Abstract
Quartz and feldspar have similar physical, chemical, and surface properties. Effectively separating them in near-neutral systems has long been a challenging research focus. This study introduces 1-Dodecyl-3-methylimidazolium bromide (DMB), an ionic liquid, as a collector in a quartz–feldspar flotation separation system to investigate its effects on the flotation behavior of quartz and feldspar. The interaction between the collector and the minerals is explained through zeta potential measurements, infrared spectroscopy analysis, and DFT calculations. The flotation test results indicate that DMB exhibits selective flotation separation properties enabling the separation of quartz from feldspar. Across the pH range of 3 to 11, DMB demonstrates high collection capability for quartz, but lower capability for feldspar. In particular, at pH levels of 7 to 8, the recovery difference between the two minerals exceeds 80%, achieving optimal selective separation. Mechanistic studies indicate that DMB primarily adsorbs on quartz and feldspar through electrostatic adsorption. The adsorption energy between DMB and quartz reaches −340.59 kJ/mol, forming a stable adsorption layer on the quartz surface. However, electrostatic repulsion arises over a broad area due to the large volume and cationic nature of DMB’s polar group and the exposed cationic Al sites on the feldspar surface, thereby hindering the interaction between DMB and feldspar. This research establishes the foundation for achieving efficient selective flotation separation of quartz and feldspar in a neutral system.
1. Introduction
Quartz is a critically important non-metallic mineral. Owing to its unique physical and chemical properties, it is extensively utilized in numerous fields, including glass production, construction materials, refractories, photovoltaic technology, and semiconductor fabrication [,,,]. In nature, quartz is often intimately associated with feldspar, and both belong to the framework silicate group. Their closely similar physicochemical and surface properties make the effective separation of quartz from feldspar a significant challenge [,,]. Therefore, developing efficient methods for their separation is imperative to ensure a reliable supply of quartz resources and to meet the stringent purity requirements of downstream industries such as glass, ceramics, and photovoltaics.
Flotation is the most widely used method for separating quartz and feldspar [,,,]. The existing flotation approaches can be categorized into three types: the HF method, the fluorine-free acid method and the fluorine-free acid-free method. The HF method employs hydrofluoric acid to activate feldspar and amine cationic collectors to float it [,,]. However, the use of hydrofluoric acid poses serious environmental risks. Although the fluorine-free acid method replaces hydrofluoric acid with strong acid, the highly corrosive environment leads to severe equipment corrosion and compromises operational continuity [,,]. In contrast, the fluorine-free and acid-free method operates under neutral or alkaline conditions without HF, making it a major research focus. Its success depends critically on the availability of collectors that exhibit both high collecting power and selectivity between the two minerals [,]. Currently, however, no collector satisfies all these requirements by maintaining high selectivity, effectiveness, and environmental safety under neutral conditions. Furthermore, traditional amine collectors (e.g., toxic dodecylamine) are plagued by issues such as high foam viscosity, poor mobility, and inadequate selectivity [], rendering them unsuitable for modern production standards. The growing urgency of environmental issues and the need for sustainable mining practices are driving interest in eco-friendly, renewable, and cost-effective collectors [,]. Therefore, developing highly selective and environmentally benign green collectors is crucial for the efficient separation and utilization of quartz and feldspar in a neutral system.
Ionic liquids, recognized as low-temperature molten salts composed entirely of ions, have recently garnered significant attention in flotation research. They offer distinct advantages over traditional amine collectors, including low vapor pressure, non-volatility, non-flammability, high thermal and chemical stability, good solubility, and ease of synthesis. Their colorless and odorless nature further positions them as environmentally friendly reagents [,,]. Typically consisting of organic cations and inorganic anions, ionic liquids exhibit high structural diversity and tunability [], rendering them highly promising for customized flotation applications. For instance, Liu et al. [] achieved effective flotation separation of muscovite from apatite using dodecyl pyridinium chloride (DPDC) as an ionic liquid collector. Li et al. [] investigated the separation of quartz and magnesite using dodecyl trimethyl ammonium-diethylphosphonic acid (N12111-DEPA). Their single mineral flotation tests revealed that N12111-DEPA exhibited strong collecting power toward quartz. Under optimized conditions, a concentrate with a MgO grade of 41.21% and a recovery of 97.45% was obtained. In another study, Zhou et al. [] prepared quaternary ammonium ionic liquid (referred to as 1401) from tetradecyldimethylbenzyl ammonium chloride and sodium isobutylxanthate, and applied it in the reverse flotation desulfurization and desilication of bauxite, demonstrating its significant effectiveness. Collectively. These studies underscore the considerable potential of ionic liquids as high-performance flotation collectors.
Imidazole-based ionic liquids possess a large, positively charged polar group that facilitates electrostatic interaction with silicate mineral surfaces. This paper introduces the imidazolium-based ionic liquid DMB as a collector for quartz-feldspar flotation system. The flotation performance of both minerals under neutral conditions was investigated through flotation experiments and compared with the traditional cationic collector dodecylamine. To elucidate the underlying mechanism of DMB’s selective adsorption on quartz, we employed a combination of zeta potential measurements, FTIR analysis, and DFT calculations. The integrated findings reveal how DMB achieves selective quartz adsorption under neutral conditions, enabling effective separation from feldspar. This work provides a new perspective and a promising candidate for developing high-efficiency flotation reagents for quartz-feldspar separation.
2. Experiments and Methods
2.1. Materials and Reagents
All pure mineral samples used in this study were obtained from Yunnan, China. The chemical compositions of the samples are summarized in Table 1, and their XRD patterns are presented in Figure 1, confirming that the quartz and feldspar samples were of high purity, with grades of 98.5% and 97.4%, respectively, thus meeting the requirements for flotation tests. The raw minerals were subjected to crushing, grinding, and dry screening to obtain a fraction of −200 to +400 mesh for the flotation experiments. A portion of the −400 mesh material was further ground in an agate mill for 1.5 h to produce a −5 μm fraction for subsequent infrared spectroscopic and zeta potential analyses. The pH modifiers (hydrochloric acid and sodium hydroxide) and collectors (DMB and DDA) were all analytical grade and supplied by Shanghai Macklin Biochemical Co., Ltd., Shanghai, China. The CAS number of DMB is 61546-00-7, and the molecular structures of both collectors are illustrated in Figure 2. Deionized water, prepared in our laboratory, was used throughout all experiments.

Table 1.
Chemical Element Analysis of Quartz and Feldspar.
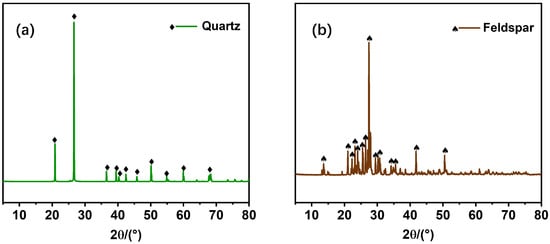
Figure 1.
XRD spectra of (a) quartz and (b) feldspar.

Figure 2.
Chemical structure of collectors DMB and DDA.
2.2. Micro-Flotation Experiments
Single-mineral flotation tests were performed in a 40 mL XFG-type mechanical flotation cell at room temperature. Optimal operational parameters, identified through preliminary experiments, were applied. In a typical procedure, a 2 g mineral sample was mixed with 40 mL of deionized water and agitated at 1996 rpm for 2 min to form a homogenized slurry. The pH of the pulp was then adjusted to the target value using dilute HCl or NaOH solutions, followed by 2 min of conditioning. Subsequently, a predetermined amount of collector was added, and the pulp was conditioned for another 2 min. Finally, flotation was carried out by scraping the froth for 1 min to collect the concentrate, leaving the tailings in the cell. Both the concentrate and tailings were filtered, dried, and weighed to calculate the flotation recovery. All micro-flotation tests were conducted in triplicate, and the results are reported as mean ± standard deviation.
For the artificial mixed mineral flotation, composite samples with a total mass of 4 g were prepared by mixing quartz and feldspar at different mass ratios. The flotation procedure described above was precisely replicated. The recoveries of SiO2 and K2O were calculated based on chemical assays of SiO2 and K2O in the concentrate and tailing products.
2.3. FTIR Analysis
The samples were analyzed using an IRAffinity Fourier Transform Infrared Spectrometer manufactured by Shimadzu Instrument Company, Kyoto, Japan. The sample was prepared according to the following procedure: (1) A 2 g portion of the pure mineral was placed in a beaker. (2) 40 mL of deionized water was added to form a suspension. (3) The collector was introduced, and the slurry was conditioned by stirring for 1.5 h to facilitate adequate adsorption. (4) The solid product was then collected by filtration, rinsed with deionized water to remove any unadsorbed reagent, and dried in a vacuum oven at 45 °C. Finally, the dried sample was homogenized with spectroscopic-grade KBr at a 1:100 mass ratio, finely ground using an agate mortar, and pressed into a transparent pellet for FTIR analysis.
2.4. Zeta Potential Measurements
In this experiment, Nano ZS90 zeta potential analyzer of Malvern Company (Singapore) was used to measure the surface potential change in quartz and feldspar during the experiment. The zeta potential samples were prepared according to the following procedure: (1) A 20 mg sample of quartz or feldspar (−5 μm) was dispersed in 40 mL of deionized water under stirring to form a stable suspension. (2) The pH of the slurry was adjusted to the target value with 0.01 mol/L NaOH or HCl solutions. (3) A predetermined amount of collector was then added to the system. (4) The mixture was conditioned with a magnetic stirrer for 5 min to ensure complete interaction. (5) After allowing the suspension to settle for 5 min, the supernatant was carefully extracted using a syringe for zeta potential analysis. Each sample was measured three times, and the results are presented as the mean value.
2.5. DFT Calculations
The computational methodology involved two main components. First, the collector molecule was modeled using GaussView 6.0, and its geometry was fully optimized using Gaussian 09W at the DFT/B3LYP/6-31G level of theory. This optimization provided the molecule’s stable configuration, from which key electronic structure parameters—including the electrostatic potential, frontier molecular orbitals, and relevant bond lengths and angles—were derived. Second, the adsorption behaviors of DMB on the quartz (001) and feldspar (001) surfaces were simulated. The adsorption energies and equilibrium configurations were calculated using the CASTEP module within Materials Studio 2020, based on the following formula:
where ΔEadsorption is the adsorption energy, Etotal is the total energy of the adsorption model (kJ/mol), Emineral and Ereagent are the energy of minerals and reagents (kJ/mol).
The calculation parameters are as follows: exchange–correlation functional GGA-PBE; the k-point sampling grid 2 × 2 × 1; maximum force 0.03 eV/Å; maximum stress 0.05 GPa; maximum displacement 0.001 Å; cut-off energy 450 eV. All computations in this work were performed on the High-Performance Computing Center of Central South University.
3. Results and Discussion
3.1. Micro-Flotation Results
3.1.1. Effect of pH on Flotation Recovery
Figure 3 illustrates the flotation recovery of quartz and feldspar as a function of pH using the collectors DMB and DDA.

Figure 3.
Effect of pH on Flotation Recovery (collector dosage = 10mg/L).
Figure 3 compares the flotation performance of traditional collector dodecylamine (DDA) and the ionic liquid DMB. When using DDA, the recoveries of both quartz and feldspar first increase and then decrease with increasing pH. At neutral pH (pH = 7), their recoveries are very similar (67.43% for quartz and 59.06% for feldspar), indicating the poor selectivity of DDA and the difficulty of achieving effective separation under these conditions.
In stark contrast, DMB exhibits exceptional performance and selectivity. At pH 7, the quartz recovery reaches 99.46%, indicating nearly complete flotation, while the feldspar recovery remains below 20% across the entire pH range studied. Although the quartz recovery with DMB decreases in strongly acidic and alkaline environments, it is consistently higher than that achieved with DDA under comparable conditions. The decline in recovery under strong acidity is likely due to the attenuated surface electronegativity of quartz, which reduces the electrostatic adsorption of the cationic DMB collector. The pronounced difference in recovery between the two minerals demonstrates the superior selectivity of DMB, establishing a solid foundation for the fluorine-free and acid-free flotation separation of quartz and feldspar, particularly at neutral pH.
3.1.2. Effect of Collector Dosage on Flotation Recovery
The influence of collector dosage on the flotation recovery of quartz and feldspar at neutral pH was systematically investigated, with the results presented in Figure 4.

Figure 4.
Effect of collector dosage on flotation recovery (pH = 7).
As shown in Figure 4, the flotation recovery of quartz increases sharply with the DMB dosage, whereas that of feldspar rises only marginally. As the dosage increased from 2 to 10 mg/L, quartz recovery surged from 10.34% to 99.82%, representing near-complete flotation, while feldspar recovery increased by a mere 14.66 percentage points. A further increase in dosage had little effect on quartz recovery but led to a continued, undesirable rise in feldspar recovery, indicating a reduction in selectivity at higher loadings. In contrast, when the conventional collector DDA was employed, the recoveries of both quartz and feldspar increased in parallel with the dosage, showing nearly identical trends and thus rendering effective separation unfeasible. Consequently, DMB demonstrates markedly superior selectivity compared to DDA. It creates a significant recovery gap between the two minerals, thereby facilitating their highly efficient separation within an optimal dosage range.
3.1.3. Mixed Mineral Flotation
Mixed mineral experiments were conducted at pH 7 to investigate the effect of DMB dosage on the flotation performance. The mass ratio of quartz to feldspar was 1:1. The results are shown in Figure 5.
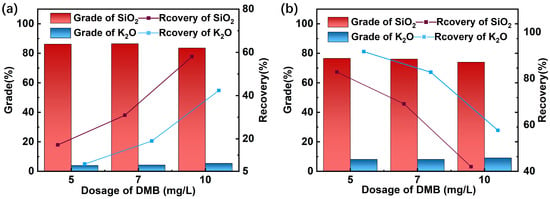
Figure 5.
Flotation results of the artificial mixed mineral. (pH = 7, (a) Concentrate, (b) Tailings).
The flotation results of the artificial mixed minerals clearly demonstrated the efficacy of DMB in separating quartz from feldspar. As shown in Figure 5, the recovery of quartz consistently exceeded that of feldspar across all collector dosages investigated. The K2O grade in the concentrate remained below 5%, while it reached approximately 10% in the tailings, indicating effective enrichment of feldspar in the tailings stream. Furthermore, the impact of varying the quartz-to-feldspar ratio in the feed on separation performance was systematically investigated, with the key results summarized in Table 2.

Table 2.
Effect of the quartz-to-feldspar mass ratio on flotation performance. (collector dosage = 7 mg/L, pH = 7).
As shown in the table, the collector DMB effectively achieves the preferential flotation of quartz from mixed minerals of different compositions. The separation efficiency improves significantly with an increase in the quartz content of the feed minerals, ultimately yielding a high-quality quartz concentrate with a grade exceeding 96%. Crucially, regardless of the feed composition, the SiO2 grade in the concentrate is consistently higher than that in the feed, while the K2O grade is consistently lower, directly confirming DMB’s selective collection of quartz. When the quartz proportion in the feed increased from 10% (1:9 ratio) to 90% (9:1 ratio), the SiO2 grade of the concentrate rose steadily from 70.18% to 96.09%, demonstrating its capability to produce high-grade concentrates from quartz-rich feedstocks. Concurrently, the K2O grade in the tailings was consistently higher than in the feed, indicating the effective enrichment of feldspar in the tailings. The distinct difference in recovery between SiO2 and K2O in the concentrate underscores the selective collecting power of DMB, which becomes more pronounced with quartz-rich feed.
3.2. Zeta Potential Measurements
The adsorption of collectors on mineral surfaces is known to alter their surface potential; hence, zeta potential measurements can provide critical insights into adsorption behaviors [,]. To elucidate the differential adsorption of DMB on quartz and feldspar, the zeta potentials of both minerals were measured under various conditions, as summarized in Figure 6.

Figure 6.
Zeta potential as a function of pH ((a) Quartz; (b) Feldspar; collector dosage = 10 mg/L).
The zeta potentials of quartz and feldspar under various conditions are presented in Figure 6. As shown in Figure 6a, the quartz surface is negatively charged across the tested pH range in the absence of a collector. The addition of DMB induces a pronounced positive shift in the quartz zeta potential. Notably, at pH 7, this shift reaches +10.4 mV, confirming substantial adsorption of the cationic DMB onto the quartz surface. This strong electrostatic interaction directly explains the high quartz recovery observed in flotation tests.
In contrast, the response of feldspar to DMB is markedly different (Figure 6b). Although the pristine feldspar surface also carries a negative charge, the introduction of DMB results in only a minor potential change. At pH 7, the shift is a mere +2.4 mV, indicating negligible adsorption of the collector. This minimal interaction underpins the poor flotation recovery of feldspar when using DMB.
In summary, the zeta potential data reveal a clear selectivity: DMB causes a significant positive shift on the quartz surface while exerting a negligible effect on feldspar. These results are fully consistent with the flotation performance, unequivocally confirming the selective adsorption of DMB on quartz, which is the fundamental mechanism enabling their efficient separation.
3.3. FTIR-Spectra Analysis
FTIR spectroscopy was utilized to further elucidate the adsorption mechanism of the collector on the mineral surfaces. The spectrum of pure DMB is presented in Figure 7. The characteristic absorption bands are identified as follows: 2916.3 cm−1 and 2850.7 cm−1 are attributed to the symmetric stretching vibrations of -CH3 and -CH2 groups, respectively. The peaks at 1573.9 cm−1 and 1473.6 cm−1 correspond to C=C stretching vibrations [], while the band at 2058.9 cm−1 is assigned to the C≡N stretching vibration. Additional features include the -CH symmetric vibration at 1473.6 cm−1, the =CH in-plane bending vibration at 1317.3 cm−1, the -CH2- scissoring vibration at 1450.4 cm−1, and the vibrational bending of the long alkyl chain -(CH2)n- at 715.5 cm−1 [].

Figure 7.
Infrared Spectrum of Collector DMB.
The FTIR spectra of quartz and feldspar, both before and after DMB treatment, are compared in Figure 8. The characteristic peaks of pristine quartz (Figure 8a) are observed at 1080 cm−1 and 796.6 cm−1 (Si-O-Si stretching vibration) and at 694.3 cm−1 (Si-O-Si bending vibration) [,]. Similarly, the spectrum of untreated feldspar (Figure 8b) shows its distinctive peaks at 1008.7 cm−1, 771.5 cm−1, and 582.5 cm−1.

Figure 8.
Infrared Spectra of (a,c) Quartz and (b,d) Feldspar Surfaces Before and After DMB Treatment.
Critically, after interaction with DMB, the quartz spectrum (Figure 8c) reveals the emergence of two new peaks at approximately 2922 cm−1 and 2856 cm−1. These are unequivocally assigned to the C-H stretching vibrations of methyl and methylene groups in the DMB molecule, providing direct evidence of its adsorption on the quartz surface. The absence of shifts in the inherent quartz peaks, coupled with the zeta potential findings, indicates that the adsorption mechanism is predominantly physical, driven by electrostatic attraction between the positively charged imidazole group of DMB and the negatively charged quartz surface.
In stark contrast, the FTIR spectrum of feldspar after DMB treatment (Figure 8d) shows no detectable new peaks, confirming the absence of significant DMB adsorption. This result aligns perfectly with the zeta potential analysis and collectively explains the flotation selectivity.
3.4. DFT Calculation
Density functional theory (DFT) is a robust and widely adopted computational approach for investigating the electronic structure and properties of flotation reagents [,,].
DFT calculations were performed to visualize the frontier molecular orbitals (FMOs) and molecular electrostatic potential (MEP) of the DMB collector, with the results presented in Figure 9 and Table 2. As listed in Table 2, the low-lying HOMO energy of DMB implies a low tendency to donate electrons, suggesting that chemical interactions with minerals are unlikely. The spatial distributions of the FMOs, shown in Figure 9a, provide further insight: the HOMO is localized on the long alkyl chain, while the LUMO is predominantly centered on the methylimidazole ring, particularly the nitrogen atoms. This distinct separation indicates that the polar headgroup (methylimidazole) acts as an electron acceptor. The MEP map in Figure 9b, where blue regions represent positive electrostatic potential, clearly shows a strong positive potential (deep blue) over the polar headgroup. This finding corroborates that the collector can engage in electrostatic attraction with negatively charged mineral surfaces.

Figure 9.
Schematic diagram of the HOMO and LUMO orbitals (a) and MEP map (b) of DMB.
Furthermore, research findings suggest a positive correlation between the geometric dimensions of the polar group in a molecule and the strength of the collector’s selectivity [,]. The van der Waals volume describes the geometric size of functional groups in space. Assuming each atom contributes a volume approximating that of a sphere with a radius equal to its van der Waals radius, the van der Waals volume of a molecule is obtained by summing the volumes of all atoms and subtracting the overlapping volumes between bonds and atoms []. The present study obtained bond lengths, bond angles, and atomic van der Waals radii of the collector molecules through DFT calculation. Additionally, the van der Waals volumes of polar groups were computed. The results are presented in Table 3.

Table 3.
Relevant data of DMB.
The table shows that the polar group van der Waals volume of DMB is 36.61 Å3, indicating that DMB possesses a relatively large van der Waals volume. Consequently, when interacting with feldspar, the exposed aluminum active sites on the feldspar surface generate extensive electrostatic repulsion with the DMB collector. This repulsion impedes the adsorption of DMB on the feldspar surface, leading to an overall weak interaction between them. Furthermore, the adsorption configurations of DMB on the quartz (001) and feldspar (001) surfaces were simulated, and the corresponding interaction energies were calculated using Materials Studio. The results are shown in Figure 10. The distance between the hydrogen atom on the -CH3 group of the methylimidazole ring in DMB and the oxygen atom on the quartz surface is 5.125 Å, while the distance to the oxygen atom on the feldspar surface is 9.137 Å. Previous studies have established that the adsorption distance between a reagent and a mineral surface serves as a key indicator of the interaction strength []. It can be seen that DMB exhibits stronger interaction capabilities with quartz surfaces, making it more prone to electrostatic interactions with quartz. Furthermore, the adsorption energy between the reagent and the mineral surface also reflects the intensity of their mutual interaction; the more negative the adsorption energy, the greater the likelihood of adsorption occurring []. As shown in Figure 10, the adsorption energy between DMB and quartz is −340.59 kJ/mol, while that between DMB and feldspar is −94.55 kJ/mol. The adsorption energy of DMB on quartz surfaces is significantly lower than that on feldspar surfaces, indicating a stronger tendency for DMB to adsorb onto quartz surfaces. DFT calculations by Zhang et al. [] reported an adsorption energy of −194.9 kJ/mol for DDA on quartz, which is significantly less negative than that computed for DMB in the present study. This indicates that DMB forms a more stable complex with the quartz surface and possesses a stronger driving force for adsorption compared to DDA. This finding correlates well with the results from flotation experiments and zeta potential measurements. Figure 11 illustrates the interaction mechanism of DMB with quartz and feldspar. The strong electrostatic repulsion between DMB and the exposed aluminum sites on feldspar prevents the collector from adsorbing onto the surface, leading to a weak overall interaction.

Figure 10.
Adsorption configurations: (a) Quartz (001) + DMB; (b) Feldspar (001) + DMB.

Figure 11.
Mechanism Diagram.
4. Conclusions
This study investigated the ionic liquid DMB as a novel collector for the flotation separation of quartz and feldspar. A multi-faceted approach, including micro-flotation tests, zeta potential measurements, FTIR spectroscopy, and DFT calculations, was employed to elucidate the interaction mechanisms between DMB and the two minerals. The main findings are summarized as follows:
(1) DMB exhibits high selectivity and strong recovery capacity for quartz. At pH 7 and 10 mg/L DMB, quartz flotation recovery reaches 99.46%. At this point, the feldspar recovery is only 14.66%, yielding a recovery difference of 84.8 percentage points. The artificial mixed minerals flotation tests also confirmed the selective collecting power of DMB, which was more pronounced with quartz-rich feed.
(2) The Zeta potential test results indicate that the surface potential of quartz shifted significantly toward positive values after DMB treatment, while the surface potential of feldspar exhibited only a slight shift. This demonstrates that DMB adsorbed extensively on the quartz surface but adsorbed minimally on the feldspar surface. Infrared spectroscopy testing further confirms that DMB adsorbs onto the quartz surface via electrostatic forces. The infrared spectrum of quartz treated with DMB exhibits stretching vibration peaks characteristic of methyl and methylene groups, while other peaks show no shift. This indicates that the adsorption of the collector on the quartz surface is electrostatic adsorption in nature.
(3) DFT calculations indicate that the polar group of the collector DMB carries a positive charge, enabling electrostatic interactions between the collector and negatively charged quartz and feldspar surfaces resulting in adsorption. The adsorption energy between DMB and quartz is −340.59 kJ/mol, while that between DMB and feldspar is −94.55 kJ/mol. The results indicate that DMB exhibits a more negative adsorption energy on quartz surfaces, reflecting stronger electrostatic interactions with quartz. This is attributed to the larger polar van der Waals volume of DMB. When interacting with feldspar, the exposed Al on the feldspar surface generates electrostatic repulsion with DMB over a broader range, thereby weakening the interaction between DMB and feldspar.
Beyond demonstrating effective separation, this work establishes DMB as a promising green collector candidate that operates efficiently under a neutral pH regime, eliminating the need for hazardous modifiers. Its practical applicability, however, hinges on further validation through bench-scale flotation tests using complex natural ores and a comprehensive assessment of its environmental footprint and economic viability. Future work will therefore focus on these scaling and sustainability metrics, alongside synthesizing structural analogs of DMB to systematically elucidate the structure–activity relationships that govern selectivity in ionic liquid collectors.
Author Contributions
Conceptualization, G.G. and Y.C.; methodology, G.G. and S.C.; software, X.Y. and S.C.; formal analysis, Y.C. and G.G.; resources, G.G. and H.H.; data curation, S.C.; writing—original draft preparation, S.C.; writing—review and editing, G.G. and Y.C.; visualization, X.Y.; supervision, G.G.; project administration, G.G. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Provincial Special Project for the Construction of the National Sustainable Development Agenda Innovation Demonstration Zone in Chenzhou (No. 2023sfq49); The National Natural Science Foundation of China (No. 52074358).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge financial support from Provincial Special Project for the Construction of the National Sustainable Development Agenda Innovation Demonstration Zone in Chenzhou (No. 2023sfq49); The National Natural Science Foundation of China (No. 52074358).
Conflicts of Interest
Author Yuan Chen was employed by the Shunshui Environmental Governance Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, M.; Wang, G.F.; Zhao, F.Y.; Li, W.F.; Zhu, G.; Liang, G.C.; Jian, W.; Liao, L.B.; Lv, G.C. Advances in purification technologies and applications of high-purity quartz resources. Prog. Nat. Sci. Mater. Int. 2025, 35, 51–64. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, J.G.; Wu, Z.C.; Zhang, H.Q.; Tan, X.M.; Yi, Y.J.; Tan, Q.; Liu, L. Characteristics of high-purity quartz raw materials for crucibles and exploration of key purification technologies. Miner. Eng. 2025, 231, 109446. [Google Scholar] [CrossRef]
- Vegliò, F.; Passariello, B.; Abbruzzese, C. Iron removal process for high-purity silica sands production by oxalic acid leaching. Ind. Eng. Chem. Res. 1999, 38, 4443–4448. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Q.; Ku, J.A.; Shang, H.L.; Shen, Z.C. Purification Technologies for High-Purity Quartz: From Mineralogy to Applications. Sep. Purif. Rev. 2025. [Google Scholar] [CrossRef]
- Hu, X.; Luo, X.P.; Liu, Z.S.; Zhang, Y.B.; Zhou, H.P.; Yang, Z.Z.; Tang, X.K. Flotation separation of feldspar from quartz using sodium fluosilicate as a selective depressant. Rare Met. 2024, 43, 1288–1300. [Google Scholar] [CrossRef]
- Huang, H.J.; Li, S.H.; Gou, H.R.; Zhang, N.; Liu, L.M. Efficient Recovery of Feldspar, Quartz, and Kaolin from Weathered Granite. Minerals 2024, 14, 300. [Google Scholar] [CrossRef]
- Tan, Y.P.; Li, S.K.; Xu, X.P.; Chen, P.; Gao, Z.Y.; Sun, W.; McFadzean, B.; Cao, J. Utilization of crown ether as selective collector for the flotation separation of pollucite from feldspar and quartz. Miner. Eng. 2025, 220, 109085. [Google Scholar] [CrossRef]
- Fan, X.Y.; Xiao, T.T.; Zhou, C.Y.; Wang, H.R.; Pan, Z.Q.; Wu, H.J.; Zhou, H. Mixed surfactants with solubilization behaviors: Separation of feldspar and quartz by self-assembly flotation. Miner. Eng. 2025, 221, 109130. [Google Scholar] [CrossRef]
- Gong, W.Q.; He, J.F.; Yang, B.; Xu, H.L.; Shan, Y.H.; Fu, L.X.; Wu, J.W. Interfacial synergistic mechanism of an effective combined collector sodium oleate/cetyltrimethyl ammonium chloride and its enhanced flotation separation of K-feldspar and quartz. Surf. Interfaces 2025, 60, 106017. [Google Scholar] [CrossRef]
- Lu, J.W.; Wang, N.L.; Li, S.K.; Lin, Z.Q.; Meng, Q.B.; Li, L.X. Atomic insight into the activation mechanism of feldspar by sodium oleate in flotation separation of quartz and feldspar: XPS, AFM, and molecular dynamics. Sep. Sci. Technol. 2023, 58, 2493–2504. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.D.; Ge, P.; Sun, W.; Xu, L.H.; Tang, H.H.; Wang, L. Selective flotation of quartz from feldspar using hydroxypropyl starch as depressant. Miner. Eng. 2023, 195, 108022. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Mohanty, K.; Oliva, J.; Alfonso, P.; Sampaio, C.H.; Anticoi, H. A Comparative Study of Quartz and Potassium Feldspar Flotation Process Using Different Chemical Reagents. Minerals 2024, 14, 167. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Study on Comparative Test for the Application of Purified Quartz from a Gold Ore Tailing. Compr. Util. Miner. Resour. 2021, 42, 159–162. [Google Scholar]
- Vidyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Li, S.; Kong, J.; Yang, C.; Bao, S. Experiment Study on Extraction of SiO2 from Gold Tailings by High Magnetic Separation Floatation Technology. Met. Mine 2018, 47, 184–188. [Google Scholar]
- Li, P.Y.; Ren, Z.J.; Xie, E.J.; Duan, S.T.; Gao, H.M.; Wu, J.X.; He, Y.H. Application of mixed collectors on quartz-feldspar by fluorine-free flotation separation and their interaction mechanism: A review. Physicochem. Probl. Miner. Process. 2021, 57, 139–156. [Google Scholar] [CrossRef]
- Jiang, X.S.; Chen, J.; Ban, B.Y.; Song, W.F.; Chen, C.; Yang, X.Y. Application of competitive adsorption of ethylenediamine and polyetheramine in direct float of quartz from quartz-feldspar mixed minerals under neutral pH conditions. Miner. Eng. 2022, 188, 107850. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, W.; Zhang, X.; Wang, R.; Zhu, G.; Cao, Y. Effects and Mechanism of a Novel Gemini Collector on Flotation Separation of Quartz from Albite. Multipurp. Util. Miner. Resour. 2025, 1–14. [Google Scholar]
- Zhang, W.D.; Ren, Q.L.; Tu, R.Y.; Liu, S.; Qiu, F.H.; Guo, Z.H.; Liu, P.; Xu, S.H.; Sun, W.; Tian, M.J. The application of a novel amine collector, 1-(dodecylamino)-2-propanol, in the reverse flotation separation of apatite and quartz. J. Mol. Liq. 2024, 399, 124377. [Google Scholar] [CrossRef]
- Aarab, I.; Amari, K.E.; He, D.; Fu, Y.H.; Boujounoui, K.; Etahiri, A. Eco-friendly apatite flotation: Unlocking the potential of spent coffee grounds as a bio-based collector. Miner. Eng. 2025, 233, 109652. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Baçaoui, A. Direct flotation of low-grade Moroccan phosphate ores: A preliminary micro-flotation study to develop new beneficiation routes. Arab. J. Geosci. 2020, 13, 1252. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Maciel, R. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Berezianko, I.A.; Kostjuk, S. Ionic liquids in cationic polymerization: A review. J. Mol. Liq. 2024, 397, 124037. [Google Scholar] [CrossRef]
- Yadav, S.; Baweja, K.; Kumar, C.; Sarkar, A.; Tomar, R. A Review: Applications of Ionic Liquids in Medicinal Chemistry. Chemistry Africa-A J. Tunis. Chem. Soc. 2024, 7, 2975–2988. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Liu, C.; Han, S.T.; Zhu, Y.G.; Xu, W.; Yang, S.Y. Novel Collector of a Dodecylpyridinium Chloride Ionic Liquid in the Reverse Flotation Separation of Muscovite from Apatite. Langmuir 2025, 41, 2834–2842. [Google Scholar] [CrossRef]
- Li, W.C.; Liu, W.G.; Liu, W.B.; Zhang, R.R.; Wang, S.C. Application of novel ionic liquids in flotation separation of quartz and magnesite and its mechanism. Powder Technol. 2024, 447, 120218. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Mei, G.J.; Yu, M.M.; Song, X.W. Effect and mechanism of quaternary ammonium salt ionic liquid as a collector on desulfurization and desilication from artificial mixed bauxite using flotation. Miner. Eng. 2022, 181, 107523. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, Y.L.; Guo, X.Y.; Liao, Z.H.; Huang, J.H. The role of phosphate in inhibiting the activation of quartz flotation induced by Mg2+. J. Mol. Liq. 2024, 398, 124278. [Google Scholar] [CrossRef]
- Kiefer, J.; Fries, J.; Leipertz, A. Experimental vibratnional study of imidazolium-based ionic liquids: Raman and infrared spectra of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and 1-ethyl-3-methylimidazolium ethylsulfate. Appl. Spectrosc. 2007, 61, 1306–1311. [Google Scholar] [CrossRef]
- Warsi, F.; Usman, M.; Ali, M. Modulating aggregation behaviour and surface properties of cationic & anionic surfactant with surface active ionic liquid 1-decyl-3-methylimidazolium chloride C10mim Cl: Role of surfactant head group. J. Mol. Liq. 2022, 365, 120093. [Google Scholar]
- He, J.F.; Chen, H.; Zhang, M.M.; Chen, L.H.; Yao, Q.Y.; Dai, Y.P.; Zhu, L.T.; Liu, C.G. Combined inhibitors of Fe3+, Cu2+ or Al3+ and sodium silicate on the flotation of fluorite and quartz. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 643, 128702. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jiao, F.; Qin, W.Q.; Zhu, H.L.; Jia, W.H. Activation effect of lead ions on scheelite flotation: Adsorption mechanism, AFM imaging and adsorption model. Sep. Purif. Technol. 2019, 209, 955–963. [Google Scholar] [CrossRef]
- Miao, Y.Q.; He, J.Y.; Zhu, X.R.; Zhu, G.L.; Cao, S.H.; Fan, G.X.; Li, G.S.; Cao, Y.J. Hardness of surface hydroxyls and its pivotal role in the flotation of cassiterite from quartz via lead ions activation. Sep. Purif. Technol. 2024, 347, 127565. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sun, W.; Chen, D.X.; Lin, S.Y.; Zhang, C.Y. Effects of Interfacial Hydroxylation Microstructure on Quartz Flotation by Sodium Oleate. Langmuir 2023, 39, 2182–2191. [Google Scholar] [CrossRef]
- Ouyang, L.Y.; Huang, Z.Q.; Wang, H.L.; He, G.C.; Yu, X.Y.; Burov, V.E.; Poilov, V.Z.; Li, F.X.; Liu, R.K.; Li, W.Y.; et al. Adsorption study of 3-tetradecylamine propyl amidoxime onto rhodochrosite surface: Implications for rhodochrosite-calcite flotation separation. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 678, 132469. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.A. Recent progress of quantifying substituent effects. Sci. Sin. Chim. 2013, 43, 801–828. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Xu, X.; Liu, W. Effect of methyl substituents on flotation performance of cationic collectors. Chin. J. Eng. 2023, 45, 1247–1253. [Google Scholar]
- Cheng, T.Y.; Xing, D.Q.; Shen, Z.C.; Ma, S.; Shi, S.X.; Deng, J.Y.; Deng, J.S. Study on expanding flotation performance differentiation of quartz and magnesite by amino-trimethylphosphonic acid in dodecylamine system. J. Mol. Liq. 2024, 409, 125286. [Google Scholar] [CrossRef]
- Ma, J.; Wu, J.; Chen, Y.; Zhong, H.; Chen, X.P.; Huang, Z.Q.; Burdonov, A.E.; Vchislo, N.V.; Bavuu, C.; Ouyang, L.Y. Adsorption Mechanism of 3-Tetradecylamine Propyl Amidoxime in the Reverse Flotation Separation of Quartz from Magnetite. Langmuir 2025, 41, 21021–21031. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).