Abstract
The increasing demand for lithium and the limited availability of high-grade resources have accelerated interest in lithium-bearing clays as a promising alternative, despite their relatively lower lithium content. Lithium extraction from such clay minerals typically requires thermal treatment or acid leaching to disrupt the clay crystal lattice and enhance lithium solubility. The enrichment tailings from the Kırka Boron Processing Plant in Türkiye consist predominantly of dolomite-rich clay minerals and contain approximately 900–1200 ppm Li. Considering the substantial quantities of these tailings currently stored on-site, recovering lithium and converting these materials into a valuable resource would be of significant economic importance. However, due to their mineralogical composition, conventional acid leaching of these tailings demands relatively high sulfuric acid consumption (1.5–2.0 M H2SO4). This leads to excessively low solution pH and the generation of highly acidic waste streams, while also promoting the co-dissolution of iron (Fe) and aluminum (Al) ions at pH levels below 2, which negatively affects lithium recovery and downstream processing. In this study, mechanical activation was applied to the tailings prior to acid leaching. As a result, the acid requirement to achieve lithium extraction efficiencies of 90% and above was successfully reduced from 1.5 M to 1.0 M H2SO4. Moreover, solution pH was maintained near neutral (~7), and the undesirable dissolution of Fe and Al ions was effectively suppressed and kept under control.
1. Introduction
Global lithium production relies mainly on brine operations and hard-rock pegmatites such as spodumene and lepidolite, with Australia, Chile, and Argentina accounting for over 80% of supply [1]. The rapid expansion of lithium-ion battery demand has intensified pressure on conventional sources, directing attention toward abundant lithium-bearing clay deposits, which are widely distributed and hold potential to supplement future supply despite their generally lower lithium grades [2,3,4].
Processing lithium from clays is challenging because conventional methods require high-temperature roasting (≈900 °C) and/or strong acid leaching (>1 M), leading to extensive gangue dissolution (Al3+, Mg2+, Ca2+, Na+, K+, Fe3+). This not only increases acid consumption and operating costs but also generates highly acidic waste streams that pose environmental risks [5]. Impurity co-dissolution further complicates downstream recovery, reducing lithium selectivity. For example, Na+ and K+ decrease lithium carbonate purity during precipitation, while Mg2+ and Ca2+ interfere with evaporation and chloride conversion steps, requiring Mg2+/Li+ ratios below 6 to achieve efficient extraction [6,7,8,9]. Boron species, which often co-occur with lithium in clays, also disrupt solution chemistry and promote co-precipitation, hindering the production of battery-grade lithium salts [10,11].
Mechanical activation has emerged as an effective pretreatment to mitigate these issues. High-energy milling disrupts the clay crystal lattice, generates amorphous domains, and increases structural defects, thereby enhancing leachability and reducing the activation energy required for lithium dissolution [12,13]. Previous studies show that mechanical activation combined with salt-assisted water leaching can significantly reduce acid consumption while improving lithium selectivity [8,14,15,16]. Despite recognized differences among planetary, stirred-media, and vibratory mills in promoting amorphization, practical comparisons for industrially relevant conditions are still limited [13].
Turkey hosts substantial lithium-bearing clay reserves associated with borate deposits, particularly in Kırka, yet research remains limited [17]. At the Kırka Boron Plant, approximately 3,000,000 tons of Tincal are produced (about 60% of Feed) annually, of which nearly 40% (about 2,000,000 tons) is disposed of as waste. Considering long-term production, these large quantities of lithium-bearing solid wastes represent a significant potential lithium reserve.
Conventional high-acid leaching or roasting approaches have achieved lithium recoveries of ~77%–99%, while alternative sulfate-roasting processes yielded 82%–97% recovery [17,18,19,20,21,22]. However, challenges such as excessive acid consumption, impurity co-leaching, and adverse effects of boron remain unresolved [23].
In this study, initial mechanical activation is proposed as a sustainable pretreatment strategy to reduce acid consumption and enhance selective lithium dissolution from clay-rich boron processing waste in Kırka. Three high-energy milling technologies—planetary, stirred-media, and vibratory mills—were evaluated for their ability to modify the structure of the surface and make it akin to industrial applications. Dissolution experiments focused on samples activated by stirred-media milling due to its suitability for continuous large-scale operations. The findings provide insight into relatively low-acid reducing acid concentration, high-selectivity lithium extraction, eliminating or preventing the dissolution of competing ions such as Ca2+, Mg2+, Al3+, and Fe3+, and demonstrate a practical approach for valorizing boron-related waste while minimizing environmental impact by producing wastes with a low acidic characteristic.
2. Materials and Methods
Material
The lithium-bearing material investigated in this study was obtained from the tailings of the Kırka Boron Plant, Türkiye, where it exists in a fine, mud-like form. The processing of run-of-mine (ROM) boron ore in the plant consists of dissolution in hot water (96 ± 2 °C), followed by sedimentation, decantation, and crystallization. In this process, boron minerals are selectively dissolved, while the dolomitic clay gangue remains undissolved. Solid–liquid separation is achieved through a two-stage operation involving dissolution sluices and decanter centrifuges. The fine sediments generated from these operations are combined and discharged into a designated tailings storage area. Representative samples were collected from this site for experimental investigation. The chemical composition of the boron plant tailings, as presented in Table 1, indicates the presence of significant amounts of dolomitic clay minerals with minor boron residues, suggesting their potential as secondary lithium resources.

Table 1.
Chemical analysis of the boron plant waste material.
As shown in Table 1, the waste sample contains approximately 900 ppm lithium, along with other alkali and alkaline earth elements, including 2.15% Na, 0.62% K, 11.16% Ca, and 10.16% Mg. The loss on ignition (LOI) value of 34.08% suggests the presence of significant carbonate and clay constituents. XRD analysis of the sample (Figure 1) confirms that dolomite is the dominant mineral phase (70%–75%), consistent with the high Ca and Mg contents and the elevated LOI value. Minor phases such as tincalconite (10.1%), illite (1.2%), clinochlore (5.4%), zeolite, muscovite, and halite were also identified as accessory minerals.
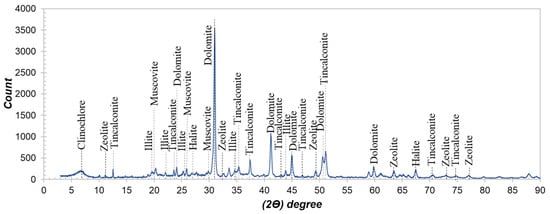
Figure 1.
XRD results of the boron processing plant waste sample.
As is well known, dolomite is a carbonate mineral composed of Ca and Mg, and it exhibits a strong buffering capacity, leading to high acid consumption, particularly during acid-based processing. It is critically important during the recovery of critical metals from such mineral compositions to minimize acid consumption through additional control strategies. Reduced acid usage not only improves economic efficiency but also contributes to the generation of solid wastes with a lower acidic characteristic. Furthermore, operating under lower acid concentrations plays a key role in limiting and preventing the dissolution of competing ions such as Ca2+, Mg2+, Fe3+, and Al3+ into the solution, thereby enhancing process selectivity.
3. Method
Representative samples with a particle size of −50 mm, collected in the form of thick slurry from the waste storage area, were taken and reduced to smaller quantities using standard cone-and-quartering and riffling methods, and then were dried under open-air conditions and subsequently in an oven. After drying, the material was crushed to −2 mm using a laboratory-scale jaw crusher prior to the mechanical activation and leaching experiments (Figure 2).
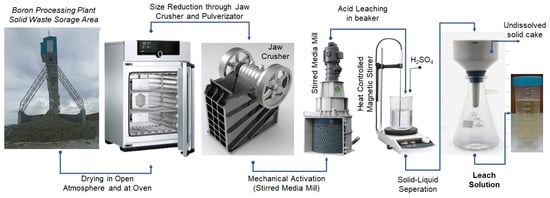
Figure 2.
Flowsheet of experimental procedure.
It is acknowledged that homogenization and manual subsampling of relatively small sample masses may introduce a certain degree of experimental uncertainty. To minimize this effect, all samples were thoroughly homogenized prior to subsampling, and identical sampling protocols were applied consistently throughout the experimental program. The mass of solid samples used in each leaching test was carefully controlled, and selected experiments were repeated to verify reproducibility. The observed trends in lithium recovery, pH evolution, and impurity dissolution were found to be consistent across repeated tests, indicating that the overall conclusions are robust despite the inherent limitations associated with small-scale batch experiments.
As part of the experimental procedure, the samples were first subjected to mechanical activation (dry process) using three types of high-energy mills: planetary mill (PM) (Rantek Co., Ankara, Türkiye)), stirred mill (SM) (Union Process, Akron, OH, USA), and vibratory mill (VM) (Figure 3). The operational parameters and milling conditions used for sample activation are summarized in Table 2. The degree of crystallinity (DoCr, %) of the raw and mechanically activated samples was determined from the XRD patterns based on the ratio of crystalline peak area to total diffraction area, and the results are presented in Table 3. As seen from Table 3, no significant change was observed in the crystallinity of the dominant dolomite phase, whereas noticeable disorder was detected in the clay minerals.
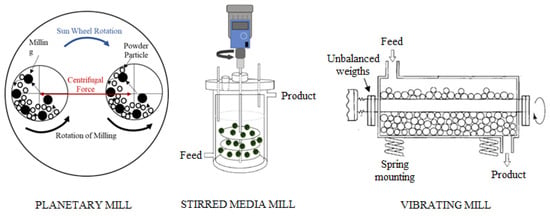
Figure 3.
High-energy mills used in this study.

Table 2.
Operating variables, mill chamber specifications, and ball characteristics of the mills used for mechanical activation.

Table 3.
Particle size parameters, degree of crystallinity, and semi-quantitative mineralogical indicators of raw and mechanically activated samples.
Following mechanical activation, both the raw and activated samples were subjected to acid leaching experiments conducted on a heat-controlled magnetic stirrer. After leaching, the resulting pulp was filtered, and the filtrate was analyzed for lithium content. Lithium concentrations in the leachates were determined using ICP-OES. All measurements were performed in triplicate, and the relative standard deviation was below 2.5%.
Acid leaching experiments were performed at a liquid-to-solid ratio (L/S) of 5:1, a leaching duration of 30 min at 500 rpm stirring speed, and a temperature of 80 °C. Dried powders (10–15 g) were added to deionized water maintained at the specified temperature in a 250 mL glass beaker. After leaching, the slurry was allowed to cool to room temperature and then vacuum filtered to obtain the leachate for lithium analysis using ICP-OES. Lithium recovery was calculated based on the lithium concentration in the leachate using Equation (1):
Accordingly, the lithium mass balance between the initial solid, leach solution, and residual solid was closed based on the measured lithium concentration in the leachate and the known initial lithium content of the feed.
The surface morphology and chemical composition of the raw material, mechanically activated powders, and solid leach residues were examined using scanning electron microscopy (SEM; HITACHI SU3500) and surface elemental analysis by energy-dispersive spectroscopy (EDS; Oxford AZtech). Phase identification of all samples was carried out using X-ray diffraction (XRD; RIGAKU Miniflex 600) with a Cu Kα radiation source (λ = 1.5406 Å) over a 2θ range of 3–90°, at a scan step size of 0.02°.
Characterization of Active Powders
Figure 4 illustrates the surface modifications and ion accumulation differences observed on the surfaces of samples activated by different high-energy milling systems. The surface characteristics of the mechanically activated samples were examined using backscattered electron (BS) imaging coupled with energy-dispersive X-ray spectroscopy (EDS/EDX), and the corresponding results are presented in Figure 4. Because low-magnification BS–EDS images (1–5 µm scale) offer limited interpretive value for the activated powders due to their predominantly amorphous nature, larger-scale BS–EDS mosaics were employed. This imaging approach provides a clearer representation of the surface features and effectively highlights the variations in elemental distribution resulting from mechanical activation.
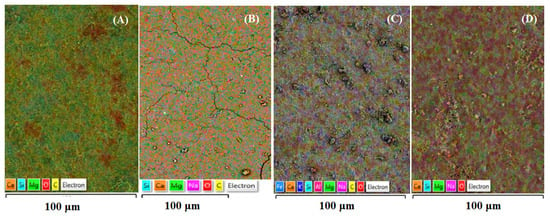
Figure 4.
BS-EDS images of raw (A) and activated products through SM (B), VM (C), and PM (D).
Figure 4 clearly demonstrates that Mg2+ ions (shown in green), which are widely distributed over the surface of the raw material (A), are largely replaced by Ca2+ and Na+ ions following stirred-media (SM) activation (B). Additionally, Fe2+ ions originating from the steel grinding media appear on the surface after mechanical activation (C), while Na+ ions become dominant in both SM- and VM-activated samples (C and D). This variation in surface elemental distribution, particularly the substitution and enrichment of specific ions, is attributed to the degree and type of mechanical activation. As previously described, planetary milling (PM) primarily induces mechanochemical activation, whereas stirred milling (SM) promotes surface activation, and vibratory milling (VM) produces a combination of lower-intensity mechanochemical and surface activation effects.
FTIR analyses were conducted on the raw sample and powders activated by planetary (PM), stirred-media (SM), and vibratory (VM) mills. The resulting FTIR spectra are presented in Figure 5. As shown in Figure 4, the O–H stretching peaks associated with crystal and adsorbed water, as well as the B–O–H, B–O/C–O, and CO3 vibrational peaks, are diminished or absent following mechanical activation. This behavior can be attributed to partial amorphization, loss of crystallinity, and transformation of structural OH groups into adsorbed water [24,25]. The intensity of these peaks decreases progressively according to the mechanical activation intensity, following the order raw > VM > PM > SM. Such structural modifications induced by varying degrees of mechanical activation facilitate the dissolution of ions from the solid matrix, thereby enhancing leach recoveries.
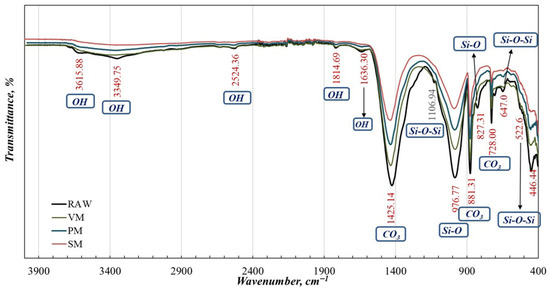
Figure 5.
FTIR analysis of raw and activated products by PM, SM, and VM.
The reduced intensity of carbonate bands in FTIR spectra after mechanical activation does not indicate complete decarbonation, but rather structural disordering and partial amorphization of carbonate phases. High-energy milling induces lattice distortion, defect formation, and short-range disorder within carbonate minerals, which leads to band broadening and intensity attenuation in vibrational spectra without altering the overall chemical stoichiometry [13,26]. Such mechanochemically induced modifications weaken the buffering behavior of carbonates, resulting in a reduced and more gradual CO2 evolution during subsequent acid leaching. This phenomenon reflects kinetic suppression rather than chemical decomposition, as carbonates remain present but react more slowly and less violently with acid due to disrupted crystal structure and reduced reactive surface coherence [12,27].
The XRD patterns of the activated powder shown in Figure 6 indicate that mechanical activation with any of the high-energy mills induces a collapse of most characteristic diffraction peaks of minerals such as clinochlore, muscovite, illite, tincalconite, and halite within the 2θ range of 5–22°, along with a notable reduction in the intensity of dolomite reflections. This behavior can be attributed to the transformation of these mineral structures from a crystalline to an amorphous state.
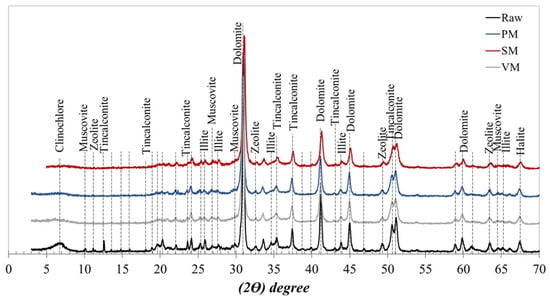
Figure 6.
XRD patterns of raw and activated products by PM, SM, and VM.
4. Results and Discussion
4.1. Effect of Leach Parameters on Li Recovery
4.1.1. Effect of H2SO4 Concentration
In sulfuric acid leaching experiments performed for lithium recovery from Kırka Boron Plant wastes, untreated raw waste and mechanically activated samples produced using VM, PM, and SM milling were evaluated. In the first stage of the study, the effect of acid concentration on lithium leaching efficiency was investigated for both untreated and mechanically activated samples (Figure 7).
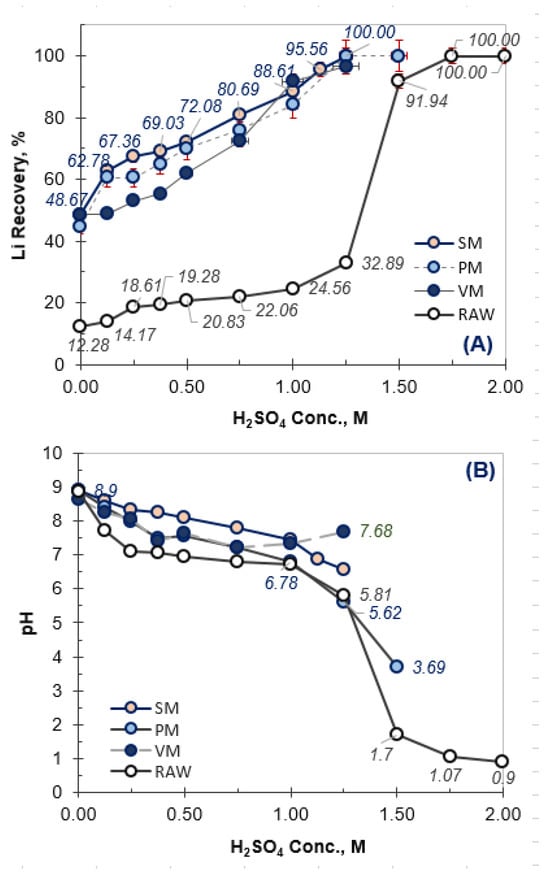
Figure 7.
Effect of acid concentration on Li recovery (A) from raw and MA powders (leach time 30 min, leach temp: 60 °C, L/S ratio: 5) and corresponded pHs of the final leach solutions (B).
As illustrated in Figure 7, lithium extraction from the untreated (raw) waste remained extremely low, reaching only 32.89% even at a sulfuric acid concentration of 1.25 M H2SO4. The Kırka boron process residue is predominantly dolomite-rich, in which the carbonate phases in dolomite consume a significant portion of the added acid. As a result, an insufficient amount of free acid remains in the solution for efficient lithium dissolution. This observation is further supported by the relatively high leach solution pH of 5.81 recorded at 1.25 M acid concentration. When higher acid concentrations (>1.25 M) were applied, a sharp increase in lithium leaching efficiency was obtained, reaching ~91% initially and ultimately approaching 100% dissolution. However, under these conditions, the solution pH decreased from 8.9 (natural pH before acid addition) to below 2.0, indicating strong acid consumption by carbonate minerals and also the buffer effect of borate ions prior to lithium release. Therefore, the leaching behavior of the untreated sample demonstrates that effective lithium extraction can only occur after substantial neutralization of the acid by carbonate phases, followed by structural breakdown of the mineral lattice.
In contrast, the mechanically activated samples showed significantly enhanced leaching performance. Although the degree of structural activation varied depending on the type and energy intensity of milling, lithium dissolution efficiencies above 90% were achieved even at a reduced acid concentration of 1.0 M, while the leach solution pH remained in the range of 5.5–6.0. This improvement indicates that mechanical activation alters the crystal structure, reduces the carbonate (CO32−) content (see FTIR results in Figure 5), and consequently lowers the acid demand of the material. Therefore, high lithium extraction efficiencies can be attained under milder leaching conditions due to the enhanced reactivity of the mechanically activated products.
The reduction in active carbonate content after mechanical activation was further quantified using the pH–acid concentration relationship shown in Figure 7. Carbonate minerals exhibited a buffering effect against acid addition, maintaining the solution pH until carbonate neutralization was completed, after which a sharp decrease in pH was observed. Accordingly, the sulfuric acid concentration at which a sudden pH drop occurred was defined as the carbonate neutralization breakpoint. For the raw sample, this breakpoint was identified at approximately 1.45 M H2SO4, whereas for the mechanically activated samples it shifted to lower acid concentrations, with an average value of approximately 1.05 M. Based on this shift, the reduction in active carbonate content was calculated using the relative decrease in the breakpoint acid concentration, resulting in an estimated reduction of approximately 28%. This decrease in active carbonate content explains the lower acid consumption and the improved lithium leaching efficiency observed for the mechanically activated samples.
The lithium leaching efficiencies of the waste samples subjected to different mechanical activation conditions were found to be nearly identical. Among the high-energy milling methods applied, the stirred media mill produced slightly higher lithium extraction efficiencies and holds greater potential for industrial-scale implementation. Therefore, further parametric investigations, including leaching duration, leaching temperature, and L/S ratio, were carried out exclusively on the stirred-media-milled sample.
Since lithium extraction efficiencies of SM approaching 90% (88.61%) were achieved using 1.0 M H2SO4, subsequent experiments on the effects of leaching time, temperature, and liquid-to-solid (L/S) ratio were conducted at a reduced acid concentration of 0.75 M H2SO4 corresponding to 80.69% recovery. The results of these experiments are presented in Figure 8.
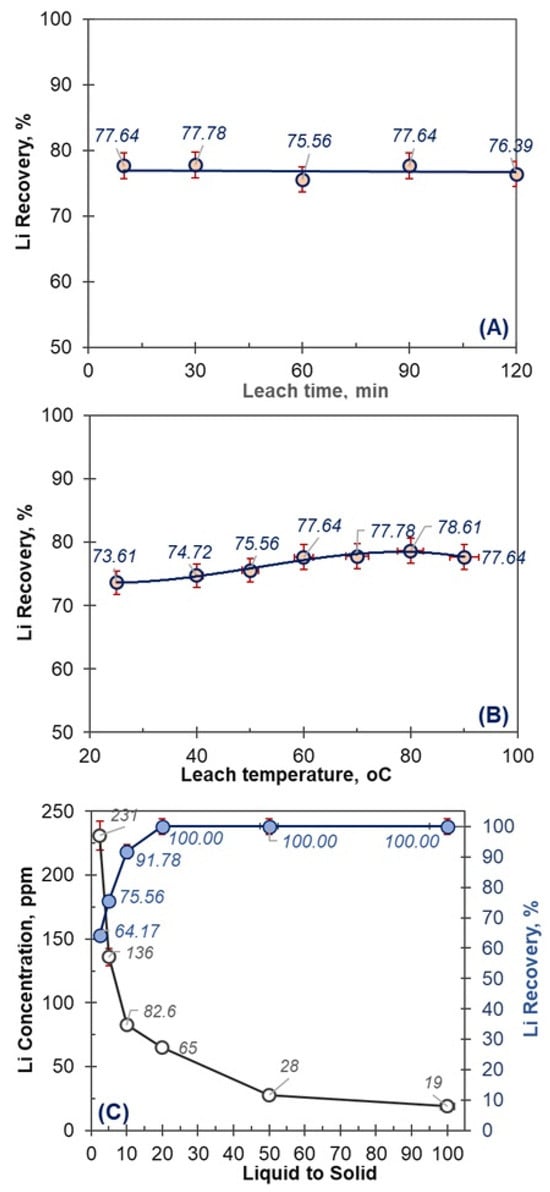
Figure 8.
The effect of leach time (A), leach temperature (B), and L/S (C) on Li recovery from MA powders (H2SO4 conc.: 0.75 M, leach time 30 min, leach temp: 60 °C, L/S ratio: 5).
4.1.2. Effect of Leach Time
At an acid concentration of 0.75 M H2SO4 and a leaching temperature of 60 °C, the effects of different leaching times on lithium dissolution were investigated using SM milled waste sample. The resulting lithium extraction efficiencies are presented in Figure 8A. As can be observed in Figure 8A, no significant variation in leaching efficiency was detected within the time range of 5–120 min, indicating that the leaching process occurs rapidly. This behavior confirms that mechanical activation induces structural amorphization of the raw waste material, rendering lithium ions more accessible for dissolution even within a very short reaction period.
4.1.3. Effect of Leach Temperature
The relationship between lithium recovery and leaching temperature was investigated by performing leaching experiments at 0.75 M H2SO4 for a fixed duration of 30 min using mechanically activated waste samples. The lithium extraction efficiencies as a function of temperature are presented in Figure 8B. As shown in Figure 8B, even at room temperature, lithium recovery reached approximately 73.61%, and increasing the leaching temperature to 60 °C enhanced the lithium recovery to the range of 77%–79%; this indicates that moderate temperature elevation improves the leaching efficiency, although significant lithium dissolution occurs even under ambient conditions.
4.1.4. Effect of Liquid to Solid Ratio
The effect of the liquid-to-solid (L/S) ratio was investigated under the conditions of 0.75 M H2SO4, a leaching time of 30 min, and a leaching temperature of 60 °C, using mechanically activated waste samples. The influence of the L/S ratio on lithium recovery is presented in Figure 8C. As shown in Figure 8C, at a low L/S ratio of 2.5, lithium recovery reached only 64.17%, whereas increasing the L/S ratio to 20 resulted in complete lithium recovery of nearly 100%.
Previous studies have indicated that the solid waste samples contain approximately 10%–12% B2O3, and even when using only water as the solvent at room temperature, more than 10,000 ppm B2O3 can dissolve into the solution. However, due to the dissolution of carbonates from dolomite (>60%) in the waste, the ionic strength of the solution increases significantly, which adversely affects lithium solubility at low L/S ratios (<10).
4.2. Discussion
In the context of this study, mechanical activation is defined as a pretreatment technique aimed at inducing physicochemical changes in the mineral structure through high-energy milling, including partial amorphization, reduction in crystallinity, and modification of carbonate reactivity. Unlike conventional milling approaches focused primarily on particle size reduction or energy efficiency comparisons, mechanical activation is employed here to alter mineral surface properties and internal lattice structures in order to enhance subsequent acid leaching performance. The use of different high-energy milling systems is therefore intended to represent varying activation intensities rather than to establish a direct comparison of milling technologies.
The experimental findings clearly demonstrate that mechanical activation plays a critical role in enhancing the lithium dissolution efficiency of dolomite-rich boron process waste. In the untreated samples, lithium recovery remained limited to approximately 10%–12%, even in the absence of acid, which can be attributed to residual lithium retained within the liquid of the waste during boron processing steps. As shown in Figure 9 (Point A), when sulfuric acid was introduced, a substantial portion of the acid was initially consumed by dolomite-associated carbonate minerals. This strong buffering effect maintained the leach solution pH above 5.8 even at 1.25 M H2SO4 (Point B in Figure 9), preventing lithium release due to insufficient proton availability for structural breakdown. Only after exceeding this carbonate neutralization threshold did lithium extraction increase sharply, reaching >90% at 1.5 M H2SO4 (Point C in Figure 9). These results confirm that lithium remains structurally locked within the mineral matrix until carbonate dissolution is completed.
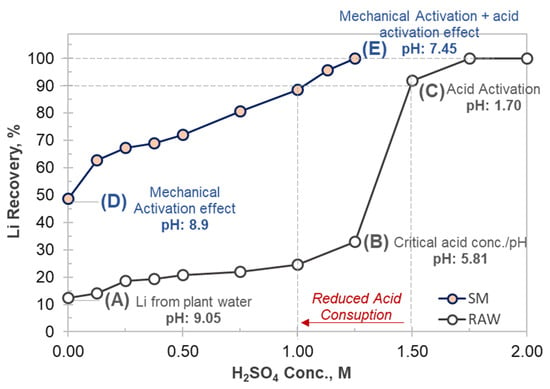
Figure 9.
The critical Li recovery points illustrating the effect of mechanical activation on lithium recovery.
In contrast, mechanical activation significantly modified the dissolution mechanism by inducing amorphization, decreasing crystallinity, and partially removing carbonate phases, as supported by FTIR and XRD analyses. The reduction in crystallinity and the development of structural disorder after mechanical activation, as evidenced by the DoCr values in Table 4, contribute to the enhanced accessibility of lithium and the improved dissolution kinetics observed in leaching experiments. These structural changes facilitated proton accessibility into the mineral layers, enabling lithium release at substantially lower acid concentrations. Even in water alone, the activated material yielded nearly 48% lithium dissolution (Point D in Figure 9), confirming enhanced lithium mobility and weakened Li–mineral bonding. Lithium extraction improved almost linearly with increasing acid concentration without exhibiting a buffering-controlled plateau, and >90% dissolution was achieved at only 1.0 M H2SO4 (Point E in Figure 9), while maintaining a final pH above 7.45. The absence of a carbonate-governed consumption regime indicates that mechanical activation effectively suppresses the neutralization reactions that dominate raw waste leaching. This behavior is consistent with the partial chemical transformation of carbonate phases accompanying structural disordering, rather than solely physical size reduction. Although mechanical activation generally enhances the reactivity of mineral phases, it reduces the apparent reactivity of carbonate minerals in the present system through several concurrent mechanisms. High-energy milling induces partial decarbonation and structural disorder in dolomite, leading to a decrease in the amount of chemically active carbonate species available for acid neutralization. In addition, mechanical activation promotes amorphization and the formation of secondary Ca–Mg–Si/B-rich surface layers, which partially passivate carbonate surfaces and hinder direct acid–carbonate interactions. As a result, the buffering capacity and acid consumption associated with carbonate minerals are significantly reduced. Consequently, the added acid becomes more effectively available for the dissolution of lithium-bearing phases, resulting in enhanced lithium extraction at lower acid concentrations.

Table 4.
The concentrations of co-dissolved ions during the dissolution of raw and SM treated waste samples.
The increased accessibility of lithium and accelerated kinetics of proton attack also resulted in rapid dissolution performance. ≥90% lithium extraction was achieved within 5–30 min at 60 °C, demonstrating that lithium release is no longer diffusion-limited but controlled primarily by reaction kinetics. Such fast leaching behavior contrasts sharply with conventional acid processes applied to clay-type lithium resources, which typically require prolonged reaction times, high temperatures, or roasting pretreatments. Furthermore, as shown in Table 4, increasing acid concentration in the untreated waste led to substantial co-dissolution of Mg2+, Al3+, and Fe3+ ions, whereas their dissolution was significantly suppressed in mechanically activated samples due to lower acid demand and the higher final solution pH.
The continuous increase in Ca2+ and Mg2+ concentrations observed at sulfuric acid concentrations higher than 1.0 M can be attributed to the progressive exhaustion of carbonate buffering and the subsequent availability of free acid in the leaching system. Once lithium-bearing phases are readily dissolved at approximately 1.0 M H2SO4, further increases in acid concentration primarily promote the dissolution of residual dolomitic and Mg-bearing silicate phases. In addition, the lower solution pH at higher acid concentrations suppresses the precipitation of Ca and Mg sulfate phases, allowing these ions to remain in solution and accumulate. Therefore, the increase in Ca2+ and Mg2+ at acid concentrations above 1.0 M reflects non-selective mineral dissolution rather than enhanced lithium recovery.
Mechanical activation provided a substantial advantage in impurity suppression. While high-acid leaching of the untreated waste resulted in the co-dissolution of Mg2+, Al3+, and Fe3+ ions—reaching up to 17,600 ppm, 201.6 ppm, and 72.4 ppm, respectively— lower acid use in activated samples prevented the release of Al3+ and Fe3+ entirely due to the final pH remaining above the neutral pH levels. It should be noted that reduced impurity entrainment eliminates the need for additional downstream treatments such as selective precipitation or solvent extraction, thus improving overall process efficiency and economics.
The comparisons on leaching recoveries were carried out using only the results reported in the literature for studies conducted on the same sample. The results of acid leaching and roasting studies performed on the same sample in the literature are presented in Table 5. As can be seen from Table 5, when sulfuric acid is used for direct acid leaching, acid concentrations higher than 1.5 M are required. The use of 0.5 M oxalic acid has been reported to suppress the dissolution of Mg2+ ions and to achieve lithium recoveries close to 100%. However, oxalic acid is more expensive than sulfuric acid, and detailed information regarding solution pH values and the release of other ions is not available in those studies.

Table 5.
Comparison of our results with the studies in the literature for Kırka Boron Plant Tailings.
For roasting processes, lithium recoveries in the range of 77%–97% have been reported. Nevertheless, the pH values of the leach solutions were not provided in those studies. Our roasting experiments on the same sample showed that water leaching at a liquid-to-solid ratio of 10:1 produced strongly alkaline solutions, with pH values of around 12.0 or higher. This indicates that the storage of such leach residues would pose a serious environmental risk due to their highly alkaline character.
Based on this comparative evaluation, the acid consumption reported in the literature for this material has been effectively reduced to approximately 1.0 M through the mechanical activation pretreatment proposed in the present study. In addition, a significant decrease in Mg2+ ion levels was achieved. The solution pH was maintained at around 7.5, and consequently, the environmental risks associated with the leach residues were minimized.
Overall, the results demonstrate that mechanical activation offers a highly promising and environmentally favorable strategy for lithium recovery from boron processing waste. The approach not only minimizes acid consumption and waste acidity but also improves selectivity and dissolution kinetics. These findings indicate strong industrial feasibility, particularly for large-scale implementation in Kırka boron facilities, where significant volumes of lithium-bearing tailings are generated annually. By transforming a currently undervalued waste into a valuable critical-metal feedstock, the proposed method may significantly contribute to the security of domestic lithium supply and circular resource utilization efforts.
4.3. Conceptual Leaching Mechanism of Mechanical Activation-Assisted Lithium Recovery
Based on the combined FTIR, XRD, final pH, and lithium recovery results, a conceptual leaching mechanism is proposed to describe the role of mechanical activation in lithium extraction. In the untreated waste, well-crystallized dolomite phases dominate the leaching behavior, resulting in rapid acid neutralization and a pronounced carbonate-buffered regime, as reflected by high final pH values and low lithium recovery. Mechanical activation induces lattice distortion, defect formation, and partial amorphization of carbonate phases, as evidenced by reduced crystallinity in XRD patterns and attenuated carbonate bands in FTIR spectra. These mechanochemically induced structural modifications kinetically suppress carbonate–acid reactions, leading to reduced buffering capacity and more gradual acid consumption. Consequently, protons become more readily available for attacking lithium-bearing phases, enabling high lithium recovery at lower acid concentrations while maintaining higher final pH values. The stirred-media mill provides more homogeneous and effective activation due to sustained energy transfer and higher collision frequency, resulting in a more uniform structural disorder compared to impact-dominated planetary and vibratory mills. At sulfuric acid concentrations above 1.0 M, continued increases in Ca2+ and Mg2+ concentrations are attributed to secondary dissolution of residual dolomitic and Mg-bearing silicate phases under excess acid conditions, reflecting non-selective mineral dissolution rather than enhanced lithium recovery. This conceptual framework consistently explains the observed relationships between structural modification, solution chemistry, and leaching performance.
5. Conclusions
This study demonstrates that mechanical activation is an effective and environmentally advantageous pretreatment for enhancing lithium extraction from dolomite-rich boron processing waste. Rather than focusing on a direct comparison of high-energy mill operating parameters, total energy consumption, or specific impact energy values among different milling systems, the primary objective of this work was to highlight the critical role of mechanical activation as a pretreatment step in acid leaching of such wastes. In particular, the application of high-energy milling is emphasized as a means to reduce acid consumption, mitigate potential environmental risks associated with acidic residues, and improve ionic selectivity during dissolution.
The untreated waste exhibited extremely low lithium dissolution efficiencies due to the strong buffering effect of carbonate minerals, requiring ≥1.5 M H2SO4 to achieve >90% lithium recovery. In contrast, mechanical activation significantly modified the mineralogical structure, suppressed carbonate reactivity, and promoted lithium mobility, enabling >90% lithium dissolution using only 1.0 M H2SO4 while maintaining a final leach solution pH above 6.5.
Mechanical activation notably reduced acid consumption and prevented the co-dissolution of major impurities such as Al3+ and Fe3+, thereby improving process selectivity and minimizing the need for downstream purification. Rapid dissolution kinetics were achieved, with lithium extraction reaching ≥90% within 5–30 min at moderate temperatures of 60 °C, indicating favorable conditions for industrial implementation.
It should be noted that the present study is limited to laboratory-scale batch experiments, and detailed evaluations of energy consumption, long-term milling behavior, media wear, and continuous operation were beyond the scope of this work. Therefore, further investigations at pilot scale are recommended, particularly focusing on continuous stirred mill activation, acid recycling, solid–liquid separation efficiency, and long-term pH stability of leach residues.
From an economic perspective, the proposed process offers significant advantages, including the elimination of high-temperature roasting, reduced acid consumption, short leaching durations, and moderate operating temperatures. These features suggest strong potential for cost-effective scalability, especially when integrated into existing boron processing infrastructure.
Overall, the integration of mechanical activation with dilute acid leaching represents a promising and cost-effective strategy for valorizing boron tailings as a secondary lithium resource. Considering the large volumes of such waste generated in Türkiye, the proposed approach may contribute to sustainable waste management and domestic critical mineral supply security, while providing a realistic pathway toward future pilot-scale and industrial-scale implementation.
Author Contributions
Conceptualization, F.B., O.Ö. and M.S.C.; Methodology, F.B., G.E.A. and O.Ö.; Validation, F.B.; Investigation, F.B., G.O.T. and G.E.A.; Resources, O.G.; Data curation, F.B., G.E.A., O.Ö. and O.G.; Writing—original draft, F.B., G.O.T., O.Ö. and O.G.; Writing—review & editing, M.S.C.; Supervision, F.B. and M.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Scientific Research Projects Unit of Istanbul Technical University for their financial support with the grants of MED-2025-46777 and MGA-2024-45340.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request due to the our internal policy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives—A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Büyükburç, A.; Maraşlıoğlu, D.; Bilici, M.S.U.; Köksal, G. Extraction of lithium from boron clays by using natural and waste materials and statistical modelling to achieve cost reduction. Miner. Eng. 2006, 19, 515517. [Google Scholar] [CrossRef]
- Seredin, V.V.; Tomson, I.N. The West Primorye noble-rare metal zone: A new Cenozoic metallogenic taxon in the Russian Far East. Dokl. Earth Sci. 2008, 421, 745–750. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Hoeck, M.; Bertau, M. Lithium market research-global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- King, H.E.; Salisbury, A.; Huijsmans, J.; Dzade, N.Y.; Plümper, O. Influence of Inorganic Solution Components on Lithium Carbonate Crystal Growth. Cryst. Growth Des. 2019, 19, 6994–7006. [Google Scholar] [CrossRef]
- Peiró, L.T.; Méndez, G.V.; Ayres, R.U. Lithium: Sources, production, uses, and recovery outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Kentish, S.; Nghiem, L.D.; Hai, F.I. Lithium enrichment from a simulated Salt Lake brine using an integrated nanofiltration-membrane distillation process. J. Environ. Chem. Eng. 2019, 7, 103395. [Google Scholar] [CrossRef]
- Battaglia, G.; Berkemeyer, L.; Cipollina, A.; Cortina, J.L.; de Labastida, M.F.; Rodriguez, J.L.; Winter, D. Recovery of Lithium Carbonate from Dilute Li-Rich Brine via Homogenous and Heterogeneous Precipitation. Ind. Eng. Chem. Res. 2022, 61, 13589–13602. [Google Scholar] [CrossRef]
- Bonin, L.; Deduytsche, D.; Wolthers, M.; Flexer, V.; Rabaey, K. Boron extraction using selective ion exchange resins enables effective magnesium recovery from lithium rich brines with minimal lithium loss. Sep. Purif. Technol. 2021, 275, 119177. [Google Scholar] [CrossRef]
- Guo, Z.; Ji, Z.; Chen, Q.; Liu, J.; Zhao, Y.; Li, F.; Liu, Z.; Yuan, J. Prefractionation of LiCl from Concentrated seawater/Salt Lake brines by electrodialysis with monovalent selective ion Exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Balaz, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sun, S.; Ye, F.; Song, F.; Li, Y.; Wang, J.; Yu, J. Extraction of Lithium from Salt Lake Brine and Mechanism Research. Chin. J. Inorg. Chem. 2011, 27, 439–444. [Google Scholar]
- Ushak, S.; Gutierrez, A.; Galazutdinova, Y.; Barreneche, C.; Cabeza, L.F. Grágeda, Influence of alkaline chlorides on thermal energy storage properties of bischofite. Int. J. Energy Res. 2016, 40, 1556–1563. [Google Scholar] [CrossRef]
- Tesla Inc. Selective Extraction of Lithium from Clay Minerals. Patent US20210207243A1, 8 July 2021. [Google Scholar]
- Mordoğan, H.; Helvacı, C.; Malayoğlu, U. Lithium Occurrence in Borate Deposit Clays and Modern Lakes: Evaluation Possibilities. In Proceedings of the Industrial Raw Materials Symposium, İzmir, Turkey, 21 April 1995. [Google Scholar]
- Akyıldız, S. Evaluation of the Clays and Processing Wastes of the Kırka Borax Mine in Terms of Lithium Content. Master’s Thesis, Graduate School of Natural and Applied Sciences, Dokuz Eylul University, İzmir, Turkey, 2015. [Google Scholar]
- Celep, O.; Yazıcı, E.Y.; Deveci, H. Recovery of lithium from ores and brines. Mining 2022, 61, 105–120. [Google Scholar] [CrossRef]
- Ulusoy, M. Is Lithium the Oil of the Future? Metalurji 2016, 178, 45–48. [Google Scholar]
- Yörükoğlu, A.; Akkurt, F.; Karakaş, S.; Özkasapoğlu, S. Recovery of lithium from boron wastes and its economical evaluation. In Proceedings of the IMPC Eurasia Conference, Antalya, Turkey, 31 October 2019. [Google Scholar]
- Yücel, A.; Sarıkaya, M.; Yılmaz, H.C.; Depci, T. Extraction of lithium from boron ore wastes and precipitation as lithium carbonate. Can. Metall. Q. 2025, 64, 2437–2452. [Google Scholar] [CrossRef]
- Obut, A.; Ehsani, İ.; Aktosun, Z.; Yörükoğlu, A.; Girgin, İ.; Temel, A.; Deveci, H. Leaching behavior of lithium, cesium and rubidium from a clay sample of Kırka borate deposit in sulfuric acid solutions. J. Boron 2020, 5, 170–175. [Google Scholar]
- Yao, G.; Zang, H.; Wang, J.; Wu, P.; Qiu, J.; Lyu, X. Effect of mechanical activation on the pozzolanic activity of muscovite. Clays Clay Miner. 2019, 67, 209–216. [Google Scholar] [CrossRef]
- Manosa, J.; Calvo-de la Rosa, J.; Silvello, A.; Maldonado-Alameda Chimenos, A.J.M. Kaolinite structural modifications induced by mechanical activation. Appl. Clay Sci. 2023, 238, 106918. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177–189. [Google Scholar] [CrossRef]
- Mucsi, G. A Review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Aladağ, M.; Erdem, M. Selective lithium leaching from dolomite-hosted clay-based boron extraction waste with oxalic acid to obtain a pregnant solution with a low Mg/Li ratio. Sep. Purif. Technol. 2025, 377, 134428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.