Abstract
Phosphate ores, which are regarded as critical mineral resources, play an important role in various industrial fields. Apatite is the main source of phosphate mineral resources and must be concentrated before it is processed into industrial products. Flotation is the most commonly employed method for apatite concentration. However, as the proportion of fine apatite increases, the challenge of separating it from gangue minerals intensifies, due to the resemblance in surface characteristics between apatite and gangue. Interfacial regulation during flotation is fundamental to the process, including the regulation of the mineral/water interface wettability by flotation reagents (collectors and modifiers), the control of interactions between mineral particles, and the regulation of interactions between mineral particles and bubbles. This article introduces the surface characteristics of apatite and its main gangue minerals. It discusses innovative work on flotation reagents (primarily collectors and depressants) and their action mechanisms on mineral surfaces. It reviews the current development of theories on the regulation of interactions between interparticles and between particles and bubbles. Finally, the study outlook the future research on interfacial regulation in apatite flotation. This study is intended to offer references for the continued advancement of apatite flotation.
1. Introduction
Phosphate ores have been classified as critical mineral resources by several countries and regions, including China, the European Union, and the United States. Data have shown that the global phosphate ore reserves total 74 billion tons, with the majority being located in Africa [1]. Morocco holds the largest phosphate reserves, amounting to 50 billion tons, which accounts for 67.57% of the global total. China follows with reserves of 3.8 billion tons. In 2023, the global production of phosphate ore reached 2.2 billion tons, with China leading the world in production at 900 million tons. Morocco and the United States followed [1]. Phosphorus is extensively consumed in agriculture and is considered irreplaceable and non-recyclable. It is essential for ensuring food security, fostering economic growth, and advancing societal well-being [1,2,3,4]. Figure 1 displays the global allocation of phosphorus reserves, the varieties of phosphorus deposits, and the applications of phosphorus ores. Among them, collophane is characterized by low grade, high levels of harmful impurities, and poor consistency. Apatite, with its good flotation properties, has emerged as a vital resource for phosphorus extraction and the manufacturing of agricultural phosphate fertilizers, making it crucial in the processing of phosphate minerals [5,6,7]. Recently, the phosphate import and export trade has been impacted by international factors, heightening the urgency for countries to develop and utilize phosphate ore resources [8].
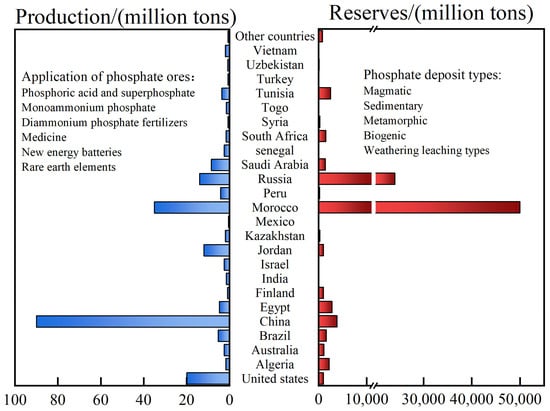
Figure 1.
Distribution and application of world phosphorus resources [9,10,11,12,13].
In addition to phosphate minerals, phosphate ores also include multiple accompanying gangue minerals, including clay, quartz, carbonate-based minerals (primarily calcite and dolomite), organic material, iron oxides, and pyrite [14]. Depending on the chemical composition and content of the gangue minerals in the ore, apatite can be classified into siliceous, calcareous, or a mixture of both [15]. The beneficiation techniques for apatite ores vary depending on the specific apatite variety and associated gangue minerals. Apatite is non-magnetic and has a density similar to that of the gangue minerals; so, gravity and magnetic separation methods are seldom applied for its enrichment, while flotation remains the predominant technique for apatite beneficiation [16,17]. The basic principle of mineral separation through froth flotation is to control the surface properties and hydrophobicity of the ore particles using surfactants, thereby amplifying the differences in mineral floatability [18,19,20]. During flotation, the equipment produces air bubbles that preferentially adhere to hydrophobic mineral particles, carrying them upward to the pulp surface, whereas hydrophilic particles stay suspended in the slurry [21]. This differential bubble adhesion capitalizes on variations in the mineral surface’s hydrophobicity and hydrophilicity, enabling effective apatite concentration [22]. It can be seen that factors such as particle diameter, contact angle, and bubble diameter significantly influence the effectiveness of flotation in this process [23].
Flotation was initially applied to the beneficiation of siliceous ores. Technological progress and enhanced knowledge of flotation chemistry and surface characteristics have enabled its application in processing both siliceous–carbonaceous and carbonaceous apatite ores [14,24,25]. In the context of the flotation enrichment of apatite, the most challenging aspect is the separation from gangue minerals such as quartz (SiO2), calcite, and dolomite, particularly the separation from calcium-containing minerals like calcite and dolomite. The presence of calcium ions with comparable structural configurations in these minerals leads to similar surface chemical behaviors and dissolution patterns within the slurry medium. This phenomenon limits the selectivity of adsorption of flotation reagents on surfaces containing calcium-rich minerals. Consequently, the separation process through flotation becomes challenging [26,27,28,29]. In addition, the ongoing development and utilization of mineral resources has resulted in the gradual depletion of high-grade phosphate deposits, leading to a decline in ore grades and a decrease in grain size. This necessitates the implementation of finer grinding techniques for monomer dissociation, thereby generating a substantial number of fine apatite particles [30,31,32]. Fine apatite particles (−20 μm) are characterized by their small size and low kinetic energy, which decreases the likelihood of contact and attachment with conventional flotation bubbles. Consequently, this leads to a diminished separation performance during the flotation process. Additionally, fine mineral particles possessing increased surface area demand higher dosages of flotation chemicals for effective treatment and frequently encounter challenges, including higher foam viscosity and mechanical entrapment of gangue minerals [33,34,35]. Furthermore, the presence of high levels of impurities in low-grade phosphate ores results in the complicated separation of apatite, reduced recovery rates at beneficiation plants, and increased separation costs [36].
Flotation represents a multiphase separation process encompassing solid particles, liquid media, and gas bubbles, governed by numerous chemical interactions and physical parameters. Chemical factors regulate the wettability of mineral surfaces through flotation reagents, while physical factors include ore properties, such as particle size and degree of liberation, as well as equipment-related factors such as aeration rate and bubble size [37]. Interfacial regulation in mineral flotation forms the foundation for addressing flotation challenges and mainly includes: (1) regulation of particle interfacial properties, primarily wettability and surface charge, and (2) regulation of interfacial interactions, including interactions between solid particles and the liquid phase (i.e., adsorption of reagents on mineral surfaces), interactions between solid particles (dispersion and aggregation), and interactions between solid particles and bubbles. It is, thus, imperative to conduct a comprehensive review of the key issues on the flotation process of apatite, incorporating a detailed analysis of its physicochemical properties, the reagents employed, the reagent reaction mechanisms, and interactions interparticles and between particles and bubbles. The present paper thus summarizes the crystal structure characteristics and surface physicochemical properties of apatite. A systematic review of the research progress on apatite flotation reagents, including collectors and depressants, is then conducted, followed by an analysis of the mechanisms of action of different reagent types. Furthermore, it summarizes the research advancements in understanding interparticles and particle–bubble interactions during apatite flotation. The overarching objective of this analysis is twofold: first, to comprehensively examine the challenges and underlying driving forces that govern the apatite flotation process; and second, to critically appraise the extant research conducted by the scientific community on these matters. The final aim is to propose future avenues for exploration in the field of apatite flotation separation theory.
2. Basic Properties of Apatite
The crystal-chemical principles of mineral flotation are predicated on the relationship between the mineral’s crystal structure, chemical composition, surface properties, and floatability. A comprehensive understanding of the crystal chemistry and surface characteristics of minerals is essential for elucidating the mechanisms of mineral flotation.
2.1. Crystal Structure Characteristics of Apatite
The Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA-CNMNC) has determined the classification of apatite into three distinct types based on the predominant anions present (Cl−, F−, and OH−). This classification system identifies chlorapatite (Ca5(PO4)3Cl), fluorapatite (Ca5(PO4)3F), and hydroxylapatite (Ca5(PO4)3OH) as the three primary types of apatite [38]. Fluorapatite is the most stable and prevalent form of apatite mineral [39]. Apatite, a mineral that is commonly found in nature, is known to undergo isomorphous substitution. Ideal pure apatite is seldom encountered [40]. The components fluorine (F), chlorine (Cl), and hydrogen oxide (OH) exhibit mutual substitutability, enabling complete solid solution formation. Within sedimentary phosphate deposits, the replacement of PO43− by CO32− and OH− groups can result in the formation of carbonate-fluorapatite. This substitution form is the most prevalent in sedimentary deposits and is commonly designated as francolite [41].
The most predominant form of apatite mineral is fluorapatite. According to the American Mineralogist Crystal Structure Database (AMCSD), the ideal fluorapatite crystal has a complex hexagonal prismatic structure, with each unit cell containing 42 atoms. As shown in Figure 2, within fluorapatite crystalline structures, two distinct calcium position variants exist: Ca1 and Ca2. The Ca1 site is located between two layers of phosphate groups and is bonded to nine surrounding oxygen atoms. The Ca2 site is positioned within the same layer as the phosphate group. In this configuration, the Ca2 site is bonded to six adjacent oxygen atoms and a single fluorine atom [39]. Within the unit cell, two sets of Ca1 atoms (with two atoms per set) form atomic columns perpendicular to the (001) plane, while two sets of Ca2 atoms (with three atoms per set) form end faces perpendicular to the c-axis [42]. Research has indicated that the Ca1 site is more active and more likely to form unsaturated bond fractures. The fracture surfaces of apatite have been observed to exhibit two distinct types of bond fracture: Ca-O and Ca-F. Furthermore, extensive substitution has been observed in natural apatite crystals, including alkali metals (K and Na), transition metal ions (Mn, Ni, Cu, Co, and Zn), alkaline earth metals (Sr and Ba), and rare-earth elements at the Ca sites [42]. The (001) crystal face of apatite is the most common cleavage plane. This plane primarily exposes calcium (Ca) atoms and phosphate (PO43−) groups in the sub-layer, with the outermost layer exposing only calcium (Ca) atoms. This facilitates the adsorption of flotation reagents [43]. Natural apatite crystals exhibit extensive substitution, including alkali metals (K and Na), transition metal ions (Mn, Ni, Cu, Co, and Zn), alkaline earth metals (Sr and Ba), along with rare-earth elements occupying calcium lattice positions [42].
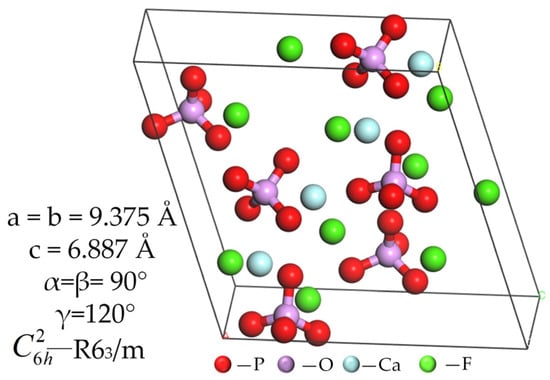
Figure 2.
Fluorapatite crystal structure (a, b, c, α, β, γ: cell parameters; C6h2—R63/m: space group, C6h: a six-fold rotational axis, and a horizontal mirror plane perpendicular to the six-fold rotational axis; R: rhombus; m: a mirror plane symmetry element) [44,45].
2.2. Surface Properties
Most flotation processes depend on the targeted attachment of collectors (organic surface-active agents) to mineral surfaces in aqueous environments. Detailed knowledge of mineral interfacial characteristics is essential for optimizing reagent formulations and improving separation efficiency. Measures of solid/liquid interface conditions, including contact angle measurements and zeta potential values, directly correlate with interfacial behaviors that manifest the intricate interactions occurring at the solid–liquid–gas boundary [46].
2.2.1. Surface Wettability
The hydrophobicity or hydrophilicity of mineral surfaces is quantified through contact angle analysis. The measurement of this parameter can be conducted employing multiple techniques, including the sessile drop approach, captive bubble analysis, and Washburn capillary rise (WCR) methodology. The regulation of mineral surface wettability by flotation reagents (mainly collectors) is the foundation of flotation separation. The contact angle of natural apatite surfaces is approximately 10°. However, due to the substitution of various ions and the deposition and impregnation of organic matter and impurities in the complex sedimentary environment, the contact angle on the surface of fluorapatite in sedimentary phosphate ores increases to some extent. The contact angle of apatite surfaces in sedimentary phosphate ores ranges from 50° to 66° [47]. Calcite and dolomite represent the dominant carbonate gangue in apatite deposits, while quartz serves as the principal silicate gangue mineral coexisting with apatite [48]. Since apatite, calcite, and dolomite share the presence of Ca2+ ions on their surfaces, as well as the deposition of calcium ions on the surface of quartz, they exhibit similar surface properties [32,49,50]. Through adjustments to the interfacial reactions between flotation chemicals and mineral surfaces, the flotation characteristics of minerals can be modified, generating significant variations in surface wettability that immediately influence separation effectiveness in flotation operations [51]. Table 1 presents measured contact angle values for apatite surfaces following treatment with various chemical reagents.

Table 1.
Contact angle reported for apatite conditioned with some collectors.
Additionally, studies have shown that changes in the mineral surface roughness caused by grinding, as well as the dissolution of metal ions, are also important factors influencing wettability [55,56]. Some studies demonstrated that, for surfaces with a hydrophilic character, an increase in surface roughness resulted in an enhancement in hydrophilicity. Conversely, for surfaces characterized by hydrophobic properties, an increase in surface roughness led to an augmentation of hydrophobicity [57]. Jiang et al. [58] utilized density functional theory calculations to demonstrate that fluorapatite crystals containing impurities, such as Mg, Al, and Fe, manifest novel impurity energy levels. Moreover, these foreign elements induce a displacement of fluorapatite’s density of states distribution relative to the Fermi energy level, thereby enhancing the hydrophobic character of the phosphate mineral surface. Observations have been made of the reaction between H2PO4- and free Ca2+ and Mg2+ ions in the slurry. The creation of hydrated Ca(H2PO4)2 has been demonstrated to substantially reduce phosphate rock surface hydrophobicity, whereas soluble Mg(H2PO4)2 produces a modest suppressing effect on calcite [55,59,60,61]. Figure 3 illustrates how dissolved metal ions influence mineral surface wettability. Furthermore, multiple investigations have documented that interactions between SO42− and Ca2+ in slurry result in CaSO4(s) precipitation, which subsequently adheres to fluorapatite surfaces and hinders NaOL adsorption [62,63,64].
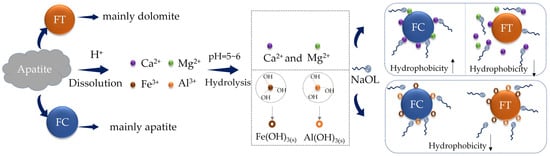
Figure 3.
The effect of dissolved metal ions on the surface wettability of minerals [58].
2.2.2. Surface Electrical Properties
The zeta potential serves as an indicator of the electrical double layer and colloidal behavior of nanoparticles, offering insights into their stability, duration in circulation, interactions with proteins, membrane penetration capacity, and biological compatibility. This analytical technique is routinely employed to evaluate the electrical properties at mineral particle surfaces [65]. The positioning ions present on apatite surfaces are Ca2+, CaOH+, HPO42−, H2PO4−, PO43−, H+, and OH−. Given the variable geological environments and mineral compositions of apatite from different sources, as well as the mutual substitution of various cations and anions, the point of zero charge (PZC) of apatite exhibits significant variation, from pH = 1 to 8.7 [40,66,67,68,69,70,71,72,73]. The isoelectric point (IEP) is defined as the negative logarithm of the concentration of location ions when the zeta potential is zero [74]. The electrical properties at a mineral’s surface or in mineral–reagent complexes depend on multiple factors: the inherent mineral makeup (including mineral variety and impurity content) along with the particular reagents employed (like depressants and collectors), with consideration to both their molecular configuration and concentration levels [75]. In the region to the left of the IEP, the mineral surface carries a positive charge and can be captured by anionic collectors. Conversely, in the right section of the IEP, the mineral surface acquires a negative charge and becomes amenable to collection by positively charged reagents. When the mineral surface and collector molecules share identical charge characteristics, the polar functional groups of the reagent undergo chemical interaction with active sites on the mineral surface, resulting in their attachment to the mineral [51].
Flotation reagents can modify the electrical properties of mineral surfaces (Table 2). For instance, andiroba oil, at a pH of 7.5 and a collector concentration of 20 mg/L, has been demonstrated to induce a negative shift in fluorapatite’s IEP curve throughout the complete examined pH spectrum [76]. According to Table 2, each of these collectors caused a reduction in the IEP value and increased negative zeta potential, demonstrating significant reactivity with the target mineral surfaces [77]. In addition, It is evident from the extant literature that metal ions in solution have varying degrees of impact on the surface potential of minerals [78,79]. Liao’s study examined how typical chloride-based electrolyte solutions containing Na+, Mg2+, and Ca2+ cations influence the zeta potential of finely ground apatite particles. The study revealed that the surface potential of apatite in the electrolyte solution was significantly higher than that of apatite in deionized water, with divalent Ca2+ and Mg2+ providing a more positive potential than the monovalent Na+ [80]. Jiang et al. conducted a study on the effects of Al3+ and Fe3+ on the surface electrical properties of fluorapatite. The study revealed that the additions of Al3+ and Fe3+ substantially enhanced the surface charge of apatite across the pH spectrum from 3 to 11 [58].

Table 2.
The IEP of apatite with and without the addition of collectors.
3. Flotation Reagents
Researchers utilized density functional theory (DFT) to probe the adsorption properties of a single water molecule at metal sites on the surface of apatite. The apatite surface contains unsaturated Ca metal active sites, where water molecules can strongly adsorb. The oxygen and hydrogen atoms of the water molecule form hydroxylated metal active sites and hydrogen bonds of varying strengths with the metal atoms and oxygen on the mineral surface. The surface reconstruction caused by the hydroxylation and hydrogen bonding of water molecules on the mineral surface is the essence of apatite exhibiting hydrophilic properties in natural systems [87]. Typically, negatively charged surfactants are applied in positive flotation to separate phosphate minerals from calcium-containing waste minerals, whereas positively charged surfactants are utilized in negative flotation to eliminate silica-based impurities from phosphate deposits. During positive flotation operations, the pulp’s alkalinity is maintained at around pH 9.5 through soda ash addition, while controlled doses of sodium silicate serve to inhibit the floating of silica minerals. Subsequently, the process involves the utilization of anionic surfactants to facilitate the flotation of phosphate minerals. Conversely, in the reverse flotation process, cationic surfactants are utilized to facilitate the flotation of silicates at a weakly acidic or neutral pH, thereby enabling the recovery of phosphate minerals from the tailings product [88].
Apatite, dolomite, and calcite are all calcium-containing minerals. When these minerals dissociate, the surface exposes Ca active sites, which are typically the adsorption sites for flotation reagents. In natural systems, dolomite and calcite exhibit hydrophilic properties similar to those of apatite. Therefore, the differentiation of apatite from calcium-containing minerals like dolomite and calcite is frequently accomplished through an inverse flotation approach, employing fatty acid collectors and suitable apatite depressants. Alternatively, a direct flotation process can be used, employing depressants that selectively attach to dolomite surfaces [89,90]. In the flotation system of apatite and quartz, cationic collectors are commonly utilized. Apatite has higher O and Ca activity, and the Ca atoms on the surface carry a relatively large positive charge. The Ca 3d states in the valence band exhibit strong metallic activity, making it difficult for apatite to interact with cationic collectors [91]. In comparison with apatite, the quartz surface demonstrates a higher absolute value of negative zeta potential. Therefore, cationic collectors exhibit stronger electrical attraction to quartz surfaces, thereby enhancing their interaction with quartz particles in the slurry relative to their behavior with apatite. This improves the efficiency of inverse flotation for separating apatite from quartz [48]. The present review systematically categorizes both collectors and depressants employed in the beneficiation of apatite from its principal associated minerals—dolomite, calcite, and quartz. A particular focus is placed on recently developed reagents. Furthermore, the mechanisms of action of the diverse reagents are analyzed.
3.1. Collector
Collectors, known as “surfactants”, are amphiphilic molecules that serve as crucial components in mineral separation by flotation. The “head” of the surfactant is capable of binding to the mineral surface, while the “tail” is hydrophobic and facilitates binding with bubbles, thus enabling the surfactant to rise to the surface [92]. Collectors, functioning as surface-active agents, are classified based on their polar group charge characteristics: anionic (negatively charged), cationic (positively charged), amphoteric (charge varies with pH), and nonionic (electrically neutral) [93]. Figure 4 shows the effect of pH on the dissociation of amphoteric surfactants. At pH values ranging from 6 to 7, the collector manifests amphoteric properties. Consequently, when the pH exceeds 7, the amphoteric collector predominantly behaves as an anion. Numerous chemical reagents serve distinct roles in phosphate ore flotation systems, acting as collectors, depressants, and modifying agents for both positive and reverse flotation operations. During direct flotation, phosphate components are recovered under basic pH environments through the application of negatively charged collectors alongside gangue-depressing chemicals. In contrast, reverse flotation employing cationic amine collectors at near-neutral pH values selectively removes silicate impurities from phosphate matrices. Conversely, anionic reverse flotation describes the use of anionic collectors to float carbonate minerals under acidic pH conditions, while appropriate depressants are employed to suppress phosphate minerals [24]. This section summarizes the different types of apatite collectors and their action mechanisms, primarily utilizing illustrative examples of newly emerging collectors. Table 3 provides a concise list of apatite flotation collectors, with a particular focus on those that have emerged in recent years.

Table 3.
Collectors used in apatite ore flotation.
Table 3.
Collectors used in apatite ore flotation.
| Ionic Property | Collector | pH * | Concentration | Ref. | Year |
|---|---|---|---|---|---|
| Anionic | FrOC | 9 | 1000 g/t | [85] | 2024 |
| 2-Cl-9-ODA | 9 | 3 × 10−4 mol/L | [94] | 2024 | |
| SNLS | 10 | 5 × 10−6 mol/L | [95] | 2023 | |
| CSFA | 7 | 40 mg/L | [96] | 2021 | |
| BHA | 9 | 1.2 × 10−4 mol/L | [97] | 2019 | |
| Cationic | DHDB | 4.5 | 15 mg/L | [98] | 2019 |
| LPDC | 6.5 | 25 mg/L | [99] | 2023 | |
| HDMEA | 6.43 | 20 mg/L | [54] | 2022 | |
| Amphoteric | DDALA | 6 | 3 × 10−4 mol/L | [48] | 2024 |
| Lecithin | 8.5 | 40 mg/L | [100] | 2023 | |
| C12Giy | 5 | 30 mg/L | [101] | 2022 | |
| Nonionic | NI-EP | 4.5~5.2 | 125 g/t | [102] | 2025 |
| oxyethylenated cetyl ether | 10 | 360 g/t | [103] | 2022 |
*: The initial pH in slurry, measured before flotation.
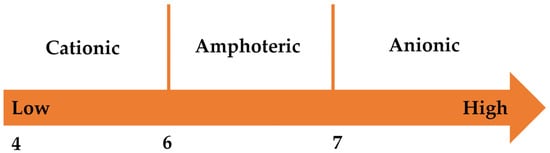
Figure 4.
Effect of pH on the dissociation of amphoteric surfactants [104].
3.1.1. Anionic Collector
Fatty acid-based collectors have shown effectiveness in increasing the hydrophobicity of both phosphate minerals and calcium-bearing gangue minerals, including dolomite and calcite. Sodium oleate (NaOL) and oleic acid (HOl), serving as characteristic fatty acid salts and fatty acids, respectively, represent the most commonly employed negatively charged collectors for calcium-bearing minerals, including phosphate minerals [66,105,106]. Fatty acids are classified as carboxylic acid-type collectors, with a general chemical structure formula of R-COOH (Na+ or K+). In the context of density functional theory, the electronic structure of oleic acid, a prevalent fatty acid collector, was examined. It was demonstrated that the oxygen atoms present in both the oleic acid molecule and the oleate ion exhibit high levels of reactivity. The oxygen atom is identified as the bonding atom in the interaction between oleic acid and the oxide mineral surface [107]. It has been established that the ionization of a substantial quantity of RCOO− and H(RCOO)2− results in the occurrence of chemical adsorption at the Ca active sites on the mineral surface. This, in turn, leads to a modification in the surface’s hydrophobicity [108]. However, because RCOO− and H(RCOO)2− ions can indiscriminately undergo non-specific adsorption with metal active sites such as Ca2+, Fe3⁺, and Mg2+, and due to the simple geometric configuration of the carboxyl group, which cannot differentiate the coordination environment of cations on mineral surfaces through steric hindrance effects, these reagents lack selectivity [109,110]. In addition, a high dosage of collectors is required, and factors such as low temperature, the presence of cations, and slimes have a significant impact on their performance. Moreover, their performance tends to fluctuate, as their composition—being derived from natural sources—may vary with the seasons [26,111,112]. The chemical alteration of fatty acids (including sulfidation, sulfonation, and esterification processes) and combining diverse fatty acid configurations with negatively charged, zwitterionic, and uncharged collectors are regarded as viable strategies [24,113,114]. By modifying fatty acids to incorporate a greater variety of functional groups, their selectivity and solubility can be improved. This modification can also enhance the dispersion state of fatty acids and promote their adsorption onto mineral surfaces [24,115]. The combined use of reagents with diverse molecular structures can diversify adsorption mechanisms and simultaneously improve frothing characteristics. Figure 5 illustrates the process by which mixed reagents strengthen collector attachment to mineral surfaces [116,117].
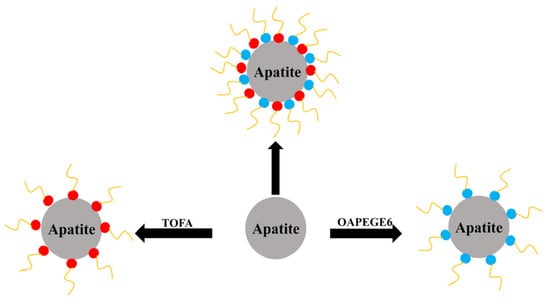
Figure 5.
Adsorption of mixed reagents on the surface of apatite. TOFA: Tall oil fatty acid; OAPEGE6: Oleic acid polyethylene glycol ester [32].
3.1.2. Cationic Collectors
Cationic collectors are mainly employed in the reverse flotation separation of silica-based minerals, but they can also be used to float dolomite and calcareous minerals from phosphate ores [24]. Amine-based cationic collectors, including dodecylamine (DDA) and its derivatives such as dodecylamine hydrochloride, are conventional cationic collectors that have been extensively utilized for the treatment of silicate minerals [118,119]. There are three distinct adsorption mechanisms for DDA on the quartz surface. First, monolayer adsorption occurs at low concentrations. Second, surface micelle formation is observed at moderate concentrations. Third, multilayer adsorption is evident at high concentrations, accompanied by the precipitation of molecular amines [120]. The DDA’s hydrocarbon chain adopts a near-vertical orientation relative to the apatite surface, enabling the effective dispersion of its non-polar segment in the flotation pulp. This configuration facilitates DDA accumulation on apatite through combined hydrophobic effects and intermolecular forces [121]. Figure 6 illustrates the proposed adsorption configuration of DDA on apatite. However, challenges including excessive froth formation, difficult foam control, and DDA’s poor mineral specificity often result in unsatisfactory separation outcomes [122,123,124]. These limitations have stimulated growing research interest in advanced amine-type collector development. Studies have shown that, when ester and ether groups are inserted into amines, the interaction strength increases. In addition, the binding energy generally rises with longer alkyl chains but stabilizes beyond a specific carbon count [125]. The isopropyl group separating the hydroxyl and secondary amine in DDAIP creates spatial constraints that inhibit concurrent coordination of the molecule’s oxygen and nitrogen atoms with surface Ca2+ sites on phosphate minerals. Consequently, DDAIP demonstrates superior target mineral specificity compared to DDA when separating phosphate from quartz through reverse flotation processes [126].
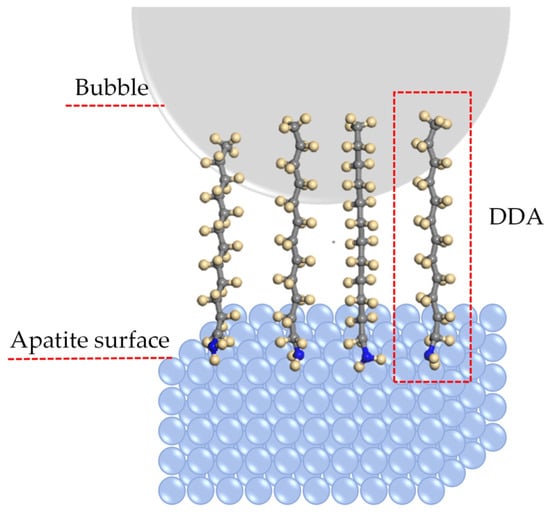
Figure 6.
Schematic of the adsorption model of DDA on the apatite surface [121].
3.1.3. Amphoteric Collectors
In previous flotation experiments, researchers found that amphoteric surfactants exhibited greater selectivity than sodium oleate and were less sensitive to dissolved ions [127]. Most amphoteric surfactants in solution exist in three distinct forms (cationic, amphoteric, and anionic) when undergoing dissociation, and their relative concentrations change with variations in the solution pH [100]. Indeed, amphoteric surfactants predominantly behave as cations at low pH values [104]. Therefore, under acidic conditions (pH 4–5), zwitterionic molecules become the dominant species in solution and attach to quartz’s negatively charged surfaces through their protonated amine groups. Consequently, at a pH smaller than 6, the amphoteric ions can act as cations, thereby reversing the surface charge of quartz and electrostatically adsorbing. In contrast, minerals such as calcite, which possess a positive surface charge, experience greater repulsion and consequently adsorb less [101].
Consequently, quartz flotation is reduced, while carbonate flotation is increased. Lecithin, an environmentally friendly amphoteric foaming agent, functions as a collecting agent in the reverse flotation beneficiation of phosphate ores [100]. Under conditions of pH 8.5 and 40 mg/L lecithin dosage, successful differentiation between phosphate-bearing apatite and carbonate impurities (calcite and dolomite) can be attained, resulting in a P2O5 grade of 32.39% with 96% mineral recovery [100]. Figure 7 shows the separation mechanism of phosphate rock using lecithin-based reagents. The newly developed amphoteric collector, N-dodecyl-β-alanine (DDALA), structurally modified from dodecylamine through the introduction of a propionic acid functional group, predominantly binds to quartz surfaces via its positively charged DDALA+ species. For apatite and dolomite surfaces, the neutral DDALA molecular form serves as the primary active agent. Flotation experiments reveal that DDALA, when combined with a xanthogenate (XG) depressant, successfully enhances the inverse flotation differentiation between apatite and quartz. Additionally, in conjunction with oleate collectors, DDALA shows exceptional performance in differentiating apatite from dolomite via reverse flotation techniques [48].
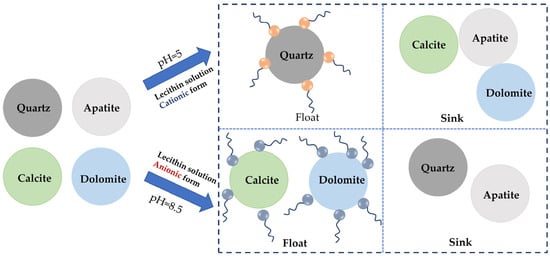
Figure 7.
Separation mechanism of phosphate ore using a lecithin solution [100].
3.1.4. Nonionic Collector
Nonionic collectors may consist of unmodified aliphatic alcohols or their chemically altered variants, such as ethoxylated alcohols [24]. Nonionic collectors are typically combined with other ionic collectors to enhance flotation performance. The prevailing opinion among researchers is that the improved flotation performance achieved using these mixed collector systems stems from the simultaneous adsorption of oppositely charged or neutral collectors on mineral surfaces. This phenomenon decreases charge-based repulsion among adsorbed collectors while enhancing both collector adsorption density and intermolecular hydrophobic interactions between adjacent hydrocarbon tails. This leads to a denser packing of the collectors [128,129,130]. Studies have found that, after adding fatty alcohols, the fatty alcohols co-adsorb with oleic acid on the mineral surface, with their quantity significantly higher than when fatty alcohols are used alone, while the adsorption of oleic acid remains similar. Consequently, the recovery of fluorapatite and calcite during oleic acid flotation is significantly improved after the co-adsorption of fatty alcohols [102,131]. Simultaneously, the amalgamation of amine collectors and fatty alcohols has shown effectiveness in improving the flotation performance of quartz [102]. In addition, ether and ester nonionic surfactants, such as polyethylene glycol alkyl ethers and Tween 80, can also improve the effectiveness of fatty acid collectors in the apatite flotation system [103,132].
3.2. Depressant
As previously stated, apatite and its gangue minerals (e.g., dolomite, calcite, and quartz) are all hydrophilic. Furthermore, the calcium-active sites shared by apatite with dolomite and calcite, in addition to the calcium ions dissolved from apatite being able to adhere to the surface of quartz minerals, serve to reduce the selectivity of collectors. Consequently, the utilization of depressants within the apatite flotation system becomes paramount [133]. The basic principle for selecting depressants is to modify the hydrophobic groups of the collector to hydrophilic groups based on the specific mineral being targeted. Depending on the flotation process and gangue type, the main types of depressants include carbonate depressants, silicate depressants, and phosphate depressants (Table 4) [24,134].

Table 4.
Depressants used in the flotation of apatite.
3.2.1. Depressors of Apatite
Phosphate depressants mainly function in apatite reverse flotation to suppress apatite. Commonly used inhibitors include sulfuric acid, phosphoric acid, and their related compounds [55]. The depressant mechanism is attributed to the ionization of sulfuric acid and phosphoric acid into H2PO4− and HSO4− in the acidic slurry environment, which bind to the Ca2+ exposed on the apatite surface, creating a hydrophilic coating that increases the mineral’s water affinity. This simultaneously reduces the number of accessible reactive centers on the apatite surface, consequently diminishing collector attachment [135,145,146,147,148,149,150]. From the above, it can be seen that the formation of Ca(H2PO4)2 and Ca(HSO4)2 enhances apatite hydrophilicity, leading to its depression. It can be concluded that sulphate and phosphate can also act as depressants for apatite [136,151,152]. However, it is worth noting that, although sulfuric acid and its derivatives have a strong depressant effect on phosphate minerals and their advantage is their relatively low cost, they can cause pipeline blockage due to gypsum formation [134].
With the increasing awareness of environmental protection, researchers have begun to focus on the development of environmentally friendly depressants, such as organic acids, polymers, and their derivatives, including nitrotrimethylphosphonic acid, modified starch, and polyacrylamide [153,154,155]. Dai et al. studied a novel depressant, TG, which is a polysaccharide. They investigated its depressant effect on apatite and its mechanism in a sodium oleate (NaOL) system. The enhanced depressing action of TG on apatite results from more robust and durable chemical bonds established between the –COOH group of TG and the Ca2+-binding sites on the apatite surface. This potent TG–apatite association effectively blocks subsequent NaOL adsorption [138]. In their investigation, Oulkhir et al. examined the depressant effect of phosphorylated starch (PS) extracted from potato waste, a material that has been posited as a green and effective depressant. PS exhibits significant surface attachment to apatite via chemical bonding between its phosphate functional groups and reactive calcium centers on the mineral surface [137].
3.2.2. Depressors of Carbonate Minerals
Calcite and dolomite are the primary carbonate gangue minerals present in apatite ores. Common carbonate mineral depressants include lignosulfonates, sulfonated phenolic resins, nitrated humic acid salts, sulfonated naphthalene (S711), and sulfonated anthracene (S808). Recent studies have increasingly concentrated on the development of new silicate depressants that exhibit high selectivity, environmental friendliness, and cost-effectiveness. Polysaccharides, recognized for their environmental sustainability, have become a significant focus of investigation within the domain of bio-based depressants. The presence of -OH and -COO groups in polysaccharides has been demonstrated to enable complexation with metallic cations (particularly Ca2+ and Mg2+) present on mineral surfaces in phosphate deposits, including apatite, calcite, and dolomite [134,156]. Examples include sodium alginate, carboxymethyl cellulose, pectin, gum Arabic (GA), xanthan gum (XG), and carboxymethyl chitosan derivatives [139,140,141,157,158]. Figure 8 shows several molecular structural formulas of typical bio-based depressants. Figure 9 presents a schematic representation of the molecular interactions occurring between polysaccharide compounds and the surface structures of calcite, dolomite, and apatite minerals.
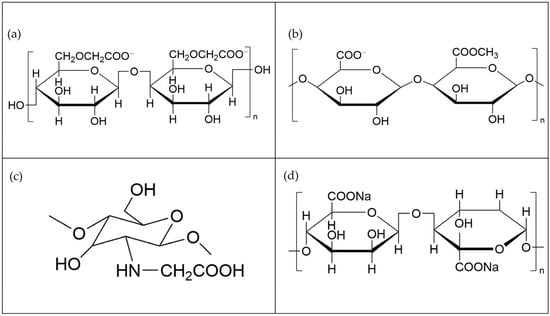
Figure 8.
Molecular structural formulas of typical bio-based depressors [95]. (a) Carboxymethyl cellulose; (b) pectin; (c) carboxymethyl chitosan; and (d) sodium alginate.
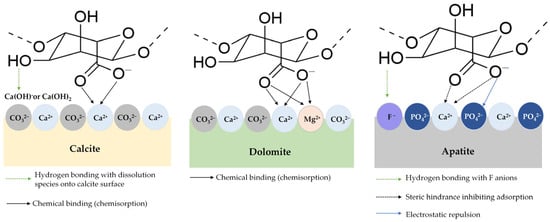
Figure 9.
The schematic illustration of molecular interactions occurring between polysaccharide compounds and the surfaces of calcite, dolomite, and apatite minerals [156].
The surfaces of bacteria are covered with various structures, including peptidoglycan, teichoic and teichuronic acids, lipoproteins and lipopolysaccharides, surface protein arrays, and bacterial capsule polymers, which are typically polysaccharides or polypeptides. These structures are capable of binding with metal ions [159]. The use of microorganisms as depressants for carbonate minerals in apatite flotation has gradually become a research hotspot. Bacteria such as Bacillus subtilis, Mycobacterium, Rhodococcus erythropolis CD 130, Pseudomonas fluorescens, Escherichia coli, Bacillus subtilis, C. albicans, and Escherichia coli have all been shown to preferentially adsorb on the dolomite’s surface, competing with the collectors for adsorption on dolomite to achieve selective suppression in the apatite flotation process [145,160,161].
3.2.3. Depressors of Silicate Minerals
Sodium silicate represents the most widely employed depressing agent for siliceous apatite, used to suppress silicate minerals such as quartz. However, Wang et al. demonstrated that, in a fatty acid flotation system utilizing sodium silicate depression, achieving high-purity apatite concentrates proves challenging [162]. The primary rationale for this phenomenon pertains to the attachment of calcium ions (Ca2+) that have been dissolved from the apatite mineral to the surface of quartz. This process serves to activate the quartz, thereby diminishing the selectivity of the collector. Hence, the selection of an effective depressant to inhibit Ca2+-activated quartz is imperative. In the context of the apatite flotation system, the mechanism of action of silicate depressants can be primarily divided into two distinct aspects. Firstly, they compete for adsorption on the quartz surface, reducing the adsorption of the collector. Secondly, they interact with Ca2+, reducing its adsorption on the quartz surface, decreasing the activation of the quartz surface, and hindering the interaction between the collector and the quartz surface [162]. Sayilgan’s research examined the impact of carbonates on the flotation behavior of quartz under alkaline conditions, utilizing amine collectors and sodium oleate in the presence of calcium ions [163]. The research results show that carbonates form precipitates with Ca2+ ions in the solution, significantly reducing the Ca2+ concentration and decreasing the activation of quartz. Wang used citric acid (CA) as a depressant for sodium NaOL flotation of calcium-activated quartz and analyzed its depression mechanism [143]. CA’s inhibitory effect on activated quartz operates through dual pathways: initially, CA anions remove surface-adsorbed Ca2+ ions from quartz, decreasing available attachment sites for NaOL; subsequently, CA molecules bound to residual Ca2+ sites block further NaOL adsorption on the quartz surface. In recent years, many depressants for calcium gangue minerals have been studied to explore their depressing effect on quartz, such as sodium alginate, carboxymethyl chitosan, modified starch, pectin, and carboxymethyl cellulose [164,165,166,167,168].
4. Interface Regulation of Fine Apatite
The beneficiation of phosphate ore presents a challenge in the recovery of fine-grained apatite from low-grade and medium-grade collophane, weathered phosphate mud, and phosphate tailings. Studies have shown that particles of different sizes behave differently during flotation, directly affecting both the flotation recovery and flotation rate. For phosphate minerals, the optimal flotation particle size range is 60–200 µm. When the particle size falls below 20 µm, the recovery rate sharply decreases to below 70% [169]. It is imperative to strengthen the research on interface regulation for fine-grained apatite flotation to enhance the flotation process and promote the efficient utilization and sustainable development of medium- and low-grade fine phosphate resources. The inferior flotation performance of fine mineral particles principally stems from their reduced probability of contacting standard-sized flotation bubbles moving at typical velocities [169,170]. This bubble-particle attachment likelihood is principally governed by the comparative sizes of the gaseous and solid phases, illustrated in Figure 10.
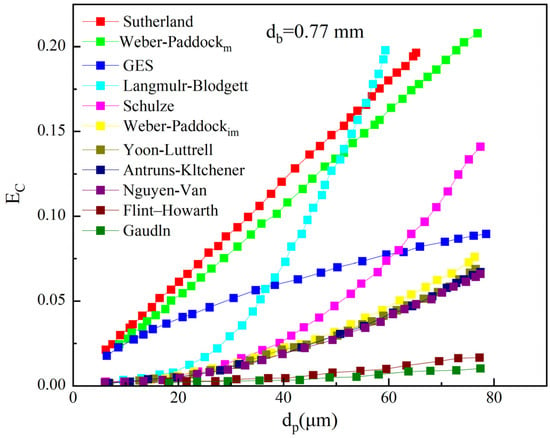
Figure 10.
Collision efficiencies as a function of particle size. Ec—Collision efficiency; dp—particle diameter; db—bubble diameter [171,172].
The probability of successful attachment rises proportionally with growing particle dimensions. Furthermore, elevated agitation rates provide greater energy input, accelerating particle movement and consequently improving particle–bubble contact frequency. And the strong energy input creates strong turbulence in the slurry, and the enhancement in turbulence further reduces the size of bubbles and also increases the probability of collision of fine particles [173]. Interface regulation during flotation is crucial, including the regulation of the mineral/water interface wettability by flotation reagents (collectors and modifiers), the regulation of interactions between mineral particles (dispersion of heterogeneous particles and hydrophobic aggregation of homogeneous particles), and the regulation of interactions between mineral particles and bubbles (collision, adhesion, and detachment) [174]. The flotation reagents have been introduced earlier, and this paper will continue to summarize recent research on the interactions between mineral particles and the interactions between mineral particles and bubbles.
4.1. Interaction Regulation Interparticle: Particle Aggregation and Stability
The targeted agglomeration of fine particles to enlarge their effective size represents a viable approach for enhancing fine mineral recovery during flotation [175]. Understanding interparticle forces is fundamental for controlling the selective aggregation processes of finely divided minerals. Ye [174] conducted a study on the dispersion and hydrophobic agglomeration regulation of fine-grained apatite particles in an apatite–quartz system under various dispersants (sodium silicate, sodium hexametaphosphate, sodium carbonate) and emulsified kerosene. The study established that, under natural conditions, fine apatite exhibited inadequate dispersion stability, while fine quartz demonstrated robust dispersion stability. In the presence of an alkaline environment, the dispersion stability of fine-grained apatite exhibited a modest enhancement. The utilization of dispersants led to a substantial augmentation in the dispersion stability of fine apatite, thereby impeding the process of heterogeneous aggregation. Their dispersion ability was ranked from highest to lowest as follows: sodium hexametaphosphate > sodium silicate > sodium carbonate. Furthermore, the micron-sized kerosene droplets prepared by mechanical stirring acted as bridging agents between the hydrophobic apatite particles induced by sodium oleate, thereby increasing the size and strength of the agglomerates and subsequently enhancing the flotation rate of fine-grained apatite. Research has identified an interdependent relationship among particle agglomeration, slurry viscosity, and interparticle forces, analogous to a “three-factor equilibrium”. Elevated slurry viscosity reflects strengthened particle interactions, predominantly resulting from intensified hydrophobic attraction. This subsequently promotes more extensive clustering of apatite particles. Moreover, as apatite aggregation progresses, a three-dimensional network develops within the pulp, impeding liquid movement and consequently raising the system’s apparent viscosity [176].
4.2. Interaction Regulation of Particle–Bubble
Flotation relies on the selective adhesion of specific mineral particles to gas bubbles, making bubble–particle attachment a critical stage in the separation process, determining the efficiency and selectivity of the process [177,178]. The process of particle capture by bubbles in the slurry is characterized by a series of physical interactions, including collision, attachment, and detachment [179,180]. Angelica investigated the influence of bubble size distribution on fine apatite particles in flotation columns. The research involved multiple flotation experiments designed to evaluate different bubble size distribution profiles. The test series included small bubbles (d32 < 300 μm), medium-sized bubbles (300 < d32 < 1000 μm), and large bubbles (d32 > 1000 μm). The optimal bubble size range for fine apatite flotation was determined. When using small bubbles (d32 < 300 μm) to float fine apatite, the highest P2O5 content could be obtained, but the P2O5 recovery rate was very low (40%) under these conditions. In contrast, using larger bubbles (d32 > 1000 μm) resulted in a lower P2O5 content, but the flotation achieved the highest P2O5 recovery. Considering both factors (P2O5 content and recovery rate), the best flotation performance was achieved using medium-sized bubbles. Various methods have been examined to enhance the collision effectiveness between bubbles and fine particles, such as modifying aeration system operating parameters, enhanced flocculation flotation techniques, pulsed aeration flotation processes, dissolved air flotation (DAF) systems, electrostatic interaction-based flotation with colloidal gas bubbles, and nanobubble-supplemented flotation approaches [179,181,182,183,184,185]. Santana et al. [182] investigated fine apatite particle separation using DAF technology. Their comparative analysis revealed DAF’s superior performance over traditional flotation methods, demonstrating extended bubble retention periods, substantially greater bubble quantities per unit air volume, and expanded bubble surface areas, which all contribut to improved particle–bubble interaction probabilities. Jiang et al. [186] investigated how impeller rotation rates influence nanobubble flotation performance. Their findings revealed that higher impeller speeds produced larger aggregate sizes, with rotational velocities between 1200 and 2800 rpm generating substantially greater quantities and sizes of nanobubble clusters compared to conventional flotation systems, which was consistent with the improvements in flotation recovery and flotation rate. Additionally, Zeng reported that nanobubbles could promote the recovery of apatite using sodium oleate, but had the opposite effect when using dodecylamine [187]. Researchers have utilized the cavitation effect and acoustic radiation force effect of ultrasound to cause bubble rupture, which impacts particles and bubbles, thereby achieving the movement, dispersion, and coalescence of particles and bubbles [188,189]. Jung et al. [190] developed a microbubble generation device and found that, within an acoustic field, adjacent microbubbles tend to cluster together, forming bubble aggregates that enhance the buoyancy of the bubbles. Gao et al. examined how varying gas supply parameters influence bubble characteristics in ultrasonic-assisted flotation systems. Their study showed that, by altering the inner diameter and angle of the gas generator, the initial morphology and motion paths of the bubbles could be modified, ultimately influencing their flotation performance [191].
5. Future Outlook
For future research on apatite flotation interface control, several considerations can be made for researchers:
- (1)
- Strengthening the development of highly selective, environmentally friendly reagents and expanding research from micro-flotation experiments to industrial trials.
- (2)
- Mixed reagents have been proven to perform better than single reagents, but their mechanisms are not yet thoroughly understood. In-depth research should be conducted using advanced detection equipment, and based on this, more reagent combinations should be proposed.
- (3)
- Research on the relationship between reagent structure and particle size in flotation systems is still insufficient, especially for fine particles or even ultra-fine particles (less than 10 μm).
- (4)
- Research on particle–particle interactions is still at the pure mineral testing stage, while the actual flotation slurry environment is much more complex. There should be more focus on the study of particle interactions in complex environments.
- (5)
- Regarding bubble size regulation, the optimization and development of micro-bubble generation equipment should be strengthened.
It should be emphasized that all research is based on the properties of mineral surfaces, and more importantly, the core of research work should focus on further studies into mineral surface properties to enhance the comprehension of reagent adsorption mechanisms and the intricate relationships between solid particles and gas bubbles.
6. Conclusions
(1) Apatite is widely used in various industrial fields, and its demand is continuously increasing. Flotation is the most commonly used process for enriching apatite. The main challenges in the flotation enrichment process of apatite are twofold. First, developing highly selective reagents to enhance the surface wettability differences between apatite and gangue minerals such as calcite, dolomite, and quartz. Second, regulating the interactions between particles and between particles and bubbles to increase the probability of particle–bubble collisions.
(2) The flotation interface regulation reagents for apatite mainly include collectors and depressants. The types of collectors primarily include anionic, cationic, amphoteric, and nonionic collectors. Developing highly selective collectors is crucial. The chemical alteration of fatty acids or combining diverse fatty acid configurations with manufactured negatively charged, zwitterionic, and uncharged collectors represents a primary strategy. Cationic collectors are widely used in silicate flotation. Studies of positively charged collectors primarily examine how varying hydrocarbon chain lengths and polar groups influence collection efficiency. Uncharged collectors are typically employed alongside ionic collectors to enable cooperative adsorption, which strengthens mineral surface hydrophobicity and enhances separation effectiveness.
(3) According to flotation processes and gangue types, the main types of depressants include carbonate, silicate, and phosphate depressants. Commonly used depressants include sulfuric acid, hydrochloric acid, lignosulfonates, and sodium silicate. With the growing awareness of green and sustainable development, organic and bio-based depressants are being continuously developed, such as modified starch, pectin, sodium alginate, and carboxymethyl chitosan. These depressants achieve their inhibitory effects through attachment to calcium-reactive surface sites via numerous -OH and -COO groups.
(4) Two primary strategies for improving fine apatite–bubble collision rates involve modifying particle–particle and particle–bubble interactions to either enlarge particle dimensions or reduce bubble size. Flotation reagents can intensify hydrophobic attraction among mineral particles, promoting hydrophobic agglomeration and effectively increasing the mineral particle size. Advanced techniques like aeration system optimization, enhancing flocculation flotation, oscillating air supply flotation, dissolved air flotation, and the use of nanobubbles can be employed to enhance the particle–bubble interaction frequency and consequently boost separation performance.
(5) Subsequent investigations should concentrate on the development of highly selective and environmentally friendly reagents, based on a thorough investigation of reagent structures and their mechanisms of action. In addition, more emphasis should be placed on the application of emerging interface regulation technologies in industrial operations, serving as a foundation for the development and optimization of interface regulation devices.
Author Contributions
Conceptualization, writing—review and editing, Z.L. (Zhe Liu); Funding acquiring, conceptualization, writing—review and editing, supervision L.L.; validation Z.L. (Zhuguo Li); formal analysis, M.W.; investigation H.M.; visualization, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52174243, and Key Research and Development Project of Liaoning Province, grant number 2024JH2/102400026.
Data Availability Statement
All data included in this study are available by contacting the corresponding author.
Conflicts of Interest
Authors Zhuguo Li and Meng Wang were employed by the company Chengde Jingcheng Mining Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MAP | Monoammonium phosphate |
| DAP | Diammonium phosphate |
| (IMA-CNMNC) | The Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association |
| AMCSD | American Mineralogist Crystal Structure Database |
| WCR | Washburn capillary rise |
| DDA | Dodecylamine |
| DDAIP | 1-(dodecylamine)-2-propanol |
| PZC | Point of zero charge |
| IEP | Isoelectric point |
| DFT | Density functional theory |
| SDS | Sodium dodecyl sulfate |
| TOFA | Tall oil fatty acid |
| OAPEGE | Oleic acid polyethylene glycol ester |
| HDMEA | N-(2-hydroxy-1, 1-dimethylethyl) |
| DTAB | Dodecyltrimethyl ammonium bromide |
| DDALA | N-dodecyl-β-alanine |
| XG | Xanthogenate |
| TG | Tragacanth gum |
| PS | Phosphorylated starch |
| CA | Citric acid |
| S711 | Sulfonated naphthalene |
| S808 | Sulfonated anthracene |
| GA | Arabic gum |
| KGM | Konjac glucomannan |
References
- Mineral Commodity Summaries. Mineral Commodity Summaries 2024; Mineral Commodity Summaries: Reston, VA, USA, 2024; p. 212. [Google Scholar]
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report. 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 31 March 2025).
- Fournie, T.; Rashwan, T.L.; Switzer, C.; Gerhard, J.I. Phosphorus Recovery and Reuse Potential from Smouldered Sewage Sludge Ash. Waste Manag. 2022, 137, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, Q.; Wang, X. New Insights on Depressive Mechanism of Citric Acid in the Selective Flotation of Dolomite from Apatite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130075. [Google Scholar] [CrossRef]
- Zhang, F.; Williamson, B.J.; Broom-Fendley, S. Apatite Texture and Composition in the Tonglushan Porphyry-Related Skarn System, Eastern China: Implications for Mineral Exploration. Ore Geol. Rev. 2023, 158, 105493. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Z.; Cao, X.; Fan, H.; Xiao, J.; Xia, Y.; Zeng, M. Geochemistry of Apatite Individuals in Zhijin Phosphorites, South China: Insight into the REY Sources and Diagenetic Enrichment. Ore Geol. Rev. 2022, 150, 105169. [Google Scholar] [CrossRef]
- Dong, L.; Cui, Y.; Qiao, L.; Lan, S.; Zheng, Q.; Shen, P.; Liu, D. A Critical Review on the Flotation of Calcium-Containing Minerals. Sep. Purif. Technol. 2025, 360, 131082. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries. Mineral Commodity Summaries 2023; Mineral Commodity Summaries: Reston, VA, USA, 2023; p. 210. [Google Scholar]
- Cloud, P. Phosphorite Sedimentation: Phosphate Deposits of the World. Science 1987, 236, 1125–1126. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.S.; Frolov, A.A.; Belov, S.V. Regularities in the Distribution and Genetic Features of Phosphate Ore Deposits. Geol. Ore Depos. 2001, 43, 150–161. [Google Scholar]
- Andersson, S.S.; Wagner, T.; Jonsson, E.; Fusswinkel, T.; Whitehouse, M.J. Apatite as a Tracer of the Source, Chemistry and Evolution of Ore-Forming Fluids: The Case of the Olserum-Djupedal REE-Phosphate Mineralisation, SE Sweden. Geochim. Et Cosmochim. Acta 2019, 255, 163–187. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, G.; Yang, X. Isolating Elemental Phosphorus from Sewage Sludge Ash by Electrochemistry. Resour. Conserv. Recycl. 2023, 190, 106815. [Google Scholar] [CrossRef]
- Marques, C.F.; Olhero, S.; Abrantes, J.C.C.; Marote, A.; Ferreira, S.; Vieira, S.I.; Ferreira, J.M.F. Biocompatibility and Antimicrobial Activity of Biphasic Calcium Phosphate Powders Doped with Metal Ions for Regenerative Medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Abouzeid, A.-Z.M.; Negm, A.T.; Elgillani, D.A. Upgrading of Calcareous Phosphate Ores by Flotation: Effect of Ore Characteristics. Int. J. Miner. Process. 2009, 90, 81–89. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M.; El-Jallad, I.S.; Orphy, M.K. Calcareous Phosphates and Their Calcined Products. Miner. Sci. Eng. 1980, 12, 73–83. [Google Scholar]
- Abouzeid, A.-Z.M. Physical and Thermal Treatment of Phosphate Ores—An Overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Raiymbekov, Y.; Besterekov, U.; Abdurazova, P.; Nazarbek, U. Review of Methods and Technologies for the Enrichment of Low-Grade Phosphorites. Rev. Inorg. Chem. 2022, 42, 385–395. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Wang, P.; Liu, D.; Han, H. A Novel Metal–Organic Complex Surfactant for High-Efficiency Mineral Flotation. Chem. Eng. J. 2021, 426, 130853. [Google Scholar] [CrossRef]
- Feng, J.; Yang, B.; Zhu, H.; Zhang, H.; Zeng, M. The Utilization of 2-Phosphonobutane-1,2,4-Tricarboxylic Acid (PBTCA) as a Novel Sphalerite Depressant in the Selective Flotation of Galena from Sphalerite. Appl. Surf. Sci. 2021, 569, 150950. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, J.; Wang, Y.; Zhang, D.; Han, Y. Surface Properties and Flotation Inhibition Mechanism of Air Oxidation on Pyrite and Arsenopyrite. Appl. Surf. Sci. 2023, 610, 155476. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Peng, T.; Liu, C.; Yang, S. Influences of Starch Depressant with the Various Molecular Structure on the Interactions between Hematite Particles and Flotation Bubbles. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 652, 129814. [Google Scholar] [CrossRef]
- Xie, H.; Jin, Y.; Zhang, P.; Liu, Y.; Gao, L.; Feng, Q.; Liu, D. Surface Modification Mechanism of Galena with H2SO4 and Its Effect on Flotation Separation Performance. Appl. Surf. Sci. 2022, 579, 152129. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Heyes, G.W.; Ilyas, S.; Kim, H. Prediction of Grade and Recovery in Flotation from Physicochemical and Operational Aspects Using Machine Learning Models. Miner. Eng. 2022, 183, 107627. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents Used in the Flotation of Phosphate Ores: A Critical Review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Somasundaran, P.; Sivakumar, A. Advances in Understanding Flotation Mechanisms. Min. Metall. Explor. 1988, 5, 97–103. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; Benzaazoua, M.; El-Bahi, A.; Ait-Khouia, Y.; Hakkou, R. Assessment of the Selective Flotation of Calcite, Apatite and Quartz Using Bio-Based Collectors: Flaxseed, Nigella, and Olive Oils. Miner. Eng. 2022, 182, 107589. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Xiao, J.; Sun, W.; Tao, D.; Zuo, Y.; Han, H. Novel Metal-Starch Depressant for the Flotation Separation of Ultrafine Apatite and Dolomite Based on Experimental and Calculational Study. Sep. Purif. Technol. 2025, 362, 131877. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xue, N.; Li, Z.; Zhu, Y.; Nie, Y. Flotation Separation of Apatite from Dolomite with Tara Gum as Depressant: An Experimental and Molecular Dynamics Simulation Study. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 136044. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, L.; Fu, W.; Chi, R.; Li, H.; Yang, S. Investigations of Amino Trimethylene Phosphonic Acid as a Green and Efficient Depressant for the Flotation Separation of Apatite from Calcite. Miner. Eng. 2022, 181, 107552. [Google Scholar] [CrossRef]
- Ruan, Y.; He, D.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q. Interaction Behavior between Coarse and Fine Particles in the Reverse Flotation of Fluorapatite and Dolomite. Langmuir 2023, 39, 12931–12943. [Google Scholar] [CrossRef]
- Yang, B.; Cao, S.; Zhu, Z.; Yin, W.; Sheng, Q.; Sun, H.; Yao, J.; Chen, K. Selective Flotation Separation of Apatite from Dolomite Utilizing a Novel Eco-Friendly and Efficient Depressant for Sustainable Manufacturing of Phosphate Fertilizer. J. Clean. Prod. 2021, 286, 124949. [Google Scholar] [CrossRef]
- George, P.; Nguyen, A.V.; Jameson, G.J. Assessment of True Flotation and Entrainment in the Flotation of Submicron Particles by Fine Bubbles. Miner. Eng. 2004, 17, 847–853. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Ndlovu, B.; Bradshaw, D. Behaviour of Swelling Clays versus Non-Swelling Clays in Flotation. Miner. Eng. 2016, 96–97, 59–66. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Etschmann, B.; Brugger, J.; Bruckard, W.; Menacho, J.; Molina, L.; Hoadley, A. Spent Lithium-Ion Battery Recycling Using Flotation Technology: Effect of Material Heterogeneity on Separation Performance. Processes 2024, 12, 1363. [Google Scholar] [CrossRef]
- Abdollahi, S.; Afraei, S.; Mehdilo, A.; Kouchakzadeh, R.; Irannajad, M. A New Mixture of Anionic Collectors for Improvement of Apatite Floatability. Miner. Eng. 2025, 222, 109129. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Chapter 12—Froth Flotation. In Wills’ Mineral Processing Technology, 8th ed.; Wills, B.A., Finch, J.A., Eds.; Butterworth-Heinemann: Boston, UK, 2016; pp. 265–380. ISBN 978-0-08-097053-0. [Google Scholar]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T.J. Nomenclature of the Apatite Supergroup Minerals. Eur. J. Mineral. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Prosanov, I.Y.; Vinokurova, O.B.; Lyakhov, N.Z. Structure and Thermal Stability of Fluorhydroxyapatite and Fluorapatite Obtained by Mechanochemical Method. J. Solid State Chem. 2020, 282, 121076. [Google Scholar] [CrossRef]
- Simukanga, S.; Lombe, W.C. Electrochemical Properties of Apatite and Other Minerals of Zambian Phosphate Ores in Aqueous Solution. Fertil. Res. 1995, 41, 159–166. [Google Scholar] [CrossRef]
- Engvik, A.K.; Golla-Schindler, U.; Berndt, J.; Austrheim, H.; Putnis, A. Intragranular Replacement of Chlorapatite by Hydroxy-Fluor-Apatite during Metasomatism. Lithos 2009, 112, 236–246. [Google Scholar] [CrossRef]
- Rulis, P.; Yao, H.; Ouyang, L.; Ching, W.Y. Electronic Structure, Bonding, Charge Distribution, and x-Ray Absorption Spectra of the (001) Surfaces of Fluorapatite and Hydroxyapatite from First Principles. Phys. Rev. B 2007, 76, 245410. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Mao, S.; Li, L.; Ke, B.; Zhang, Q. Effects of Structure of Fatty Acid Collectors on the Adsorption of Fluorapatite (0 0 1) Surface: A First-Principles Calculations. Appl. Surf. Sci. 2018, 444, 699–709. [Google Scholar] [CrossRef]
- Comodi, P.; Liu, Y.; Zanazzi, P.F.; Montagnoli, M. Structural and Vibrational Behaviour of Fluorapatite with Pressure. Part I: In Situ Single-Crystal X-Ray Diffraction Investigation. Phys. Chem. Miner. 2001, 28, 219–224. [Google Scholar] [CrossRef]
- Kim, J.Y.; Fenton, R.R.; Hunter, B.A.; Kennedy, B.J. Powder Diffraction Studies of Synthetic Calcium and Lead Apatites. Aust. J. Chem. 2000, 53, 679–686. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Pradip Zeta Potentials in the Flotation of Oxide and Silicate Minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Kholodov, V.N.; Nedumov, R.I. The Role of Black Shales in the Formation of Phosphate and Manganese Ores. Lithol. Miner. Resour. 2011, 46, 321–352. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Ren, Q.; Tu, R.; Qiu, F.; Gao, Z.; Xu, S.; Sun, W.; Tian, M. Innovating an Amphoteric Collector Derived from Dodecylamine Molecular Structure: Facilitating the Selective Flotation Separation of Apatite from Quartz and Dolomite. Miner. Eng. 2024, 215, 108819. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; Ait-khouia, Y.; Elghali, A.; Benzaazoua, M.; Hakkou, R. Enhancing Selective Calcite and Dolomite Flotation in the Phosphate Ores: Investigation, Modeling, and Automated Mineralogy Assessment. Miner. Eng. 2024, 207, 108569. [Google Scholar] [CrossRef]
- Lan, S.; Shen, P.; Zheng, Q.; Qiao, L.; Dong, L.; Liu, D. Effective Flotation Separation of Apatite from Dolomite Using a New Eco-Friendly Depressant Gallic acid. Green Chem. 2024, 26, 1627–1636. [Google Scholar] [CrossRef]
- Krasowska, M.; Zawala, J.; Bradshaw-Hajek, B.H.; Ferri, J.K.; Beattie, D.A. Interfacial Characterisation for Flotation: 1. Solid-Liquid Interface. Curr. Opin. Colloid Interface Sci. 2018, 37, 61–73. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; El-Bahi, A.; Ait-Khouia, Y.; Benzaazoua, M.; Hakkou, R. Selective Flotation of Calcite and Dolomite from Apatite Using Bio-Based Alternatives to Conventional Collectors: Castor and Mustard Oils. Miner. Eng. 2024, 208, 108597. [Google Scholar] [CrossRef]
- Liu, W.; Ge, R.; Guo, Y.; Xia, Y.; Zhao, S.; Liu, W.; Cui, B.; Shen, Y. Study on the Performance and Mechanism of Novel Hydroxyl Quaternary Ammonium Collector for Separation of Quartz from Apatite. J. Mol. Liq. 2025, 421, 126868. [Google Scholar] [CrossRef]
- Peng, X.; Liu, W.; Zhao, Q.; Liu, W.; Tong, K.; Zhao, P. Development and Utilization of a Novel Hydrogen Bonding Enhanced Collector in the Separation of Apatite from Quartz. Miner. Eng. 2022, 180, 107477. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Review of the Main Factors Affecting the Flotation of Phosphate Ores. Minerals 2020, 10, 1109. [Google Scholar] [CrossRef]
- Ulusoy, U.; Yekeler, M. Correlation of the Surface Roughness of Some Industrial Minerals with Their Wettability Parameters. Chem. Eng. Process. Process Intensif. 2005, 44, 555–563. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite. Minerals 2020, 10, 114. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, H.; Li, S.; Cao, Y.; Ao, X. Effect of Mg, Al, and Fe Impurities on the Wettability of the Fluorapatite (001) Surface. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130130. [Google Scholar] [CrossRef]
- Gao, Z.-Y.; Jiang, Z.-Y.; Sun, W.; Gao, Y.-S. Typical Roles of Metal Ions in Mineral Flotation: A Review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Parapari, P.S.; Irannajad, M.; Mehdilo, A. Modification of Ilmenite Surface Properties by Superficial Dissolution Method. Miner. Eng. 2016, 92, 160–167. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Yu, H.; Liu, M. Double Roles of Sodium Hexametaphosphate in the Flotation of Dolomite from Apatite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127080. [Google Scholar] [CrossRef]
- Abdel-Khalek, N.A. Evaluation of Flotation Strategies for Sedimentary Phosphates with Siliceous and Carbonates Gangues. Miner. Eng. 2000, 13, 789–793. [Google Scholar] [CrossRef]
- Sun, R.; Xing, D.; Deng, J.; Jin, D.; Ma, S.; Liu, J.; Li, G.; Qin, G.; Liu, X. Effect of Sulfuric Acid Pretreatment on Flotation Performance of Calcite and Fluorite. Miner. Eng. 2023, 203, 108301. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, X.; Yin, Y.; Wang, M.; Cheng, D.; Ao, X.; Xie, Y. Effect of Ca2+ Dissolution and Migration Transformation from Phosphorite on the Surface Properties of Dolomite. Miner. Eng. 2022, 188, 107824. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Tao, D.; Xu, G. Fatty Acid Collectors for Phosphate Flotation and Their Adsorption Behavior Using QCM-D. Int. J. Miner. Process. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Cheng, R.; Li, C.; Liu, X.; Deng, S. Synergism of Octane Phenol Polyoxyethylene-10 and Oleic Acid in Apatite Flotation. Physicochem. Probl. Miner. Process. 2017, 53, 1214–1227. [Google Scholar] [CrossRef]
- Perrone, J.; Fourest, B.; Giffaut, E. Surface Characterization of Synthetic and Mineral Carbonate Fluoroapatites. J. Colloid Interface Sci. 2002, 249, 441–452. [Google Scholar] [CrossRef]
- Chaïrat, C.; Oelkers, E.H.; Schott, J.; Lartigue, J.-E. Fluorapatite Surface Composition in Aqueous Solution Deduced from Potentiometric, Electrokinetic, and Solubility Measurements, and Spectroscopic Observations. Geochim. Cosmochim. Acta 2007, 71, 5888–5900. [Google Scholar] [CrossRef]
- Yuehua, H.; Chi, R.; Xu, Z. Solution Chemistry Study of Salt-Type Mineral Flotation Systems: Role of Inorganic Dispersants. Ind. Eng. Chem. Res. 2003, 42, 1641–1647. [Google Scholar] [CrossRef]
- Nduwa-Mushidi, J.; Anderson, C.G. Surface Chemistry and Flotation Behaviors of Monazite–Apatite–Ilmenite–Quartz–Rutile–Zircon with Octanohydroxamic Acid. J. Sustain. Metall. 2017, 3, 62–72. [Google Scholar] [CrossRef]
- Amankonah, J.O.; Somasundaran, P. Effects of Dissolved Mineral Species on the Electrokinetic Behavior of Calcite and Apatite. Colloids Surf. 1985, 15, 335–353. [Google Scholar] [CrossRef]
- Mishra, S.K. The Electrokinetics of Apatite and Calcite in Inorganic Electrolyte Environment. Int. J. Miner. Process. 1978, 5, 69–83. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Selective Depressive Effect of Sodium Fluorosilicate on Calcite during Scheelite Flotation. Miner. Eng. 2019, 131, 262–271. [Google Scholar] [CrossRef]
- Angélica Evangelista de Carvalho, J.; Roberto Gomes Brandão, P.; Bicalho Henriques, A.; Silva de Oliveira, P.; Zanoni Lopes Cançado, R.; Rodrigues da Silva, G. Selective Flotation of Apatite from Micaceous Minerals Using Patauá Palm Tree Oil Collector. Miner. Eng. 2020, 156, 106474. [Google Scholar] [CrossRef]
- Santos, L.H.; Otávio dos Santos, G.; Fernandes de Magalhães, L.; Rodrigues da Silva, G.; Eduardo Clark Peres, A. Application of Andiroba Oil as a Novel Collector in Apatite Flotation. Miner. Eng. 2022, 185, 107678. [Google Scholar] [CrossRef]
- Abdel-Halim, M.M.; Abdel Khalek, M.A.; Zheng, R.; Gao, Z. Sodium N-Lauroylsarcosinate (SNLS) as a Selective Collector for Calcareous Phosphate Beneficiation. Minerals 2022, 12, 829. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The Effects of Ca(II) and Mg(II) Ions on the Flotation of Spodumene Using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the Flotation of Molybdenite. Part I: Surface Properties and Floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Liao, S.; Wu, Y.; Yang, Y.; Wu, L.; Gu, G.; Wang, Y. Flotation Behavior and Mechanism of Microfine Apatite and Illite in Different Electrolyte Solution Systems. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134563. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.-M.; Forssberg, E. Mechanism of Oleate Interaction on Salt-Type Minerals, Part II. Adsorption and Electrokinetic Studies of Apatite in the Presence of Sodium Oleate and Sodium Metasilicate. Int. J. Miner. Process. 1990, 28, 59–79. [Google Scholar] [CrossRef]
- Jong, K.; Han, Y.; Ryom, S. Flotation Mechanism of Oleic Acid Amide on Apatite. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 127–131. [Google Scholar] [CrossRef]
- de Oliveira, P.; Mansur, H.; Mansur, A.; da Silva, G.; Clark Peres, A.E. Apatite Flotation Using Pataua Palm Tree Oil as Collector. J. Mater. Res. Technol. 2019, 8, 4612–4619. [Google Scholar] [CrossRef]
- Xu, W.; Mei, G.; Tian, Y.; Shi, B.; Guo, C.; Pan, W. Reverse Cationic Flotation of Low-Grade Phosphate Ore Using Quaternary Ammonium Salt as a Collector and Its Adsorption Mechanism. Green Smart Min. Eng. 2024, 1, 322–335. [Google Scholar] [CrossRef]
- El-bahi, A.; Taha, Y.; Ait-Khouia, Y.; Elghali, A.; Benzaazoua, M. Enhancing Sustainability in Phosphate Ore Processing: Performance of Frying Oil as Alternative Flotation Collector for Carbonate Removal. Int. J. Min. Sci. Technol. 2024, 34, 557–571. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Bi, Y.; Yu, P.; Dai, H.; Wen, S.; Bai, S. The Adsorption Mechanism of Synergic Reagents and Its Effect on Apatite Flotation in Oleamide-Sodium Dodecyl Benzene Sulfonate (SDBS) System. Miner. Eng. 2021, 170, 107070. [Google Scholar] [CrossRef]
- Wang, X. Surface Wettability Regulation of Minerals in Flotation of Calcium-Magnesium Phosphate Ore. Ph.D. Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Budemberg, G.; Jolsterå, R.; Chelgani, S.C. Eco-Friendly Collectors in Apatite Froth Flotation: A Review. Int. J. Min. Sci. Technol. 2025, 35, 539–551. [Google Scholar] [CrossRef]
- Lan, S.; Dong, L.; Shen, P.; Zheng, Q.; Qiao, L.; Liu, D. Synergistic Impact of Surfactant and Sodium Oleate on Dolomite Removal from Fluorapatite via Reverse Flotation. Appl. Surf. Sci. 2024, 655, 159504. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Chai, Y.; Zhang, Q. Crystal Properties and Interaction with Flotation Reagent of Fluorapatite and Dolomite. Miner. Eng. 2023, 201, 108204. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Chen, J.; Xie, J.; Xie, F.; Li, L. Electronic Properties and Amine Collectors Effect of Fluorapatite and Quartz Surface. J. Guizhou Univ. 2017, 34, 21–28. [Google Scholar]
- Dederichs, T.; Möller, M.; Weichold, O. Colloidal Stability of Hydrophobic Nanoparticles in Ionic Surfactant Solutions: Definition of the Critical Dispersion Concentration. Langmuir 2009, 25, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Veeramanoharan, A.; Kim, S.-C. A Comprehensive Review on Sustainable Surfactants from CNSL: Chemistry, Key Applications and Research Perspectives. RSC Adv. 2024, 14, 25429–25471. [Google Scholar] [CrossRef]
- Zhang, W.; Tu, R.; Ren, Q.; Liu, S.; Guo, Z.; Liu, P.; Tian, M. The Selective Flotation Separation of Apatite from Dolomite and Calcite Utilizing a Combination of 2-Chloro-9-Octadecenoic Acid Collector and Sodium Pyrophosphate Depressant. Sep. Purif. Technol. 2025, 353, 128446. [Google Scholar] [CrossRef]
- Abdel-Halim, M.M.; Fan, R.; Khalek, M.A.A.; Zheng, R.; Xu, S.; Gao, Z. Apatite–Calcite Flotation Separation Using Sodium N-Lauroylsarcosinate as a Selective Collector. Minerals 2023, 13, 970. [Google Scholar] [CrossRef]
- Ruan, Y.; Deng, B.; He, D.; Chi, R. Synergetic Effect of Cottonseed Fatty Acid Salt and Nonionic Surfactant NP-4 in the Froth Flotation of Siliceous-Calcareous Phosphate Rock. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126650. [Google Scholar] [CrossRef]
- Wang, L.; Tian, M.; Khoso, S.A.; Hu, Y.; Sun, W.; Gao, Z. Improved Flotation Separation of Apatite from Calcite with Benzohydroxamic Acid Collector. Miner. Process. Extr. Metall. Rev. 2019, 40, 427–436. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, B.; Duan, H.; Peng, X.; Chen, X.; Zhao, Q. Novel Hydroxy Polyamine Surfactant N-(2-Hydroxyethyl)-N-Dodecyl-Ethanediamine: Its Synthesis and Flotation Performance Study to Quartz. Miner. Eng. 2019, 142, 105894. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, P.; Liu, W.; Liu, W.; Guo, Y.; Tong, K.; Chen, X. Novel Hydroxyl-Containing Quaternary Ammonium Salt N-(2-Hydroxyethyl)-N, N-Dimethyl-3-[(1-Oxododecyl)Amino]-1-Propanaminium: Its Synthesis and Flotation Performance to Quartz. Minerals 2023, 13, 702. [Google Scholar] [CrossRef]
- Farid, Z.; Assimeddine, M.; Abdennouri, M.; Barka, N.; Sadiq, M. Lecithin as an Ecofriendly Amphoteric Collector Foaming Agent for the Reverse Flotation of Phosphate Ore. Chem. Eng. Res. Des. 2023, 200, 292–302. [Google Scholar] [CrossRef]
- Li, J.; Nie, G.; Li, J.; Zhu, Z.; Wang, Z. Flotation Separation of Quartz and Dolomite from Collophane Using Sodium N-Dodecyl-β-Amino Propionate and Its Adsorption Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128586. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Ardian, R.; Fornasiero, D. Ethoxylated Alcohols as Co-Collectors in Apatite, Calcite and Dolomite Flotation. Miner. Eng. 2025, 224, 109173. [Google Scholar] [CrossRef]
- Severov, V.V.; Filippova, I.V.; Filippov, L.O. Use of Fatty Acids with an Ethoxylated Alcohol for Apatite Flotation from Old Fine-Grained Tailings. Miner. Eng. 2022, 188, 107832. [Google Scholar] [CrossRef]
- Isah, A.; Arif, M.; Hassan, A.; Mahmoud, M.; Iglauer, S. Fluid–Rock Interactions and Its Implications on EOR: Critical Analysis, Experimental Techniques and Knowledge Gaps. Energy Rep. 2022, 8, 6355–6395. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Baçaoui, A. Direct Flotation of Low-Grade Moroccan Phosphate Ores: A Preliminary Micro-Flotation Study to Develop New Beneficiation Routes. Arab. J. Geosci. 2020, 13, 1252. [Google Scholar] [CrossRef]
- Gong, W.Q.; Parentich, A.; Little, L.H.; Warren, L.J. Adsorption of Oleate on Apatite Studied by Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Langmuir 1992, 8, 118–124. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Ke, B.; Mao, S. Density Functional Theory of Molecular Structure and Properties of Fatty Acid Collectors. Multipurp. Util. Miner. Resour. 2024, 45, 167–173. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, Q. The Flotation Separation of Apatite from Dolomite Using a Fatty Acid-Based Collector and the Mechanisms of Adsorption. Surf. Interface Anal. 2023, 55, 138–150. [Google Scholar] [CrossRef]
- Fuerstenau, M.C. Chapter 8—Flotation. In Principles of Mineral Processing; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2003; p. 252. ISBN 978-0-87335-167-6. [Google Scholar]
- Cook, B.K.; Gibson, C.E. A Review of Fatty Acid Collectors: Implications for Spodumene Flotation. Minerals 2023, 13, 212. [Google Scholar] [CrossRef]
- Houot, R. Beneficiation of Phosphatic Ores through Flotation: Review of Industrial Applications and Potential Developments. Int. J. Miner. Process. 1982, 9, 353–384. [Google Scholar] [CrossRef]
- de Lima, F.V.; Budemberg, G.; Cruz, S.H.; Braga, A.S. A Collector Promoter for Apatite Flotation in the Serra Do Salitre Complex. Minerals 2023, 13, 599. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Improving Froth Characteristics and Flotation Recovery of Phosphate Ores with Nonionic Surfactants. Miner. Eng. 2003, 16, 587–595. [Google Scholar] [CrossRef]
- Giesekke, E.W.; Harris, P.J. The Role of Polyoxyethylene Alkyl Ethers in Apatite Flotation at Foskor, Phalaborwa (South Africa). Miner. Eng. 1994, 7, 1345–1361. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Liu, J. Synergistic Effect of Dodecyl Sulfonate on Apatite Flotation with Fatty Acid Collector. Sep. Sci. Technol. 2016, 51, 1389–1396. [Google Scholar] [CrossRef]
- Du, W.; Li, X.; Zhang, Q. DFT Study of Coadsorption of Fatty Acid and Kerosene on Fluorapatite (001) Surface. Physicochem. Probl. Miner. Process. 2023, 59, 161890. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Y.; Lu, L.; Hu, X.; Li, S. Removal of Dolomite and Potassium Feldspar from Apatite Using Simultaneous Flotation with a Mixed Cationic-Anionic Collector. Int. J. Min. Sci. Technol. 2023, 33, 783–791. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Li, W.; Yan, X.; Zhang, H. Intensification of Interfacial Adsorption of Dodecylamine onto Quartz by Ultrasonic Method. Sep. Purif. Technol. 2019, 227, 115701. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Ren, Q.; Tu, R.; Qiu, F.; Xu, S.; Sun, W.; Tian, M. Proposing a Novel Factor Influencing Flotation Collector Abilities to Collect Minerals by Comparing Two Cationic Collectors N,N-Bis(2-Hydroxy-3-Chloropropyl) Dodecylamine and Dodecylamine. Appl. Surf. Sci. 2023, 640, 158284. [Google Scholar] [CrossRef]
- Novich, B.E.; Ring, T.A. Predictive Model for The Alkylamine-Quartz Flotation System. Langmuir 1985, 1, 701–708. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Tian, H.; Liu, J.; Guo, Z.; Liu, P.; Tian, M.; Xie, L. Experimental Evidence Supporting Distinct Adsorption Configurations of N,N-Bis(2-hydroxy-3-Chloropropyl) Dodecylamine and Dodecylamine on Apatite Surfaces: Quartz Crystal Microbalance and Atomic Force Microscopy Studies. Surf. Interfaces 2024, 51, 104681. [Google Scholar] [CrossRef]
- Luo, B.; Zhu, Y.; Sun, C.; Li, Y.; Han, Y. Flotation and Adsorption of a New Collector α-Bromodecanoic Acid on Quartz Surface. Miner. Eng. 2015, 77, 86–92. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New Insights into Selective-Depression Mechanism of Novel Depressant EDTMPS on Magnesite and Quartz Surfaces: Adsorption Mechanism, DFT Calculations, and Adsorption Model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, W.; Mei, G.; Tian, Y.; Guo, C.; Pan, W. Synthesis of a Photosensitive Quaternary Ammonium Collector and Its Flotation Performance and Mechanism for Quartz. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130936. [Google Scholar] [CrossRef]
- Rath, S.S.; Sahoo, H.; Das, B.; Mishra, B.K. Density Functional Calculations of Amines on the (101) Face of Quartz. Miner. Eng. 2014, 69, 57–64. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, Q.; Tu, R.; Liu, S.; Qiu, F.; Guo, Z.; Liu, P.; Xu, S.; Sun, W.; Tian, M. The Application of a Novel Amine Collector, 1-(Dodecylamino)-2-Propanol, in the Reverse Flotation Separation of Apatite and Quartz. J. Mol. Liq. 2024, 399, 124377. [Google Scholar] [CrossRef]
- Shao, X.; Jiang, C.L.; Parekh, B.K. Enhanced Flotation Separation of Phosphate and Dolomite Using a New Amphoteric Collector. Min. Metall. Explor. 1998, 15, 11–14. [Google Scholar] [CrossRef]
- Somasundaran, P.; Zhang, L. Adsorption of Surfactants on Minerals for Wettability Control in Improved Oil Recovery Processes. J. Pet. Sci. Eng. 2006, 52, 198–212. [Google Scholar] [CrossRef]
- Pugh, R.J. The Role of the Solution Chemistry of Dodecylamine and Oleic Acid Collectors in the Flotation of Fluorite. Colloids Surf. 1986, 18, 19–41. [Google Scholar] [CrossRef]
- Rao, C.Y.V. Magnetically Coupled Sealless Pumps. Chem. Eng. World 1997, 32, 67–69. [Google Scholar]
- Filippov, L.O.; Filippova, I.V.; Lafhaj, Z.; Fornasiero, D. The Role of a Fatty Alcohol in Improving Calcium Minerals Flotation with Oleate. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 410–417. [Google Scholar] [CrossRef]
- Liu, S.; Han, B.; Zhao, T. The Effect of Various Surfactants on Fatty Acid for Apatite Flotation and Their Adsorption Mechanizm. Physicochem. Probl. Miner. Process. 2021, 57, 46–56. [Google Scholar]
- Pawliszak, P.; Beheshti, A.; Møller, A.; Blencowe, A.; Beattie, D.A.; Krasowska, M. Increasing Surface Hydrophilicity with Biopolymers: A Combined Single Bubble Collision, QCM-D and AFM Study. J. Colloid Interface Sci. 2024, 667, 393–402. [Google Scholar] [CrossRef]
- Yu, L.; Yu, P.; Bai, S. A Critical Review on the Flotation Reagents for Phosphate Ore Beneficiation. Minerals 2024, 14, 828. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.; Li, C.; Cheng, R. Effect and Mechanism of Phosphoric Acid in the Apatite/Dolomite Flotation System. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; Arafat, Y.; Abd El-Aleem, F.A.; El-Midany, A.A. Investigating Sodium Sulphate as a Phosphate Depressant in Acidic Media. Sep. Purif. Technol. 2014, 124, 163–169. [Google Scholar] [CrossRef]
- Oulkhir, A.; Lyamlouli, K.; Oussfan, A.; Orange, F.; Etahiri, A.; Benhida, R. Efficient Flotation Separation Approach of Apatite from Calcite for Phosphate Up-Grading Using Phosphorylated Starch Macromolecules as a Selective Depressant. Carbohydr. Polym. 2025, 348, 122878. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Feng, B.; Zhang, W.; Guo, W. Study of the Effect and Mechanism of Environmentally Friendly Depressant Tragacanth Gum on Scheelite and Apatite Separation. Process Saf. Environ. Prot. 2025, 196, 106913. [Google Scholar] [CrossRef]
- Jing, L.; Xu, L.; Xue, K.; Wang, D.; Ma, Z.; Meng, J.; Shi, X.; Liu, C. Selective Depression by Using Environment-Friendly Depressant Pectin in Apatite and Dolomite Flotation System. Miner. Eng. 2023, 203, 108373. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Flotation Separation of Apatite and Calcite Using Gum Arabic as a Depressant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127723. [Google Scholar] [CrossRef]
- Wang, T.; Feng, B.; Guo, Y.; Zhang, W.; Rao, Y.; Zhong, C.; Zhang, L.; Cheng, C.; Wang, H.; Luo, X. The Flotation Separation Behavior of Apatite from Calcite Using Carboxymethyl Chitosan as Depressant. Miner. Eng. 2020, 159, 106635. [Google Scholar] [CrossRef]
- Jing, L.; Xue, K.; Tian, J.; Zhang, X.; Wang, D.; Guo, W.; Ma, Z.; Xu, L. Separation Mechanism of Apatite and Dolomite with Flotation Depressant Konjac Glucomannan. Miner. Eng. 2024, 216, 108844. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmed Khoso, S.; Luo, X.; Tian, M. Understanding the Depression Mechanism of Citric Acid in Sodium Oleate Flotation of Ca2+-Activated Quartz: Experimental and DFT Study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Dho, H.; Iwasaki, I. Role of Sodium Silicate in Phosphate Flotation. Min. Metall. Explor. 1990, 7, 215–221. [Google Scholar] [CrossRef]
- Zheng, X.; Arps, P.J.; Smith, R.W. Adhesion of Two Bacteria onto Dolomite and Apatite: Their Effect on Dolomite Depression in Anionic Flotation. Int. J. Miner. Process. 2001, 62, 159–172. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, D. Solution Chemistry: Minerals and Reagents, 1st ed.; Developments in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2006; Volume 17, ISBN 978-0-444-52059-3. [Google Scholar]
- Prasad, M.; Majumder, A.K.; Rao, T.C. Reverse Flotation of Sedimentary Calcareous/Dolomitic Rock Phosphate Ore—An Overview. Min. Metall. Explor. 2000, 17, 49–55. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Kaba, O.B.; Fornasiero, D. In-Situ Study of the Kinetics of Phosphoric Acid Interaction with Calcite and Fluorapatite by Raman Spectroscopy and Flotation. Miner. Eng. 2021, 162, 106729. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Liu, R.; Liu, C.; Liu, L.; Li, R.; Zhang, H.; Pang, J.; Liu, D. Application of Waste Acid from Phosphogysum Dam as an Eco-Friendly Depressant in Collophane Flotation. J. Clean. Prod. 2020, 267, 122184. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective Reverse Flotation of Apatite from Dolomite in Collophanite Ore Using Saponified Gutter Oil Fatty Acid as a Collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals 2018, 8, 278. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Abouzeid, A.-Z.M. Flotation of Carbonates from Phosphate Ores in Acidic Media. Int. J. Miner. Process. 1993, 38, 235–256. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Rothenberg, A.S.; Lipp, D.W.; Panzer, H.P. Low Molecular Weight Polyacrylamide-Based Polymers as Modifiers in Phosphate Beneficiation. Int. J. Miner. Process. 1987, 20, 291–308. [Google Scholar] [CrossRef]
- Wang, X.; Song, Q.; Xie, R.; Liu, J.; Zhu, Y. Selective Flotation Separation of Scheelite from Apatite by Application of ATMP as an Efficient Depressant. J. Mol. Liq. 2023, 378, 121604. [Google Scholar] [CrossRef]
- Zhang, P.; Snow, R. Evaluation of Phosphate Depressants in the Phosphate/Silica System. Min. Metall. Explor. 2009, 26, 101–104. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The Effect of Sodium Alginate on the Flotation Separation of Scheelite from Calcite and Fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Feng, B.; Zhang, L.; Chen, Y.; Gao, Z. Flotation Separation of Scheelite and Apatite by Polysaccharide Depressant Xanthan Gum. Miner. Eng. 2021, 170, 107045. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, W.; Li, F.; Liu, J.; Zhang, H. Discrepant Adsorption Behavior of Sodium Alginate onto Apatite and Calcite Surfaces: Implications for Their Selective Flotation Separation. Miner. Eng. 2022, 181, 107553. [Google Scholar] [CrossRef]
- McLean, R.J.C.; Beauchemin, D.; Clapham, L.; Beveridge, T.J. Metal-Binding Characteristics of the Gamma-Glutamyl Capsular Polymer of Bacillus Licheniformis ATCC 9945. Appl. Environ. Microbiol. 1990, 56, 3671–3677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Nie, G.H.; Jiang, Y.; Luo, G.J.; Li, J. Selective Depression of Escherichia Coli on Flotation of Collophanite and Dolomite. Physicochem. Probl. Miner. Process. 2022, 58, 150604. [Google Scholar] [CrossRef]
- Rabia, H.; Ould Hamou, M.; Kasperkiewicz, K.; Brożek, J.; Augustyniak, M. Adhesion Abilities and Biosorption of Cd and Mg by Microorganisms—First Step for Eco-Friendly Beneficiation of Phosphate Ore. Sci. Rep. 2019, 9, 12929. [Google Scholar] [CrossRef] [PubMed]
- Qun, W.; Heiskanen, K. Batch Flotation Tests by Fatty Acid on a Phosphate-Iron Oxide-Silicate Regolith Ore Sample from Sokli, Finland. Miner. Eng. 1990, 3, 473–481. [Google Scholar] [CrossRef]
- Sayilgan, A.; Arol, A.I. Effect of Carbonate Alkalinity on Flotation Behavior of Quartz. Int. J. Miner. Process. 2004, 74, 233–238. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Song, S.; Zhou, W.; Xue, N.; Nie, Y.; Feng, B. The Depressing Role of Sodium Alginate in the Flotation of Ca2+-Activated Quartz Using Fatty Acid Collector. J. Mol. Liq. 2021, 343, 117618. [Google Scholar] [CrossRef]
- Dai, L.; Feng, B.; Zhang, L.; Chen, Y.; Bayoundoula, J. Selective Flotation Separation of Spodumene and Quartz with Carboxylated Chitosan as Depressant. Miner. Eng. 2023, 203, 108343. [Google Scholar] [CrossRef]
- Xiang, Z.; Feng, B.; Zhang, L.; Bayoundoula, J.; Wang, Z. The Role of Depressant Pectin in the Flotation Separation of Spodumene and Quartz. Miner. Eng. 2023, 203, 108320. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Hu, X.; Zhang, X.; Zheng, G. Flotation Separation of Quartz from Magnesite Using Carboxymethyl Cellulose as Depressant. Trans. Nonferrous Met. Soc. China 2022, 32, 1623–1637. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.; Ge, P.; Sun, W.; Xu, L.; Tang, H.; Wang, L. Selective Flotation of Quartz from Feldspar Using Hydroxypropyl Starch as Depressant. Miner. Eng. 2023, 195, 108022. [Google Scholar] [CrossRef]
- Sokolović, J.; Mišković, S. The Effect of Particle Size on Coal Flotation Kinetics: A Review. Physicochem. Probl. Miner. Process. 2018, 54, 1172–1190. [Google Scholar]
- Zhang, D.; Ma, F.; Tao, Y. Study on Effect of Nanobubble on Ultra-Fine Flake Graphite (UFG) Flotation. Part. Sci. Technol. 2023, 41, 1062–1070. [Google Scholar] [CrossRef]
- Yoon, R.-H. The Role of Hydrodynamic and Surface Forces in Bubble-Particle Interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle-Bubble Collision Models—A Review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, H.; Li, X.; Li, D.; Wang, W.; Liang, Y.; Yan, X.; Zhang, H. Effect of Energy Input on Flotation of Particles with Different Sizes: Perspective of Hydrodynamics Characteristics. J. Environ. Chem. Eng. 2023, 11, 111272. [Google Scholar] [CrossRef]
- Ye, J. Study on Interfacial Regulation of Fine Apatite Flotation. Ph.D. Thesis, Guizhou University, Guiyang, China, 2020. [Google Scholar]
- Yang, B.; Huang, P.; Song, S.; Luo, H.; Zhang, Y. Hydrophobic Agglomeration of Apatite Fines Induced by Sodium Oleate in Aqueous Solutions. Results Phys. 2018, 9, 970–977. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q. Study of Oleic Acid-Induced Hydrophobic Agglomeration of Apatite Fines through Rheology. Miner. Eng. 2024, 218, 108911. [Google Scholar] [CrossRef]
- Basařová, P.; Hubička, M. The Collision Efficiency of Small Bubbles with Large Particles. Miner. Eng. 2014, 66, 230–233. [Google Scholar] [CrossRef]
- Cheng, G.; Shi, C.; Yan, X.; Zhang, Z.; Xu, H.; Lu, Y. A Study of Bubble-Particle Interactions in a Column Flotation Process. Physicochem. Probl. Miner. Process. 2017, 53, 17–33. [Google Scholar] [CrossRef]
- Ross, V.E. Flotation and Entrainment of Particles during Batch Flotation Tests. Miner. Eng. 1990, 3, 245–256. [Google Scholar] [CrossRef]
- Hoang, D.H.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Impact of Flotation Hydrodynamics on the Optimization of Fine-Grained Carbonaceous Sedimentary Apatite Ore Beneficiation. Powder Technol. 2019, 345, 223–233. [Google Scholar] [CrossRef]
- Waters, K.E.; Hadler, K.; Cilliers, J.J. The Flotation of Fine Particles Using Charged Microbubbles. Miner. Eng. 2008, 21, 918–923. [Google Scholar] [CrossRef]
- Santana, R.C.; Ribeiro, J.A.; Santos, M.A.; Reis, A.S.; Ataíde, C.H.; Barrozo, M.A.S. Flotation of Fine Apatitic Ore Using Microbubbles. Sep. Purif. Technol. 2012, 98, 402–409. [Google Scholar] [CrossRef]
- Li, C.; Wang, L. Improved Froth Zone and Collection Zone Recoveries of Fine Mineral Particles in a Flotation Column with Oscillatory Air Supply. Sep. Purif. Technol. 2018, 193, 311–316. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Yang, B.; Lopez-Mendoza, A. Flotation of Molybdenite Fines as Hydrophobic Agglomerates. Sep. Purif. Technol. 2012, 98, 451–455. [Google Scholar] [CrossRef]
- Santana, R.C.; Farnese, A.C.C.; Fortes, M.C.B.; Ataíde, C.H.; Barrozo, M.A.S. Influence of Particle Size and Reagent Dosage on the Performance of Apatite Flotation. Sep. Purif. Technol. 2008, 64, 8–15. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, H.; Xing, Y.; Cao, Y.; Gui, X. The Effect of Impeller Speeds on the Nanobubbles Flotation Efficiency of Ultrafine Coal Particles. Powder Technol. 2025, 449, 120431. [Google Scholar] [CrossRef]
- Zeng, M.; Li, K.; Huang, L.; Bao, S.; Liu, C.; Yang, S. Interaction Mechanism of Interfacial Nano-Micro Bubbles with Collec-tors and Its Effects on the Fine Apatite Flotation. Appl. Surf. Sci. 2025, 682, 161736. [Google Scholar] [CrossRef]
- Jung, M.U.; Kim, Y.C.; Bournival, G.; Ata, S. Industrial Application of Microbubble Generation Methods for Recovering Fine Particles through Froth Flotation: A Review of the State-of-the-Art and Perspectives. Adv. Colloid Interface Sci. 2023, 322, 103047. [Google Scholar] [CrossRef]
- Chen, Y.; Truong, V.N.T.; Bu, X.; Xie, G. A Review of Effects and Applications of Ultrasound in Mineral Flotation. Ultrason. Sonochem. 2020, 60, 104739. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.U.; Kim, Y.C.; Bournival, G.; Ata, S. An Acoustic Agglomeration Method for Segregation of Micro– to Nano–Bubbles for the Flotation of Ultrafine Particles. Sep. Purif. Technol. 2025, 361, 131290. [Google Scholar] [CrossRef]
- Gao, K.; Liu, H.; Sun, L.; Zhang, Z. Effect of Gas Input Conditions and Ultrasound on the Dynamic Behavior of Flotation Bubbles. ACS Omega 2022, 7, 22326–22340. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).