Abstract
Background: Increasing evidence exists that higher levels of androgens can be found in individuals with autism. Evidence yields to a susceptible role of Cytochrome P450 17A1 (CYP17A1) with its catalyzation of the two distinct types of substrate oxidation by a hydroxylase activity (17-alpha hydroxylase) and C17/20 lyase activity. However, to what extent steps are altered in affected children with autism versus healthy controls remains to be elucidated. Methods: Urine samples from 48 boys with autism (BMI 19.1 ± 0.6 kg/m2, age 14.2 ± 0.5 years) and a matched cohort of 48 healthy boys (BMI 18.6 ± 0.3 kg/m2, 14.3 ± 0.5 years) as well as 16 girls with autism (BMI 17.5 ± 0.7 kg/m2, age 13.8 ± 1.0 years) and a matched cohort of 16 healthy girls (BMI 17.2 ± 0.8 kg/m2, age 13.2 ± 0.8 years) were analyzed for steroid hormone metabolites by gas chromatography-mass spectrometry. Results: The activity of 17-alpha Hydroxylase increased by almost 50%, whereas activity of 17/20 Lyase activity increased by around 150% in affected children with autism. Furthermore, the concentration of Cortisol was higher as compared to the average increase of the three metabolites TH-Corticosterone, 5α-TH-Corticosterone and TH-11β-DH-Corticosterone, indicating, in addition, a stimulation by the CRH-ACTH system despite a higher enzymatic activity. Discussion: As it was shown that oxidative stress increases the 17/20-lyase activity via p38α, a link between higher steroid hormone levels and oxidative stress can be established. However, as glucocorticoid as well as androgen metabolites showed higher values in subjects affected with autism as compared to healthy controls, the data indicate, despite higher CYP17A1 activity, the presence of increased substrate availability in line with the Cholesterol theory of autism.
1. Introduction
Already, in one of the first description of children with autism from Hans Asperger in 1944, altered steroid hormones were implied [1]. Hans Asperger showed four cases of children with autism, whereby in one child, definitely dysregulated steroid hormones were present, implied by the clinical description [2]. In consequence, lots of efforts were performed to elucidate the endocrine phenotype of this disorder. Special attention was first paid to the HPAG-axis with a special interest in the CRH-ACTH system (reviewed by Taylor and Corbett [3]). Theories such as extreme male theory [4] or the Cholesterol theory of autism [5] were directly aiming at steroid hormones. Further supporting relevance can be derived that altered sex hormones are in line with the around four times higher prevalence of autism in boys as compared to girls [4,5]. A parallel with the increasing evidence of altered steroid hormones genetic studies were performed, indicating, for example, an association with fragile X-Syndrome in patients with autism in line with altered androgens [6]. Furthermore, a direct association was performed between autism and RORA as a novel candidate gene in autism, directly implying a role of Cytochrome P450 17A1 (CYP17A1; also P450c17 and P450sccII) [7,8,9,10,11]. Besides the mentioned lines of evidence, dysregulated steroid hormones were descriptively measured several times in boys as well as girls with autism [12,13]. Measurements from urine [12,13], plasma [14] or amniotic fluid [4] were performed and alterations were detected in pre-pubertal, as well as post-pubertal boys and girls. A recent systematic review and metanalysis from a respectable number of 321 boys and 64 girls with autism indicated that 17/20 Lyase activity is altered in boys as well as girls, implying a relevant role of CYP17A1 [15]. CYP17A1 is a critically important enzyme in humans that catalyzes the formation of all endogenous androgens via two steps: the 17alpha Hydroxylase step and the 17/20 Lyase step [9] (Figure 1). Through its hydroxylase activity, it catalyzes the 17alpha-hydroxylation of pregnenolone to 17alpha-OH pregnenolone [9]. Subsequently, through its C17/20lyase activity, it can further convert 17α-OH pregnenolone to the androgen dehydroepiandrosterone (DHEA), which is a precursor to androstenedione, testosterone and dihydrotestosterone [9,16] (Figure 1). To date, more than 100 mutations in the CYP17A1 gene have been described and the majority are associated with a classic phenotype of combined 17alpha-hydroxylase and 17/20-lyase deficiency [17,18,19,20,21,22]. Interestingly, the reduction of 17alpha-hydroxylase activity and/or 17/20 Lyase activity seems to be a mirror image of the alterations of the steroid hormones detected in affected subjects with autism [4,12,13,14,15,23,24]. One key question is whether CYP17A1 stops after 17α-hydroxylation or proceeds to 17/20 lyase activity, which is largely dependent on three post-translational factors [9,16]. First, 17/20 lyase activity is especially sensitive to the molar abundance of the electron-transfer protein P450 oxidoreductase (POR) [9,16]. Second, cytochrome b5 strongly promotes 17/20 lyase activity, principally by acting as an allosteric factor promoting the interaction of CYP17A1 with POR [9,16,25,26]. Third, the serine/threonine phosphorylation of CYP17A1 itself promotes 17/20 lyase activity, again apparently by promoting the interaction of CYP17A1 with POR [9,16]. The principal kinase that phosphorylates CYP17A1 to confer 17/20 lyase activity appears to be p38α (MAPK14), which increases the maximum velocity of the 17/20 lyase reaction, while having no detectable effect on the 17alpha-hydroxylase reaction [9,16].
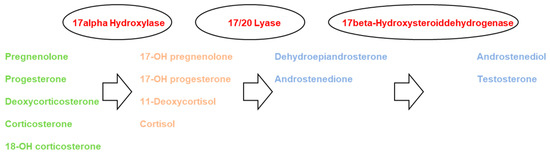
Figure 1.
Steroid hormone synthesis pathway with the two CYP17A1-dependent steps of 17alpha-Hydroxylase and 17/20 Lyase activity [4,14,28]. Green (mineralocorticoids), orange (glucocorticoids), blue (androgens).
To point out, based on different lines of evidence, a role of CYP17A1 seems crucial in autism, however, the direct description of causes and mechanisms is still missed. In this work, we try to put the focus on CYP17A1 activity and try to elucidate the roles of the two enzymes 17-alpha Hydroxylase and 17/20 Hydroxylase by comparing measurements of the relevant steroid hormones indicating potential differences in the activity of CYP17A1 in affected children with autism versus healthy controls. As this is a hypothesis with potential for falsification, it shall be stated that CYP17A1 activity is not altered in children with autism versus healthy controls [27].
2. Material & Methods
2.1. Participants
Forty-eight boys with autism (BMI 19.1 ± 0.6 kg/m2, age 14.2 ± 0.5 years) and a cohort of forty-eight individually-pairwise healthy boys were matched by BMI and age (BMI 18.6 ± 0.3 kg/m2, 14.3 ± 0.5 years), as well as 16 girls with autism (BMI 17.5 ± 0.7 kg/m2, age 13.8 ± 1.0 years) and a cohort of 16 individually-pairwise healthy girls matched by BMI and age (BMI 17.2 ± 0.8 kg/m2, age 13.2 ± 0.8 years). Individuals were included after they had given written informed consent which was also signed by their legal guardians. In the control group, autism was excluded by the Marburg questionnaire for Asperger syndrome (MBAS), as completed by the caregivers. The study was approved by the governmental ethics board of Graz, Austria (Approval Number FA8B-50.2), and registered at ClinicalTrials.gov ID NCT01197131.
2.2. Study Procedure
Children with autism and healthy control girls were recruited from the area of Leipzig (Austria). Enrolment took place from mid-2009 to mid-2012. All participants were Caucasians. Participants were excluded if they had a history of liver diseases, renal or endocrine disorders, a current infection or fever. Intellectual disability or behavioral disorders were exclusion criteria only for the control group, but were allowed as comorbid conditions in the group of children with autism, whereby one girl had to be categorized as intellectually disabled. The diagnosis was given in the first years of the children’s lives according to the diagnostic criteria of the DSM-IV (for details, see Supplementary Table S1) and was cross-checked by experienced clinicians (i.e., medical doctors and/or psychologists) during enrolment of the study (for details of diagnosis, see Supplementary Table S1). All procedures performed in the studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the governmental ethics board of Graz, Austria, and registered at ClinicalTrials.gov. Involvement in the study was voluntary and not compensated.
3. Methods
Urine samples were taken between 7 and 9 a.m. in the morning after breakfast. Urine sample preparation comprised pre-extraction, enzymatic hydrolysis, extraction from the hydrolysis mixture, derivatization and gel filtration, as previously described [29,30,31]. The recovery standard was prepared by adding 2.5 µg of medroxyprogesterone to 1.5 mL of urine. The sample was extracted on a Sep-Pak C18 column (Waters Corp., Milford, MA, USA), dried, reconstituted in 0.1 M acetate buffer, pH 4.6, and hydrolyzed with powdered Helix pomatia enzyme (12.5 mg; Sigma Chemical Co., St. Louis, MO, USA) and 12.5 µL of β-glucuronidase/arylsulfatase liquid enzyme (Roche Diagnostics, Rotkreuz, Switzerland). The resulting free steroids were extracted on a Sep-Pak C18 cartridge. A mixture of internal standards (2.5 μg each of 5α-androstane-3α, 17α-diol, stigmasterol and cholesterol butyrate, and 0.15 μg of 3β5β-tetrahydroaldosterone) was added to this extract and the sample derivatized to form the methyloxime–trimethylsilyl ethers. Analyses were performed on a Hewlett-Packard gas chromatograph 6890 (Hewlett Packard, Palo Alto, CA, USA) with a mass selective detector 5973 by selective ion monitoring. One characteristic ion was chosen for each compound measured. The derivatized samples were analyzed during a temperature-programmed run (210–265 °C) over a 35 min period. The calibration standard consisted of a steroid mixture containing known quantities of all steroid metabolites to be measured. Responses and retention times were recorded regularly. In each case the ion-peak was quantified against that of the stigmasterol internal standard. All steroid hormone metabolites measured were corrected for urinary creatinine excretion. Apparent enzyme activities were calculated as ratios of the relevant metabolites measured (Androsterone, Etiocholanolone, DHEA, Androstendione, Testosterone, TH-11β-DH-Corticosterone, TH-Corticosterone, 5α-TH-Corticosterone and Cortisol), as previously described by us and others [29,30,31].
4. Statistical Analysis
Descriptive statistics were calculated with mean and SEM for all metabolites. In addition, all metabolites were analyzed concerning the normal distribution with the Jarque–Bera test. A hypothesis of the normal distribution of all of the sets of the measured values could not be rejected, at least on the alpha = 0.10 level, except for DHEA and tetrahydrocorticosterone in girls with autism [32]. Therefore, two-sided paired t-tests with a Bonferroni correction for multiple comparisons were performed for the metabolite classes, except for DHEA and tetrahydrocorticosterone where Wilcoxon Tests were performed due to the missing normal distribution. Data are presented as a mean ± SEM. Analyses were conducted with Graphpad Prism (GraphPad Software, Inc., La Jolla, CA, USA), Microsoft Excel (Microsoft Inc., Redmond, WA, USA) and SPSS (IBM Inc., Armonk, NY, USA).
5. Results
Despite thorough age, BMI and gender matching, with no significant differences for age and BMI in the cohort of affected versus healthy children, the urinary secretion of almost all steroid hormone metabolites tended to be altered in children with autism, reaching statistical significance more frequently in boys versus girls (for details, see previous analyses [12,13]). Using the assumption of a general dysregulation of steroid hormones as a starting point to elucidate the role of CYP17A1 activity in depth, Table 1 summarizes the steroid hormones of interest for a sample of 48 boys with autism versus an individually-pairwise matched cohort of healthy boys and, in addition, 21 girls with autism versus an individually-pairwise matched cohort of healthy girls. When focusing on activities of 17-alpha Hydroxylase and 17/20 Lyase, we refer to Figure 2 which tries to capture one aspect of excessive androgen production in autism. It is indicated by a low ratio of tetrahydro-11β-dehydrocorticosterone + tetrahydrocorticosterone + 5α-tetrahydrocorticosterone to the sum of androsterone + etiocholanone ((THA + THB + 5α-THB)/(AN + ET)), suggesting increased cytochrome P450 17A1 (CYP17A1) activity (Figure 2).

Table 1.
Steroid hormone metabolites in gender-segregated comparison of autistic to control children (mean ± SEM); dehydro (DH), tetrahydro (TH), hydroxy (OH).
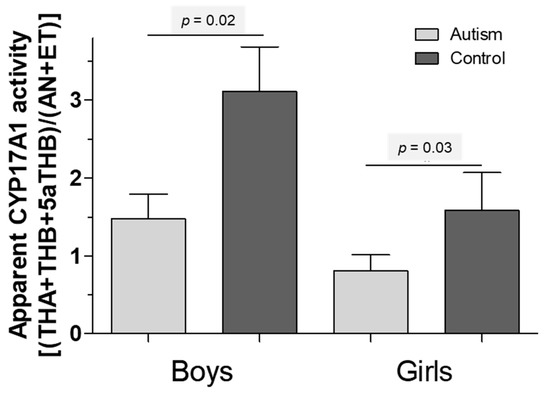
Figure 2.
A low ratio of tetrahydro-11β-dehydrocorticosterone + tetrahydrocorticosterone + 5α-tetrahydrocorticosterone to androsterone + etiocholanone ((THA + THB + 5α-THB)/(AN + ET)), suggesting an increased cytochrome P450 17A1 (CYP17A1) activity. (Grey: children with autism, black: healthy children).
Figure 3 encompasses the ratios of Cortisol to DHEA (Figure 3a), Cortisol to Androstenedione (Figure 3b) and Cortisol to Testosterone (Figure 3c). DHEA and cortisol characterize the main metabolites following 17α-hydroxylation towards either 11β- or further 20α-hydroxylation. The metabolism in children with autism is clearly shifted towards DHEA (p < 0.01). Increased ratios of testosterone to Cortisol can be detected when comparing boys with autism versus healthy controls (p < 0.01). Ratios of Androstenediol to Cortisol show about a double value for boys with autism (p < 0.01).
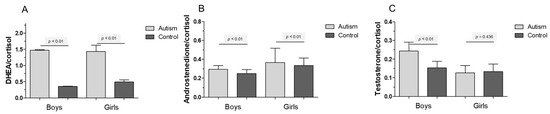
Figure 3.
(A) Ratios of dehydroepiandrosterone (DHEA) to cortisol in either control (dark grey bars) or children affected by ASD (light grey bars) for (B) Androstenedione/Cortisol and (C) Testosterone/Cortisol. High ratios in autistic children suggest a preference for DHEA over cortisol. Means ± SEM are given [33].
Figure 4 tries to capture the percent increase or decrease in boys and girls with autism versus healthy controls per metabolite. The ratios were calculated by dividing the mean concentration of a metabolite of boys and girls, respectively, with autism by the mean concentration of healthy boys and girls, respectively. Despite 5α-TH-Corticosterone in girls, the average metabolite levels are always higher in affected boys and girls with autism, respectively. By deciphering the information in detail, the concentration of Cortisol is higher compared to the average increase of the three metabolites TH-Corticosterone, 5α-TH-Corticosterone and TH-11β-DH-Corticosterone, indicating an increased 17-alpha activity and/or a higher stimulation by the CRH-ACTH system, as Cortisol is directly released by ACTH in the adrenal gland (Figure 4). The increase before was around 50% and almost the same before and after 17-alpha activity (Figure 4). Furthermore, in boys as well as girls, the average of the ratio of metabolites after 17/20 Lyase activity shows an increased activity of around 150%. In consequence, both catalyzing steps imply an increased activity of 17-alpha Hydroxylase and 17,20 Lyase, whereby the activity of 17/20 Lyase is more affected than 17-alpha Hydroxylase.
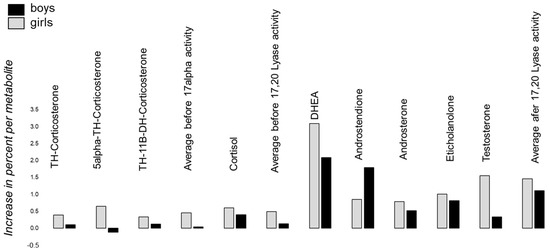
Figure 4.
The percent increase or decrease in boys (black) and girls (grey) with autism versus healthy controls per metabolite is shown. The ratios were calculated by dividing the mean concentration of a metabolite of boys and girls with autism, respectively, by the mean concentration of healthy boys and girls, respectively. The increase in Cortisol is somewhat higher than the average before 17-alpha activity, indicating that, in addition, a stimulation of HPAG via CRH-ACTH is present in affected subjects with autism.
6. Discussion
The aim of this study was to analyze potential alterations of the activity of CYP17A1 in affected boys and girls with autism versus healthy controls. The initially stated hypothesis, that there is no difference in the activity levels of CYP17A1 in children with autism versus healthy controls, seems to be wrong. As previously indicated by us and others, a dysregulation with a general dysregulation not solely of CYP17A1, but in general (not only androgens), is most conspicuous in boys and girls with autism [4,12,13,14]. Here, we focused in detail on the role of 17alpha Hydroxylase and 17/20 Lyase activity. Generally, as cortisol, as well as testosterone, shows an almost double value of steroid hormone concentrations in boys with autism as compared to the healthy cohort, a general pattern of a substantial increase in glucocorticoids and androgens seems to exist, allowing the suggestion that a principal overload of steroid metabolites is present in affected children with autism. This finding is in line with the Cholesterol Theory of autism, as cholesterol is the main precursor of steroid hormones [5] (Figure 1). In addition, it would explain the increased release of ACTH through stimulation on the level of the central nervous system by CRH (reviewed by Taylor and Corbett [3]). ACTH is a strong regulator of VEGF and, in consequence, Angiogenesis, which implies a number of effects on the nerve cell level supplying the paradigm of autism as a pervasive disorder [34,35]. However, we further believe that the disturbed HPAG-axis with an increased substrate availability is neither solely causative, nor the main cause of autism. This is indicated by the fact that the ratio of DHEA to cortisol is displaced towards the DHEA, suggesting a preference of routing precursors towards androgens instead of cortisol. Mechanistically, such conditions have been described as enhancing coenzyme or electron transfer availability via stimulated oxidoreductase activity to excess androgen exposure despite otherwise normal regulatory feedback responses [36]. This is conceivable in conditions of inherited stress susceptibility such as, for example, preterm birth, in induced stress conditions and during developmental periods with an inevitably high stimulatory impact on androgen production [36,37,38,39]. Nevertheless, alterations on the level of 17-alpha Hydroxylase and 17/20 Lyase and, in addition, the CRH-ACTH System are in line with the findings that the average increase of metabolites measured prior to 17-alpha Hydroxylase activity (TH-Corticosterone, 5α-TH-Corticosterone and TH-11β-DH-Corticosterone) is less increased in affected children with autism as compared to the increase of Cortisol, indicating, in addition, stimulation by the CRH-ACTH system (Figure 4). When focusing on the specific cascades altered, it becomes evident that both steps, the 17-alpha Hydroxylase and the 17/20 Lyase step, seem to be altered; however, the second step is more pronounced compared to the first (Figure 4). The activity of 17-alpha Hydroxylase increased by almost 50%, whereas the activity of 17/20 Lyase increased by around 150%. However, when reflecting the above-mentioned points we might forget the multiple phenotypes of autism, which might have an impact on the degree of alteration of CYP17A1 activity. For example, in our samples we had boys and girls with the previous diagnosis of DSM-IV of Kanner Syndrome, Asperger Syndrome and Atypical Autism (Supplementary Table S1). Nowadays it is well known that these diagnoses are not used anymore for different reasons, and it is simply spoken about autism spectrum disorders. In consequence, different degrees of social and communicative impairment are subsumed under one diagnosis. Thereby, the degree of social and communicative impairment might be correlated with the alterations of CYP17A1 activity. This aspect is not captured by our study design limiting the findings in consequence. Maybe in further studies it might be interesting to correlate the severity of the autism with CYP17A1 activity and to develop a cut-off of alteration, as this would allow one to implement a rational pharmacotherapy.
When searching for potential treatment options in autism, pharmaceuticals directly addressing CYP17A1 activity—especially 17/20 Lyase activity—should be considered as therapeutical targets. These pharmaceuticals, for example, abiraterone, are mainly known for the treatment of prostate cancer to reduce androgen concentrations [40,41,42]. Furthermore, Metformin might be an opportunity which is used for the metabolic consequences seen in polycystic ovary syndrome for its androgen-lowering and insulin-sensitizing properties [36]. Interestingly, the approach to use insulin-sensitizing drugs for affected subjects with autism is not new [43] and it was shown in a mouse model of autism that Metformin showed positive effects on social behavior in C57/BL6 mice [44]. Furthermore, Metformin was already used in the autistic context to lower weight gain due to antipsychotic therapy [45]. Yet, as well as for abiraterone the mechanism of action of Metformin remains unclear [46]. Two potential targets for metformin regulating steroid and glucose metabolism are AMP-activated protein kinase (AMPK) and the complex I of the mitochondrial respiratory chain, whereas the latter effect seems to be more relevant [36,47]. Similar to the in vivo situation, it was shown that metformin directly inhibited androgen production by decreasing CYP17A1 activity [36]. The effect of metformin on androgen production was dose dependent while inhibiting, especially, complex I of the respiratory chain in the mitochondria [36,47]. Interestingly, several studies have examined the electron transport chain function in the brain of children with autism, indicating alterations on different structural and functional levels of the mitochondria. One study examined eight children with autism versus eight healthy controls (4–10 years of age) and reported significantly lower electron transport chain complex activities in the cerebellum, frontal cortex and temporal cortex of the group with autism [48,49]. Another study of temporal lobe brain samples, taken from 20 individuals with autism and 25 controls, found decreased electron transport chain complex I and IV activities and protein content in the group with autism [49,50]. In one study of 15 individuals with autism and 15 controls, the mean activity of the citrate cycle enzyme aconitase was significantly decreased in the cerebellum and temporal lobe in the autism group [49,50,51]. Finally, another study of 14 individuals with autism and 12 controls reported mean reductions in electron transport chain complexes I (31%) and V (36%) activities, as well as in pyruvate dehydrogenase (35%) in the frontal cortex in the autism group, and, in addition, reported a higher mitochondrial DNA (mtDNA) copy number compared to the nuclear DNA in three different mitochondrial genes in the autism group [49,52]. As Metformin directly influences mitochondrial activity, these studies underly the possible usefulness of this pharmaceutical for the treatment of affected subjects with autism. Furthermore, Metformin inhibits oxidative stress [53] and, in addition, it is likely to suggest, that increased oxidative stress upregulates CYP17A1 activity via p38α [9,16,49,54]. Oxidant exposure significantly induced dehydroepiandrosterone production and increased p38α phosphorylation and activation, allowing one to state an association between high androgens and oxidative stress [54]. In detail, p38α inhibition attenuated the H2O2-mediated augmentation of DHEA production with relatively stable 17OHP levels, indicating that activated p38α mediates oxidative-stress-induced 17/20-lyase activation and, in consequence, stimulates androgen synthesis [54]. Interestingly, the effect of oxidative stress on 17alpha-Hydroxylase is far less clear [54], which implies its relevance for autism as 17-alpha Hydroxylase activity was only increased by around 50%, compared to 17/20 Lyase with an increase of 150% in our analyses. Furthermore, these suggestions are in line with a number of studies that indicated the relevance of oxidative stress for autism [49,55,56,57,58,59,60,61,62,63]. Genetic variations in glutathione-related pathways have been observed in affected subjects with autism [58,64,65,66] and have been correlated in some studies with autistic behavior [48,67,68]. Several case–control studies have reported lower concentrations of reduced glutathione (GSH), higher levels of oxidized glutathione (GSSG) and a decrease in the GSH/GSSG redox ratio in autism [56,57,58]. Furthermore, a lower mitochondrial GSH reserve was implied [48,69]. In addition, in some studies, lower GSH levels [70] and markers of increased oxidative stress [71] have been correlated with the severity of autistic traits [48]. Yet, these mentioned studies examined the peripheral markers of oxidative stress, including those found in blood and urine [48]. In addition, a number of studies have reported evidence of oxidative stress in post-mortem brain samples from individuals with ASD compared to controls [48,50,52,72,73,74,75,76,77]. Furthermore, the work by Gevi et al. [33] found that the Tryptophan and Purine metabolism was altered in affected subjects with autism, which has an influence on the mitochondrial activity of Melatonin as Melatonin is suggested to reduce oxidative stress [78]. It was indicated that affected subjects with autism transform tryptophan into xanthurenic acid and quinolinic acid (two catabolites of the kynurenine pathway) at the expense of kynurenic acid and, in particular, of melatonin [33]. Gevi et al., therefore, directly implied a large reduction of melatonin concentrations in affected children with autism and, in consequence, higher levels of oxidative stress [33].
To summarize, understanding the mechanisms regulating 17/20 lyase activity seems essential for the understanding of hyperandrogenic disorders, such as autism, and for the design of selective 17/20 lyase inhibitors, as this step seems mainly affected [16]. A CYP17A1 deficiency can be used as a mirror image concerning the alterations of steroid hormones while enhancing our understanding of the role in affected subjects with autism. To conclude, we suggest that the increased androgens are a result of oxidative stress and mitochondrial dysfunction, as discussed [49]. Pharmaceuticals addressing mitochondria such as Metformin might be a valid therapeutic opportunity for affected subjects with autism [49]. Studies directly elucidating markers of oxidative stress and steroid hormones might give further hints to decipher the enigma of autism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12060867/s1, Table S1: Characteristics of the clinical cohort according to DSM-IV with no significant difference for BMI and age between children with autism and healthy controls.
Author Contributions
Conceptualization, J.K.; Data curation, B.A.G. and J.K.; Formal analysis, B.A.G. and J.K.; Funding acquisition, B.A.G. and M.G.M.; Investigation, J.K.; B.A.G. and M.G.M.; Methodology, B.A.G. and J.K.; Project administration, B.A.G. and J.K.; Resources, B.D.; Software, B.A.G. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Gebauer Stiftung, Palatin Stiftung, Julia Bangerter-Rhyner Stiftung and Lindenhofstiftung.
Institutional Review Board Statement
The study was approved by the governmental ethics board of Graz, Austria (Approval Number FA8B-50.2), and registered at ClinicalTrials.gov ID NCT01197131.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is available on qualified request to the corresponding author.
Acknowledgments
We thank Gebauer Stiftung, Palatin Stiftung and Gottfried und Julia Bangerter-Rhyner Stiftung for the constant support to unsolve the enigma of autism. The funders had no involvement throughout the research process, including the decision for publication. We also thank all families and their children, who took part in the study, for their commitment and supporting our efforts to resolve the enigma of autism spectrum disease.
Conflicts of Interest
The authors declare having no conflict of interest.
References
- Asperger, H. Die Autistischen Psychopathen im Kindesalter. Arch. Für Psychiatr. Und Nervenkrankh. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Gasser, B. The Case of Hellmuth in The Autistic Psychopathy—Suffering from Cushing Syndrome? Glob. J. Intellect. Dev. Disabil. 2018, 4, 555643. [Google Scholar] [CrossRef]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Baron Cohen, S.; Auyeung, B.; Norgaard Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, C.; Fernell, E.; Kočovská, E.; Minnis, H.; Bourgeron, T.; Thompson, L.; Allely, C. The role of cholesterol metabolism and various steroid abnormalities in autism spectrum disorders: A hypothesis paper. Autism Res. 2017, 10, 1022–1044. [Google Scholar] [CrossRef]
- Niu, M.; Han, Y.; Dy, A.B.C.; Du, J.; Jin, H.; Qin, J.; Zhang, J.; Li, Q.; Hagerman, R.J. Autism Symptoms in Fragile X Syndrome. J. Child Neurol. 2017, 32, 903–909. [Google Scholar] [CrossRef]
- Sarachana, T.; Hu, V.W. Differential recruitment of coregulators to the RORA promoter adds another layer of complexity to gene (dys) regulation by sex hormones in autism. Mol. Autism 2013, 4, 39. [Google Scholar] [CrossRef]
- Sarachana, T.; Xu, M.; Wu, R.C.; Hu, V.W. Sex hormones in autism: Androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS ONE 2011, 6, e17116. [Google Scholar] [CrossRef]
- Porubek, D. CYP17A1: A biochemistry, chemistry, and clinical review. Curr. Top. Med. Chem. 2013, 13, 1364–1384. [Google Scholar] [CrossRef]
- Hu, V.W.; Sarachana, T.; Sherrard, R.M.; Kocher, K.M. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism 2015, 6, 7. [Google Scholar] [CrossRef]
- Hu, V.W.; Nguyen, A.; Kim, K.S.; Steinberg, M.E.; Sarachana, T.; Scully, M.A.; Soldin, S.J.; Luu, T.; Lee, N.H. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: Altered pathways in neuronal development and steroid biosynthesis. PLoS ONE 2009, 4, e5775. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. Are Steroid Hormones Dysregulated in Autistic Girls? Diseases 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. Steroid Metabolites Support Evidence of Autism as a Spectrum. Behav. Sci. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Janšáková, K.; Hill, M.; Čelárová, D.; Celušáková, H.; Repiská, G.; Bičíková, M.; Máčová, L.; Ostatníková, D. Alteration of the steroidogenesis in boys with autism spectrum disorders. Transl. Psychiatry 2020, 10, 340. [Google Scholar] [CrossRef]
- Gasser, B.A.; Buerki, S.F.; Kurz, J.; Mohaupt, M.G. Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. Int. J. Mol. Sci. 2021, 22, 12324. [Google Scholar] [CrossRef]
- Miller, W.L.; Tee, M.K. The post-translational regulation of 17,20 lyase activity. Mol. Cell Endocrinol. 2015, 408, 99–106. [Google Scholar] [CrossRef]
- Sun, M.; Mueller, J.W.; Gilligan, L.C.; Taylor, A.E.; Shaheen, F.; Noczyńska, A.; T’Sjoen, G.; Denvir, L.; Shenoy, S.; Fulton, P.; et al. The broad phenotypic spectrum of 17α-hydroxylase/17,20-lyase (CYP17A1) deficiency: A case series. Eur. J. Endocrinol. 2021, 185, 729–741. [Google Scholar] [CrossRef]
- Rubtsov, P.; Nizhnik, A.; Dedov, I.; Kalinchenko, N.; Petrov, V.; Orekhova, A.; Spirin, P.; Prassolov, V.; Tiulpakov, A. Partial deficiency of 17α-hydroxylase/17,20-lyase caused by a novel missense mutation in the canonical cytochrome heme-interacting motif. Eur. J. Endocrinol. 2015, 172, K19–K25. [Google Scholar] [CrossRef][Green Version]
- Yanase, T.; Simpson, E.R.; Waterman, M.R. 17Alpha-hydroxylase/17,20-lyase deficiency: From clinical investigation to molecular definition. Endocr. Rev. 1991, 12, 91–108. [Google Scholar] [CrossRef]
- Taniyama, M.; Tanabe, M.; Saito, H.; Ban, Y.; Nawata, H.; Yanase, T. Subtle 17α-hydroxylase/17,20-lyase deficiency with homozygous Y201N mutation in an infertile woman. J. Clin. Endocrinol. Metab. 2005, 90, 2508–2511. [Google Scholar] [CrossRef][Green Version]
- Yanase, T.; Kagimoto, M.; Suzuki, S.; Hashiba, K.; Simpson, E.R.; Waterman, M.R. Deletion of a phenylalanine in the N-terminal region of human cytochrome P-450(17 alpha) results in partial combined 17 alpha-hydroxylase/17,20-lyase deficiency. J. Biol. Chem. 1989, 264, 18076–18082. [Google Scholar] [CrossRef]
- Yao, F.; Huang, S.; Kang, X.; Zhang, W.; Wang, P.; Tian, Q. CYP17A1 mutations identified in 17 Chinese patients with 17α-hydroxylase/17,20-lyase deficiency. Gynecol. Endocrinol. 2013, 29, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Hill, M.; Urbanowicz, E.; Rok-Bujko, P.; Bieńkowski, P.; Namysłowska, I.; Mierzejewski, P. Marked elevation of adrenal steroids, especially androgens, in saliva of prepubertal autistic children. Eur. Child Adolesc. Psychiatry 2013, 23, 485–498. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.; Hamza, R.T.; Ayad, M.S.; Mahmoud, N.H. Hyperandrogenemia in male autistic children and adolescents: Relation to disease severity. Int. J. Adolesc. Med. Health 2014, 26, 79–84. [Google Scholar] [CrossRef]
- Katagiri, M.; Kagawa, N. The regulation of steroidogenesis by 17 alpha-hydroxylase/17,20-lyase (P450c17). Folia Pharmacol. Jpn. 1998, 112, 43–50. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Hyun, D.H.; Lee, G.H. Cytochrome b5 reductase, a plasma membrane redox enzyme, protects neuronal cells against metabolic and oxidative stress through maintaining redox state and bioenergetics. Age 2015, 37, 122. [Google Scholar] [CrossRef]
- Popper, K.R. Logik der Forschung; Mohr Siebeck: Tübingen, Germany, 1969. [Google Scholar]
- Xu, S.; Hu, S.; Yu, X.; Zhang, M.; Yang, Y. 17α-hydroxylase/17,20-lyase deficiency in congenital adrenal hyperplasia: A case report. Mol. Med. Rep. 2016, 15, 339–344. [Google Scholar] [CrossRef]
- Shackleton, C.H.L. Profiling steroid hormones and urinary steroids. J. Chromatogr. 1986, 379, 91–156. [Google Scholar] [CrossRef]
- Shackleton, C.H.L. Role of a Disordered Steroid Metabolome in the Elucidation of Sterol and Steroid Biosynthesis. Lipids 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Vogt, B.; Dick, B.; N’Gankam, V.; Frey, F.J.; Frey, B.M. Reduced 11B-hydroxysteroid dehydrogenase activity in patients with the nephrotic syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 811–814. [Google Scholar]
- Jarque, C.M.; Bera, A.K. Efficent tests for normality, homoscedasticity and serial independence of regression residuals. Econ. Lett. 1980, 6, 255–259. [Google Scholar] [CrossRef]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Mesiano, S.; Taylor, R.N.; Ferrara, N.; Jaffe, R.B. Corticotropin regulates vascular endothelial growth factor expression in human fetal adrenal cortical cells. J. Clin. Endocrinol. Metab. 1998, 83, 1342–1347. [Google Scholar] [CrossRef]
- Ishimoto, H.; Ginzinger, D.G.; Jaffe, R.B. Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: Implications for coordinated adrenal organ growth and angiogenesis. J. Clin. Endocrinol. Metab. 2006, 91, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Hahn, D.; Kempná, P.; Hofer, G.; Nuoffer, J.M.; Mullis, P.E.; Flück, C.E. Metformin inhibits human androgen production by regulating steroidogenic enzymes HSD3B2 and CYP17A1 and complex I activity of the respiratory chain. Endocrinology 2012, 153, 4354–4366. [Google Scholar] [CrossRef]
- Jacobson, L. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr. Physiol. 2014, 4, 715–738. [Google Scholar]
- Bitsika, V.; Sharpley, C.F.; Sweeney, J.A.; McFarlane, J.R. HPA and SAM axis responses as correlates of self- vs parental ratings of anxiety in boys with an Autistic Disorder. Physiol. Behav. 2014, 127, 1–7. [Google Scholar] [CrossRef]
- Markou, A.; Sertedaki, A.; Kaltsas, G.; Androulakis, I.; Marakaki, C.; Pappa, T.; Gouli, A.; Panastasiou, L.; Fountoulakis, S.; Zacharoulis, A.; et al. Piaditis, Stress-induced aldosterone hyper-secretion in a substantial subset of patients with essential hypertension. J. Clin. Endocrinol. Metab. 2015, 100, 2857–2864. [Google Scholar] [CrossRef]
- Cheong, E.J.Y.; Nair, P.; Neo, R.W.Y.; Tu, H.T.; Lin, F.; Chiong, E.; Esuvaranathan, K.; Fan, H.; Szmulewitz, R.Z.; Peer, C.J.; et al. Slow-, Tight-Binding Inhibition of CYP17A1 by Abiraterone Redefines Its Kinetic Selectivity and Dosing Regimen. J. Pharmacol. Exp. Ther. 2020, 374, 438–451. [Google Scholar] [CrossRef]
- Wróbel, T.M.; Rogova, O.; Sharma, K.; Rojas Velazquez, M.N.; Pandey, A.V.; Jørgensen, F.S.; Arendrup, F.S.; Andersen, K.L.; Björkling, F. Synthesis and Structure-Activity Relationships of Novel Non-Steroidal CYP17A1 Inhibitors as Potential Prostate Cancer Agents. Biomolecules 2022, 12, 165. [Google Scholar] [CrossRef]
- Auchus, R.J. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J. Steroid Biochem. Mol. Biol. 2016, 165, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Boris, M.; Kaiser, C.C.; Goldblatt, A.; Elice, M.W.; Edelson, S.M.; Adams, J.B.; Feinstein, D.L. Effect of pioglitazone treatment on behavioral symptoms in autistic children. J. Neuroinflamm. 2007, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A.; Kurz, J.; Senn, W.; Escher, G.; Mohaupt, M.G. Stress-induced alterations of social behavior are reversible by antagonism of steroid hormones in C57/BL6 mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wink, L.K.; Adams, R.; Pedapati, E.V.; Dominick, K.C.; Fox, E.; Buck, C.; Erickson, C.A. Brief report: Metformin for antipsychotic_induced weight gain in youth with autism spectrum disorder. J. Autism Dev. Disord. 2017, 47, 2290–2294. [Google Scholar] [CrossRef]
- DeVore, N.M.; Scott, E.E. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature 2012, 482, 116–119. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and Molecular Mechanisms of Metformin: An Overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef]
- Chauhan, A.; Gu, F.; Essa, M.M.; Wegiel, J.; Kaur, K.; Brown, W.T.; Chauhan, V. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 2011, 117, 209–220. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef]
- Tang, G.; Gutierrez Rios, P.; Kuo, S.-H.; Akman, H.O.; Rosoklija, G.; Tanji, K.; Dwork, A.; Schon, E.A.; DiMauro, S.; Goldman, J.; et al. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol. Dis. 2013, 54, 349–361. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef]
- Gu, F.; Chauhan, V.; Chauhan, A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: Alterations in the activities and protein expression of glutathione-related enzymes. Free Radic. Biol. Med. 2013, 65, 488–496. [Google Scholar] [CrossRef]
- Hartwig, J.; Loebel, M.; Steiner, S.; Bauer, S.; Karadeniz, Z.; Roeger, C.; Skurk, C.; Scheibenbogen, C.; Sotzny, F. Metformin Attenuates ROS via FOXO3 Activation in Immune Cells. Front. Immunol. 2021, 12, 581799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Han, B.; Fan, M.; Wang, N.; Wang, H.; Zhu, H.; Cheng, T.; Zhao, S.; Song, H.; Qiao, J. Oxidative stress increases the 17,20-lyase-catalyzing activity of adrenal P450c17 through p38α in the development of hyperandrogenism. Mol. Cell. Endocrinol. 2019, 484, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yorbik, O.; Sayal, A.; Akay, C.; Akbiyik, D.I.; Sohmen, T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 341–343. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [PubMed]
- James, S.J.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am. J. Clin. Nutr. 2008, 89, 425–430. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef]
- Ming, X.; Brimacombe, M.; Chaaban, J.; Zimmerman-Bier, B.; Wagner, G.C. Autism spectrum disorders: Concurrent clinical disorders. J. Child Neurol. 2007, 23, 6–13. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Yao, Y.; Walsh, W.J.; McGinnis, W.R.; Praticò, D. Altered vascular phenotype in autism: Correlation with oxidative stress. Arch. Neurol. 2006, 63, 1161–1164. [Google Scholar] [CrossRef]
- Al-Gadani, Y.; El-Ansary, A.; Attas, O.; Al-Ayadhi, L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin. Biochem. 2009, 42, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, S.; Fuchs, G.J.; Schulz, E.; Lopez, M.; Kahler, S.G.; Fussell, J.J.; Bellando, J.; Pavliv, O.; Rose, S.; Seidel, L.; et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J. Autism Dev. Disord. 2011, 42, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Boris, M.; Goldblatt, A.; Galanko, J.; James, S.J. Association of MTHFR gene variants with autism. J. Am. Phys. Surg. 2004, 9, 106–108. Available online: http://www.jpands.org/vol9no4/boris.pdf (accessed on 15 March 2022).
- Bowers, K.; Li, Q.; Bressler, J.; Avramopoulos, D.; Newschaffer, C.; Fallin, M.D. Glutathione pathway gene variation and risk of autism spectrum disorders. J. Neurodev. Disord. 2011, 3, 132–143. [Google Scholar] [CrossRef]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Bernardina, B.D.; Bonassi, S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef]
- Goin-Kochel, R.P.; Porter, A.E.; Peters, S.U.; Shinawi, M.; Sahoo, T.; Beaudet, A.L. The MTHFR 677C–>T polymorphism and behaviors in children with autism: Exploratory genotype-phenotype correlations. Autism Res. 2009, 2, 98–108. [Google Scholar] [CrossRef]
- Guo, T.; Chen, H.; Liu, B.; Ji, W.; Yang, C. Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet. Test. Mol. Biomark. 2012, 16, 968–973. [Google Scholar] [CrossRef]
- James, S.J.; Rose, S.; Melnyk, S.; Jernigan, S.; Blossom, S.; Pavliv, O.; Gaylor, D.W. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009, 23, 2374–2383. [Google Scholar] [CrossRef]
- Adams, J.B.; Baral, M.; Geis, E.; Mitchell, J.; Ingram, J.; Hensley, A.; Zappia, I.; Newmark, S.; Gehn, E.; Rubin, R.A.; et al. The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J. Toxicol. 2009, 2009, 532640. [Google Scholar] [CrossRef]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative stress and erythrocyte membrane alterations in children with autism: Correlation with clinical features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef]
- Evans, T.A.; Siedlak, S.L.; Lu, L.; Fu, X.; Wang, Z.; McGinnis, W.R.; Fakhoury, E.; Castellani, R.J.; Hazen, S.L.; Walsh, W.J.; et al. The autistic phenotype exhibits a remarkably localized modification of brain protein by products of free radical-induced lipid oxidation. Am. J. Biochem. Biotechnol. 2008, 4, 61–72. [Google Scholar] [CrossRef]
- López-Hurtado, E.; Prieto, J.J. A microscopic study of language-related cortex in autism. Am. J. Biochem. Biotechnol. 2008, 4, 130–145. [Google Scholar] [CrossRef][Green Version]
- Sajdel-Sul, E.M.; Windom, H.; McGinnis, W.; Lipinski, B.; Audhya, T. Oxidative stress in autism: Elevated cerebellar 3-nitrotyrosine levels. Am. J. Biochem. Biotechnol. 2008, 4, 73–84. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Xu, M.; Koibuchi, N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum 2009, 8, 366–372. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Xu, M.; McGinnis, W.; Koibuchi, N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD). Cerebellum 2010, 10, 43–48. [Google Scholar] [CrossRef]
- Palmieri, L.; Papaleo, V.; Porcelli, V.; Scarcia, P.; Gaita, L.; Sacco, R.; Hager, J.; Rousseau, F.; Curatolo, P.; Manzi, B.; et al. Altered calcium homeostasis in autism-spectrum disorders: Evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol. Psychiatry 2008, 15, 38–52. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.-X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).