Abstract
With the rising cost of energy and fuel oils, clean coal technologies will continue to play an important role during the transition to a clean energy future. Victorian brown coals have high oxygen and moisture contents and hence low calorific value. This paper presents an alternative non evaporative drying technology for high moisture brown coals based on osmotic dewatering. This involves contacting and mixing brown coal with anionic super absorbent polymers (SAP) which are highly crossed linked synthetic co-polymers based on a cross-linked copolymer of acryl amide and potassium acrylate. The paper focuses on evaluating the water absorption potential of SAP in contact with 61% moisture Loy Yang brown coal, under varying SAP dosages for different contact times and conditions. The amount of water present in Loy Yang coal was reduced by approximately 57% during four hours of SAP contact. The extent of SAP brown coal drying is directly proportional to the SAP/coal weight ratio. It is observed that moisture content of fine brown coal can readily be reduced from about 59% to 38% in four hours at a 20% SAP/coal ratio.
1. Introduction
Victorian brown coal is a cost effective fuel for power generation. It is very cheap to mine and low in sulphur and ash yield but the high moisture content and hence low calorific value result in high CO2 emission intensity relative to bituminous coal. This high moisture has ensured that power stations are located adjacent to their mines to minimise handling, transportation and environmental problems. Run-of-mine brown coal will require upgrading before its use in new generation thermal power plants.
Practical and economic advantages in reducing the moisture content in brown coals include enhanced handling characteristics and reduced transportation costs. Additional benefits include reduced boiler capital costs, higher combustion efficiency, lower water consumption and waste disposal costs.
1.1. Victorian Brown Coals
Brown coal is at an intermediate stage in the geochemical conversion of accumulated vegetable debris from peat into hard or bituminous coal [1]. Brown coals typically have high moisture contents, in the 30%–70% range, with Victorian brown coal at the extreme end of this range. This high moisture content has a negative impact on every thermal application for brown coal. There is an uninterrupted converse relationship between the moisture content of Victorian brown coal and useful heat accessible from combustion of the coal (the net wet specific energy).
According to Mackay [2], Victorian brown coals occur in seams of a few centimetres to over 100 m thick, extending laterally from a few metres to over 50 km. Within the seams there are bands of coal which vary in appearances and properties. These bands are formed of coals belonging to different lithotypes (rock types) which reflect different depositional environments. The samples for current study are supplied from Loy Yang brown coal mines, Gippsland, Victoria (Figure 1).
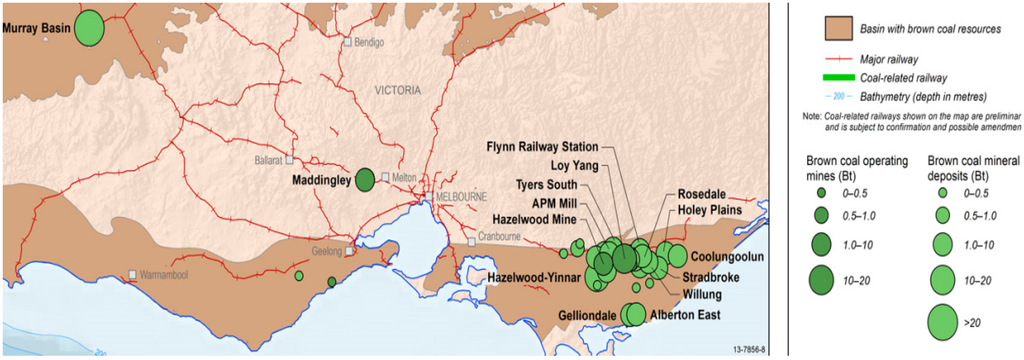
Figure 1.
Current operating brown coal mines located in Victoria website [3].
The freshly exposed surface of as mined brown coal changes in colour from red brown to dark brown, as drying or oxidation occurs. When brown coal is air dried (to moisture 10%–15%) the colour varies from yellow to black.
Verheyen and Perry [4] reported Victorian brown coals to be physically complex and heterogeneous due to their detrital origin. Their relatively low carbon aromaticity, presence of residual carbohydrates (in woody macerals), along with methoxy phenols, unsaturated diterpenoids and fatty acids all confirm that Victorian brown coals have only been exposed to very mild (diagenetic) conditions. Structural heterogeneity tends to decrease as coalification continues due to condensation and cleavage reactions.
Iyengar, Sibal and Lahiri [5] reported that the water sorbed as a monolayer on coal is attached to hydrophilic sites on the coal surface. These sites were identified as oxygen-containing functional groups. They have been confirmed for a variety of coal ranks including Victorian brown coals [6,7].
Molecular simulation techniques were applied by Kumagai, Chiba and Nakamura [8] to define a model for coal structure. They modelled the structure of Yallourn brown coal as two oligomers, namely a tetramer (molecular weight (MW) 1540) and a pentamer (MW 1924) based on a monomer of composition C21H20O7 as represented in Figure 2. The unit structure was modelled on the basis of combined data from elemental analysis (C: 65.6, H: 5.2, O: 29.2 wt %) by Schafer [7] and 13C-NMR spectroscopy. First two molecules were joined with 360 water molecules matching to 65.3% moisture content (wet basis).
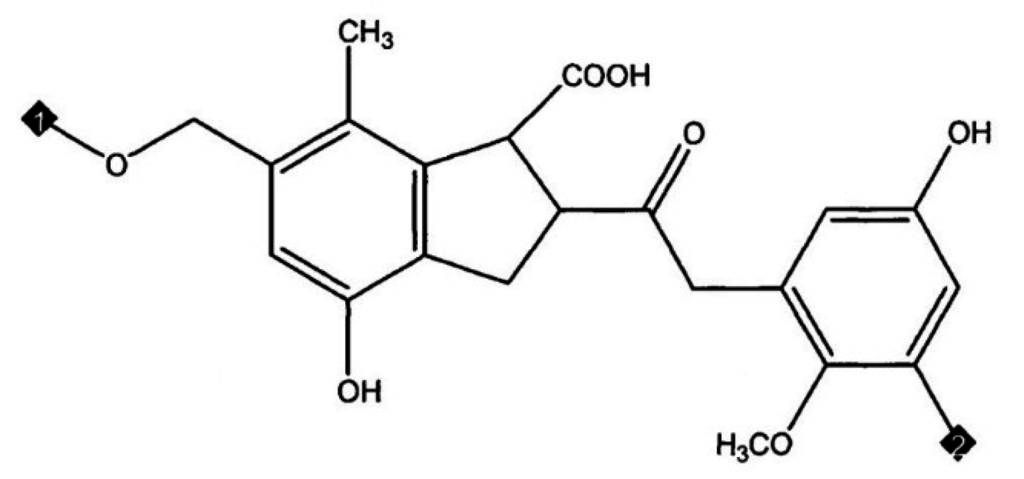
Figure 2.
Monomer structure of brown coal used as basis of Yallourn brown coal molecular model (Kumagai, Chiba and Nakamura 1999) [8] corresponds to C: 65.6, H: 5.2, O: 29.2 wt % (Molecular Weight (MW) = 384.4).
1.2. Brown Coal Drying
Processes for drying or dewatering brown coals are grouped into three wide categories: (a) evaporative (thermal); (b) non-evaporative (thermal) and (c) other non-evaporative drying processes.
The common commercial procedures include evaporative drying where heat is applied to evaporate the water from coal at atmospheric pressure. Non evaporative drying processes are attractive due to their enhanced energy effectiveness, since water is separated in liquid form and the latent heat of vaporisation is not expended.
Studies have been conducted to investigate feasibility of non-evaporative drying of brown coal such as the Fleissner process [9] which incorporates pressurised steam treatment at temperatures above 200 °C. The Evans-Siemon process is the updated Fleisssner process where pressurised hot water is used to improve thermal efficiency by avoiding a pressure cycle [10]. The Koppelman (K-fuel) process [11] involves high pressure with low operating temperature; however, this process was not demonstrated commercially. The mechanical thermal expression dewatering process [12] involves use of elevated temperatures, up to 250 °C and pressure <12.7 MPa, resulting in significant reductions in residual moisture content which may be largely attributed to the destruction of brown coal porosity. Attempts at electro dewatering brown coals [13] did not demonstrate encouraging results. Our current research aims at developing alternative technologies to dewater brown coals.
Dzinomwa, Wood and Hill [14] employed super absorbents to de-water fine black coal particles, revealed some advantages over the alternative non evaporative drying technologies, and offered an alternative lower energy osmotic water removal approach to the thermal technologies outlined above.
1.3. Super Absorbent Polymers (SAP)
SAPs comprise high molecular weight crossed linked hydrophilic polymers which absorb several tens to hundred times their individual mass of water as they expand in size, but still preserve distinct particle identity [15,16,17]. SAP can be cationic and anionic. The amount of water that a specific SAP can absorb depends on its chemical composition and morphology as well as the quality of absorbed water [18], particularly with respect to occurrence of ionic salts. The recycled water used in mining plant operations generally contains a significant concentration of salts [19] and these need to be managed to maximize the polymeric absorption potential.
Factors affecting the capacity of a SAP to absorb water are as follows:
- Swelling properties (attributed to presence of hydrophilic groups in the network).
- Cross linking density (generally higher molecular weight with lower cross linking densities exhibits higher absorption capacities).
- Structural integrity (high cross linking density is crucial to retain the structural integrity of the polymer loaded with moisture as the high cross-linking density offers high mechanical strength).
- The “availability” of target water i.e., how tightly it is bound to the drying substrate.
Figure 3 shows the mechanism of water absorption in SAP. The initial diffusion of water inside hydrophilic SAP causes ionization of neutralized acrylate groups into negative carboxylate ions and positive sodium ions. Negative electrical charges along the SAP backbone cause mutual repulsion of carboxylate ions, increasing the osmotic pressure inside the gel, thereby resulting in expansion and swelling of the SAP chains due to absorbed water. Finally, cross links between chains inhibit solubilisation of SAP (in water) thus governing the extent of swelling or absorption by restricting infinite swelling.
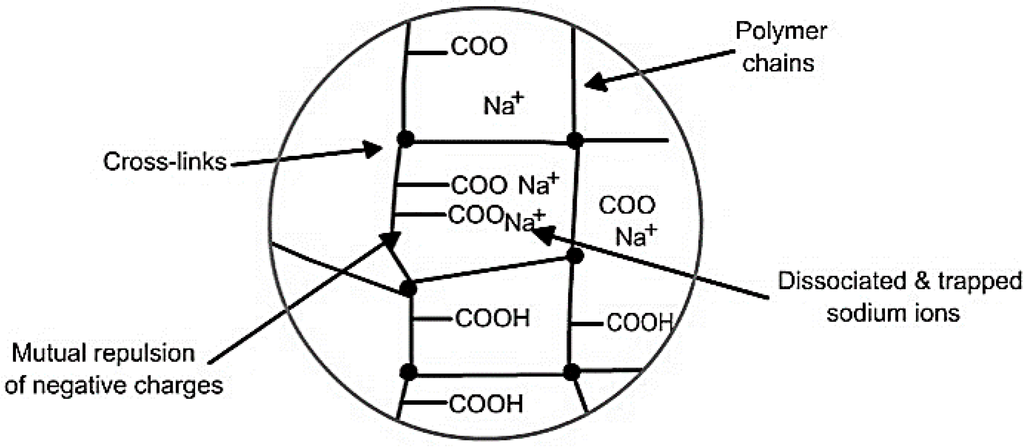
Figure 3.
Mechanism of water absorption in super absorbent polymers (SAP).
1.4. Standard Method of Moisture Determination
According to Allardice [1], determination of moisture content of brown coals is complicated by the lack of an inadequate definition for what constitutes moisture in coal. The most widely accepted definition is that the moisture content is the water present in the coal as water molecules (H2O), which can be released at 105–110 °C. This is not intended to include water from the decomposition of functional groups or chemically adsorbed water.
Allardice [1] reported on two basic types of standard moisture determination methods i.e., (a) azeotropic distillation, in an immiscible liquid such as toluene and (b) oven drying at 105 °C. In azeotropic distillation moisture content is determined directly from the volume of water collected in the condenser, in contrast to most oven drying methods where water is estimated indirectly by net weight loss. The standard method for determining moisture content of brown coals does not discriminate between water of decomposition at a temperature up to 105 °C and molecular water present in it.
2. Methodology of the SAP Dewatering Process
Figure 4 depicts an outline of the osmotic dewatering process, using SAP to decrease moisture content of brown coal. This is achieved by direct contact between SAP and sized brown coal using an end-over-end tumbler, tumbling through 360° from top to bottom providing complete mixing and a sieve shaker to affect final separation. High moisture brown coal is intimately mixed with dry SAP by shaking in air tight bottles to prevent evaporation. This batch storage approach also enabled the SAP to passively draw and absorb water from the surface of brown coal particles. Mechanical end-over-end tumbling (vertical bottle rotation at 60 rpm) for a set time at constant speed was used to maximize surface contact between SAP and brown coal particles thereby reducing the equilibration time. The apparatus provided reproducible and gentle particle contact conditions. Water absorption causes SAP to swell thus permitting the separation of swollen SAP from shrunken brown coal particles by sieving.

Figure 4.
Proposed processes for reducing water content of brown coal by treatment with SAP.
3. Experimental
3.1. Materials
3.1.1. Brown Coal
The brown coal (<80 mm) was provided by Omnia Specialities from a bulk sample collected from the AGL Loy Yang mine in 2014. It was stored in sealed plastic pails prior to analysis. Its proximate and ultimate analysis is presented in Table 1. According to [20] brown coals of Victoria, particularly from Latrobe have low ash yields, but as would be predicted from such huge volumes of coal, even separate seams have slight to substantial differences in physical properties and chemical composition.

Table 1.
Loy Yang Brown coal analysis.
| Coal Properties | Percentage (% Dry Basis) |
|---|---|
| Moisture | 59.3 * |
| Ash | 2.2 |
| Volatile matter | 50.5 |
| Carbon | 68.4 |
| Hydrogen | 5.1 |
| Sulphur | 0.3 |
* % as received.
3.1.2. Super Absorbent Polymers (SAP) Used in this Work
Aquasorb 3005 (<1000 μm) supplied by SNF (Australia) Pty Ltd. (Lara, Victoria, Australia), is a highly crossed linked, synthetic co-polymer of acryl amide and potassium acrylate. It is water insoluble with maximum water absorption of 150% w/w in 1000 ppm NaCl solution.
The polymer consists of a set of polymeric chains that are parallel to each other and regularly linked to each other by cross-linking agents, forming a network. When water comes in contact with one of these chains, it is drawn into the molecule by osmosis. Water rapidly migrates into the interior of the SAP network where it is stored. The pH of SAP is alkaline at 8.1 with a density of 1.10 g/cm3. The maximum water absorption of 400 wt % occurs for deionized water. SAP particles were sieved and those >600 μm and <850 μm were selected for use. Figure 5 presents a molecular structural representation of Aquasorb.
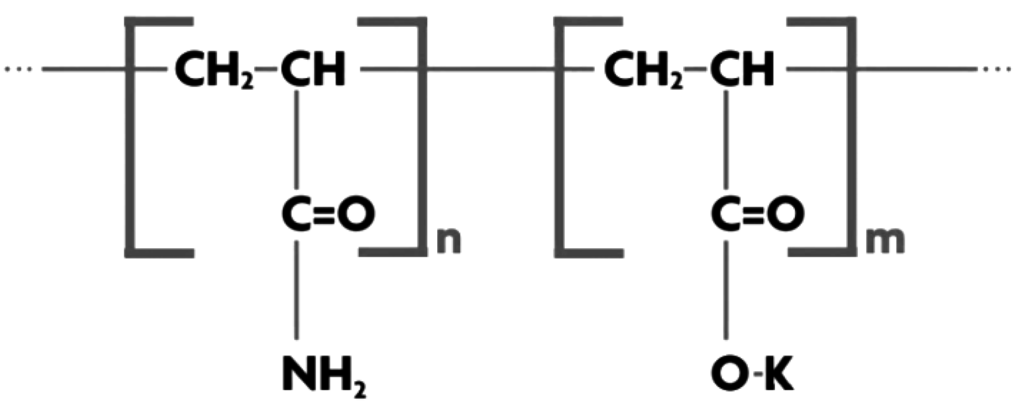
Figure 5.
Structure of Aquasorb.
3.2. Experimental Methods
3.2.1. Fourier Transform Infrared (FTIR)
Fourier transform infrared (FTIR) spectroscopy is widely used to determine/evaluate functional groups in coal structure. The reference [21] attempted structural assignments via deconvolution of particular absorbance bands associated functional groups. FTIR spectral comparison of dewatered and run of mine brown coal samples has the potential to elucidate changes in hydrogen bonding and oxygen functional groups occurring during drying.
In this project, the FTIR spectra were recorded with a FTS1000 FTIR (Varian, MA, USA) fitted with a ATR (attenuated total reflectance) accessory (Specac, Golden Gate Mark II, Orpington, UK); 128 scans were accumulated with spectral resolution of 4 cm−1, over the range of 4000–650 cm−1. The spectrometer was equipped with ATR diamond anvil cell with single reflection. After measurement of spectra, the remaining coal sample on the crystal was removed with soft paper soaked in methanol and allowed to dry. Regular background spectra (air) were collected to ensure no cross contamination. The raw reflectance spectra were corrected for frequency distortion and converted to absorbance by standard software.
Figure 6 presents the ATR-FTIR spectrum of run-of-mine moisture Loy Yang brown coal. The ATR technique probes only the surface layers of the brown coal structure. Peaks identified at 3365, 2900, 1700, 1621 and 1440 cm−1 correspond to OH groups [22], aliphatic groups, carboxylic and ketone groups, carbon oxygen double bonded aromatic rings and bending mode of H-bonded O–H groups [23] respectively (Table 2). Water in brown coal constitutes a progressive series of forms—each is more difficult to remove from apparent bulk water to that resulting from thermal decomposition of hydroxyl groups in brown coal and water of hydration of impure minerals.
The pH of the Loy Yang coal sample was found to be pH 4.05 suggesting that carboxylic groups will mainly be involved in dewatering.

Table 2.
Structural assignments for absorption bands observed in infrared spectra of brown coal.
| Frequency (cm−1) | Functional Groups | Reference |
|---|---|---|
| 3350–3600 | Stretching vibration of OH groups (water, alcohol, phenol, carbohydrates, peroxides) as well as amides (3650 cm−1) | (Liu et al., 2006) [22] Vijayalakshmi and Ravindhran, 2012 [24] |
| 2750–3000 | CH3 and CH2 (aliphatic) | (Chandarlal et al., 2014) [23] |
| 1700 | Carboxylic acid and ketone groups | (Chandarlal et al., 2014) [23] |
| 1649 | Adsorbed water | (Li et al., 2001) [24] |
| 1618–1622 | C=O, aromatic rings | (Chandarlal et al., 2014) [23] |
| 1440 | CH2, C=O bending mode of H-bonded O–H groups | (Chandarlal et al., 2014) [23] |
| 1000–1300 | Phenoxy structure, aliphatic ethers, alcohols | (Chandarlal et al., 2014) [23] |
| 1355 | Benzene or condensed benzene rings | (Chandarlal et al., 2014) [23] |
| 1180 | Sp-3 rich structure COOH and OH | (Chandarlal et al., 2014) [23] |
| 997–1130 | Stretching vibration of C–O of mono, oligo and carbo-hydrates | (Vijayalakshmi and Ravindhran, 2012) [25] |
| <1000 | C–H bending vibration from isoprenoids | (Vijayalakshmi and Ravindhran, 2012) [25] |
| 870–750 | Weak absorption due to C–H bending vibrations of olefinic and aromatic structures | (Verheyen and Perry 1991) [4] |
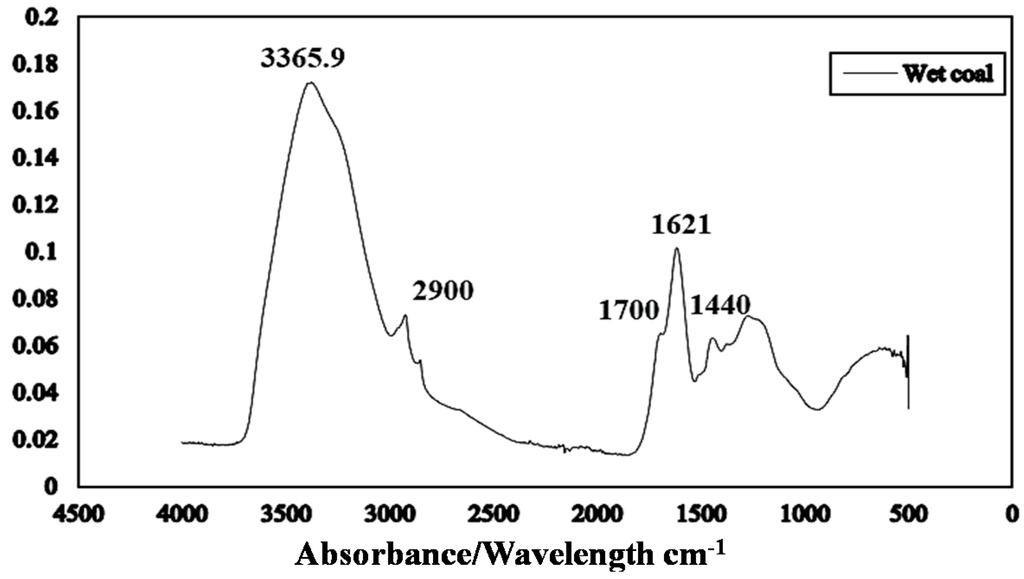
Figure 6.
Attenuated total reflectance Fourier transform infrared (ATR FTIR) spectrum of as received Loy Yang coal.
3.2.2. Dewatering Tests
A series of tests were conducted to optimise the SAP dosage and brown coal/SAP contact time, to identify the effective operating conditions to maximise the water removal.
Loy Yang coal (as received) was crushed and screened <600 μm (to ensure a smaller size than SAP) and stored in sealed containers prior to use. 20 g coal sub samples were mixed with predetermined amounts of SAP to achieve target loadings i.e., 0%, 5%, 10%, 15%, 20%, 25% and 30% SAP/coal. The SAP/coal mixtures were sealed in air tight glass 600 mL lab bottles fitted with plastic screw caps. Up to twelve bottles (replicates) were placed inside the sample box of a vertical tumbler for gentle “top over bottom” bottle agitation at 60 rpm. The sample was weighed before and after the predetermined tumbling time period to ensure no evaporative losses occurred. Contact time was monitored by determining the weight loss of brown coal (after SAP separation) until there was no further change at which point the moisture was considered to be at equilibrium. The mixture was readily separated into dewatered fine brown coal and swollen polymer via a sieve due to their size difference—SAP swells and increases in size, whilst the coal shrinks as it loses moisture. Changes in moisture were measured by a moisture balance programmed for constant weight at a temperature of 105 °C. Figure 7 shows loaded SAP after dewatering of brown coal. It was found the coal lost to the SAP was <0.2%.

Figure 7.
SAP after dewatering brown coal. Note increase in size and the dark colouration afforded by traces of humic material adsorbed onto the alkaline SAP.
4. Results and Discussion
According to Li et al. [26] there is presence of significant amount of alkali and alkaline earth metals associated with the carboxylic and phenolic functionalities in the structure of low rank coal. At low pH, ion exchange mainly takes place with carboxylic groups to form carboxylates. According to Schafer [27], acidic groups and phenolic, are primarily responsible for ion exchange properties of brown coals. At the in-situ pH prevalent in wet brown coals, carboxyl groups may interchange cations with pore water to form carboxylates. Cations normally related with these groups are calcium, magnesium, sodium and iron. Meanwhile, phenolic groups do not interchange cations to any degree until system pH is greater than pH 8. The mean acid group content of the brown coal from Loy Yang field is shown in Table 3.

Table 3.
Mean acidic group content of Loy Yang brown coal (Schafer, 1991) [27].
| Phenolic OH (Dry Basis) | COOH (Dry Basis) | COO (Dry Basis) | Phenolic Oxygen (Dry Basis) | Carboxylic Oxygen (Dry Basis) | Acidic Oxygen | Total Oxygen (Dmif) | Acidic Oxygen |
|---|---|---|---|---|---|---|---|
| meq/g | Percentage (%) | ||||||
| 3.04 | 2.39 | 0.10 | 4.86 | 7.97 | 13.0 | 24.7 | 53 |
Dmif—dry mineral and inorganic free basis.
4.1. Moisture Results
SAP dosage rates ranging from 5 to 30 wt % SAP/brown coal, and contact times between 1 to 6 h were evaluated; to determine the optimum drying conditions for our experimental setup.
Test samples of fine brown coal prior to drying had a water content of 59.3%. Figure 8 and Table 4 show the final coal moisture for different SAP doses and contact times. It was considered that dosage of 5% of SAP by weight of brown coal and a contact time of six hours would give an adequate moisture reduction while avoiding excessive cost of SAP and holding capacity. The brown coal was observed to lose maximum of 53.25% of its original moisture after six hours contact with 20% w/w SAP.
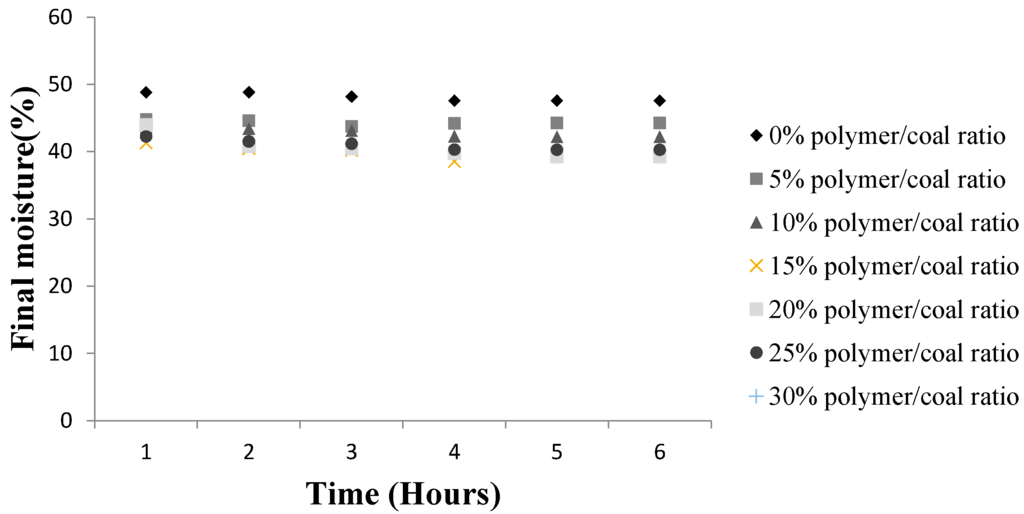
Figure 8.
Variation in Loy Yang moisture content with contact time and Super absorbent Polymer, SAP/coal.
A SAP/coal of 25 wt % caused a further decrease in moisture level. However at 30 wt % SAP dosage, there was no further reduction in coal moisture instead a slight increase was observed. This implies that higher weight percentage SAP loadings may release water from SAP which is reabsorbed on to the brown coal.
Figure 8 reveals the final Loy Yang moisture values with 20% SAP/coal achieving the optimal drying for moisture reduction. Static osmotic dewatering of brown coal i.e., without the tumbling, produced a final coal moisture of 47.3%, compared to the 38.6% with tumbling for the same contact time. This result illustrates the importance of continuously exposing fresh surfaces for maximum contact osmotic drying rates. The higher convective mass transfer and increased surface contact interactions afforded by tumbling make it possible to reduce the brown coal drying time. It was ascertained that a contact time of four hours was the optimal treatment time. Further drying via SAP contact could be achieved by increasing the pH of coal as the SAP has higher moisture affinity under basic conditions and the Loy Yang coal is acidic in nature.

Table 4.
Effect of anionic super absorbent polymers (SAP) dosage and contact time on reduction of water content of fine brown coal (initial water content of brown coal = 59.3%).
| Polymer/Coal Ratio (%) | |||||||
| - | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
| Hours | Final Moisture Content (%) | ||||||
| 0 | 59.3 | 59.3 | 59.3 | 59.3 | 59.3 | 59.3 | 59.3 |
| 1 | 58.7 | 48.8 | 44.8 | 43.6 | 41.3 | 44 | 42.2 |
| 2 | 58.7 | 48.8 | 44.6 | 43.4 | 40.4 | 40.8 | 41.5 |
| 3 | 58.4 | 48.2 | 43.7 | 43.1 | 40.2 | 40.4 | 41.7 |
| 4 | 58 | 48.5 | 44.2 | 42.3 | 38.6 | 39.7 | 40.3 |
| 5 | 58.2 | 48.6 | 44.2 | 42.2 | 39.7 | 39.3 | 40.3 |
| 6 | 58.2 | 47.6 | 44.3 | 42.2 | 39.2 | 39.3 | 40.2 |
4.2. Dewatering Kinetics
According to Szekely et al. [28] analysis of a heterogeneous reaction system must start from the recognition that reaction takes place at the interface and hence mass must be transported to and from this interface. It follows that structure, pertinent to mass transfer during and after contact plays a significant role on the overall rate of water transfer. The fraction of moisture removed from brown coal was calculated from the following equation:
where, α = fraction of moisture removed.
Figure 9 shows the fraction of moisture removed with respect to the time. It can be seen that the bulk of the drying occurs within the first two hours (Regions 1 and 2) and then attains a steady state/equilibrium (Region 3). Figure 9 indicates the maximum removal of moisture by SAP within the first two hours.
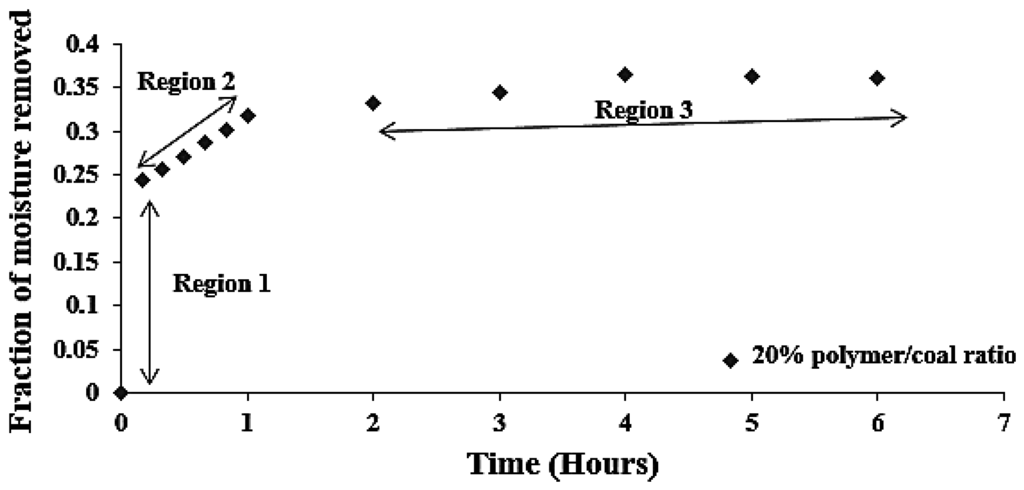
Figure 9.
Fraction of moisture removed (α) vs. time at different SAP to coal ratio.
The three different regions identified in Figure 9 are as follows:
- Region 1: Dissociation and breaking of chemically adsorbed and surface moisture (chemical process).
- Region 2: Desorption of water (bound and free water)/and breaking of liquid bridges in coal (chemical and physical process).
- Region 3: Diffusion/mass transfer process (physical process).
4.3. Surface Chemical and Diffusion Control Model
Accordingly the dewatering kinetics were tested for surface chemical model (R3 (α)) and diffusion control model (D3 (α)). Where,
R3 (α) = 1 − (1 − α) 1/3
It follows from Figure 9 that Region 1 and parts of Region 2 should follow the surface chemical model and parts of Region 2 and Region 3 should follow the diffusion model. Figure 10 shows the plots for surface chemical shrinking core model, R3 (α) vs. time and diffusion control model, D3 (α) vs. time, for 20% SAP to coal ratio. Plots for the pore diffusion model (Figure 10) indicate a better linear trend until the end of the reaction, indicating that the mass transfer-diffusion process controls the reactions. A linear trend in the Region 2 for R3 (α) plots indicates a competing chemical, and mass transfer-diffusion process controlling the dewatering process.
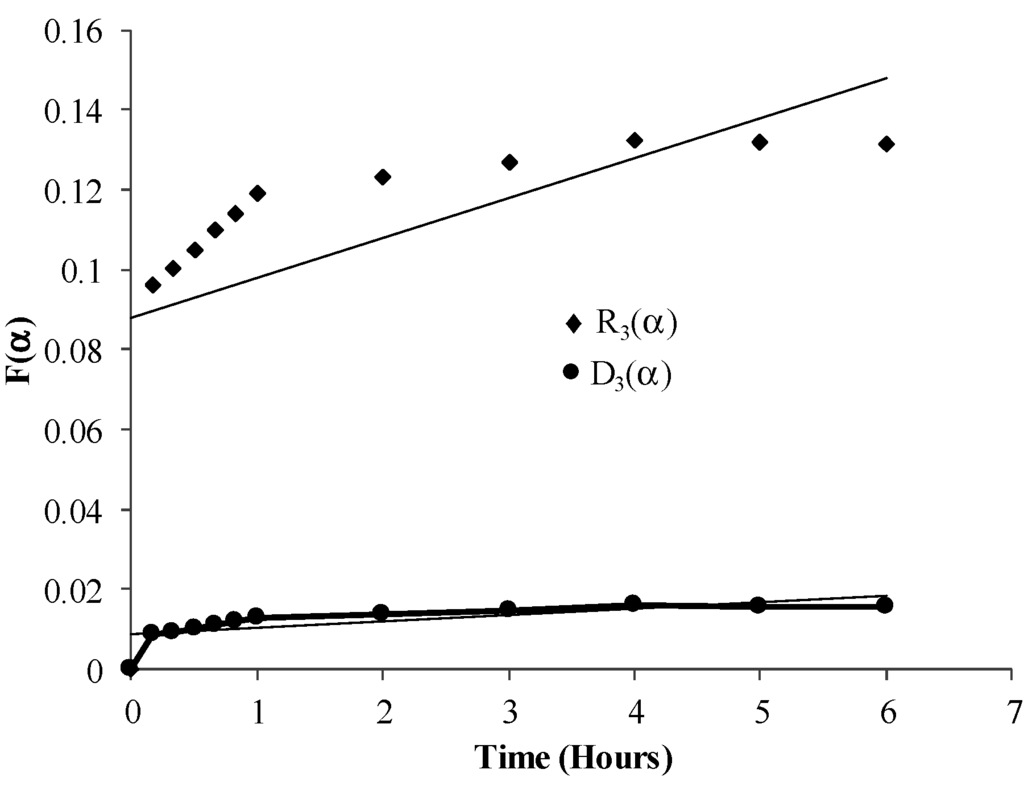
Figure 10.
Plot of diffusion control model [D3 (α)] vs. time and surface control model [R3 (α)] vs. time for 20% SAP to coal ratio.
4.4. Fourier Transform Infrared (FTIR ATR)
The Fourier transform infrared spectroscopy of the brown coal sample and SAP sample before and after dewatering over the wave number ranges 4000–600 cm−1 are presented in Figure 11 and Figure 12 respectively. The amplified difference spectrum in Figure 12 reveals that the as received and SAP treated brown coal samples have only minimal difference in absorptions due to inherent moisture i.e., positive broad H-bonding band centred at approx. 3365 cm−1 and the minor contribution to the band near 1621 cm−1. Removal of all the non-bound water at 105 °C resulted in the typical IR absorbance spectrum (Figure 11) for dried brown coal with the O–H and C–H of aromatic and aliphatic stretching, (3700–2400 cm−1) [4,29] and more pronounced absorptions due to C–H stretch near 2900 cm−1 [4,23] and bend near 1450 cm−1; carbonyl 1700 cm−1 [23] and aromatic ring (breathing mode enhanced by ring substitution) at 1621 cm−1. The fingerprint region 1440–949.2 cm−1 which incorporates a multitude of functional group contributions [23,25], including etheric oxygen and mineral salts which is also enhanced in the oven dried sample. The sensitivity (IR extinction coefficients) changes for the organic bands in the coal on removal of moisture with slightly higher band intensities observed for most organic functional groups. For the SAP treated sample as seen in Figure 11 and Figure 12 the doublet band centred near 2380 cm−1 is assigned to adsorbed CO2 on the surface of the brown coal. The variation in this band in SAP treated coal is thought to relate to both atmospheric CO2 adsorption by the sample and CO2 transfer from the SAP itself. Figure 13 and Figure 14 provide an IR spectral comparison of SAP before and after contact with Loy Yang coal. Differences in absorption in the 1440–1600 cm−1 region (Figure 14) corresponds to fine structural bands that are lost from the polymer on wetting and the spectrum is dominated by positive water bands. There is an expected increase in peaks at approximately 3335 and 1650 cm−1 for SAP after coal contact indicative of adsorbed water.
The SAP would need to be recycled in a commercial coal drying application by a non-evaporative dehydration process. This can be achieved by pH shock wherein the SAP shrinks on contact with acids such as HCl [14]. The low pH water self-drains from the SAP thereby reducing the need for thermal evaporation. Further research into SAP recycling is warranted given the encouraging results presented here for removing bulk moisture osmotically from Loy Yang coal.
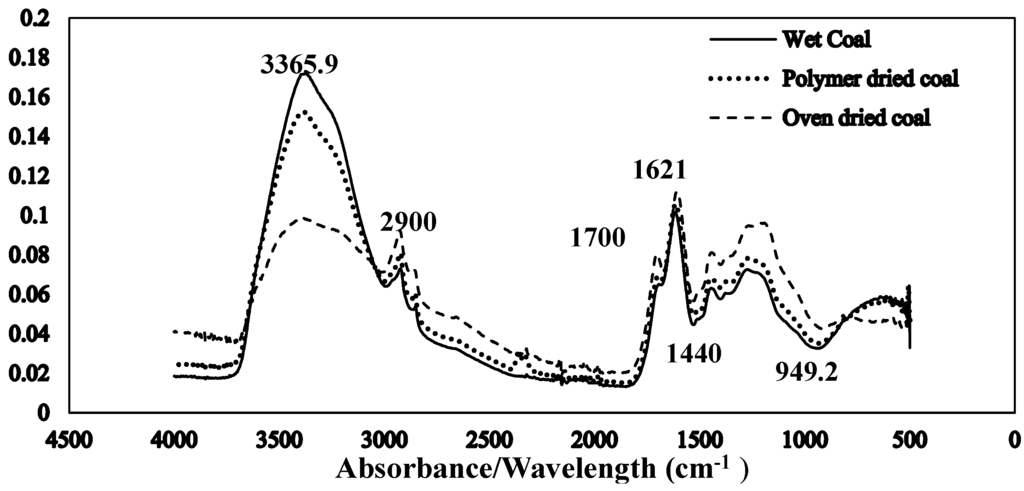
Figure 11.
Stacked ATR–IR spectra of as received (59.3% moisture) SAP treated (38.6% moisture) and oven dried (105 °C in air) Loy Yang coal. Spectra presented at the same scale.
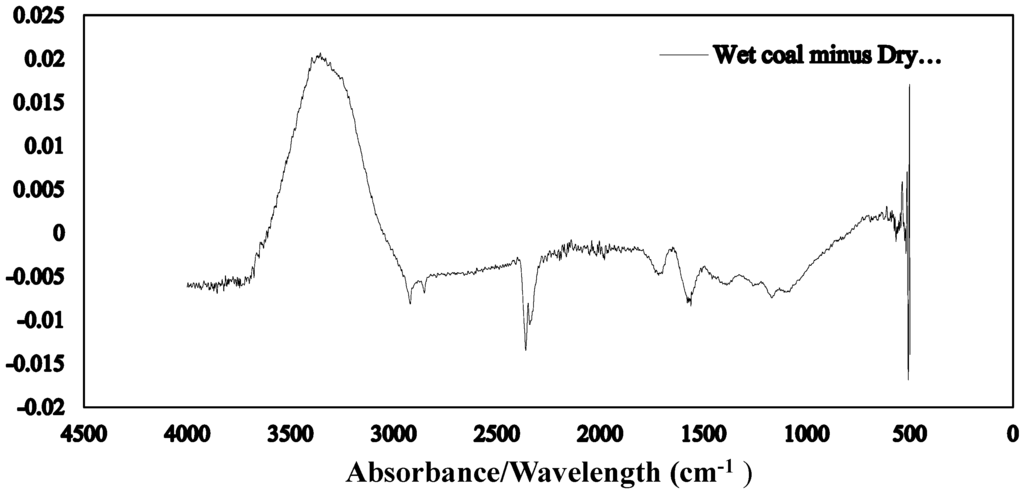
Figure 12.
ATR–IR difference spectrum for as received moisture Loy Yang coal minus its SAP treated product.
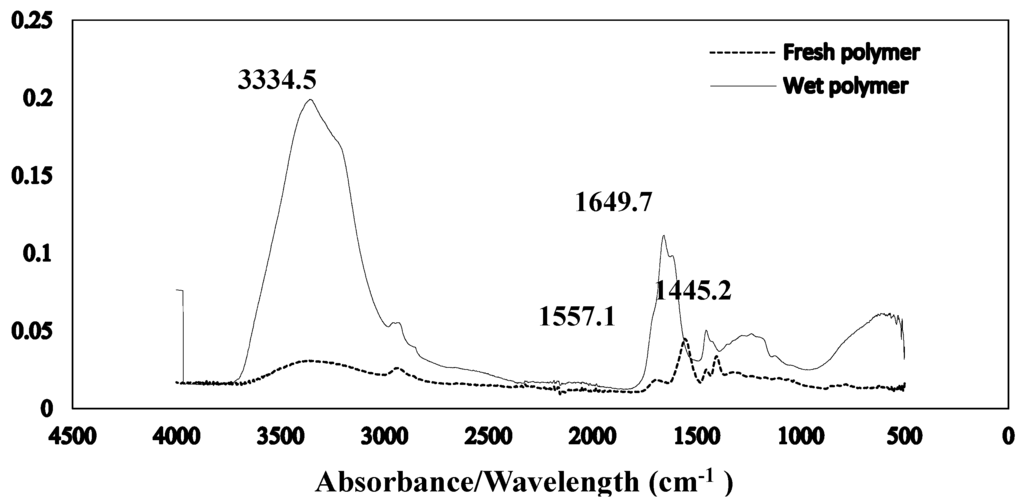
Figure 13.
Stacked ATR–IR spectra of fresh SAP and its water loaded equilibrium (with coal) moisture product. Spectra presented at the same scale.
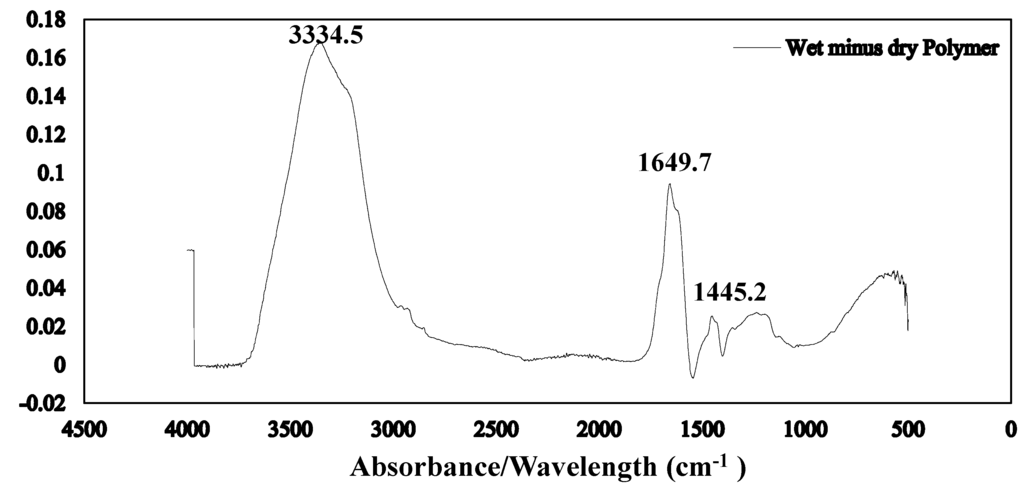
Figure 14.
ATR–IR difference spectrum for wet minus fresh SAP Note the lack of any coal derived absorbance bands despite the brown colouration of the loaded SAP.
5. Conclusion
Osmotic dewatering of brown coal using SAP indicates that it is possible to achieve reduction in Loy Yang moisture from 59.3% to 38.6% equivalent to removing 57% (on dry basis) of the initial water present. Under the tumbling contact conditions employed mixing for four hours is necessary to attain equilibrium moisture loadings between polymer and brown coal. This process is safer than direct thermal drying and will produce fine brown coal with a consistent moisture content. Laboratory tests revealed that the rate of moisture absorption remains constant between 4–6 h.
Acknowledgments
We acknowledge the CRN seed funding awarded to Sheila Devasahayam by the federation University Australia and David Hill’s (University of Queensland, Australia) technical input and discussions during the course of the project. Andrew Stranieri’s, (FoST, Federation University Australia), interest, encouragement, facilitating of the project are very much appreciated.
Author Contributions
Sheila Devasahayam is the supervisor of this student’s project. Anas Ameen, the master’s student in Mining Engineering at the Federation University, Australia carried out this project in partial fulfilment of his master’s degree, under the supervision of Sheila Devasahayam, co-supervised by Vince Verheyen (Fed Uni) and Sri Bandyopadhyay, UNSW.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Allardice, D.J.; Newell, B.S. Industrial imlication of the properties of brown coal. In The Science of Victorian Brown Coal: Structure Properties and Consequences for Utilization; Durie, R.A., Ed.; Butterworh-Heinmann Ltd.: Oxford, UK, 1991. [Google Scholar]
- Mackay, A.M.; George, G.H. Petrology. In The Science of Victorian Brown Coal: Structure, Properties and Consequences for Utilization; Durie, R.A., Ed.; Buterworth-Heinemann Ltd.: Oxford, UK, 1991. [Google Scholar]
- Australia’s Coal Industry. Available online: http://www.minerals.org.au/resources/coal/coal_mines_by_state (accessed on 24 September 2015).
- Verheyen, T.V.; Perry, G.J. Chemical structure of Victorian brown coal. In The Science of Victorian Brown Coal: Structure Properties and Consequences for Utilization; Durie, R.A., Ed.; Butterworth-Heinmann Ltd.: Oxford, UK, 1991. [Google Scholar]
- Iyengar, M.S.; Sibal, D.H.; Lahiri, A. Role of hydrogen bonds in the briquetting of lignite. Fuel 1957, 36, 76–84. [Google Scholar]
- Allardice, D.J.; Evans, D.G. The brown coal/water system: Part 2. Water sorption isotherms on bed moisture Yallourn brown coal. Fuel 1971, 50, 236–253. [Google Scholar] [CrossRef]
- Schafer, H.N.S. Factors effecting the equilibrum moisture content of low rank coals. Fuel 1972, 51, 4–9. [Google Scholar] [CrossRef]
- Kumagai, H.; Chiba, T.; Nakamura, K. Change in physical and chemical characteristics of brown coal along with a progress of moisture release. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1999; Volume 218. [Google Scholar]
- Fleissner, H. The Drying of Fuels and the Australian Coal Industry. Sonderdruck Spartwirtschaft, 1927; Nos.10 and 11. [Google Scholar]
- Higgins, R.S.; Allardice, D.J. Development of Brown Coal Dewatering; Reserach and Deveopment Report; State Electricity Commission of Victoria (SECV): Victoria, Australia, 1973. [Google Scholar]
- Murray, R.G. Stable high enery solid fuel from lignite. Coal Process Tech. 1979, 5, 211–214. [Google Scholar]
- Hulston, J.; Favas, G.; Chaffee, A.L. Physico-chemical properties of Loy Yang lignite dewatered by mechanical thermal expression. Fuel 2005, 84, 1940–1948. [Google Scholar] [CrossRef]
- Reuter, F.; Molek, H.; Kockert, W.; Lange, W. Laboratory desalination of brown coal by elecrto-osmosis. Neue Bergbautechnik 1981, 11, 186–187. [Google Scholar]
- Dzinomwa, G.P.T.; Wood, C.J.; Hill, D.J.T. Fine coal dewatering using pH-sensitive and temeprature sensitive super absorbent polymers. Polym. Adv. Technol. 1997, 8, 767–772. [Google Scholar] [CrossRef]
- Masuda, K.; Iwata, H.; Masuda, K.; Iwata, H. Dewatering of particulate materials utilizing highly water-absorptive polymer. Powder Technol. 1990, 63, 113–119. [Google Scholar] [CrossRef]
- Moody, G.M. Role of polyacrylamides and related products in treatment of mineral processing effluent. Trans. Inst. Min. Metall. 1990, 99, C136. [Google Scholar]
- SadeghiI, M.; Hosseinzadeh, H. Synthesis and properties of collagen-g-poly(sodium acrylate-co-2-hydroxyethylacrylate) superabsorbent hydrogels. Braz. J. Chem. Eng. 2013, 30, 379–389. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Sun, X.W.; Xie, Z.X.; Qin, L. Study on synthesis and property of anti-salt super absorbent resin. Adv. Mater. Res. 2013, 873, 683–688. [Google Scholar] [CrossRef]
- Laftha, W.A.; Hashim, S.; Ibrahim, A.N. Polymers hydrogel: A review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Gloe, C.S.; Holdgate, G.R. Geology and Resources. In The Science of Victorian Brown Coal: Structure, Properties and Consequences for Utilization; Durie, R.A., Ed.; Butterworh-Heinmann Ltd.: Oxford, UK, 1991. [Google Scholar]
- Painter, P.C.; Starsinic, M.; Coleman, M.M. Fourier Transform Infrared Spectroscopy Applications to Chemical Systems; Ferraro, J.R., Basile, J.L., Eds.; Academic Press: Waltham, MA, USA, 1985. [Google Scholar]
- Liu, H.; Sun, S.Q.; Lv, G.H.; Chan, K.K. Study on Angelica and its different extracts by Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. Spectrochim. Acta Part A 2006, 64, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Chandarlal, N.; Mahapatra, D.; Shome, D.; Dasgupta, P. Behaviour of low rank high mositure coal in small stockpile under controlled ambient condition—A approach. Am. Int. J. Res. Form. Appl. Nat. Sci. 2014, 6, 98–108. [Google Scholar]
- Li, H.; Khor, K.A.; Cheang, P. HVOF Sprayed Hydroxyapatite Coatings: Powders’ Smelting State and Mechanical Properties. In Thermal Spray 2001: New Surface for a New Millenium; Berndt, C.C., Khor, K.A., Lugscheider, E.F., Eds.; ASM International: Geouga County, OH, USA, 2001; pp. 99–104. [Google Scholar]
- Vijayalakshmi, R.; Ravindhran, R. Comparitive fingureprint and extraction yield of diospyrus ferrea(wild.) Bakh. root with phenol compounds (gallic acid), as determined by UV-Vis and FT-IR spectroscopy. Asian Pac. J. Trop. Biomed. 2012, 2, 1367–1371. [Google Scholar] [CrossRef]
- Hayashi, J.; Li, C.-Z. Structure and properties. In Advances in the Science of Victorian Brown Coal, 1st ed.; Li, C.Z., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; pp. 11–78. [Google Scholar]
- Schafer, H.N.S. Functional groups and ion exchange prooperties. In The Science of Victorian Brown Coal; Durie, R.A., Ed.; Butterworh-Heinmann Ltd.: Oxford, UK, 1991. [Google Scholar]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid Reaction; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Meekum, U. Study of the molecular srain of polymerizable cyclic oligocarbonates using the spectroscopic techique. Suranaree J. Sci. Technol. 2005, 12, 107–113. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).