Abstract
Selenium compounds are relatively rare as minerals; there are presently only 118 known mineral species. This work is intended to codify and systematize the data of mineral systems and the thermodynamics of selenium minerals, which are unstable (selenides) or formed in near-surface environments (selenites), where the behavior of selenium is controlled by variations of the redox potential and the acidity of solutions at low temperatures and pressures. These parameters determine the migration of selenium and its precipitation as various solid phases. All selenium minerals are divided into four groups—native selenium, oxide, selenides, and oxysalts—anhydrous selenites (I) and hydrous selenites and selenates (II). Within each of the groups, minerals are codified according to the minimum number of independent elements necessary to define the composition of the mineral system. Eh–pH diagrams were calculated and plotted using the Geochemist’s Workbench (GMB 9.0) software package. The Eh–pH diagrams of the Me–Se–H2O systems (where Me = Co, Ni, Fe, Cu, Pb, Zn, Cd, Hg, Ag, Bi, As, Sb, Al and Ca) were plotted for the average contents of these elements in acidic waters in the oxidation zones of sulfide deposits. The possibility of the formation of Zn, Cd, Ag and Hg selenites under natural oxidation conditions in near surface environments is discussed.
1. Introduction
Selenium release and pollution is a worldwide phenomenon that results from a wide variety of anthropogenic activities, such as agriculture, mining, and other process industries [1]. Selenium is a potentially toxic element, and mining-related selenium release was a major concern during the last decade as high concentrations were reported at some mine sites [2,3]. Selenium contamination is vast, affecting both aquatic and terrestrial ecosystems, and has therefore attracted the attention of natural resource and water quality regulators around the world [4,5]. Due to the high mobility of selenium in oxidizing geochemical environments, the behavior of selenium is also important in safety analyses of radioactive waste repositories [6,7]. Khamkhash et al. [1] reported a brief introduction to selenium chemistry and toxicity, presented a detailed review of currently available techniques for removing selenium from industrial/mining wastewater, and discussed mining-related selenium contamination from Alaskan mines. Se mobility, bioavailability and toxicity are controlled by the various possible oxidation states (the most common being −2, 0, +4 and +6) that prevail under various conditions. These characteristics of selenium are affected by the redox conditions and the pH, which also plays a crucial role in selenium behavior in near-surface environments.
The oxidation zone of selenide or selenium-bearing sulfide deposits is one of the prime contributors of selenium release into the environment. The mining of coal, precious metals (gold and silver), and metallic sulfides are key contributors of selenium from mining operations [2] exposing selenium-bearing compounds to air and water. This mobilizes selenium into aquatic systems where it bioaccumulates in the food chain, thereby escalating its hazardous effects [1].
Previously, we have characterized the selenium oxysalts (selenites and selenates) and evaluated the accuracy of the thermodynamic constants of the selenites [8]. The objective of this paper is to characterize all selenium minerals as natural mineral systems and to review the thermodynamic constants of the selenium minerals, which are unstable or formed during the oxidation of selenides and selenium-bearing sulfide ores, and to propose data at the standard state (25 °C, 1 bar pressure).
2. Materials and Methods
2.1. Mineral Systems of Selenium Minerals
The selenium minerals are relatively rare in nature; only 118 mineral species are presently known (Supplementary Table S1). As shown [9,10], any mineral can be assigned to a specific system, each component of which is a species-defining chemical element (cf. [11,12,13]) determined by the rules of the new mineral species definition [14,15,16]. For mineral coding, we used the sequence of species-defining chemical element symbols according to the so-called “thermochemical” sequence of chemical elements (Figure 1) [9,10]. For example, giraudite, Cu6Cu4Zn2(AsSe3)4S, responds to the system SSeAsZnCu, while prewittite, K2Pb3Zn2Cu12(SeO3)4O4Cl20, responds to the system OClSePbZnCuK.
Figure 1.
Thermochemical sequence of chemical elements and corresponding single-component systems.
As in all contemporary mineral classifications, selenium minerals is clearly divided into four groups—native selenium, oxide, selenides, and oxysalts—anhydrous selenites (I) and hydrous selenites and selenates (II). Within each of these groups, minerals can be classified according to the minimum number of components required for their formation [9,10]. The proposed classification of selenium mineral systems is given in Table 1. An important advantage of this classification of selenium mineral systems is the ability to use computer technology to organize, store and retrieve thermodynamic data.

Table 1.
Mineral systems and ΔfG0298 of selenium minerals.
2.2. Thermodynamics
The physico-chemical modeling of mineral equilibria is based on the thermodynamic constants of minerals and aqueous species (values of the Gibbs energy of formation, ΔfG0298). These data are mostly calculated from calorimetric measurements (e.g., [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]) or experimental determinations of solubility (e.g., [35,36,37,38,39,40,41,42,43,44]). Such works are very numerous; their results are not always consistent with each other. A detailed analysis of all thermodynamic data for selenium minerals is beyond the scope of this article; this can be found, for example, in the review [45], and primarily in the reference book [46], which presents a scrupulous critical review of all the published experimental measurements. We used this reference book [46] in the preparation of Table 1, which contains the values of the Gibbs energy of formation (ΔfG0298) for selenium minerals and their synthetic analogues. Additional thermodynamic data (ΔfG0298 for solid phases not containing selenium and aqueous species) for the calculation of diagrams have been taken from [47]. In addition, some of our recent articles were used as sources of ΔfG0298 for certain selenites [17,18,48,49]. It should be emphasized that the values of the Gibbs energy of formation are known only for 25 (Table 1) of the 118 selenium minerals.
3. Results and Discussion
Thermodynamic modeling of the mineral-forming processes in the oxidation zone of ore deposits is based on the analysis of Eh–pH diagrams. In this study, the calculation and construction of Eh–pH diagrams were carried out by means of the Geochemist’s Workbench software (GMB 9.0) [50]. The calculation of the diagrams was predated by the introduction of new thermodynamic data and some elements (Cd, Sb, Bi) into the database and the specification of some constants. The activity coefficients were calculated with the Debye–Hückel equation. Eh–pH diagrams of Me–Se–H2O systems (where Me = Co, Ni, Fe, Cu, Pb, Zn, Cd, Hg, Ag, Bi, As, Sb, Al and Ca) have been constructed for the average contents of these elements in acidic waters of the oxidation zones of sulfide deposits [51]. It should be noted that Eh–pH diagrams of Me–Se–H2O systems (where Me = Co, Ni, Fe, Cu, Pb, Zn, Al and Ca) have been discussed in detail previously [8] and therefore we present here only their brief description.
3.1. Thermodynamic System Co–Se–H2O
In this system, three selenides (bornhardtite, CoCo2Se4; freboldite, CoSe; and trogtalite, CoSe2) and one selenite (cobaltomenite, CoSeO3∙2H2O) were reported. For cobalt selenides the thermodynamic data are known only for trogtalite and freboldite (Table 1). These data were used for plotting the Eh–pH diagram of the Co–Se–H2O system (Figure 2). The thermal stability, solubility and thermochemical calorimetric investigations were carried out on a synthetic analogue of cobaltomenite [49,52,53]. The Eh–pH diagram of the Co–Se–H2O system (Figure 2) contains, in addition to the stability field of native selenium, a stability field of Co3O4, which is unknown in nature, and the stability field of trogdalite. Cobaltomenite appears in the environment covering the pH range of 4.5 to 7.5, while Eh is not too high.
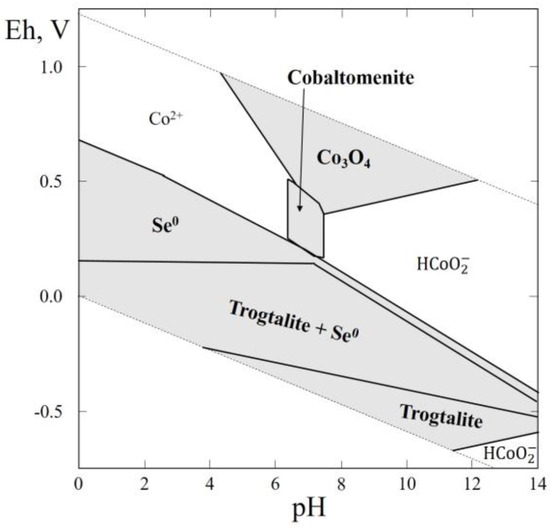
Figure 2.
Eh–pH diagram of the system OHSeCo (Co–Se–H2O) at 25 °C and the activities of the components: aΣSe = 10−4, aΣCo = 10−3. Here and in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14 and Figure 15, the solid lines are boundaries of the stability fields of the solid phases.
3.2. Thermodynamic System Ni–Se–H2O
In this system six selenides (sederholmite, and mäkinenite, NiSe; penroseite, and kullerudite, NiSe2; trüstedtite, and wilkmanite, NiNi2Se4) were reported, but the thermodynamic data are known only for penroseite and sederholmite (Table 1). These data were used for plotting the Eh–pH diagram of the Ni–Se–H2O system (Figure 3). The thermal stability, the solubility and the thermochemical calorimetric investigations were carried out on a synthetic analogue of ahlfeldite [49,52,53]. The Eh–pH diagram of the Ni–Se–H2O system (Figure 3) contains the stability fields of native selenium, penroseite and sederholmite, and a wide field of bunsenite. Ahlfeldite appears in the environment covering the pH range of 4.3 to 8.2, while the Eh is not too high at the temperature fluctuations corresponding to the environmental conditions.
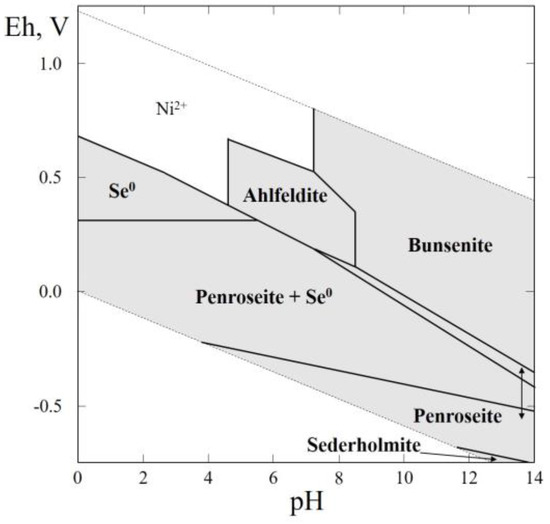
Figure 3.
Eh–pH diagram of the system OHSeNi (Ni–Se–H2O) at 25 °C and the activities of the components: aΣSe = 10−4, aΣNi = 10−2.
3.3. Thermodynamic System Fe–Se–H2O
The Table 1 shows that in this system, three selenides (achávalite, FeSe; dzharkenite, and ferroselite, FeSe2), native selenium, and one selenite, mandarinoite (Fe2(SeO3)3·6H2O), were reported. The composition, solubility, and the thermal behavior of iron selenite were discussed earlier [8]. Figure 4 presents Eh–pH diagrams of the Fe–Se–H2O system, which contains wide stability fields of native selenium, hematite and ferroselite, and small fields corresponding to the crystallization of oxides (magnetite, wüstite). The field of mandarinoite appears in acid areas at a high positive Eh.
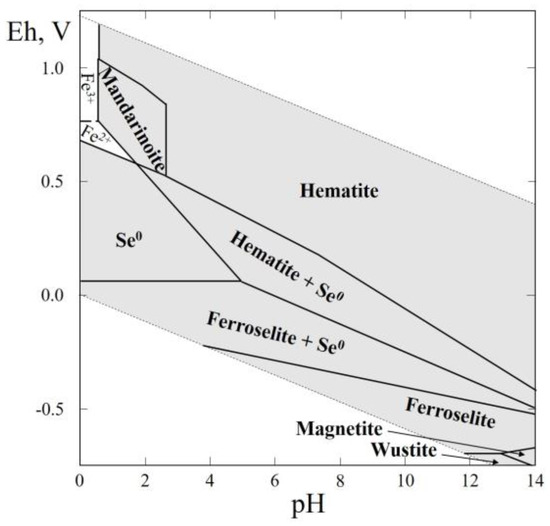
Figure 4.
Eh–pH diagram of the OHSeFe (Fe–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣFe = 10−2. The dotted line is the boundary between fields of Fe2+ and Fe3+.
3.4. Thermodynamic System Cu–Se–H2O
In this thermodynamic system, nine selenides (klockmannite, CuSe; krut’aite, petříčekite, and bambollaite, CuSe2; bellidoite, and berzelianite, Cu2Se; umangite, Cu3Se2; athabascaite, Cu5Se4; geffroyite, Cu9Se8) were reported, but the thermodynamic data are only available for CuSe (Table 1) and it was used for plotting the Eh–pH diagram of the Cu–Se–H2O system (Figure 5). Also in this system, two natural polymorphous modifications exist among water-containing selenites without additional anions. They have the CuSeO3·2H2O formula: chalcomenite (orthorhombic) and clinochalcomenite (monoclinic). Chalcomenite is the more abundant mineral; clinochalcomenite is poorly documented and remains a controversial mineral species. The thermal stability, the solubility and thermochemical calorimetric investigations were carried out on a synthetic analogue of chalcomenite [17,54,55].
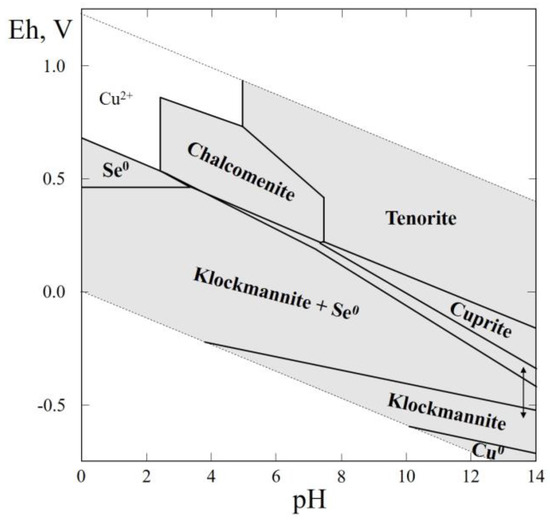
Figure 5.
Eh–pH diagram of the OHSeCu (Cu–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣCu = 10−2.
The Eh–pH diagram of the Cu–Se–H2O system is shown in Figure 5. It is seen that klockmannite, copper oxides (cuprite, tenorite), native selenium, and native copper (in the alkaline region of negative Eh) are stable. Chalcomenite (CuSeO3·2H2O) occurs in a slightly acid environment and at a rather high positive Eh.
3.5. Thermodynamic System Pb–Se–H2O
In this thermodynamic system, one selenide (clausthalite, PbSe), and two anhydrous lead selenites (molybdomenite, PbSeO3, and plumboselite, Pb3(SeO3)O2) were reported (Table 1), but the thermodynamic data are only available for PbSe, and PbSeO3 (Table 1). These data were used for plotting the Eh–pH diagram of the Pb–Se–H2O system (Figure 6). The greatest part of the diagram contains stability fields of solid phases and is characterized by the fields of native selenium, claustalite (PbSe), plattnerite (PbO2), litharge (Pb3O4) and massicot (PbO). It is noteworthy that a wide stability field of molybdomenite (PbSeO3) appears covering the pH range of 4 to 9.5.
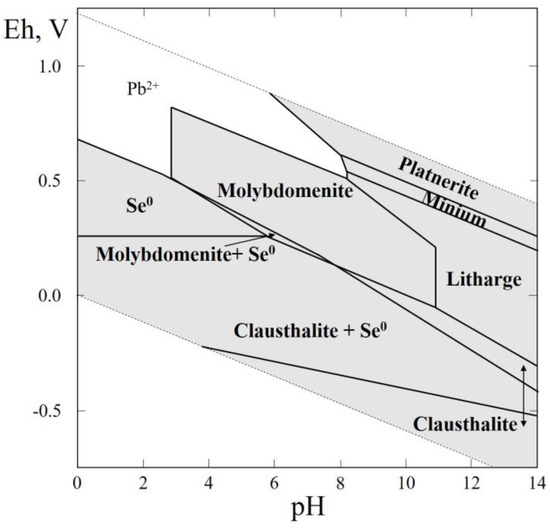
Figure 6.
Eh–pH diagram of the OSePb (Pb–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣPb = 10−4.
3.6. Thermodynamic System Zn–Se–H2O
In this system only one selenide, stilleite (ZnSe), was reported (Table 1). Natural hydrous Zn selenites have not yet been found, but their formation in nature is quite possible, for example, in the oxidation zones of Se-bearing sulfide ores, in which sphalerite or stilleite (ZnSe) is a source of Zn. The rarity and difficulty of their identification may explain why hydrous zinc selenites have not yet been detected in nature. However, Pekov et al. [56] have recently found a new anhydrous Zn selenite mineral (zincomenite, ZnSeO3) in fumarole products in the Tolbachik volcano.
The solubility and the thermal behavior of synthetic ZnSeO3·2H2O, and ZnSeO3·H2O were discussed in [55,57]. It was shown that ZnSeO3·2H2O is a more stable phase than ZnSeO3·H2O under environmental conditions. The monohydrate appears to be a metastable phase. The thermochemical calorimetric investigations were carried out on synthetic zinc selenites, ZnSeO3·2H2O and ZnSeO3·H2O [18].
Finally, the Eh–pH plot of the Zn–Se–H2O system is shown in Figure 7. This system attracts interest because it allows the estimation of the physico-chemical parameters of the formation of hydrous Zn selenite. As we see in Figure 7, the diagram contains the stability fields of native selenium, stilleite, zincite (ZnO), and zinc selenite (ZnSeO3·2H2O). As follows from this diagram, the physico-chemical parameters of zinc selenite stability—pH, Eh, and the activities of Zn and Se—are close to those of cobalt and nickel selenites (ahlfeldite and cobaltomenite) [49], and ZnSeO3·2H2O is the stable phase at the temperature fluctuations corresponding to environmental conditions.
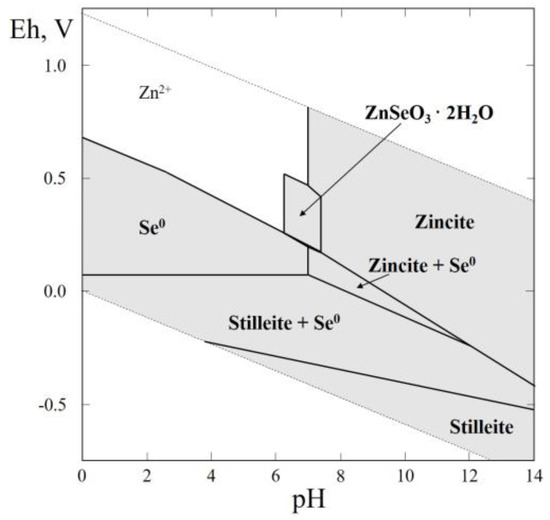
Figure 7.
Eh–pH diagram of the OHSeZn (Zn–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣZn = 10−2.
3.7. Thermodynamic System Cd–Se–H2O
In this thermodynamic system, only one selenide (cadmoselite, CdSe) was reported (Table 1). Cadmoselite was found in Ust’ Uyok deposit (Turan District, Tuva Republic, Eastern-Siberian Region, Russia) as fine xenomorphic disseminations cementing sandstone associated with ferroselite, clausthalite, amorphous selenium, Cd-bearing sphalerite, and pyrite [58]. The thermodynamic constants of synthetic hydrous cadmium selenite were reported [18]. These data were used for plotting the Eh–pH diagram of the Cd–Se–H2O system (Figure 8). The greatest part of the diagram contains stability fields of solid phases and is characterized by the fields of native selenium, cadmoselite and hydrous Cd selenite. It is noteworthy that a wide stability field of hydrous Cd selenite (CdSeO3·H2O) appears covering the pH range of 2 to 12.5. Therefore, in terms of geochemistry, hydrous cadmium selenite is theoretically able to form in the oxidation zones of Se-bearing sulfide ores, in which Cd-bearing sphalerite (Zn, Cd)S or Cd-bearing is a source of Cd. The rarity and difficulty of their identification may explain why hydrous cadmium selenites have not yet been detected in nature.
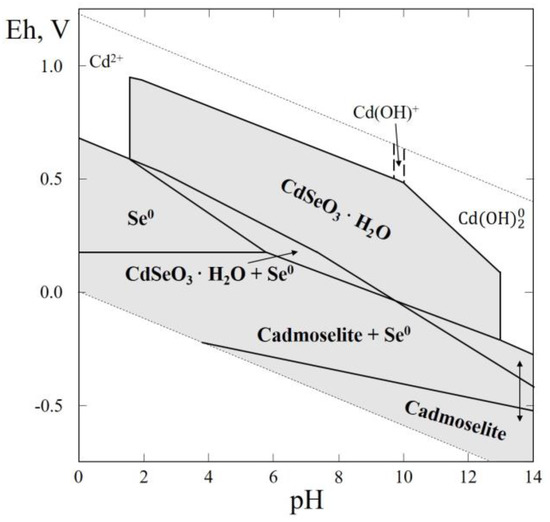
Figure 8.
Eh–pH diagram of the OHSeCd (Cd–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣCd = 10−2. The dotted lines are the boundaries between fields of Cd2+, Cd(OH)+ and Cd(OH)20.
3.8. Thermodynamic System Hg–Se–H2O
In this thermodynamic system, one selenide, tiemannite (HgSe) was reported. Natural Hg selenites have not yet been found in nature. Tiemannite was described for the first time at the St Lorenz Mine, Burgstatt veins, Clausthal-Zellerfeld, Harz, Lower Saxony, Germany [59] in association with clausthalite, berzelianite, naumannite, pyrite, sphalerite, galena, quartz, bournonite. Tiemannite is a common mineral, it has been found in 75 localities (according www.mindat.org), associated with low-sulfur hydrothermal deposits with other selenides, and also in mercury deposits. The Eh–pH plots of the Hg–Se–H2O system are shown in Figure 9. This system is interesting because it allows the estimation of the physico-chemical parameters of the formation of anhydrous Hg selenites. The greatest part of the diagram contains the stability fields of tiemannite, mercury and anhydrous Hg selenites (HgSeO3, Hg2SeO3).
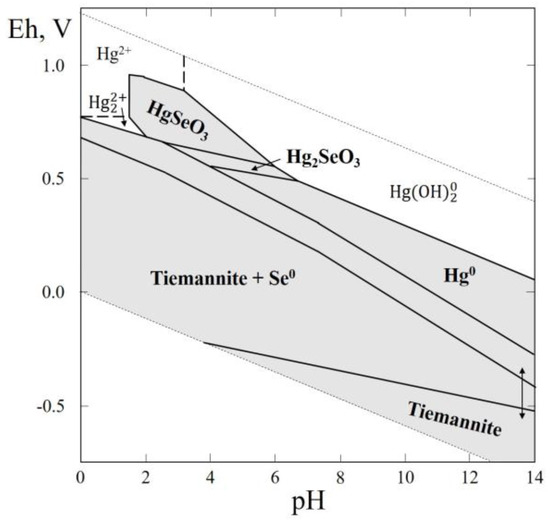
Figure 9.
Eh–pH diagram of the OHSeHg (Hg–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣHg = 10−5. The dotted lines are the boundaries between fields of Hg2+, Hg22+, and Hg(OH)20.
3.9. Thermodynamic System Ag–Se–H2O
In this thermodynamic system, only one mineral, naumannite (Ag2Se) was reported. Naumannite was found in Germany, in the Harz Mountains, at Tilkerode [60], in hydrothermal veins deficient in sulfur, associated with other selenides, quartz, and carbonates. Natural Ag selenites have not yet been found, but their formation in nature is quite possible in the oxidation zones of Se-bearing sulfide ores, because naumannite is a widespread mineral (according to mindat.org it was discovered in 156 localities). The thermodynamic data recommended in the directory [46] was used for plotting the Eh–pH diagram of the Ag–Se–H2O system (Figure 10). The greatest part of the diagram contains stability fields of naumannite, native selenium and silver, with small field of Ag2O in extremely alkaline areas at a high positive Eh. It is noteworthy that a small stability field of Ag selenite (Ag2SeO3) appears covering the pH range of 6 to 7.5.
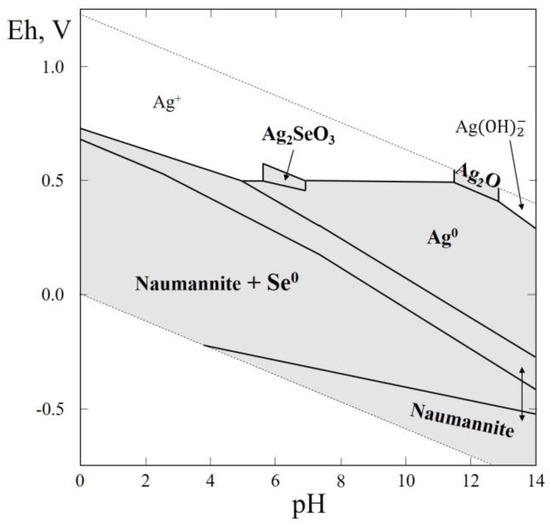
Figure 10.
Eh–pH diagram of the OHSeAg (Ag–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣAg = 10−5.
3.10. Thermodynamic System Bi–Se–H2O
The Table 1 shows that in this system four mineral species—nevskite (BiSe), guanajuatite (Bi2Se3), paraguanajuatite (Bi2Se3), and laitakarite (Bi4Se3)—were reported, but only for two of them (nevskite and paraguanajuatite) are the thermodynamic data available (Table 1). Both minerals occur in hydrothermal deposits of low to medium temperatures. The greatest part of the Eh–pH diagram of Bi–Se–H2O system is characterized by the fields of paraguanajuatite, native selenium, and a small bismuth stability field. It is noteworthy that a wide stability field of paraguanajuatite appears covering the wide pH range (Figure 11).
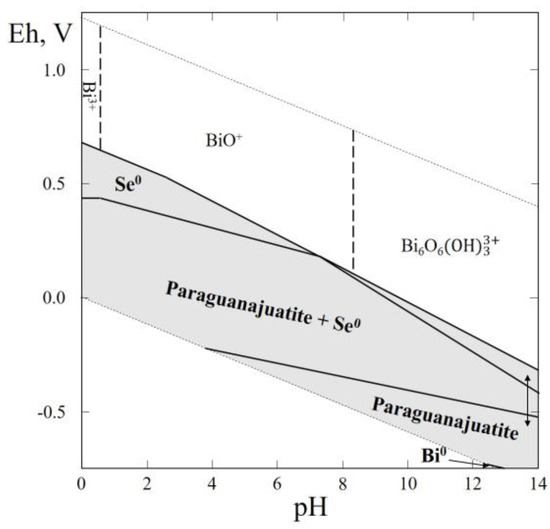
Figure 11.
Eh–pH diagram of the OHSeBi (Bi–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣBi = 10−5. The dotted lines are the boundaries between fields of Bi3+, BiO+ and Bi6O6(OH)33+.
3.11. Thermodynamic System As–Se–H2O
In this system only one selenide mineral (laphamite, As2Se3) was reported. Laphamite was found in one locality (Burnside, Northumberland County, PA, USA) as a secondary incrustation, probably by sublimation, on a burning pile of waste material from an anthracite coal mine in association with arsenolite, bararite, downeyite, galena, orpiment, selenium, and sulfur [61].
The Eh–pH diagram of the As–Se–H2O system (Figure 12) shows that the larger part of the diagram contains stability fields of native selenium and laphamite. A small stability field of arsenic corresponds to the alkaline region with a negative Eh.
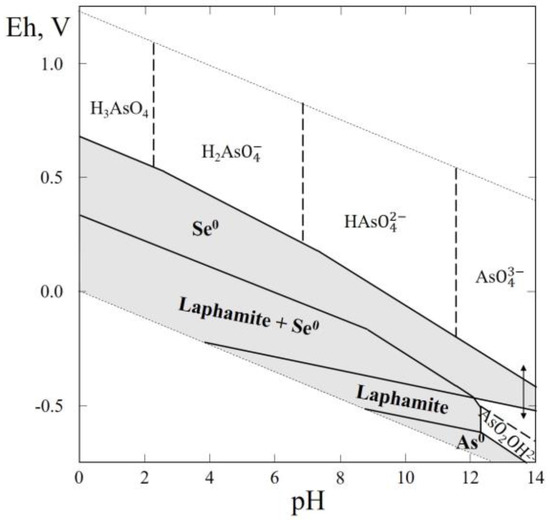
Figure 12.
Eh–pH diagram of the OHSeAs (As–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣAs = 10−3. The dotted lines are the boundaries between fields of H3AsO40, H2AsO4−, HAsO42− and AsO43−.
3.12. Thermodynamic System Sb–Se–H2O
In this system only one selenide, antimonselite (Sb2Se3), was reported (Table 1). Antimonselite was found in uraniferous calcite veins in a hydrothermal U–Hg–Mo polymetallic deposit in association with pyrite, sphalerite, galena, ferroselite, clausthalite, uraninite, cinnabar, hematite, and calcite (near Kaiyan, Guizhou Province, China) [62].
The Eh–pH diagram of the Sb–Se–H2O system (Figure 13) shows that the larger part of the diagram contains stability fields of antimonselite and native selenium.
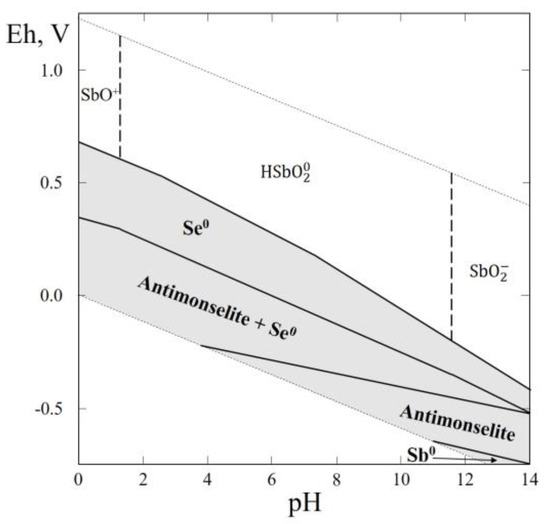
Figure 13.
Eh–pH diagram of the OHSeSb (Sb–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣSb = 10−5. The dotted lines are the boundaries between fields of SbO+, HSbO20 and SbO2.
3.13. Thermodynamic System Al–Se–H2O
Recently, the compound Al2(SeO3)3·6H2O was found in nature as the mineral alfredopetrovite (Table 1). The mineral occurs in the El Dragón mine, Antonio Quijarro Province, Potosí Department, Bolivia. The El Dragón mine is situated in a telethermal deposit consisting of a single selenide vein hosted by sandstones and shales. Selenide oxidation has produced a wide range of secondary rare selenites (ahlfeldite, chalcomenite, mandarinoite, favreauite, molybdomenite, olsacherite, schmiederite and others), one of which is alfredopetrovite [63].
The Eh–pH diagram of the Al–Se–H2O system is shown in Figure 14. At the background concentration of Al and Se, the larger section of the diagram contains stability fields of gibbsite (Al(OH)3) and native selenium. A small stability field corresponds to alfredopetrovite in the diagram’s acidic section, with a high positive Eh.
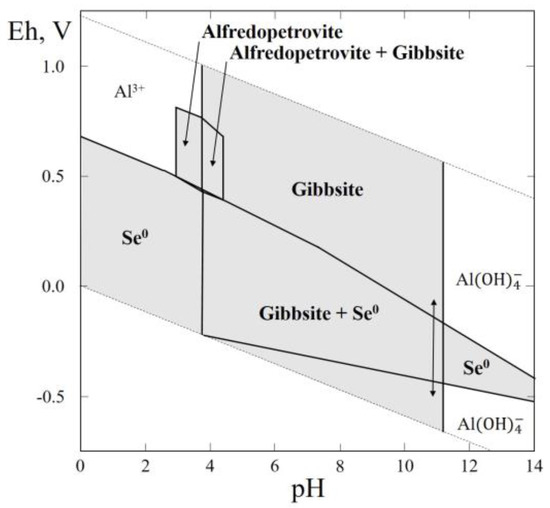
Figure 14.
Eh–pH diagram of the OHSeAl (Al–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣAl = 10−3.
3.14. Thermodynamic System Ca–Se–H2O
In this system, hydrous calcium selenite (CaSeO3·H2O) has recently been found in nature as the mineral nestolaite (Table 1). Specimens containing nestolaite were collected in the Little Eva mine, Yellow Cat District, Grand County, UT, USA. The mineral is very rare and occurs as light violet, round aggregations on sandstone associated with cobaltomenite, gypsum, orschallite, ferroselite, native selenium and others. Nestolaite was formed due to the supergene oxidation of primary Se minerals, such as native selenium and ferroselite [64].
The Eh–pH diagram of the Ca–Se–H2O system is shown in Figure 15. The larger part of the diagram contains stability fields of native selenium and portlandite, Ca(OH)2. A stability field of nestolaite corresponds to the alkaline region with a positive Eh.
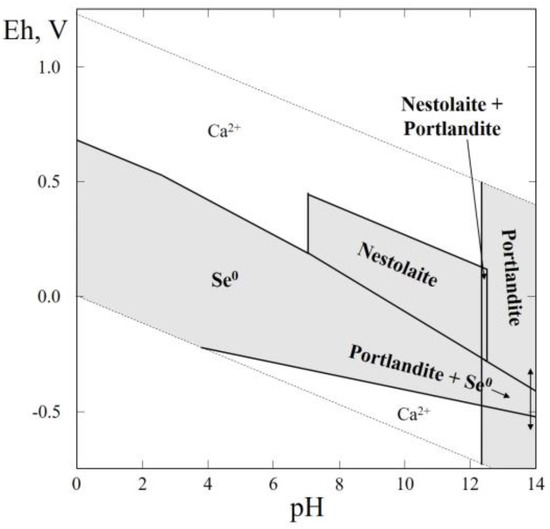
Figure 15.
Eh–pH diagram of the OHSeCa (Ca–Se–H2O) system at 25 °C and the activities of the components: aΣSe = 10−4, aΣCa = 10−2.
3.15. Selenates
In conclusion, we dwell on the probable formation of natural Co, Ni, Fe, Cu, Zn and Pb selenates. We calculated the Eh–pH diagrams within a wide range of activities of the components and found values corresponding to the appearance of the stability fields of CoSeO4·6H2O, CuSeO4·5H2O, NiSeO4·6H2O, PbSeO4 and ZnSeO4·6H2O [65].
The calculations indicate that, among all the discussed systems, selenates are formed only in the Pb–Se–H2O and Cu–Se–H2O systems at 25 °C with more or less real (although high) activities of metals and Se in the solution: aΣSe = 10−3 and aΣPb = 10−3; aΣSe = 10−1 and aΣCu = 10−1.
The activities of Co, Ni and Zn necessary for the formation of their selenate hydrates are extremely high and cannot be encountered in nature. The Eh–pH diagrams of the Cu–Se–H2O and Pb–Se–H2O systems show crystallization fields of CuSeO4·5H2O and PbSeO4 [65]. The results are consistent with the list of the three known mineral species containing selenate ions: schmiederite (Pb2Cu2(SeO3)(SeO4)(OH)4); olsacherite (Pb2(SeO4)(SO4)), which is found at the Dragon and Pacajake deposits in Bolivia and the Baccu Locci deposit in Sardinia, Italy together with selenites; and carlosruizite (K6Na10Mg10(IO3)12(SeO4)12·12H2O), a very specific mineral differing in its formation conditions, which are found at a niter deposit in association with nitratine, fuenzalidaite, and halite [66]. Kerstenite, PbSeO4 [67], a single simple selenate, has turned out to be a molybdomenite after a detailed study [68].
3.16. Thermodynamic Data
Unfortunately, for most selenium minerals no data on the thermodynamic functions of the formation have been reported. Additional experimental investigations are needed in order to produce reliable and accurate standard thermodynamic data, which will enable researchers to adequately describe Se migration behavior in the oxidation zones of sulfide and selenide ores and contaminated areas. It should be noted that the experimental determination of the thermodynamic data of rare minerals in general, and of the title compounds in particular, on the basis of studying their solubility or by calorimetric measurements, can hardly rely on natural samples, because these usually do not occur in sufficient amounts, forming only tiny crystals which may incorporate inclusions, be covered by weathering crusts, and almost inevitably contain impurities. All these defects influence many properties of the samples studied and certainly their thermodynamic parameters. Therefore, the investigation of the thermodynamic properties of selenium minerals is carried out on their synthetic analogues.
4. Conclusions
The obtained data show that the behavior of selenium, the nearest geochemical counterpart of sulfur, in the surface environment can be quantitatively explained by variations of the redox potential and the acidity–basicity of the mineral-forming medium. Precisely these parameters determine the migration ability of selenium compounds and their precipitation in the form of various solid phases.
Supplementary Materials
The following are available online at www.mdpi.com/2075-163X/7/10/188/s1, Table S1: Selenim minerals: Chemical formula, type locality (TL) and number of localities (NL).
Acknowledgments
The three anonymous reviewers are thanked for their useful comments, which led to many important clarifications. This study was supported by St. Petersburg State University (Grants No. 3.38.286.2015; 3.42.732.2017) and the Russian Foundation for Basic Research (Grant No. 16-05-00293). The St. Petersburg State University Resource Centers “X-Ray Diffraction Methods” and “Geomodel” made their equipment available for this study.
Author Contributions
Vladimir Krivovichev and Marina Charykova wrote the article. Marina Charykova and Andrey Vishnevskiy collected the thermodynamic data and calculated the Eh–pH diagrams.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khamkhash, A.; Srivastava, V.; Ghosh, T.; Akdogan, G.; Ganguli, R.; Aggarwal, S. Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge. Minerals 2017, 7, 46. [Google Scholar] [CrossRef]
- Lemly, A.D. Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef]
- Sandy, T.; DiSante, C. Review of Available Technologies for the Removal of Selenium from Water; Technical Report; CH2M Hill: Englewood, CO, USA, June 2010; pp. 2–223. [Google Scholar]
- Plant, J.A.; Bone, J.; Voulvoulis, N.; Kinniburgh, D.G.; Smedley, P.L.; Fordyce, F.M.; Klinck, B. Arsenic and selenium. In Environmental Geochemistry; Treatise on Geochemistry; Holland, H.D., Turekain, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 11, pp. 13–57. [Google Scholar]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Biogeochemistry of selenium: A review. Environ. Chem. Lett. 2014, 13, 49–58. [Google Scholar] [CrossRef]
- Séby, F.; Potin-Gautier, M.; Giffaut, E.; Donard, O.F.X. Assessing the speciation and the biogeochemical processes affecting the mobility of selenium from a geological repository of radioactive wastes to the biosphere. Analysis 1998, 26, 193–198. [Google Scholar] [CrossRef]
- Chen, F.; Burns, P.C.; Ewing, R.C. 79Se: Geochemical and crystallo-chemical retardation mechanisms. J. Nucl. Mater. 1999, 275, 81–94. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, V.G. Mineral systems and the thermodynamics of selenites and selenates in the oxidation zone of sulfide ores—A review. Mineral. Petrol. 2017, 111, 121–134. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Charykova, M.V. Classification of Mineral Systems; St.-Petersburg University Press: Saint-Petersburg, Russia, 2013; 196p. (In Russian) [Google Scholar]
- Krivovichev, V.G.; Charykova, M.V. Number of minerals of various chemical elements: Statistics 2012 (a new approach to an old problem). Geol. Ore Depos. 2014, 56, 553–559. [Google Scholar] [CrossRef]
- Hawthorne, F.C. The use of end-member charge-arrangements in defining new mineral species and heterovalent substitutions in complex minerals. Can. Mineral. 2002, 40, 699–710. [Google Scholar] [CrossRef]
- Hatert, F.; Burke, E.A.J. The IMA-CNMNC dominant-constituent rule revised and extended. Can. Mineral. 2008, 46, 717–728. [Google Scholar]
- Christy, A.G. Causes of anomalous mineralogical diversity in the Periodic Table. Mineral. Mag. 2015, 79, 33–49. [Google Scholar]
- Nickel, E.H. The definition of a mineral. Can. Mineral. 1995, 33, 689–690. [Google Scholar]
- Nickel, E.H.; Grice, J.D. The IMA Commission on New Minerals and Mineral Names: Procedures and guidelines on mineral nomenclature. Can. Mineral. 1998, 36, 913–926. [Google Scholar]
- Mills, S.J.; Hatert, F.; Nickel, E.H.; Ferraris, G. The standardisation of mineral group hierarchies: Application to recent nomenclature proposals. Eur. J. Mineral. 2009, 21, 1073–1080. [Google Scholar] [CrossRef]
- Charykova, M.V.; Lelet, M.I.; Krivovichev, V.G.; Ivanova, N.M.; Suleimanov, E.V. A calorimetric and thermodynamic investigation of the synthetic analogue of chalcomenite, CuSeO3∙2H2O. Eur. J. Mineral. 2017, 29, 269–277. [Google Scholar] [CrossRef]
- Lelet, M.I.; Charykova, M.V.; Krivovichev, V.G.; Efimenko, N.M.; Platonova, N.V.; Suleimanov, E.V. A Calorimetric and Thermodynamic Investigation of Zinc and Cadmium Hydrous Selenites. J. Chem. Thermodyn. 2017, 115, 63–73. [Google Scholar] [CrossRef]
- Boone, S.; Kleppa, O.J. Enthalpies of formation for group IV selenides (GeSe2, GeSe2(am), SnSe, SnSe2, PbSe) by directcombination drop calorimetry. Thermochim. Acta 1992, 197, 109–121. [Google Scholar] [CrossRef]
- Gattow, G.; Schneider, A. Die Bildungsenthalpien im System Kupfer-Selen. Z. Anorg. Allg. Chem. 1956, 286, 296–306. (In German) [Google Scholar] [CrossRef]
- Grønvold, F. High-temperature reaction calorimeter. Enthalpy of formation of iron selenides and nickel selenides at 1050 K. Acta Chem. Scand. 1972, 26, 2085–2099. [Google Scholar] [CrossRef]
- Grønvold, F.; Westrum, E.F., Jr. Heat capacities and thermodynamic functions of iron disulphide (pyrite), iron diselenide, and nickel diselenide from 5 to 350 K. The estimation of standard entropies of transition metal chalcogenides. Inorg. Chem. 1962, 1, 36–48. [Google Scholar] [CrossRef]
- Leshchinskaya, Z.L.; Selivanova, N.M. Thermodynamic properties of calcium selenite. Tr. Inst.-Mosk. Khim.-Tekhnol. Inst. Im. D.I. Mendeleeva. 1963, 44, 37–40. (In Russian) [Google Scholar]
- Leshchinskaya, Z.L.; Selivanova, N.M. Thermodynamic properties of copper selenites. Zh. Fiz. Khim. 1965, 39, 2430–2434. (In Russian) [Google Scholar]
- Leshchinskaya, Z.L.; Selivanova, N.M.; Maier, A.I.; Strel’tsov, I.S.; Muzalev, E.Y. Heat of formation of nickel and cobalt selenites. Zh. Vses. Khim. O-va Im. D. I. Mendeleeva. 1963, 8, 577–578. (In Russian) [Google Scholar]
- Maekawa, T.; Yokokawa, T.; Niwa, K. Enthalpies of mixing in the liquid state. IV. Bi + Se and Sb + Se. J. Chem. Thermodyn. 1972, 4, 873–878. [Google Scholar] [CrossRef]
- Nasar, A.; Shamsuddin, M. Thermodynamic properties of zinc selenide. Z. Metallkd 1990, 81, 244–246. [Google Scholar]
- O’Hare, P.A.G.; Lewis, B.M.; Susman, S.; Volin, K.J. Standard molar enthalpies of formation and transition at the temperature 298.15 K and other thermodynamic properties of the crystalline and vitreous forms of arsenic sesquiselenide (As2Se3). Dissociation enthalpies of As-Se bonds. J. Chem. Thermodyn. 1990, 22, 1191–1206. [Google Scholar] [CrossRef]
- Petrova, Z.K.; Kofman, N.A. Heat capacity and some thermodynamic properties of cadmium selenide. Dokl. Akad. Nauk BSSR 1976, 20, 688–690. (In Russian) [Google Scholar]
- Selivanova, N.M.; Leshchinskaya, Z.L.; Klushina, T.V. Physicochemical properties of selenites. I. Thermodynamic properties of silver selenite. Zh. Phiz. Khim. 1962, 36, 1349–1352. (In Russian) [Google Scholar]
- Selivanova, N.M.; Leshchinskaya, Z.L. Physical-chemical properties of selenites. Thermodynamic properties of lead selenite. Tr. Inst.-Mosk. Khim.-Tekhnol. Inst. Im. D. I. Mendeleeva. 1962, 38, 37–42. (In Russian) [Google Scholar]
- Selivanova, N.M.; Leshchinskaya, Z.L.; Maier, A.I.; Strel’tsov, I.S.; Muzalev, E.Y. Thermodynamic properties of nickel selenite dehydrate. Zh. Fiz. Khim. 1963, 37, 1563–1567. (In Russian) [Google Scholar]
- Sirota, N.N.; Petrova, Z.K.; Solovoskii, T.D. Heat capacity of zinc selenide in the 4,2–300 K region. Dokl. Akad. Nauk BSSR 1980, 24, 214–217. (In Russian) [Google Scholar]
- Stolyarova, T.A.; Gavrilov, N.M.; Nekrasov, I.Y. Thermodynamic properties of bismuth selenides. Geokhimiya 1990, 9, 1367–1374. (In Russian) [Google Scholar]
- Chukhlantsev, V.G.; Tomashevsky, G.P. The solubility of the selenites of certain metals. Zh. Anal. Khim. 1957, 12, 296–302. (In Russian) [Google Scholar]
- Chukhlantsev, V.G. Solubility product of selenite of some metals. Zh. Neorg. Khim. 1956, 1, 2300–2305. (In Russian) [Google Scholar]
- Gospodinov, G.; Barkov, D. Das System HgO-SeO2-H2O bei 25 und 100 °C. Z. Phys. Chem. (Munich) 1991, 171, 241–247. (In German) [Google Scholar] [CrossRef]
- Slavtscheva, J.; Popova, E.; Gospodinov, G. Untersuchung von Löslichkeit und Löslichkeitsprodukt bei Seleniten der Elemente der IV. Gruppe des Periodensystems. Z. Chem. 1984, 24, 105–106. (In German) [Google Scholar] [CrossRef]
- Slavtscheva, J.; Popova, E.; Gospodinov, G. Untersuchung der Löslichkeit und Bestimmung des Löslichkeitsproduktes von Kupfer-und Silberselenit. Monatsh. Chem. 1993, 124, 1115–1118. (In German) [Google Scholar] [CrossRef]
- Popova, E.; Slavtscheva, J.; Gospodinov, G. Untersuchung der Löslichkeit einiger Phasen des Systems M2O3-SeO2-H2O der Elemente der 3. Gruppe des Periodensystems der Elemente. Z. Chem. 1986, 26, 342–343. (In German) [Google Scholar] [CrossRef]
- Rai, D.; Felmy, A.R.; Moore, D.A. The solubility product of crystalline ferric selenite hexahydrate and the complexation constant of FeSeO3+. J. Solution Chem. 1995, 24, 735–752. [Google Scholar] [CrossRef]
- Ripan, R.; Vericeanu, G. Conductimetric determination of the solubility product of some selenites. Stud. Univ. Babes-Bolyai Chem. 1968, 13, 31–37. (In Romanian) [Google Scholar]
- Mehra, M.C.; Gubeli, A.O. The complexing characteristics of insoluble selenides. 1. Silver selenide. Can. J. Chem. 1970, 48, 3491–3497. [Google Scholar] [CrossRef]
- Savenko, V.S. Solubility product of magnesium and calcium selenites. Zh. Neorg. Khim. 1995, 40, 1254–1256. (In Russian) [Google Scholar]
- Séby, F.; Potin-Cautier, M.; Giffaut, E.; Borge, G.; Donard, O.F.X. A critical review of thermodynamic data for selenium species at 25 °C. Chem. Geol. 2001, 171, 173–194. [Google Scholar] [CrossRef]
- Olin, A.; Nolang, B.; Osadchii, E.G.; Ohman, L.-O.; Rosen, E. Chemical Thermodynamics of Selenium; Elsevier: Amsterdam, The Netherlands, 2005; 851p. [Google Scholar]
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L.; Nuttall, R.L. The NBS tables of chemical thermodynamic properties: Selected values for inorganic and C1 and C2 organic substances in (SI) units. J. Phys. Chem. Ref. Data 1982, 11 (Suppl. 2), 1–392. [Google Scholar]
- Charykova, M.V.; Krivovichev, V.G.; Depmeier, W. Thermodynamics of arsenates, selenites and sulphates in oxidising zone of sulphides ore deposits. Part I: Thermodynamic properties at standard conditions. Geol. Ore Depos. 2010, 52, 759–770. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, V.G.; Lelet, M.I.; Yakovenko, O.S.; Suleimanov, E.V.; Depmeier, W.; Semenova, V.V.; Zorina, M.L. A calorimetric and thermodynamic investigation of the synthetic analogues of cobaltomenite, CoSeO3∙2H2O, and ahlfeldite, NiSeO3∙2H2O. Am. Mineral. 2014, 99, 742–748. [Google Scholar] [CrossRef]
- Bethke, C.M.; Yeakel, S. The Geochemist’s Workbench (Release 9.0); LLC: Champaign, IL, USA, 2011; 130p. [Google Scholar]
- Krivovichev, V.G.; Charykova, M.V.; Yakovenko, O.S.; Depmeier, W. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. IV. Eh–pH Diagrams of the Me–Se–H2O Systems (Me = Co, Ni, Fe, Cu, Zn, Pb) at 25 °C. Geol. Ore Depos. 2011, 53, 514–527. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, V.G.; Yakovenko, O.S.; Semenova, V.V.; Semenov, K.N.; Depmeier, W. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. VI. Solubility of Synthetic Analogs of Ahlfeldite and Cobaltomenite at 25 °C. Geol. Ore Depos. 2012, 54, 638–646. [Google Scholar] [CrossRef]
- Charykova, M.V.; Fokina, E.L.; Krivovichev, V.G.; Yakovenko, O.S.; Klimova, E.V.; Semenova, V.V. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. X. Thermal Stability and Dehydration Features of Synthetic Analogs of the Cobaltomenite–Ahlfeldite Solid Solution Series. Geol. Ore Depos. 2015, 57, 570–578. [Google Scholar] [CrossRef]
- Fokina, E.L.; Klimova, E.V.; Charykova, M.V.; Krivovichev, V.G.; Platonova, N.V.; Semenova, V.V.; Depmeier, W. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. VIII. Field of Thermal Stability of Synthetic Analog of Chalcomenite, its Dehydration and Dissociation. Geol. Ore Depos. 2014, 56, 538–545. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, V.G.; Ivanova, N.M.; Semenova, V.V. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. XI. Solubility of Synthetic Chalcomenite Analog and Zinc Selenite at 25 °C. Geol. Ore Depos. 2015, 57, 691–698. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Britvin, S.N.; Chukanov, N.V.; Lykova, I.S.; Sidorov, E.G.; Pushcharovsky, D.Y. Zincomenite, ZnSeO3, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Eur. J. Mineral. 2016, 28, 997–1004. [Google Scholar] [CrossRef]
- Charykova, M.V.; Fokina, E.L.; Klimova, E.V.; Krivovichev, V.G.; Semenova, V.V. Thermodynamics of Arsenates, Selenites, and Sulfates in the Oxidation Zone of Sulfide Ores. IX. Physico-chemical Formation Conditions and Thermal Stability of Zinc Selenites. Geol. Ore Depos. 2014, 56, 546–552. [Google Scholar] [CrossRef]
- Bur’yanova, E.Z.; Kovalev, G.A.; Komkov, A.I. The new mineral cadmoselite. Zap. Vsesojuz. Miner. Obshch. 1957, 86, 626–628. (In Russian) [Google Scholar]
- Naumann, C.F. XI. Classe. Galenoide oder Glanze. B. Selenische Glanze. 531. Selenmercur oder Tiemannit. In Elemente der Mineralogie; Wilhelm Engelmann: Leipzig, Germany, 1855; p. 425. (In German) [Google Scholar]
- Haidinger, W. Zweite Klasse: Geogenide. XIV. Ordnung. Glanze. III. Silberglanz. Naumannit. In Handbuch der Bestimmenden Mineralogie; Braumüller and Seidel: Vienna, Austria, 1845; pp. 563–570. (In German) [Google Scholar]
- Dunn, P.J.; Peacor, D.R.; Criddle, A.J.; Finkelman, R.B. Laphamite, an arsenic selenide analogue of 557 orpiment, from burning anthracite deposits in Pennsylvania. Mineral. Mag. 1986, 50, 279–282. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Li, D.; Wang, G. Antimonselite, a new mineral. Acta Mineral. Sin. 1993, 13, 7–11, (In Chinese with English abstract). [Google Scholar]
- Kampf, A.R.; Mills, S.J.; Nash, B.P.; Thorne, B.; Favreau, G. Alfredopetrovite: A new selenite mineral from the El Dragόn mine. Eur. J. Mineral. 2016, 28, 479–484. [Google Scholar] [CrossRef]
- Kasatkin, A.V.; Plášil, J.; Marty, J.; Agakhanov, A.A.; Belakovskiy, D.I.; Lykova, I.S. Nestolaite, CaSeO3·H2O, a new mineral from the Little Eva mine, Grand County, Utah, USA. Mineral. Mag. 2014, 78, 497–505. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, V.G. Thermodynamics of environmentally important natural and synthetic phases containing selenium. In Biogenic-Abiogenic Interactions in Natural and Anthropogenic Systems; Springer-Verlag: Heidelberg, Germany, 2016; pp. 145–155. [Google Scholar]
- Krivovichev, V.G.; Depmeier, W. Selenates and selenites: Systems Se-S-H2O, Pb-Se-S-H2O, U-Se-H2O and U-Se-I-H2O—Thermodynamical analysis and geological applications. Zap. Ross. Mineral. Obshch. 2005, 134, 1–14. (In Russian) [Google Scholar]
- Mandarino, J.A. Natural and synthetic selenites and selenates and their Gladstone-Dale compatibility. Eur. J. Mineral. 1994, 6, 337–349. [Google Scholar] [CrossRef]
- Burke, E.A.J. A Mass Discreditation of GQN Minerals. Can Mineral. 2007, 44, 1557–1560. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).