Abstract
Rocks and soil excavated at construction sites can contain naturally occurring toxic substances. One low-cost means of managing the environmental burden posed by leaching of these substances is the attenuation layer method, which uses an adsorbent positioned between the fill and ground. Evaluation of adsorbent performance based on sorption tests is important for designing and optimizing attenuation layer methods; however, few studies have examined the effect of coexisting ions on sorption performance. Here, we examined the effects of these ions contained in soil extract solutions on the fluoride sorption performance of a commercial layered double-hydroxide (LDH)–based adsorbent used in the attenuation layer method. Batch and column sorption tests showed that the distribution coefficients in the presence of coexisting ions were 29%–72% lower than those in tests conducted without coexisting ions. Furthermore, the results of a solid-state analysis and various ion analyses suggest that competition for the sorption sites of LDH by sulfate ions in the soil extract solution was the cause of the reduced sorption performance. These findings imply that reliance only on deionized water-based sorption tests may overestimate the real-world sorption performance of LDH-based adsorbents.
1. Introduction
From the perspective of environmental sustainability, rocks and soils generated at construction sites should be used as fill or backfill material instead of being disposed of as waste. However, such excavated rocks and soils may contain naturally occurring arsenic, lead, fluorine, selenium, and other toxic substances [1,2]. This is particularly relevant in Japan, which is located along a volcanic belt, meaning the strata and minerals produced by volcanic activity contain arsenic and fluorine. Indeed, soils excavated in Japan often contain concentrations of these naturally occurring toxic substances that slightly exceed the standards specified in Japan’s Soil Contamination Countermeasures Law [3]; therefore, appropriate countermeasures and management approaches must be applied to these soils [4,5,6,7,8].
There are various approaches to the sustainable use of contaminated rocks and soils. For example, the Netherlands uses a risk-based approach in which soil is classified into three categories: uncontaminated, mildly contaminated, and severely contaminated, with mildly contaminated soil managed in a sustainable manner by being reused based on risk-based and land use-specific criteria [9]. As an alternative to this approach, in Japan the attenuation layer method is used as a low-cost, low-environmental-impact solution for soil containing naturally occurring hazardous substances that slightly exceed standard values or background levels [10,11]. This method aims to prevent the infiltration of harmful substances, such as naturally occurring heavy metals, leached from excavated rocks and soils into the ground by installing an attenuation layer under the excavated rocks and soils (Figure 1). The attenuation layer is usually a mixture of a base material and an artificial adsorbent material that has excellent sorption properties [10,12], but natural soil that has sorption properties can also be used [2,13]. When leachate from the excavated rocks and soils through the attenuation layer, heavy metals contained in the infiltrated water are adsorbed by the attenuation layer. As a result, heavy metals and other substances leached from the excavated materials are adsorbed, precipitated and co-precipitated in the attenuation layer, and are thereby stably retained in the attenuation layer for a long period of time. The attenuation layer is not intended to be replaced or collected, and will remain in place with the heavy metals retained.
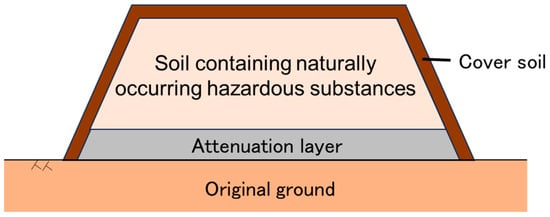
Figure 1.
Attenuation layer method.
When designing attenuation layer approaches, batch and column sorption tests are widely used to evaluate the ability of adsorbent materials and soils to retain hazardous substances. Batch tests are easy to perform and require only a few days, but they involve a higher liquid-to-solid ratio than is found under real-world conditions. In contrast, column tests more closely replicate actual environmental conditions but require longer durations, typically a month or more. Therefore, the test method for a given sample is usually selected according to the overall test objectives. Currently, there are several guidelines available that address the batch sorption testing of soil and adsorbents against toxic elements (Table 1). For the solvents used in the tests, the selection of which is the focus of the present study, the Organisation for Economic Co-operation and Development [14] and US Environmental Protection Agency [15] methods recommend a 0.01 mol/L calcium chloride solution to improve centrifugation and minimize cation exchange, while the Japanese Ministry of the Environment guidelines [16] recommend deionized water, for tests designed to evaluate the adsorption characteristics of soil. Notably, the Hokkaido Environmental Conservation Technology Association guideline [17] and the JIS A1291-1 standard [18] for evaluating the adsorption characteristics of adsorbent materials emphasize that, where possible, a soil extract solution obtained from soil containing naturally occurring hazardous substances should be used for batch sorption tests. This is because the leachate that comes into contact with the attenuation layer at real-world construction sites is water that has passed through the soil and therefore contains not only toxic substances but also ions such as silicate ions and carbonate ions that have eluted from the soil [1,2]. The preparation of large volumes of soil extract solution for use in adsorption tests may require considerable effort; therefore, the use of soil extract is not mandatory in these standards, but is recommended. Among the aforementioned standards and guidelines, column sorption tests are only mentioned in JIS A1291-1 standard, in which the solvent used for column sorption tests is water because of the difficulty of preparing large quantities of soil extract solution [18].

Table 1.
Guideline test parameters for batch sorption tests against.
The presence of coexisting ions in the sorption tests solution can either decrease [11] or increase [19] adsorbent performance, depending on the adsorbent or ion. For example, layered double hydroxide (LDH) has been extensively studied as an adsorbent for arsenic, fluorine, copper, and lead [20,21,22]. However, competing ions have been reported to reduce removal rates [11,22,23,24].
In the evaluation of adsorbent performance, only one study has been conducted using soil extract solutions. Arima et al. conducted sorption tests using soil extracts, adsorbent (Mg-based materials) and soils and reported the effects of coexisting ions on the fluoride removal rates, sorption capacities, and mechanisms [11]. The results confirmed that the influence of soil extract solutions on the adsorbent was limited. However, adsorbents used in attenuation layer methods include not only the Mg-based adsorbents examined by Arima et al. but also other types, such as LDH and iron, and evaluating their effects is important. LDH adsorbs harmful substances via exchange of harmful substances with the interlayer ion, but sulfate is also known to exchange with interlayer ion in the same way. Therefore, it may be more sensitive to coexisting ions than Mg-based adsorbents. Furthermore, many studies have reported column sorption tests using various toxic substances, soils, and adsorbents [10,25,26,27,28,29,30], but all of these studies used calcium chloride or deionized water. No studies have examined sorption using aqueous solutions extracted from rocks or soils, which could better simulate real-world conditions.
In the present study, we aimed to evaluate the effect of soil extractions on the adsorption performance of LDH-based adsorbents and to assess the extent of this effect in column tests under conditions that are closer to those in the real world. Batch and column sorption tests were conducted using deionized water and soil extract solution as solvents to evaluate (i) the effects of coexisting ions in the solvent on adsorbent performance, and (ii) the differences in these effects between test methods. Fluoride, a toxic substance widely found in nature, was used as the target ion, and a commercial LDH-based material commonly used in the attenuation layer method was used as the adsorbent. Distribution coefficients were determined to assess the influence of different solvents and test methods on the sorption results. Finally, the mechanisms underlying the changes in the distribution coefficient were considered based on the results of X-ray diffraction, scanning electron microscopy, and ion analyses.
2. Materials and Methods
Batch and column sorption tests were conducted for fluoride with either deionized water or soil extract solution as the solvent. As the test material, either an LDH-based adsorbent (LDH-BA) alone and/or a mixture of an LDH-based adsorbent and silica sand—referred to as ALM (attenuation layer material)—was used. The silica sand used in the ALM was selected because it simulates highly permeable soil mixed with the adsorbent in the attenuation layer and has low adsorption performance for fluoride ions. The test method was based on JIS A1291-1 [18]. In addition, the distribution coefficient when the equilibrium concentration of fluoride ions was 8 mg/L, which is 10 times the standard value stipulated in Japan’s Soil Contamination Countermeasures Act, was calculated using the results of the sorption test and the performance of the adsorbent was evaluated. The soil extractant solvent was prepared by conducting batch leaching tests on actual contaminated soil. The conditions for each test are summarized in Table 2.

Table 2.
Summary of the batch and column test conditions. LDH-BA is LDH-based adsorbent and AML is mixture of an LDH and silica sand.
2.1. Sample Preparation
2.1.1. Materials
LDH is a type of natural or synthetic anionic clay and is also a hydrotalcite analog. The general chemical composition of LDH is [M2+1−xM3+x(OH)2]x+(An−)x/n·mH2O, where M2+ and M3+ are metallic cations occupying the octahedral sites in the hydroxide layer, An− is an exchangeable anion, and x represents the M3+/(M2+ + M3+) ratio. The LDH used in this study was provided by a domestic adsorbent manufacturer in Japan.
To prepare ALM, silica sand was mixed with the LDH adsorbent so that the adsorbent content was 5% ± 0.3%. A Japanese commercial silica sand, Toyoura Silica Sand (Toyoura Keiseki Kogyo Co., Ltd., Yamaguchi, Japan), was used. It was selected because it was expected not to adsorb fluoride ions.
2.1.2. Preparation of Soil Extract Solutions
Rocks obtained from construction sites in Tohoku-region, Japan were used to produce the soil extract solution. After collection, the rocks were air-dried until their weight remained constant, crushed using an alumina crusher, passed through a sieve (pore size, 2 mm), and then used to produce the soil extract solution. The mineral and chemical compositions of the ground rocks after being passed through the sieve were determined by grain size measurement [31], X-ray diffraction (Rigaku RINT-2500 installed at the GSJ-Lab, AIST, Rigaku, Tokyo, Japan), scanning electron microscopy (HITACHI SU-3500 installed at the GSJ-Lab, AIST, Hitachi High-Technologies, Tokyo, Japan), and X-ray fluorescence analysis (Supermini 200, Rigaku, Tokyo, Japan).
To prepare the extract solutions, a 1 L polypropylene bottle was filled with 500 mL of deionized water and 50 g of soil to achieve a liquid-to-solid ratio of 10. The bottle was then capped and agitated in the horizontal position at 200 rpm (TAITEC TS-20) for 6 h at ambient temperature and atmospheric pressure. After agitation, the soil was filtered through a membrane filter (pore size, 0.45 μm) and the filtrate was used as the soil extract solution. The soil extract solutions were analyzed for pH and electrical conductivity (EC) by using a portable multi-water quality meter and the relevant electrode (MM-42DP, TOADKK, Tokyo, Japan), Si concentration by inductively coupled plasma mass spectrometry (7900 ICP-MS, Agilent, the detection limits of Si was 0.05 mg/L), anion concentration (F−, Cl−, SO42−) by ion chromatography (Dionex Integrion HPIC, Thermo Fisher Scientific, Massachusetts, USA, the detection limits of F−, Cl−, and SO42− were 0.6, 0.5 and 1.4 mg/L, respectively.), total organic carbon (TOC) by using a TOC-V CSH analyzer (SHIMADZU, Kyoto, Japan, the detection limits of TOC was 0.1 mg/L), and alkalinity (Kyoto Electronics Manufacturing Co., Ltd., Kyoto, Japan, AT-710M) [32]. Si and TOC were measured through outsourced analysis (Propha Engineering Consultants Co., Ltd., Gunma, Japan). Each analysis was preceded by a blank test. Calibration curves were also created using control samples.
2.2. Fluroride Sorption Tests
2.2.1. Adsorbent Batch Sorption Tests
Fluoride sorption tests using the LDH adsorbent were conducted at liquid-to-solid ratios of 100, 250, 500, 1000, 2500, and 5000. All tests for every liquid-to-solid ratio were conducted in duplicate. The performance of the adsorbent was evaluated using the distribution coefficient at an equilibrium concentration of 8 mg/L of fluoride ions, so the concentration of fluoride ions in the batch sorption test was set at 24 mg/L with the expectation that the equilibrium concentration after the test would be around 8 mg/L [18]. The standard for fluoride ion leaching from soil in Japan is 0.8 mg/L. We therefore assumed a fluoride ion concentration 10 times that standard in the evaluation of the performance of the adsorbents, resulting in an apparent distribution coefficient of 8 mg/L. The solvent used to prepare the fluoride solution for the tests was either deionized water or soil extract solution, and the solutions were prepared by dilution of fluoride ion standard solution (Kanto Chemical Co., Inc., Tokyo, Japan). The pH of the fluoride solution was not adjusted.
The adsorbent and 500 mL of the fluoride solution were capped in a 1 L polypropylene container at the specified liquid-to-solid ratio, and the container was agitated horizontally at 200 rpm for 24 h at ambient temperature and atmospheric pressure. After agitation, centrifugation and solid–liquid separation using a membrane filter (pore size, 0.45 μm) were performed to obtain the test solution. The fluoride solution and the post-sorption test solution were analyzed using the same method as described in Section 2.1.2. The morphology of the adsorbent before and after the sorption test was observed by X-ray diffraction and scanning electron microscopy.
As a blank test, LDH adsorbent and deionized water without fluoride were agitated for 24 h under the same conditions as used in the sorption test. The blank test was conducted only once.
2.2.2. Attenuation Layer Material (ALM) Batch Sorption Tests
Fluoride sorption tests were also performed using ALM at liquid-to-solid ratios of 100, 250, 500, 1000, 2500, and 5000. All tests for every liquid-to-solid ratio were conducted in duplicate. Note that the liquid-to-solid ratio was the ratio of the solution to the adsorbent, not the ratio of the solution to ALM. The fluoride concentration in the fluoride solution was set at 24 mg/L. The solvents were deionized water, soil extract solution, or soil extract solution diluted four-fold, and the test solutions were prepared by dilution of the fluoride standard. Testing of soil extract solution diluted four-fold was conducted under the same conditions as the column test for comparison. The extractant solution was diluted because of the difficulty in preparing a sufficient volume of solution for the column tests. The pH of the solutions was not adjusted. After the adsorption test, the apparent distribution coefficient for the adsorbent, not ALM as a whole, was calculated, and the results were compared with those of the other tests.
Fluoride removal rates were calculated using the following formula:
where C0 is the concentration of fluoride ions in the solution before the test (mg/L) and C is the concentration of fluoride ions in the solution after the test (mg/L).
2.2.3. Column Sorption Tests
Up-flow column sorption tests were conducted using an ALM. The test was conducted once only under each condition. The fluoride concentration in the water was set at 8 mg/L. The performance of the adsorbent was evaluated using the distribution coefficient at an equilibrium concentration of 8 mg/L of fluoride ions. In the column sorption tests, the equilibrium concentration of the solution used in the test is the same as that after the test, so the concentration of fluoride ions was set at 8 mg/L. The solvents were deionized water or soil extract solution diluted four-fold, and the test solutions were prepared by dilution of the fluoride standard. The extractant solution was diluted because of the difficulty in preparing a sufficient volume of solution for the column tests. The pH of the solutions was not adjusted.
ALM was packed into a 5 cm-diameter cylindrical column in five layers, (Figure 2). Each layer was compacted using a 125 g rammer. After loading the sample, deionized water was passed through the column at a flow rate of 72 mL/h using a constant flow pump. Immediately after the deionized water reached the top of the column, the pump was stopped, and the column was allowed to stand for about 24 h for initial saturation. After initial saturation was complete, the water was passed through at a flow rate of approximately 72 mL/h. The water was continuously pumped until the cumulative liquid-to-solid ratio reached 250, after which the pump was stopped. The cumulative liquid-to-solid ratio in the column test was calculated from the ratio of the solution collected and ALM used in the column test. Samples of the solution from the top of the column were collected every 2 h from the start of water flow up to 6 h, and every 12 or 24 h thereafter. Liquid samples were filtered through a membrane filter (pore size, 0.45 μm). The pH, EC, and fluoride concentrations of the liquid samples were measured after filtration. After the sorption test, the apparent distribution coefficient was calculated for the adsorbent alone not for ALM as a whole, and the results were compared with those of the other tests.
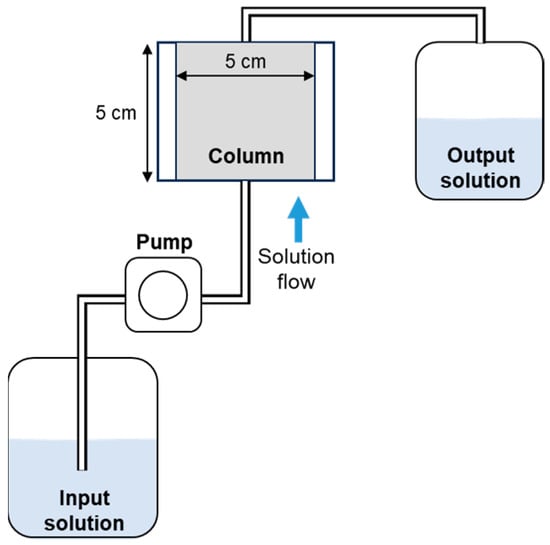
Figure 2.
Schematic diagram of column test apparatus.
2.3. Caluculation of Charge Balance During Ion Exchange and Saturation Sorption in Sorption Reaction
Ion sorption and emission per kilogram of adsorbent in equivalent amounts were calculated with Equations (2)–(4):
where M(mol) is the amount sorbed in each test (mol/kg), Mad(mol) is the amount sorbed in the sorption test (mol/kg), Mbl(mol) is the amount sorbed in the blank test (mol/kg), M(mol)′ is the net amount sorbed in the sorption test (mol/kg), Meq is the amount sorbed in terms of equivalent volume (eq/kg), C(mol) is the ion concentration in the test solution after the test (mol/L), C0(mol) is the ion concentration in the solution before the test (mol/L), and Val is the valence of the ion. LS is the liquid–solid ratio of the test (L/kg). In the pH range (7.2–8.6) used in the present study, it was assumed that dissolved carbon dioxide exists as bicarbonate ions, so it was calculated as a monovalent ion. If the net amount sorbed is negative, it is the amount released, not the amount sorbed. Equivalent weights were calculated for sulfate ions as divalent ions and other ions as monovalent ions.
2.4. Calculation of Distribution Factors
Distribution coefficients were obtained for a fluoride concentration of 8 mg/L by using the following method, with reference to JIS A1291 [18].
2.4.1. Calculation of Distribution Coefficients for the Batch Sorption Tests
The amount of sorbed fluoride in each test was determined from the concentration of fluoride in the test solution after the test and the mass of adsorbent used in the test:
where M(mg/L) is the amount of sorbed fluoride (mg/kg), C0 is the concentration of fluoride in the solution before the test (mg/L), C is the concentration of fluoride in the solution after the test (mg/L), V is the amount of solution used in the test (L), and m is the mass of the adsorbent used in the test (kg). Next, in which the degree of sorption increases with increasing concentration of fluoride in the test solution after the test, we plotted the degree of sorption versus the concentration of the test solution after the test, and selected three liquid-to-solid ratios for which the concentration of the test solution after the test was close to 8 mg/L (Figure S1). Since three points containing 8 mg/L were plotted on the graph for the test with water, a line of best fit was drawn, and from the slope and intercept the amount of sorption was calculated for a post-test solution concentration of 8 mg/L. Finally, the sorption amount obtained was divided by eight, which was the concentration of the test solution after the test, to obtain the apparent distribution coefficient for the batch sorption test. Since the test with the soil extract solution did not yield three points that contained 8 mg/L, the largest sorption value plotted was divided by 8 to obtain the apparent distribution coefficient for the batch sorption test.
2.4.2. Calculation of Distribution Coefficients for the Column Sorption Tests
The amount of sorption for each solution type was determined from the liquid-to-solid ratio and the concentration of fluoride in the test solution at each sampling interval (Figure S2, Equations (6) and (7)):
where M(i) is the amount of fluoride sorbed during the sampling interval (mg/kg), M(clm) is the total amount of fluoride sorbed in the column test (mg/kg), C0 is the concentration of fluoride in the solution before the test (mg/L), C(i) is the concentration of fluoride in the water samples (mg/L), and LS(i) is the liquid–solid ratio during the sampling interval (L/kg). The apparent distribution coefficient was then calculated by dividing the cumulative amount sorbed by 8 mg/L. For the sampling interval where the specific concentration exceeded 1, the sorption amount was considered negative, but all values were totaled to obtain the total sorption amount regardless of whether the sorption amount was positive or negative.
3. Results
3.1. Properties of the Soil and Soil Extract Solution
Grain size analysis of the soil used to prepare the soil extract solution showed that the coarse sand content was 34.4%, medium sand content was 29.1%, fine sand content was 14%, and silt and clay content was 22.5%. X-ray diffraction analysis confirmed the presence of quartz, anorthite, pyrite, and chalcopyrite. Table 3 shows the chemical composition of the soil, as determined by X-ray fluorescence analysis.

Table 3.
Chemical composition of contaminated soils, as determined by X-ray fluorescence analysis.
Table 4 shows the properties of the soil extract solution prepared from the soil.

Table 4.
Soil extract solution properties.
3.2. Batch Sorption Test Results for Adsorbent
3.2.1. Fluoride Removal and Adsorbent Characteristics
In the batch sorption test using deionized water, the fluoride removal rate was about 90% at liquid-to-solid ratios of 100, 250, and 500 but decreased thereafter until it was only 16% at a liquid-to-solid ratio of 5000 (Figure 3a). While the removal rate of the soil extract solution was similar to that of deionized water at a liquid-to-solid ratio of 100, the removal rate decreased rapidly from a liquid-to-solid ratio of 250, with the removal rate deviating substantially from that of deionized water at the higher liquid-to-solid ratios. The largest difference in removal rate was observed at a liquid-to-solid ratio of 500, where the removal rate of the soil extract solution was 30% compared to 88% for deionized water. The distribution coefficient of the adsorbent at a fluoride concentration of 8 mg/L, calculated by using the sorption isotherms shown in Figure 3b, was 2260 L/kg for the test with deionized water and 596 L/kg for the test with the soil extract solution. Parameters obtained by fitting the test results to the Henry model and the Freundlich model are shown in Figure S3 and Table S1.
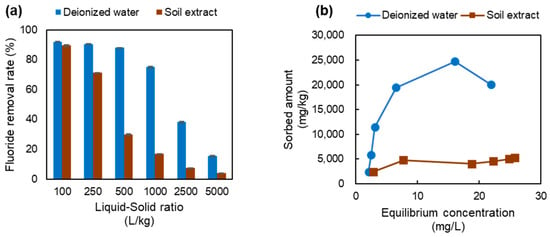
Figure 3.
Fluoride removal rates (a) and sorption isotherms (b) for batch tests using the adsorbent only. The coefficient of variation was less than 2%.
The pH of the post-test solution was 7.48–8.19 for deionized water and 7.41–8.56 for the soil extract solution, with no marked differences between the two at any liquid-to-solid ratio (Figure S4a). X-ray diffraction and scanning electron microscopy analyses of the adsorbent showed no change in the adsorbent before and after the sorption test, regardless of whether deionized water or the soil extract solution was used in the test (Figure 4 and Figure 5). In the blank test, the concentration of fluoride in the post-test solution was below the detection limit.
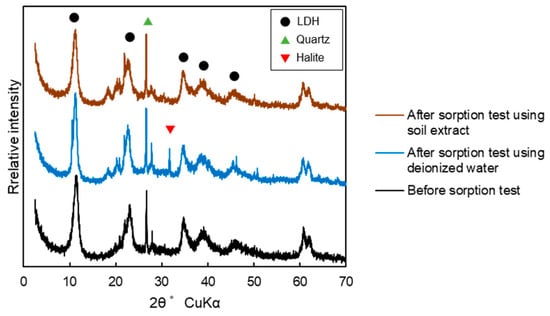
Figure 4.
X-ray diffraction results for the LDH-based adsorbent (LDH-BA).

Figure 5.
Scanning electron micrographs of the LDH-based adsorbent (LDH-BA) before the sorption test (a), after the sorption test using deionized water (b), and after the sorption test using soil extract solution (c).
3.2.2. Co-Existing Ion Concentrations
Before and after the batch sorption tests using the LDH adsorbent alone, the concentrations of various ions in the test solution were determined (Figure 6).
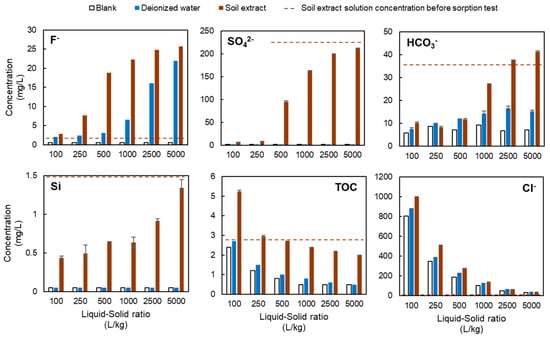
Figure 6.
Solution ion concentrations in the batch sorption tests using the LDH-based adsorbent (LDH-BA) alone.
As the liquid-to-solid ratio was increased, the fluoride concentration increased. More fluoride ions were detected in tests using soil extractions than in tests using water. In tests using water, the concentration rose sharply at a liquid-solid ratio of 2500, while in tests using soil extract, the concentration rose sharply at a liquid-solid ratio of 500.
In the deionized water, the sulfate concentration remained below the limit of quantification both before and after the test. In the soil extract solution, the sulfate ion concentration was approximately 230 mg/L before the test, but was reduced to less than 10 mg/L in the tests with low liquid-to-solid ratios (100 and 250). However, as the liquid-to-solid ratio was increased, the sulfuric acid concentration increased, and at liquid-to-solid ratios above 2500, the concentration approached the pre-test concentration.
The bicarbonate ion concentration was 37.2 mg/L in the soil extract solution before the test and was decreased after testing at liquid-to-solid ratios of 100 to 1000. However, when a liquid-to-solid ratio of 2500 or 5000 was used, the bicarbonate ion concentration in the test solution was increased, possibly due to an increase in pH during the sorption test. In tests using water, the concentration increased approximately twofold as the liquid-solid ratio increased.
In the tests of deionized water, the silica concentration remained below the limit of quantification both before and after the test. In the soil extract solution, the concentration of silica was 3.3 mg/L before the test, but was reduced to less than 0.5 mg/L in the tests with liquid-to-solid ratio of 100. The concentration increased as the liquid-solid ratio increased, but the maximum concentration was about 1.3 mg/L.
TOC concentrations exhibited the same trend for the soil extract solution, deionized water, and blank tests. Concentrations of TOC were highest when the liquid-to-solid ratio was 100, then decreased as the liquid-to-solid ratio was increased. The TOC concentrations at the same liquid-to-solid ratio decreased in the order of soil extract solution > deionized water > blank test. In the test using the soil extract solution, TOC was 2.8 mg/L before the test, and it was eluted during the batch sorption test at a liquid-to-solid ratio of 100 or 250 but sorbed at a liquid-to-solid ratio of 500 or higher.
At all liquid-to-solid ratios, the chloride ion concentrations decreased in the order of soil extract solution > deionized water > blank test. The highest concentrations were observed at a liquid-to-solid ratio of 100, followed by a decrease in concentration as the liquid-to-solid ratio was increased. The chloride ion concentrations in the pre-test solutions were all less than 0.5 mg/L, and more chloride was eluted in the test using the soil extract solution than in the other tests.
3.3. Batch Sorption Test Results Using the Mixed Attenuation Layer Material (ALM)
Overall, the fluoride removal rates and sorption isotherms were similar to those observed for the batch sorption tests using the LDH adsorbent alone (Figure 7). In the sorption tests using deionized water, the fluoride removal rate was about 90% at liquid-to-solid ratios of 100, 250, and 500, but decreased thereafter until at a liquid-to-solid ratio of 5000 the removal rate was 16%. Tests using four-fold diluted soil extract solution showed a similar trend as that observed for deionized water, although the removal rate at a liquid-to-solid ratio of 5000 was 10.0%, which was lower than that with deionized water.
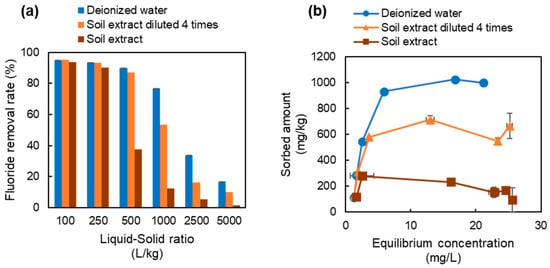
Figure 7.
(a) Fluoride removal rates and (b) sorption isotherms for the batch sorption tests using the mixed attenuation layer material (ALM).
The fluoride removal rate of the soil extract solution was similar to that of deionized water at liquid-to-solid ratios of 100 and 250, but the removal rate was markedly decreased at liquid-to-solid ratios of 500 and higher. The largest difference in sorption was observed in the test with a liquid-to-solid ratio of 500, where the removal rate with deionized water was 90% but that with the soil extract solution was 38%. The distribution coefficient of the adsorbent at a fluoride concentration of 8 mg/L was 2140 L/kg for deionized water, 1530 L/kg for four-fold diluted soil extract solution, and 730 L/kg for soil extract solution.
The pH of the post-test solutions ranged from 7.49 to 8.15 for deionized water, 7.25–7.79 for the soil extract solution diluted four-fold, and 7.52–8.57 for the soil extract solution (Figure S4b).
3.4. Column Sorption Tests Results
In the tests using deionized water, the specific concentration (specific concentration is the value obtained by dividing the concentration of fluoride in the sampled solution by the concentration of fluoride in the flowing solution) was as low as 0.01 until the cumulative liquid-to-solid ratio reached about 60 L/kg, after which it increased until it reached a value of 1 at a liquid-to-solid ratio of 140 L/kg (Figure 8). Similarly, in the test using four-fold diluted soil extract solution, the specific concentration was 0.01 up to a liquid-to-solid ratio of about 26 L/kg, after which it increased until it reached a value of 1 at a liquid-to-solid ratio of 50 L/kg. The distribution coefficient of the adsorbent at a fluoride concentration of 8 mg/L was 1600 L/kg for deionized water and 309 L/kg for the four-fold diluted soil extract solution.
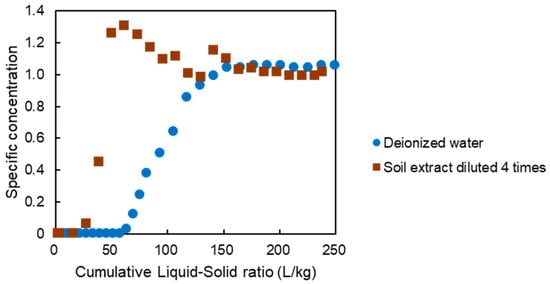
Figure 8.
Specific concentration of fluoride in the column sorption test solution.
3.5. Charge Balance During Ion Exchange and Saturation Sorption in Sorption Reaction
In the sorption tests using deionized water, fluoride was the ion sorbed on the LDH, and chloride and bicarbonate were the ions released from the LDH (Figure 9). The sorption and release at the same liquid-to-solid ratio were similar. In contrast, in the tests with the soil extract solution, the quantity of ions sorbed on LDH ranged from 0.63 eq/kg at a liquid-to-solid ratio of 100 to 1.11–1.80 eq/kg at a liquid-to-solid ratio of 250 and higher (Figure 10). As mentioned earlier, chloride is released from LDH, and fluoride, sulfate, carbonate, and silicon ions are sorbed. In particular, sulfate ions accounted for more than 70% of the sorption, and fluoride accounted for only about 15%, indicating that the coexisting ions in the soil extract solution had an effect on the sorption performance.
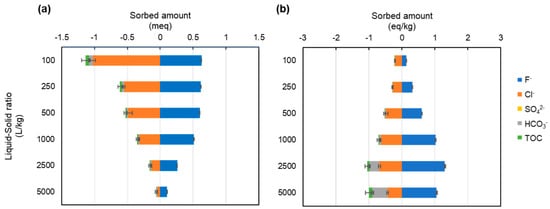
Figure 9.
Amounts of ions sorbed in the tests using the LDH-based adsorbent (LDH-BA) alone and deionized water per test (a) and per mass of adsorbent (b). If the net amount sorbed is negative, it is the amount released, not the amount sorbed.
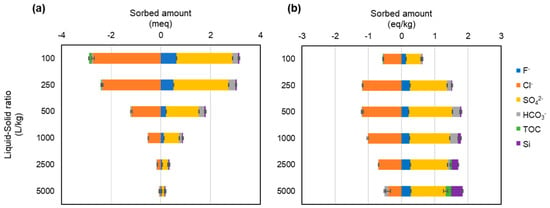
Figure 10.
Amounts of ions sorbed in the tests using the LDH-based adsorbent (LDH-BA) alone and soil extract solution per test (a) and per mass of adsorbent (b). If the net amount sorbed is negative, it is the amount released, not the amount sorbed.
In the tests at a liquid-to-solid ratio of 500 or more, the sorption sites of LDH are saturated with fluoride and coexisting ions, which is supported by the present observations that the removal rate of fluoride was decreased markedly at a liquid-to-solid ratio of 250 in tests using soil extract (Figure 3), and that the total amount of ions sorbed and released were comparable for the tests with liquid-to-solid ratios of 100 and 250 before the LDH sorption sites were saturated; however, for liquid-to-solid ratios of 500 or more, the total amount of ions released was higher. Furthermore, in the tests with a liquid-to-solid ratio higher than 1000, the total amount of ions sorbed was decreased, while the total amount of ions released remained almost constant, resulting in the total amount of ions released (mainly chloride ions) being 1.8–4 times higher than the total amount of ions sorbed.
Although the underlying cause of this phenomenon is unknown, it is possible that the lower ionic strength of the solution in the blank test compared with that of the soil extract solution caused more LDH to dissolve in the blank test, resulting in elution of more chloride ions. In the tests using the soil extract solution, the sorption isotherm of fluoride (Figure 3b) and the sorption amounts of the various ions (Figure 10b) at liquid-to-solid ratios of 500 or higher indicate that the sorption amount reaches saturation at a liquid-to-solid ratio of 500 or higher. Therefore, it is plausible that the ions were either exchanged with non-chloride ions or removed through mechanisms unrelated to ion exchange.
3.6. Distribution Coefficients
The apparent distribution coefficients of fluoride were calculated from the results of the various tests (Table 5). The distribution coefficients of fluoride in the batch sorption tests with deionized water were comparable at 2260 L/kg and 2140 L/kg for the adsorbent alone and the mixed adsorbent layer material, respectively. The distribution coefficient of fluoride in the column sorption test with deionized water was 1600 L/kg, which was 76% of that in the batch sorption test.

Table 5.
Apparent distribution coefficient of adsorbent in each test. ALM is attenuation layer material.
4. Discussion
4.1. Fluoride Sorption Mechanism of the LDH-Based Adsorbent (LDH-BA) and the Influence of Coexisting Ions
In the present study, an increase in chloride ions was observed after the sorption test in both the deionized water and soil extract solution tests compared to the blank test (Figure 6). This was presumably due to the release of chloride ions when fluoride and other anions are incorporated into the interlayer of the LDH. In the test using the soil extract solution, the elements sorbed on LDH in place of chloride ions in addition to fluoride were thought to be sulfate and bicarbonate ions. The concentrations of these ions were lower after the test than before the test at liquid-to-solid ratios of less than 1000 (Figure 6). In particular, the sulfate ion concentration was 228 mg/L in the solution before the tests using liquid-to-solid ratios of 100 and 250, but was reduced to less than 10 mg/L after the tests, with more than 90% being sorbed by the LDH. As shown in Figure 10b, in addition to sulfate ions, carbonate ions appear to be adsorbed at liquid-to-solid ratios of 500 and 1000, whereas at ratios of 2500 and 5000, silicon shows adsorption comparable to that of fluoride, suggesting competitive interactions with fluoride. In the test using soil extract solution, the fluoride removal rate (i.e., sorption performance) was more than 70% lower at a liquid-to-solid ratio of 250 than in the test using deionized water (Figure 3). This is thought to be due to the greater sorption of sulfate ions in the soil extract solution onto the LDH, which inhibited the sorption of fluoride. This result is consistent with the previous finding that sulfate ions are more selectively sorbed than is fluoride in the interlayer of LDH [23]. Therefore, in the present study, it was assumed that fluoride and sulfate ions are competing to be sorbed between the layers of LDH, as shown schematically in Figure 11.
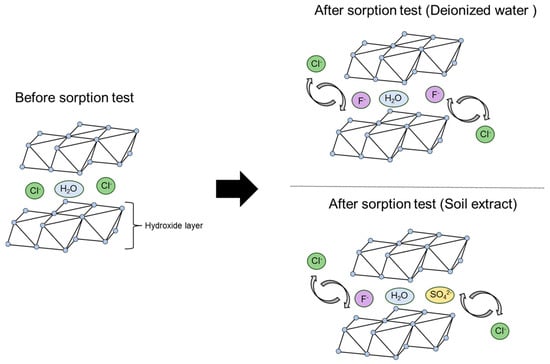
Figure 11.
Schematic diagram of the LDH sorption reaction.
4.2. Comparison of Distribution Coefficients by Test Method
The effect of competing ions on anion sorption by LDH has been studied, mainly with respect to the treatment of industrial wastewater [24,33,34,35]. Miyata reported that the anion sorption capacity of LDH increases in the order of I− < NO3− < Br− < Cl− < F− < OH− < SO42− < CO32− [23]. Lv et al., investigated the effects of fluoride removal from industrial wastewater by Mg-Al LDH and inhibition by artificially added coexisting ions (Cl−, SO42−, CO32−) [35]. They found that inhibition by sulfate ion was the most marked, reporting declines in performance of 20% at 100 mg/L and 45% at 200 mg/L. The differences from the present study are the concentration of fluoride ions, the relative concentration of coexisting ions to fluoride ions, and the fact that the coexisting ions were independently added to the fluoride solution. In the tests conducted by Lv et al., the fluoride concentration was 1000 mg/L, and the concentrations of the coexisting ions were less than half of that of the fluoride ions. In contrast, the concentration of fluoride ions contained in the leachate from construction-derived soil is much lower than that in industrial wastewater, and the relative concentration of fluoride ions to the coexisting ions is also lower. Therefore, there is greater concern that the influence of coexisting ions will be more significant. Dadwhal examined the effect of coexisting ions on the sorption of arsenic by LDH, reporting that phosphate ions have the greatest effect on reducing arsenic sorption, followed by sulfate ions; fluoride ions were reported to have the least effect on arsenic sorption [36].
The apparent distribution coefficients of fluoride (Table 5) are consistent with the results of previous sorption tests using boron with fly ash by Öztürk & Kavak, where the sorption capacity from the batch test was about half that from the column test [25]. Notably, in all tests, the removal rate of fluoride with the soil extract solution was smaller than that with deionized water. In the batch tests, the distribution coefficient for the soil extract solution (731 L/kg) was 27% that for the deionized water (2260 L/kg). This trend was more pronounced in the column test, where the distribution coefficient was 309 L/kg for the four-fold diluted soil extract solution, or about 19% of the 1600 L/kg observed for the deionized water. This is presumed to be because the fluoride concentration in the solution used for the column test was lower, resulting in a lower relative concentration compared to the coexisting ions, and thus a greater influence from them. Therefore, when considering the effect of soil extract solution on sorption performance, the relative concentration between the coexisting ions and the target hazardous ion is presumed to be important.
4.3. Implication
In general, the sorption performance of adsorbents and soils is studied by modeling the results of batch or column sorption tests [37,38]. When tests are conducted using deionized water or calcium chloride solutions, various variables can be obtained by fitting the sorption isotherm to previously reported experimental values. However, in general, not only in this study but also in other studies using soil extract solutions, the shape of the sorption isotherm may show repeated increases and decreases in the amount of sorption, as shown in Figure 2 and Figure 4, or a decrease in the amount of sorption after a certain concentration, thus making regression using the sorption isotherm equation difficult.
In the present study, the inhibition of fluoride sorption by sulfate ions led to an overestimation of adsorbent performance, as measured in the batch and column tests with deionized water compared to that with soil extract solution. Sulfate ions easily leach out from soil containing naturally occurring hazardous substances [1,2,39]. Sulfate ions are produced by the oxidation of pyrite (Equations (8)–(10)):
FeS2(s) + 3.5O2(aq) + H2O → Fe2+ + 2H+ + 2SO42−
Fe2+ + 0.25O2(aq) + H+ → Fe3+ + 0.5H2O
FeS2(s) + 14Fe3+ + 8H2O → 15Fe2+ + 2SO42−+ 16H+
Since the soil used to prepare the soil extract solution used in the present study also contained pyrite, it is thought that sulfate ions were generated by a similar process. Therefore, when evaluating the performance of adsorbents used in the field, evaluating the influence of coexisting ions by evaluating the sorption performance using actual soil extract solutions is desirable, in addition to conducting batch and column tests using deionized water. However, conducting all tests using soil extract would require a great deal of effort and cost. To reduce the number of the batch sorption tests using soil extract, we propose the following hybrid test using deionized water and soil extract: Tests using deionized water should be conducted under all conditions, and tests using soil extract should be conducted only under three liquid-to-solid ratios (100, 500 or 1000, 5000) with N = 1. The maximum percentage decrease in the sorption rate of the soil extract solution to deionized water is calculated from the three liquid-to-solid ratios for which data is available for both test solutions (100, 500 or 1000, 5000). The distribution coefficient for the soil extract solution is calculated by multiplying the maximum decrease in the sorption rate by the distribution coefficient obtained using deionized water.
5. Conclusions
Fluoride sorption tests using an LDH-BA were conducted to investigate the influence of coexisting ions in soil extract solution on adsorbent performance in both batch and column tests. Tests with the soil extract solution demonstrated a decrease in fluoride sorption performance compared with those using deionized water, which we attributed to the inhibition of fluoride uptake by sulfate and other ions present in the soil extract. The column tests were more susceptible to this effect than the batch tests, likely because the fluoride concentration in the column test solution was lower, resulting in a lower relative concentration with respect to the coexisting ions and thus a greater influence from them. These findings underscore the importance of finding an appropriate balance between the concentrations of the target hazardous element and the coexisting ions. Therefore, in addition to conducting batch and column tests with deionized water, it is advisable to include sorption tests using actual soil extract solution—at least for a subset of experiments—to more accurately evaluate the effects of coexisting ions.
The coexisting ion concentrations in extract solutions from soil and rock generated during construction work vary widely. Furthermore, various types of adsorbents are used in the attenuation layer method and include various iron-based adsorbents as well as LDH- and Mg-based adsorbents. Therefore, further knowledge of the relationship between the concentration of coexisting ions in the soil extract solution and the decrease in sorption performance of the adsorbent should be collected.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15090937/s1: Figure S1. Calculation of distribution coefficients for batch sorption test. Figure S2. Calculation of distribution coefficients for column sorption test. The area of the blue-colored part corresponds to the cumulative sorption amount. Figure S3. Adsorption isotherms of tests using only adsorbents and approximate straight lines of (a) Henry type and (b) Freundlich type. Each parameter is shown in Table S1. Figure S4. Test solution pH of (a)after adsorbent batch sorption tests, (b) attenuation layer material (ALM) batch sorption tests and (c) column sorption tests. Figure S5. Sorption isotherms for fluoride and chloride ions tests using deionized water and soil extract solutions. If the sorbed amount is negative, it is the amount released, not the amount sorbed. Table S1. Parameters of Henry and Freundlich models of adsorption isotherms for tests using only adsorbents. M is amount of sorbed fluoride (mg/kg), C is the concentration of fluoride (mg/L).
Author Contributions
M.N.: conceptualization, formal analysis, visualization, writing—original draft. Y.H.: validation, supervision, writing—reviewing and editing. K.F.: formal analysis. T.K.: formal analysis, validation, writing—reviewing and editing T.Y.: conceptualization, validation, writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number JP22H00227.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
In promoting this research, we would like to thank the members of the Committee for Standardization of Testing Methods for Materials Used in Attenuation Layer Methods for their input. In addition, we would like to express our gratitude to the adsorbent manufacturer for providing us with the test material.
Conflicts of Interest
The authors declare no conflicts of interest associated with this manuscript.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Tabelin, C.B.; Basri, A.H.M.; Igarashi, T.; Yoneda, T. Removal of arsenic, boron, and selenium from excavated rocks by consecutive washing. Water Air Soil Pollut. 2012, 223, 4153–4167. [Google Scholar] [CrossRef]
- Tatsuhara, T.; Arima, T.; Igarashi, T.; Tabelin, C.B. Combined neutralization-adsorption system for the disposal of hydrothermally altered excavated rock producing acidic leachate with hazardous elements. Eng. Geol. 2012, 139–140, 76–84. [Google Scholar] [CrossRef]
- Ministry of the Environment, Soil Environment Standards, Appended Table. Available online: https://www.env.go.jp/kijun/dt1.html (accessed on 30 May 2025). (In Japanese).
- Inoue, T.; Tanaka, S.; Ito, Y. Current Status and Issues of Countermeasures for Naturally Occurring Heavy Metals in Hokkaido. In Proceedings of the 55th Hokkaido Development Technology Research and Presentation Meeting, Hokkaido, Japan, 21–23 February 2012. (In Japanese). [Google Scholar]
- Itabashi, T.; Li, J.; Hashimoto, Y.; Ueshima, M.; Sakanakura, H.; Yasutaka, T.; Imoto, Y.; Hosomi, M. Speciation and Fractionation of Soil Arsenic from Natural and Anthropogenic Sources: Chemical Extraction, Scanning Electron Microscopy, and Micro-XRF/XAFS Investigation. Environ. Sci. Technol. 2019, 53, 14186–14193. [Google Scholar] [CrossRef]
- Ito, H.; Katsumi, T. Leaching characteristics of naturally derived toxic elements from soils in the western Osaka area: Considerations from the analytical results under the Soil Contamination Countermeasures Act. J. Geotech. Eng. 2020, 15, 119–130. (In Japanese) [Google Scholar] [CrossRef]
- Tokyo Metropolitan Government. Soil Contamination Countermeasures Review Committee in 2023. 2024. Available online: https://www.kankyo.metro.tokyo.lg.jp/documents/d/kankyo/dojou-kentou_r5siryou-1 (accessed on 30 May 2025). (In Japanese).
- Hashimoto, Y.; Imoto, Y.; Nishikata, M.; Suga, H.; Wang, S.L.; Yasutaka, T. Unveiling the potential mobility and geochemical speciation of geogenic arsenic in the deep subsurface soil of the Tokyo metropolitan area. J. Hazard. Mater. 2025, 484, 136580. [Google Scholar] [CrossRef]
- Swartjes, F.A.; Rutgers, M.; Lijzen, J.P.A.; Janssen, P.J.C.M.; Otte, P.F.; Wintersen, A.; Brand, E.; Posthuma, L. State of the art of contaminated site management in The Netherlands: Policy framework and risk assessment tools. Sci. Total Environ. 2012, 427–428, 1–10. [Google Scholar] [CrossRef]
- Kato, T.; Gathuka, L.W.; Okada, T.; Takai, A.; Katsumi, T.; Imoto, Y.; Morimoto, K.; Nishikata, M.; Yasutaka, T. Sorption-desorption column tests to evaluate the attenuation layer using soil amended with a stabilising agent. Soils Found. 2021, 61, 1112–1122. [Google Scholar] [CrossRef]
- Arima, T.; Yokobori, N.; Mufalo, W.; Nakajima, K.; Tabelin, C.B.; Igarashi, T. Fluoride leaching from tuff breccia and its removal by natural and commercial adsorbents. Chemosphere 2024, 354, 141735. [Google Scholar] [CrossRef]
- Mo, J.; Flores, G.; Inui, T.; Katsumi, T. Hydraulic and sorption performances of soil amended with calcium-magnesium composite powder against natural arsenic contamination. Soils Found. 2020, 60, 1084–1096. [Google Scholar] [CrossRef]
- Arima, T.; Sasaki, R.; Yamamoto, T.; Tabelin, C.B.; Tamoto, S.; Igarashi, T. Effects of environmental factors on the leaching and immobilization behavior of arsenic from mudstone by laboratory and in situ column experiments. Minerals 2021, 11, 1220. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals, Section 1, Test No. 106: Adsorption—Desorption Using a Batch Equilibrium Method; OECD: Paris, France, 2000. [Google Scholar]
- US EPA. Fate, Transport and Transformation Test Guidelines—OPPTS 835.1230 Adsorption/Desorption (Batch Equilibrium); US EPA United States Environmental Protection Agency: Washington, DC, USA, 2008; pp. 1–55.
- Ministry of the Environment, Japan. Guidelines for Contaminated Soil Treatment Industry (Revision 4.3) Appendix 13; Ministry of the Environment, Japan: Tokyo, Japan, 2024. (In Japanese)
- Hokkaido Environmental Conservation Technology Association. No. 6 Design Manual for Adsorption Layer Method; Hokkaido Environmental Conservation Technology Association: Hokkaido, Japan, 2012. (In Japanese) [Google Scholar]
- JIS A 1291; Test Method for Adsorption Performance of Attenuation Layer. Japanese Industrial Standards Committee: Tokyo, Japan, 2025.
- Nozawa, S.; Sato, T.; Otake, T. Effect of Dissolved Silica on Immobilization of Boron by Magnesium Oxide. Minerals 2018, 8, 76. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, X.; Zhang, Y.; Zhang, Q.; Pan, B. Enhanced fluoride removal by La-doped Li/Al layered double hydroxides. J. Colloid Interface Sci. 2018, 509, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Luengo, C.V.; Lopez, N.A.; Ramos, C.P.; Avena, M.J. Highly efficient arsenic adsorption onto Mg/Al/Fe layered double hydroxides: Kinetics, isotherm, XPS and Mössbauer spectroscopies. J. Water Process Eng. 2023, 56, 104542. [Google Scholar] [CrossRef]
- González, M.A.; Pavlovic, I.; Rojas-Delgado, R.; Barriga, C. Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide-humate hybrid. Sorbate and sorbent comparative studies. Chem. Eng. J. 2014, 254, 605–611. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. (In Japanese) [Google Scholar] [CrossRef]
- Morimoto, K.; Anraku, S.; Hoshino, J.; Yoneda, T.; Sato, T. Surface complexation reactions of inorganic anions on hydrotalcite-like compounds. J. Colloid Interface Sci. 2012, 384, 99–104. [Google Scholar] [CrossRef]
- Öztürk, N.; Kavak, D. Adsorption of boron from aqueous solutions using fly ash: Batch and column studies. J. Hazard. Mater. 2005, 127, 81–88. [Google Scholar] [CrossRef]
- Plassard, F.; Winiarski, T.; Petit-Ramel, M. Retention and distribution of three heavy metals in a carbonated soil: Comparison between batch and unsaturated column studies. J. Contam. Hydrol. 2000, 42, 99–111. [Google Scholar] [CrossRef]
- Chotpantarat, S.; Ong, S.K.; Sutthirat, C.; Osathaphan, K. Competitive modeling of sorption and transport of Pb2+, Ni2+, Mn2+ AND Zn2+ under binary and multi-metal systems in lateritic soil columns. Geoderma 2012, 189–190, 278–287. [Google Scholar] [CrossRef]
- Refaey, Y.; Jansen, B.; Parsons, J.R.; de Voogt, P.; Bagnis, S.; Markus, A.; El-Shater, A.H.; El-Haddad, A.A.; Kalbitz, K. Effects of clay minerals, hydroxides, and timing of dissolved organic matter addition on the competitive sorption of copper, nickel, and zinc: A column experiment. J. Environ. Manag. 2017, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Zhu, W.; Xu, H.; Fan, X.; Wu, S.; Shi, J.; Song, J. A new method for determination of heavy metal adsorption parameters in compacted clay by batch tests. Ecotoxicol. Environ. Saf. 2019, 181, 114–120. [Google Scholar] [CrossRef]
- Wan Zuhairi, W.Y.; Nurita, R. Assessment of heavy metal attenuation and mobility in compacted soil columns. Sains Malays. 2019, 48, 2463–2472. [Google Scholar] [CrossRef]
- JIS A 1204; The Method for Particle Size Distribution of Soils. Japanese Standards Association: Tokyo, Japan, 2020.
- Rounds, S.A.; Wilde, F.D. Alkalinity and Acid Neutralizing Capacity (USGS Numbered Series No. 09-A6.6); USGS: Reston, VA, USA, 2012; Chapter A6, Section 6.6.
- Tezuka, S.; Chitrakar, R.; Sakane, K.; Sonoda, A.; Ooi, K.; Tomida, T. The synthesis and phosphate adsorptive properties of Mg(II)-Mn(III) layered double hydroxides and their heat-treated materials. Bull. Chem. Soc. Jpn. 2004, 77, 2101–2107. [Google Scholar] [CrossRef]
- Yang, L.; Shahrivari, Z.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. Removal of trace levels of arsenic and selenium from aqueous solutions by calcined and uncalcined layered double hydroxides (LDH). Ind. Eng. Chem. Res. 2005, 44, 6804–6815. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Zhou, Z. Treatment of high fluoride concentration water by MgAl-CO3 layered double hydroxides: Kinetic and equilibrium studies. Water Res. 2007, 41, 1534–1542. [Google Scholar] [CrossRef]
- Dadwhal, M.; Sahimi, M.; Tsotsis, T.T. Adsorption isotherms of arsenic on conditioned layered double hydroxides in the Presence of various competing ions. Ind. Eng. Chem. Res. 2011, 50, 2220–2226. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Pandi, T.A.; Matis, K.A. Chromium(VI) Removal from Aqueous Solutions by Mg-Al-CO3 Hydrotalcite: Sorption-Desorption Kinetic and Equilibrium Studies. Ind. Eng. Chem. Res. 2004, 43, 2209–2215. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Z.; Feng, C.; Li, M.; Chen, R.; Sugiura, N. Investigations on the batch and fixed-bed column performance of fluoride adsorption by Kanuma mud. Desalination 2011, 268, 76–82. [Google Scholar] [CrossRef]
- Kamata, A.; Ueshima, M.; Sakanakura, H.; Miura, T.; Katoh, M. The effects of redox conditions on arsenic re-release from excavated marine sedimentary rock with naturally suppressed arsenic release. Environ. Geochem. Health 2022, 44, 4157–4171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).