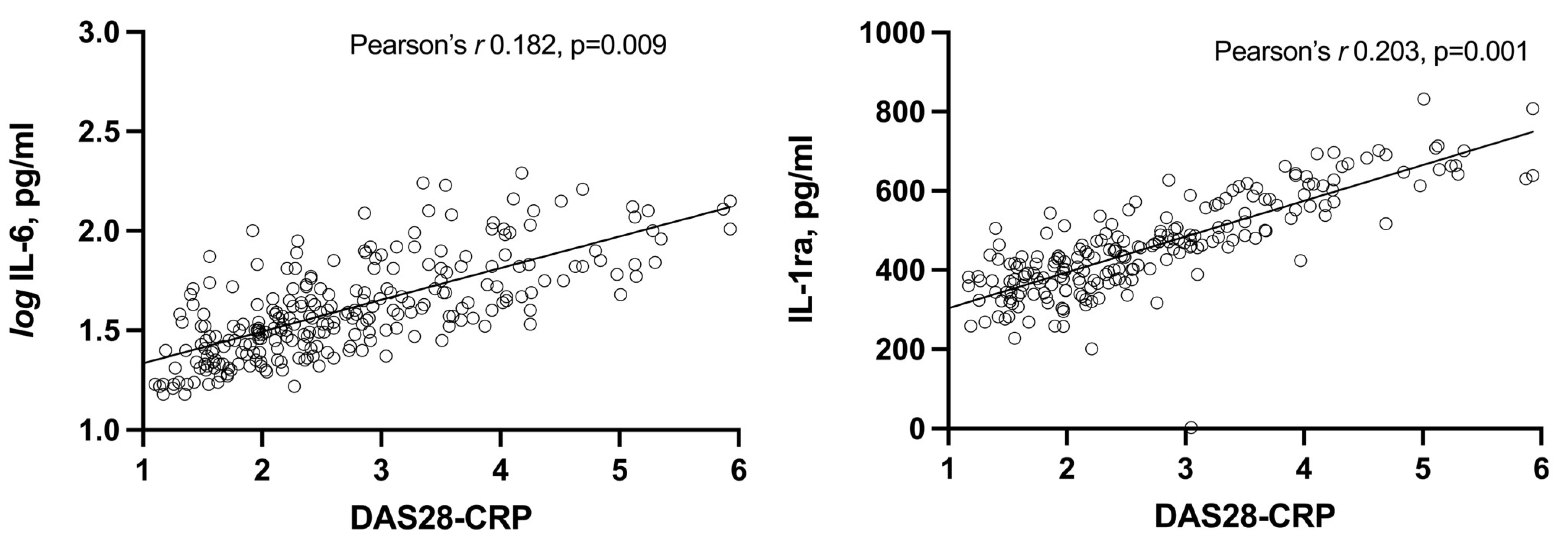
3.3. Correlation of IL-6 and IL-1ra with Each Other and with Disease Activity Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yap, H.Y.; Tee, S.Z.Y.; Wong, M.M.T.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Moreland, L.W.; Cush, J.J.; Greenwald, M.W.; Block, S.; Shergy, W.J.; Hanrahan, P.S.; Khraishi, M.M.; Patel, A.; Sun, G.; et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann. Rheum. Dis. 2004, 63, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.H. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, i103–i108. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Arend, W.P. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002, 13, 323–340. [Google Scholar] [CrossRef]
- Favalli, E.G. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatol. Ther. 2020, 7, 473–516. [Google Scholar] [CrossRef]
- Srirangan, S.; Choy, E.H. The role of Interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef]
- Deon, D.; Ahmed, S.; Tai, K.; Scaletta, N.; Herrero, C.; Lee, I.-H.; Krause, A.; Ivashkiv, L.B. Cross-Talk Between IL-1 and IL-6 Signaling Pathways in Rheumatoid Arthritis Synovial Fibroblasts. J. Immunol. 2001, 167, 5395–5403. [Google Scholar] [CrossRef]
- Tosato, G.; Jones, K.D. Interleukin-1 Induces Interleukin-6 Production in Peripheral Blood Monocytes. Blood 1990, 75, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Kandere-Grzybowska, K.; Letourneau, R.; Kempuraj, D.; Donelan, J.; Poplawski, S.; Boucher, W.; Athanassiou, A.; Theoharides, T.C. IL-1 Induces Vesicular Secretion of IL-6 without Degranulation from Human Mast Cells 1. J. Immunol. 2003, 171, 4830–4836. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santiago, C.; Quevedo-Abeledo, J.C.; Hernández-Hernández, V.; de Vera-González, A.; Gonzalez-Delgado, A.; González-Gay, M.Á.; Ferraz-Amaro, I. Interleukin 1 receptor antagonist relation to cardiovascular disease risk in patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 13698. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.L.; Van’T Hof, M.A.; Kuper, H.H.; Van Leeuwen, M.A.; Van De Putte, L.B.A.; Van Riel, P.L.C.M. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Breedveld, F.C.; Schiff, M.H.; Kalden, J.R.; Emery, P.; Eberl, G.; van Riel, P.L.; Tugwell, P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003, 42, 244–257. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): A review of their usefulness and validity in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, S100-8. [Google Scholar]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Cahill, C.M.; Rogers, J.T. Interleukin (IL) 1β Induction of IL-6 Is Mediated by a Novel Phosphatidylinositol 3-Kinase-dependent AKT/IκB Kinase α Pathway Targeting Activator Protein-1. J. Biol. Chem. 2008, 283, 25900. [Google Scholar] [CrossRef]
- Martin, M.U.; Wesche, H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta Mol. Cell Res. 2002, 1592, 265–280. [Google Scholar] [CrossRef]
- Helal, A.M.H.; Shahine, E.M.; Hassan, M.M.; Hashad, D.I.; Moneim, R.A. Fatigue in rheumatoid arthritis and its relation to interleukin-6 serum level. Egypt. Rheumatol. 2012, 34, 153–157. [Google Scholar] [CrossRef]
- Milman, N.; Karsh, J.; Booth, R.A. Correlation of a multi-cytokine panel with clinical disease activity in patients with rheumatoid arthritis. Clin. Biochem. 2010, 43, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Masedu, F.; Alvaro, S.; Airò, P.; Battafarano, N.; Cantarini, L.; Cantatore, F.P.; Carlino, G.; D’Abrosca, V.; Frassi, M.; et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. 2019, 16, e1002901. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2020, 93, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Luotola, K. IL-1 Receptor Antagonist (IL-1Ra) Levels and Management of Metabolic Disorders. Nutrients 2022, 14, 3422. [Google Scholar] [CrossRef]
| Rheumatoid Arthritis | |
|---|---|
| (n = 407) | |
| Age, years | 56 ± 10 |
| Female, n (%) | 329 (81) |
| BMI, kg/m2 | 28 ± 5 |
| Waist circumference, cm | 97 ± 13 |
| Cardiovascular data | |
| CV risk factors, n (%) | |
| Current smoker | 88 (22) |
| Obesity | 130 (32) |
| Hypertension | 140 (34) |
| Diabetes Mellitus | 53 (13) |
| Dyslipidemia | 190 (47) |
| Statins, n (%) | 129 (32) |
| Disease-related data | |
| Disease duration, years | 8 (4–15) |
| hsCRP at time of study, mg/L | 2.9 (1.4–6.3) |
| ESR at time of study, mm/1st hour | 18 (8–34) |
| IL-6, pg/mL | 4.8 (3.1–7.5) |
| IL-1ra, pg/mL | 472 (248–586) |
| Rheumatoid factor, n (%) | 286 (72) |
| ACPA, n (%) | 238 (65) |
| DAS28-ESR | 3.2 ± 1.4 |
| DAS28-CRP | 2.7 ± 1.1 |
| SDAI | 13 (7–20) |
| CDAI | 8 (4–14) |
| History of extraarticular manifestations, n (%) | 32 (9) |
| Erosions, n (%) | 151 (41) |
| Current drugs, n (%) | |
| Prednisone | 147 (36) |
| Prednisone doses, mg/day | 5 (3–5) |
| NSAIDs | 179 (44) |
| DMARDs | 362 (89) |
| Methotrexate | 306 (75) |
| Leflunomide | 91 (22) |
| Hydroxychloroquine | 44 (11) |
| Salazopyrin | 27 (7) |
| Anti-TNF therapy | 83 (20) |
| Rituximab | 7 (2) |
| Abatacept | 12 (3) |
| JAK inhibitors | 20 (5) |
| Data represent percentages, mean ± SD, or median (IQR) when data were not normally distributed. | |
| hsCRP: high-sensitivity C-reactive protein; ACPA: anti-citrullinated protein antibodies; CV: cardiovascular. General population values of IL-6 and IL-1ra have been described as, respectively, 5.186 pg/mL (95%CI 4.631, 5.740) and 248 (95%CI: 244, 252) pg/mL. | |
| NSAID: nonsteroidal anti-inflammatory drugs; DMARD: disease-modifying antirheumatic drug. | |
| TNF: tumor necrosis factor; ESR: erythrocyte sedimentation rate. | |
| BMI: body-mass index; DAS28: Disease Activity Score in 28 joints. | |
| CDAI: Clinical Disease Activity Index; SDAI: Simple Disease Activity Index. | |
| IL-6: interleukin-6; IL-1ra: interleukin-1 receptor antagonist. | |
| log IL-6, pg/mL | IL-1ra, pg/mL | |||
|---|---|---|---|---|
| beta coefficient (95% CI), p | ||||
| Age, years | 0.006 (−0.003–0.2) | 0.18 | −3 (−7–1) | 0.16 |
| Female | −0.1 (−0.3–0.1) | 0.39 | −7 (−117–104) | 0.90 |
| BMI, kg/m2 | 0.004 (−0.01–0.02) | 0.61 | 18 (10–26) | <0.001 |
| Waist circumference, cm | 0.004 (−0.003–0.01) | 0.31 | 6 (3–10) | <0.001 |
| Cardiovascular data | ||||
| CV risk factors | ||||
| Current smoker | 0.2 (−0.02–0.4) | 0.080 | −45 (−144–54) | 0.37 |
| Obesity | 0.09 (−0.09–0.3) | 0.31 | 145 (57–234) | 0.001 |
| Hypertension | 0.1 (−0.04–0.3) | 0.13 | −2 (−88–84) | 0.96 |
| Diabetes Mellitus | −0.1 (−0.4–0.2) | 0.45 | 108 (−14–229) | 0.082 |
| Dyslipidemia | 0.2 (0.01–0.4) | 0.035 | −29 (−112–54) | 0.49 |
| Statins | −0.07 (−0.3–0.1) | 0.46 | 24 (−65–113) | 0.60 |
| Disease-related data | ||||
| Disease duration, years | 0.005 (−0.004–0.01) | 0.29 | −5 (−9–−0.7) | 0.024 |
| log hsCRP, mg/L | 0.2 (0.08–0.2) | <0.001 | 44 (8–80) | 0.015 |
| ESR, mm/1st hour | 0.008 (0.004–0.01) | <0.001 | 3 (0.7–5) | 0.010 |
| Rheumatoid factor | −0.09 (−0.3–0.1) | 0.41 | −86 (−182–10) | 0.079 |
| ACPA | 0.01 (−0.2–0.2) | 0.90 | −60 (−160–40) | 0.24 |
| DAS28-ESR | 0.05 (−0.01–0.1) | 0.13 | 61 (30–92) | <0.001 |
| Remission | ref. | - | ref. | - |
| Low activity | −0.05 (−0.3–0.2) | 0.66 | 14 (−101–129) | 0.81 |
| Moderate and high activity | 0.2 (0.009–0.4) | 0.041 | 152 (61–244) | 0.001 |
| DAS28-CRP | 0.09 (0.01–0.2) | 0.023 | 67 (30–104) | <0.001 |
| Remission | ref. | - | ref. | - |
| Low activity | 0.2 (−0.07–0.4) | 0.17 | 35 (−81–151) | 0.56 |
| Moderate and high activity | 0.3 (0.07–0.5) | 0.008 | 35 (−81–152) | 0.001 |
| SDAI | 0.003 (−0.002–0.008) | 0.19 | 3 (0.2–5) | 0.036 |
| CDAI | 0.007 (−0.004–0.02) | 0.20 | 7 (2–12) | 0.005 |
| History of extraarticular manifestations | 0.4 (0.1–0.7) | 0.007 | −10 (−169–148) | 0.90 |
| Erosions | 0.03 (−0.2–0.2) | 0.78 | −53 (−142–36) | 0.24 |
| Current drugs | ||||
| Prednisone | 0.08 (−0.1–0.3) | 0.37 | −32 (−118–55) | 0.47 |
| Prednisone doses, mg/day | 0.01 (−0.03–0.05) | 0.53 | −12 (−29–5) | 0.16 |
| NSAIDs | 0.03 (−0.1–0.2) | 0.71 | −19 (−103–64) | 0.65 |
| DMARDs | −0.001 (−0.3–0.3) | 0.99 | −154 (−298–−11) | 0.035 |
| Methotrexate | 0.04 (−0.2–0.2) | 0.69 | −56 (−155–43) | 0.27 |
| Leflunomide | 0.1 (−0.07–0.3) | 0.20 | −35 (−131–62) | 0.48 |
| Hydroxychloroquine | 0.07 (−0.2–0.3) | 0.57 | −137 (−261–−12) | 0.032 |
| Salazopyrin | 0.3 (0.02–0.6) | 0.036 | −101 (−255–52) | 0.19 |
| Anti-TNF therapy | 0.09 (−0.1–0.3) | 0.42 | −7 (−111–96) | 0.89 |
| Rituximab | −0.6 (−1.6–0.5) | 0.27 | −85 (−463–294) | 0.66 |
| Abatacept | 0.4 (−0.1–0.8) | 0.12 | −19 (−261–223) | 0.88 |
| JAK inhibitors | −0.2 (−0.6–0.1) | 0.20 | −116 (−298–66) | 0.21 |
| Log IL-6 and IL-1ra are the dependent variables in this analysis. Significant p-values are depicted in bold. | ||||
| CV: cardiovascular; hsCRP: high-sensitivity C-reactive protein; ESR: erythrocyte sedimentation rate; ACPA: anti-citrullinated protein antibodies. PCR is natural-log-transformed. JAK: Janus kinase. | ||||
| NSAID: nonsteroidal anti-inflammatory drugs; DMARD: disease-modifying antirheumatic drug. | ||||
| TNF: tumor necrosis factor; BMI: body-mass index; DAS28: Disease Activity Score in 28 joints. | ||||
| CDAI: Clinical Disease Activity Index; SDAI: Simple Disease Activity Index. | ||||
| log IL-6, pg/mL | IL-1ra, pg/mL | |||
|---|---|---|---|---|
| r | p | r | p | |
| Correlation | ||||
| log hsCRP, mg/L | 0.242 | <0.001 | 0.134 | 0.015 |
| ESR, mm/1st hour | 0.243 | <0.002 | 0.155 | 0.010 |
| DAS28-ESR | 0.091 | 0.133 | 0.213 | <0.001 |
| DAS28-CRP | 0.136 | 0.024 | 0.195 | <0.001 |
| SDAI | 0.078 | 0.19 | 0.117 | 0.036 |
| CDAI | 0.077 | 0.20 | 0.157 | 0.005 |
| Partial correlations | ||||
| Controlled by IL-1ra | Controlled by IL-6 | |||
| log hsCRP, mg/L | 0.236 | <0.001 | 0.112 | 0.071 |
| ESR, mm/1st hour | 0.242 | <0.001 | 0.127 | 0.068 |
| DAS28-ESR | 0.076 | 0.22 | 0.210 | <0.001 |
| DAS28-CRP | 0.130 | 0.037 | 0.204 | 0.001 |
| SDAI | 0.070 | 0.264 | 0.116 | 0.061 |
| CDAI | 0.070 | 0.261 | 0.168 | 0.007 |
| Controlled by IL-1ra +hsCRP and ESR | Controlled by IL-6 +hsCRP and ESR | |||
| DAS28-ESR | 0.062 | 0.38 | 0.194 | 0.006 |
| DAS28-CRP | 0.170 | 0.016 | 0.233 | <0.001 |
| SDAI | 0.075 | 0.29 | 0.190 | 0.007 |
| CDAI | 0.077 | 0.28 | 0.210 | 0.003 |
| Controlled by IL-1ra +hsCRP and ESR +age, sex, and ACPA and RF | Controlled by IL-6 +hsCRP and ESR +age, sex, and ACPA and RF | |||
| DAS28-ESR | 0.098 | 0.16 | 0.172 | 0.006 |
| DAS28-CRP | 0.182 | 0.009 | 0.203 | 0.001 |
| SDAI | 0.098 | 0.16 | 0.169 | 0.007 |
| CDAI | 0.098 | 0.16 | 0.182 | 0.004 |
| hsCRP: high-sensitivity C-reactive protein; ESR: erythrocyte sedimentation rate; IL: interleukin. | ||||
| ACPA: anti-citrullinated protein antibodies; RF: rheumatoid factor. PCR is natural-log-transformed. | ||||
| CDAI: Clinical Disease Activity Index; SDAI: Simple Disease Activity Index. | ||||
| DAS: Disease activity score. Significant values are depicted in bold. r refers to Pearson’s coefficient. Correlations between disease activity indices and ILs are controlled/adjusted by the mentioned variables. Significant p-values are depicted in bold. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Santiago, C.; Quevedo-Abeledo, J.C.; Hernández-Hernández, M.V.; de Vera-González, A.; González-Delgado, A.; González-Gay, M.Á.; Ferraz-Amaro, I. Disease Activity Is More Associated with IL-1 Than with IL-6 in Patients with Rheumatoid Arthritis. Life 2023, 13, 82. https://doi.org/10.3390/life13010082
Almeida-Santiago C, Quevedo-Abeledo JC, Hernández-Hernández MV, de Vera-González A, González-Delgado A, González-Gay MÁ, Ferraz-Amaro I. Disease Activity Is More Associated with IL-1 Than with IL-6 in Patients with Rheumatoid Arthritis. Life. 2023; 13(1):82. https://doi.org/10.3390/life13010082
Chicago/Turabian StyleAlmeida-Santiago, Cristina, Juan Carlos Quevedo-Abeledo, María Vanesa Hernández-Hernández, Antonia de Vera-González, Alejandra González-Delgado, Miguel Ángel González-Gay, and Iván Ferraz-Amaro. 2023. "Disease Activity Is More Associated with IL-1 Than with IL-6 in Patients with Rheumatoid Arthritis" Life 13, no. 1: 82. https://doi.org/10.3390/life13010082
APA StyleAlmeida-Santiago, C., Quevedo-Abeledo, J. C., Hernández-Hernández, M. V., de Vera-González, A., González-Delgado, A., González-Gay, M. Á., & Ferraz-Amaro, I. (2023). Disease Activity Is More Associated with IL-1 Than with IL-6 in Patients with Rheumatoid Arthritis. Life, 13(1), 82. https://doi.org/10.3390/life13010082







