Abstract
Laboratory kinetic leach column (KLC) tests were carried out to define the conditions required to control acid generation from a highly reactive, potentially acid-forming (PAF) iron ore waste rock. It was found that lime addition (0.1 wt % blended) plus either blending of silicates (25 wt % K-feldspar and 25 wt % chlorite), or addition of a non-acid forming (NAF) top cover containing about 10% dolomite (PAF:NAF = 5:1 wt %), when watered/flushed with lime-saturated water, greatly reduced acid generation as compared to the control KLC (PAF alone, watered/flushed with Milli-Q water), but did not result in circum-neutral pH as required for pyrite surface passivation and effective acid and metalliferous drainage (AMD) mitigation. In contrast, the combined use of these treatments—blended lime and silicates with an NAF cover and watering/flushing with lime-saturated water—resulted in leachate pH of ≈12 (up to 24 weeks). Mass balance calculations for Ca2+ and scanning electron microscopy (SEM) analyses suggest that calcite or gypsum may have formed in the NAF-amended KLCs and lime with added silicate KLC. Although the combined approach in the form trialled here may not be practical or cost-effective, control of a highly reactive natural PAF waste by pyrite surface passivation appears to be possible, and an improved treatment methodology (e.g., slightly increased lime blending without the need for further lime watering/flushing) could usefully be examined in the future.
1. Introduction
Acid and metalliferous drainage (AMD) from both operating and inactive/abandoned mines is a global environmental problem. In most cases, AMD is triggered naturally by the oxidation of sulfide minerals, in particular pyrite—the most abundant sulfide mineral on Earth—through chemical and microbially-mediated reactions with both surface water and oxygen [1]. Acidic leachates containing toxic metals/metalloids, such as Mn, Zn, Cd, Pb, As and Se, can be released. AMD is a complex environmental issue, due to both the severity and long-lasting nature of its impacts. For the mining industry and governments, AMD remediation is expensive, with costs in the US and Canada alone estimated to be in the tens of billions of dollars [2]. Accordingly, cost-effective and sustainable strategies for AMD prevention and remediation are required.
Pyrite surface passivating layers, incorporating silicate from the natural dissolution of reactive silicate minerals in real waste rocks, can be preserved in a continuous, coherent, and stable form at pH ≥ 6, and have been found to reduce the pyrite oxidation rate by 50–95% [3,4]. However, in some cases the establishment of these stable surface layers requires initial short-term treatment with greater levels of alkalinity than can be obtained from limestone covers. After passivation layers are established, the acid generation rate (AGR) can be reduced significantly so that some carbonates and reactive silicates such as limestone, anorthite feldspar, and hornblende, all commonly found in waste rocks, provide the required matching acid neutralising rate (ANR) [5]. This formed the basis of our experimental approach to treat highly reactive potentially acid-forming (PAF) iron ore waste (Mt McRae shale) from the Pilbara region of Western Australia.
The aim of this study was to determine whether, in conjunction with lime-saturated watering/flushing, the following treatments could maintain circum-neutral pH as required for the establishment and maintenance of pyrite passivation: (1) lime blending; (2) combined use of silicates (chlorite + K-feldspar) and lime blending; and (3) application of a dolomitic non-acid forming (NAF) cover in conjunction with lime blending or (4) with both lime and silicate blending.
2. Materials and Methodology
2.1. Kinetic Leach Columns (KLC)
Quartz, K-feldspar and chlorite (Geo Discoveries, New South Wales, Australia) were crushed, pulverised and dry-sieved to <4 mm size for the KLC experiments as per Qian et al. [6]. Setup and daily operation for the KLCs were based on Smart et al. [7], except that different solutions were used for watering/flushing, and KLCs other than the control were flushed with lime-saturated water (supernatant after dissolving >95% pure Ca(OH)2 in Milli-Q water until saturation; alkalinity: ≈1900 mg CaCO3 L−1) after flush 1. A summary of the setup for the five KLCs is given in Table 1.

Table 1.
The composition (wt %) and watering/flushing of the KLCs.
2.2. Sample Characterisation
All mineral samples (dry-ground to <38 μm) were examined using powder X-ray diffraction analysis (XRD, Bruker D4 Endeavor diffractometer, Bruker, Billerica, MA, USA) with Co Kα radiation (λKα1 = 1.78897 Å; λKα2 = 1.79285 Å) at 35 kV and 40 mA. 15 wt % corundum (α-Al2O3; <38 μm) was added as an internal standard for quantification of amorphous phase(s). Phase identification was carried out using the DiffracPlus EVA software (Version 3.0; Bruker, Billerica, MA, USA) with the COD database (Crystallography Open Database). Quantitative X-ray diffraction analysis (QXRD) was performed using TOPAS (Version 4.2; Bruker). Owing to the complex mineralogy and significant amounts of amorphous phases present within the real waste samples (based on QXRD), quantitative evaluation of minerals by scanning electron microscopy (QEM-SCAN) and bulk sample assay were also carried out to further characterise mineralogy and elemental composition of each sample. Chromium reducible sulfur (CRS) analysis for sulfide-S was as per Schumann et al. [8].
Leached mineral samples were examined using scanning electron microscopy (Quanta 450 Environmental SEM, FEI, Hillsboro, OR, USA), coupled with energy dispersive spectrometry (EDS), using both backscattered and secondary electron modes. The operating voltage was set to 15 kV, or 20 kV to observe heavier elements. EDS analysis was carried out to obtain semi-quantitative chemical compositions. For samples possibly containing calcite (CaCO3) precipitates (as predicted by mass balance calculations and pH conditions, see Results and Discussion), SEM analysis was conducted under low vacuum conditions without sample surface carbon coating.
Leachate pH and Eh (collected after each four-weekly flush) was measured, using Ag/AgCl pH and Eh electrodes (saturated KCl-filled) calibrated with standard pH buffers (TPS, Brendale, Qld, Australia) and oxidation–reduction potential (ORP) standards (EUTECH Instruments, Singapore). All pH and Eh measurements were completed within several hours of leachate collection.
The concentration (±10%) of major ions in KLC leachates was analysed via inductively coupled plasma optical emission spectroscopy (ICP-OES; Perkin Elmer Optima 5300 V, Perkin Elmer, Waltham, MA, USA) analysis. Calculations of mineral saturation indices were performed using the PHREEQC software [9], with the Lawrence Livermore National Laboratory database (llnl.dat), Eh, pH, and solution chemistry of leachates.
3. Results and Discussion
3.1. Sample Characterisation
QEM-SCAN results were broadly in agreement with QXRD analysis (Table 2), with some variations in mineral composition. It should be noted that amorphous content is not identified as such using QEM-SCAN but is assigned to the mineral phase of closest stoichiometry. A comparison between QEM-SCAN and QXRD results suggests that in the PAF sample, the amorphous component may contain some potassium aluminosilicates and iron oxides. Bulk assay and CRS results are provided in Table 3. CRS concentrations for both the PAF and NAF are equivalent to 7.2 wt % and 1.1 wt % pyrite, respectively, generally consistent with the wt % of pyrite based on both QXRD and QEM-SCAN analyses. The wt % of inorganic carbon in NAF, determined by the bulk sample assays, also agrees with the wt % of dolomite determined by both QXRD and QEM-SCAN.

Table 2.
Mineralogical composition of the PAF and NAF wastes.

Table 3.
Bulk assay results for NAF and PAF wastes (all data in ppm).
All samples were subjected to net acid generation (NAG) and acid base accounting (ABA) tests (Table 4). Net acid producing potential (NAPP) results (Table 4) indicate that the PAF and NAF samples have been characterised appropriately.

Table 4.
ABA and NAG test results of PAF and NAF (in kg H2SO4 t−1).
3.2. Leachate Properties
The pH of the KLC leachates indicates that blending with 0.1 wt % lime alone or with silicates (KLCs lime and lime + silicate, Figure 1A) provided insufficient neutralisation to maintain circum-neutral pH as required for the formation and maintenance of a pyrite surface passivating layer. The greater leachate pH for lime + silicate as compared to lime, especially over weeks 8–12, suggests that the added silicates may have neutralised a minor amount of acidity from the PAF waste during the 24 weeks.
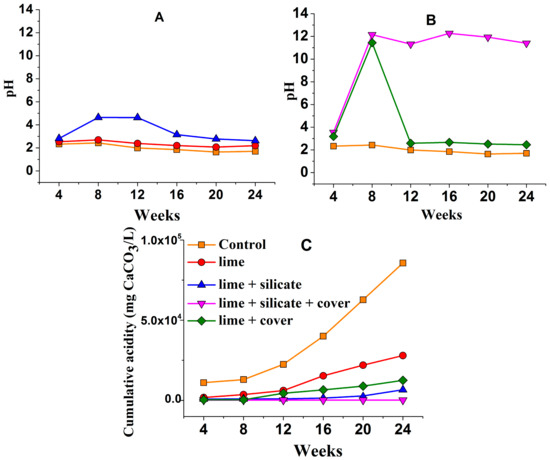
Figure 1.
KLC leachate pH and cumulative acidity profiles during 24 weeks of operation (see Table 1 for details of each KLC). Figure legend in (C) is common to (A,B).
The blending of lime and silicates into the PAF waste in addition to an NAF top cover (lime + silicate + cover) resulted in basic pH, but the application of the same NAF cover to the lime-blended KLC (lime + cover) gave rise to acidic pH (around 2.5) after 12 weeks (Figure 1B). This again demonstrates the positive role of silicates through the neutralisation of acid generated from pyrite oxidation.
Figure 1C shows that the cumulative acidity is greater from the lime and lime + cover KLC tests over 24 weeks than from lime + silicate and lime + silicate + cover. This also demonstrates that the blended silicates play a significant role in decreasing the acidity released from the KLC tests. In addition, the NAF cover (lime + cover and lime + silicate + cover) decreased the total leachate acidity across the 24 weeks, as compared to the absence of an NAF cover—lime and lime + silicate, respectively—despite the acidic pH for lime + cover after 12 weeks. The blended lime combined with an NAF cover substantially reduced the total cumulative leachate acidity by around 65% in comparison to the addition of lime alone. Only the combined use of blended lime + silicate and an NAF cover with lime-saturated watering/flushing completely inhibited acidity generation across the 24 weeks (>90%) (Figure 1C).
Figure 2 shows the proton and metal acidities calculated based on pH and solution ICP metal concentrations, respectively. It is clear that the proton acidity only accounted for a small proportion (<5%) of the total acidity. It was found that 0.1 wt % lime addition alone to lime and lime + silicate in conjunction with watering/flushing with lime-saturated water after flush 1 significantly reduced the metal acidity (and also the proton acidity) as compared to the control (Figure 2). It was observed that the blended silicates into the PAF waste in lime + silicate and lime + silicate + cover also contributed to the reduction of the metal acidity (>70%), in comparison with lime and lime + cover (both with no silicates), respectively. Similarly, the addition of an NAF cover to lime + cover and lime + silicate + cover resulted in a significantly lower metal acidity than those of lime and lime + silicate, respectively. The results clearly indicate that both silicates and NAF play positive roles in reducing primarily the metal acidity generated from PAF, and that only a combined use of lime addition, NAF covers and silicates blending (with saturated lime watering/flushing) can maintain circum-neutral/basic pH as required for the establishment and maintenance of pyrite surface passivation.
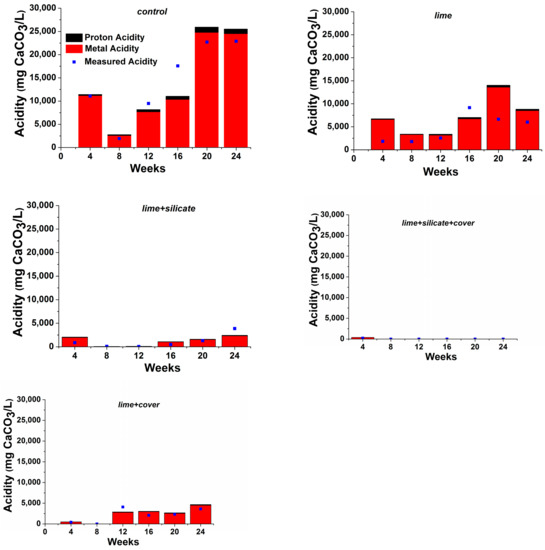
Figure 2.
Measured acidity alongside calculated proton and metal acidities (mg CaCO3 L−1) for all KLCs.
3.3. Calculation of Acid Neutralising Rate (ANR) and Acid Generation Rate (AGR)
ANR and AGR (Table 5) were calculated based on ICP metal concentrations (Na, Mg, Al, Ca) and S (sulfate) present in the leachate [12]. For the three KLCs without an NAF cover (Figure 1A) for the periods 8–12 and 16–24 weeks, pH variations were small and thus their ANR and AGR (Table 5) were relatively constant. For the two KLCs with an NAF cover (Figure 1B) the period 12–24 weeks maintained almost constant pH (stable ANR and AGR) for ANR and AGR calculations.

Table 5.
ANR and AGR (mmol H2SO4 week−1) calculated using the concentration of cations and S in leachates during different periods of time.
Between weeks 8–12, the AGR of the control, lime and lime + silicate was greater than the ANR, consistent with the acidic leachates. The total Ca2+ (31.4 mmol) added to lime + silicate from combined watering and flushing (i.e., 1.4 L per month) with lime-saturated water, assuming a lime solubility of ≈1.66 g L−1, was much greater than that released (13.5 mmol) in the leachate, suggesting that the Ca2+ retained (17.9 mmol) in the KLC may have precipitated. PHREEQC calculations suggested that leachates were saturated with respect to gypsum (Table 6).

Table 6.
Gypsum saturation indices calculated for lime + silicate and lime + cover using PHREEQC.
SEM-EDS analysis of samples taken from lime + silicate within this time period clearly showed rod-like euhedral crystals containing Ca, O, and S, providing further evidence that gypsum precipitation occurred (Figure 3). The precipitation of gypsum can result in inaccurate estimations of both AGR based on S concentrations and ANR based on Ca concentrations, but does not affect the rate difference between ANR and AGR.
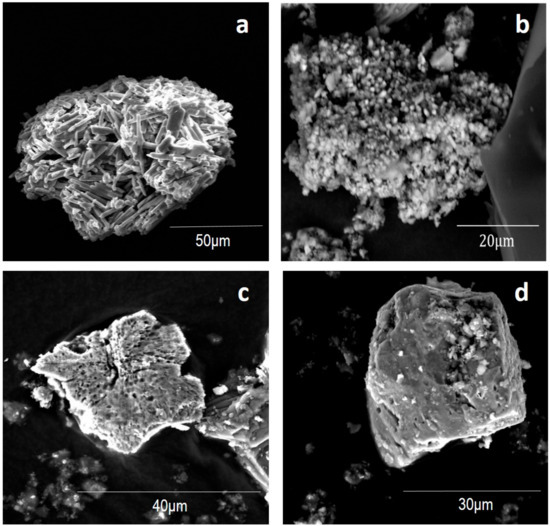
Figure 3.
Secondary electron SEM images of secondary Ca precipitates and pyrite: Ca sulfate (possibly gypsum) found at week 12 in lime + silicate (a); Ca carbonate (possibly calcite) found at week 20 in lime + silicate + cover (b); pyrite sampled from the control at week 20 (around pH 2) (c); pyrite sampled from lime + silicate + cover at week 20 (around pH 12) (d).
Assuming a lime solubility of 1.66 g L−1, nearly 94.2 mmol Ca2+ was applied to the lime + cover and lime + silicate + cover KLCs through watering and flushing with lime-saturated water (9 watering and 3 flushing, totaling 4.2 L; Table 7). For lime + cover (pH < 3 during weeks 12–24), 63.0 mmol Ca2+ was retained in the KLC, suggesting the possible formation of Ca-containing secondary minerals. It was found that gypsum was the only Ca-containing mineral phase with positive saturation indices (Table 6). Similar to lime + silicate, rod-like euhedral crystals (similar to those in Figure 3a) containing Ca, O, and S were also found in lime + cover by SEM-EDS analysis.

Table 7.
Mass balance calculations of Ca2+ (mmol) for lime + cover and lime + silicate + cover between weeks 12–24.
During the period of weeks 12–24, lime + silicate + cover gave rise to the lowest AGR and was the only KLC with alkaline leachate. The total amount of Ca2+ in the leachates from lime + silicate + cover was 37.0 mmol, indicating that 57.2 mmol Ca2+, possibly in the form of calcite (SEM-EDS analysis found Ca carbonate; Figure 3b; EDS data not shown) and/or other Ca-containing phases, were retained in this KLC. Note that the sample from lime + silicate + cover was not coated by carbon prior to the environmental SEM-EDS analysis, and thus the detection of Ca, C, and O suggests the presence of calcium carbonate, possibly calcite.
3.4. Pyrite Morphology
Pyrite from KLC tests with acidic leachate pH (e.g., pyrite from the control; Figure 3c) contained numerous micro-sized pores which may accelerate pyrite oxidation rate by increasing the reactive surface area. In contrast, pyrite from lime + silicate + cover with basic leachate pH showed a completely different morphology, with relatively compact surface structures (Figure 3d). SEM-EDS analysis suggested that pyrite surfaces (50.1 S, 44.9 Fe, 5.0 O; all in wt %) from the control were possibly covered to some degree with iron (oxy)hydroxide, and that pyrite (47.4 S, 34.5 Fe, 14.9 O, 3.2 Si; all in wt %) from lime + silicate + cover may be coated by silicate-doped iron (oxy)hydroxide layers. These observations are largely consistent with the preposition that pyrite surfaces may be passivated by silicate-stabilised surface iron (oxy)hydroxide layers under alkaline pH conditions in this study [3,6,13]. Further analysis, such as focused ion beam SEM and transmission electron microscopy, will be carried out to examine the nature of these oxidation layers.
4. Conclusions
Using laboratory-scale KLC tests, combinations of PAF waste blended with lime and silicates and the application of natural NAF materials (readily available on-site) as top covers, in conjunction with saturated lime-water watering/flushing, were employed to determine the conditions required to control acid generation from a highly reactive natural PAF waste material. It was found that an initial 0.1 wt % lime addition to the PAF waste, even with addition of silicates or an NAF top cover, was not able to maintain leachate pH near or above neutral during 24 weeks of operation. In contrast, the application of both silicates and an NAF cover, together with 0.1 wt % initial lime blending and flushing/watering with lime-saturated water, maintained a pH of around 12 up to 24 weeks.
The blending of lime combined with an NAF cover substantially reduced the cumulative leachate acidity by approximately 65%, as compared to lime addition alone. Similarly, the addition of both lime and silicates in conjunction with an NAF cover significantly reduced cumulative leachate acidity by >90%, relative to the acidity resulting from the same treatment but without silicates addition. This clearly demonstrates the positive role of the blended silicates in reducing acid generation. SEM-EDS analysis confirmed the formation of gypsum between weeks 8–12 in lime + silicate. Similarly, gypsum and calcite were formed between weeks 12–24 in lime + cover and lime + silicate + cover, respectively, consistent with the SEM-EDS findings. SEM analysis also revealed that pyrite from lime + silicate + cover (pH ≈ 12) was possibly coated by silicate-doped iron (oxy)hydroxide layers with relatively compact surface structures, distinctly different from porous pyrite surfaces from the control with an acidic pH of around 2.
This study suggests that the treatment of highly reactive PAF wastes via blending of lime (greater than 0.1 wt %), silicates, and the addition of an NAF cover, when combined with other control measures (e.g., construction of waste rock emplacements using layered and compacted methods), could substantially reduce or eliminate acidity and metal loads in mine drainage. These results highlight the potential for the beneficial use of on-site neutralising waste materials and/or lithologies for cost-effective AMD control and mitigation strategies. The KLC tests are currently ongoing to test the efficacy of our methodology in the longer term.
Acknowledgments
This project was financially supported by BHP Billiton (Australia), and the Australian Research Council (ARC) via an ARC Linkage project LP140100399. AMIRA International and P933B project staff Gray Bailey and Chris Ward are also acknowledged for their support.
Author Contributions
Y.Z. performed all experimental work, instrumental analysis and data interpretation. A.R.G., R.S.C.S., R.C.S. and J.L. conceived and designed the experiments. G.Q., J.L. and M.D.S. supervised this work. A.R.G., R.C.S., J.L., M.D.S. and G.Q. edited the paper. All authors contributed to paper writing, data interpretation and discussion.
Conflicts of interest
The authors declare no conflict of interest.
References
- Erguler, G.K.; Erguler, Z.A.; Akcakoca, H.; Ucar, A. The effect of column dimensions and particle size on the results of kinetic column test used for acid mine drainage (AMD) prediction. Miner. Eng. 2014, 55, 18–29. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of acid mine drainage-impacted water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Zeng, S.; Li, J.; Schumann, R.; Smart, R. Effect of pH and Dissolved Silicate on the Formation of Surface Passivation Layers for Reducing Pyrite Oxidation. Comput. Water Energy Environ. Eng. 2013, 2, 50–55. [Google Scholar] [CrossRef]
- Fan, R.; Short, M.D.; Zeng, S.; Qian, G.; Li, J.; Schumann, R.; Kawashima, N.; Smart, R.S.C.; Gerson, A.R. The formation of surface passivating layers on pyrite for reduced acid rock drainage. Environ. Sci. Technol. 2017. submitted. [Google Scholar]
- Smart, R.S.C.; Ciccarelli, J.; Zeng, S.; Fan, R.; Li, J.; Kawashima, N.; Gerson, A.; Schumann, R. Assessment of acid neutralization rate from site rock for AMD control. In Proceedings of the 10th International Conference on Acid Rock Drainage & IWMA Annual Conference, Santiago, Chile, 21–24 April 2015. [Google Scholar]
- Qian, G.; Schumann, R.C.; Li, J.; Short, M.D.; Fan, R.; Li, Y.; Kawashima, N.; Zhou, Y.; Smart, R.S.C.; Gerson, A.R. Strategies for Reduced Acid and Metalliferous Drainage by Pyrite Surface Passivation. Minerals 2017, 7, 42. [Google Scholar] [CrossRef]
- Smart, R.; Skinner, W.; Levay, G.; Gerson, A.; Thomas, J.; Sobieraj, H.; Schumann, R.; Weisener, C.; Weber, P.; Miller, S. ARD Test Handbook: Project P387. A Prediction and Kinetic Control of Acid Mine Drainage; AMIRA: Melbourne, Australia, 2002. [Google Scholar]
- Schumann, R.; Stewart, W.; Miller, S.; Kawashima, N.; Li, J.; Smart, R.S.C. Acid–base accounting assessment of mine wastes using the chromium reducible sulfur method. Sci. Total Environ. 2012, 424, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Users’s Guide to Phreeqc (Version 2)—A Computer Program for Speciation, Batch-Reaction. In One—Dimensional Transport, and Inverse Geochemical Calculations; US Geological Society: Denver, CO, USA, 1999; pp. 1–326. [Google Scholar]
- Qian, G.; Li, Y.; Li, J.; Gerson, A.R. Consideration of enthalpic and entropic energy contributions to the relative rates of chalcopyrite dissolution in the presence of aqueous cationic impurities. Int. J. Miner. Process. 2017, 159, 42–50. [Google Scholar] [CrossRef]
- Geelhoed, J.S.; Meeussen, J.C.; Hillier, S.; Lumsdon, D.G.; Thomas, R.P.; Farmer, J.G.; Paterson, E. Identification and geochemical modeling of processes controlling leaching of Cr(VI) and other major elements from chromite ore processing residue. Geochim. Cosmochim. Acta 2012, 66, 3927–3942. [Google Scholar] [CrossRef]
- Miller, S.D.; Stewart, W.S.; Rusdinar, Y.; Schumann, R.E.; Ciccarelli, J.M.; Li, J.; Smart, R.S.C. Methods for estimation of long-term non-carbonate neutralisation of acid rock drainage. Sci. Total Environ. 2010, 408, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Schumann, R.; Kawashima, N.; Li, J.; Miller, S.; Smart, R.S.C.; Stewart, W.S. Passivating Surface Layer Formation on Pyrite in Neutral Rock Drainage. In Proceedings of the 8th International Conference on Acid Rock Drainage (8ICARD), Skelleftea, Sweden, 22–26 June 2009; pp. 22–26. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).