Abstract
Proteomic analyses of extinct moa (Dinornithidae; ~800–1000 years) bone tissue previously revealed preserved collagens (I, II, and V), as well as several biological post-translational modifications (PTMs) and diagenetic peptide sequence alterations. The diagenetiforms detected in that study provided a baseline of PTM preservation in degraded tissues, identifying sequence alterations that could be accounted for in bioinformatic data searches (e.g., carboxymethyllysine). Subsequently, an improved extraction and sample preparation methodology, coupled with higher resolution mass spectrometry analyses, identified a wealth of previously unidentified non-collagenous proteins (NCPs) from the specimen. Here, in-depth analyses of the PTMs preserved in the expanded data set provide a detailed look at the types of PTMs (i.e., biological, diagenetic, and potential experimental artifacts) that occur in degraded tissues, the proteins they occur on, and the amino acids they modify. In total, 10 biological PTMs (e.g., ubiquitylation) and 18 diagenetic PTMs, including two advanced glycation end products (e.g., dihydroxy methylglyoxal adduction) and 12 types of oxidative damage (e.g., pyrrolidone formation from proline), were detected. In addition, peptides displaying diagenetic backbone cleavage (hydrolysis) were frequently observed to possess unidentified, variable mass shifts at their broken terminus, which search software would attempt to erroneously identify as different PTMs. The modifications characterized in the bones of this specimen, both in collagens and in NCPs, provide insight into patterns of preservation and degradation that paleoproteomic studies can utilize when searching and interpreting data sets from fossil tissue.
1. Introduction
In 2015, proteomic analyses of extinct moa bone [1] revealed preserved collagens (I, II, and V) as well as several peptide modifications, some of which had previously been unidentified in paleoproteomic studies. Identified sequence alterations included both post-translational modifications (PTMs), interpreted to be preserved from in vivo biological processes (hydroxylation, alkylation, methylation, demethylation, and fucosylation) and diagenetic modifications, interpreted to have been induced from the geochemical environment to which the specimen was subjected (backbone cleavage, deamidation, dehydroxylation, and carboxymethylation) [1]. Subsequently, the same specimen was reanalyzed using more optimized methodology [2], including an extraction technique designed to purify and enrich paleoproteomic samples abundant with humic substances, and high-resolution mass spectrometry analyses at both the precursor and fragment ion level. The result of this reanalysis was an expanded proteome for this taxon [2], even when the data were conservatively searched with software not optimized for the identification of unspecified PTMs.
Because typical bioinformatic analysis of proteomic data is reliant on a database of known proteins for the identification of peptides from an experimental sample, the detection of preserved proteins can be hampered by the presence of unanticipated mass shifts on constituent peptides [3]. Whether these unexpected mass differences from the in silico comparison data are caused by phylogenetic differences in the fossil taxa and in silico taxa, biological modification in vivo, geochemical alteration by the environment during diagenesis, or the experiment itself, these changes can prevent the detection of endogenous peptides [3,4,5] and lead researchers to assume a fossil is more molecularly degraded than it actually is. Thus, the expanded proteome recovered for the moa after optimized analyses [2] is an opportunity to revisit this specimen and characterize the patterns of preservation and degradation revealed by its PTMs at higher resolution. Here, the expanded moa data set is evaluated with bioinformatic software more amenable to unspecified PTMs (PEAKS [6]), and the proteins detected are rigorously evaluated for sequence alterations. These modifications and their placement are then discussed and characterized by their likely origin (i.e., biological, diagenetic, or artifact), including a discussion of modifications that are mislabeled by the search software because of chemical processes that cannot be inferred from the PSM alone. These data may serve as a guide to researchers looking to interpret paleoproteomic data, providing information on alterations that can be included in proteomic search parameters and software errors that may occur.
2. Materials and Methods
2.1. Specimen and Prior Mass Spectrometry Analysis
Moa (Dinornithidae) cortical bone (MOR OFT255; 800–1000 yr old) from cave deposits in New Zealand [7] was analyzed [2]. Previous immunological testing via enzyme-linked immunosorbent assay (ELISA) detected collagen I, hemoglobin, and osteocalcin, suggesting the preservation of these proteins in this specimen [8]. Subsequent mass spectrometry analyses after this initial study reported substantial coverage (>60%) for collagen I (both alpha 1 and 2), as well as peptides of collagen II (alpha 1) and collagen V [1]. These sequences also retained both biological and diagenetic post-translational modifications (PTMs) [1]. Proteomic reanalysis was then conducted using a FASP workflow modified to concentrate proteins and separate large quantities of humic substances [2]. For full details of the protein extraction and mass spectrometry analysis parameters used to generate the data set interpreted here, see Schroeter et al., 2019 [2].
2.2. Bioinformatics Analyses
Raw files from MS analyses of various solubilization fractions [2] (3 injections of each fraction) were searched as a combined sample in PEAKS [9] (version 8.0). Spectra were searched against the full SwissProt database (downloaded 26 May 2020), NCBI Archosauria (downloaded 24 June 2020), and NCBI Aves (downloaded 24 June 2020) using the following parameters: precursor mass tolerance 10 ppm and fragment ion mass tolerance 0.02 Da; four missed cleavages allowed-; non-specific cleavage allowed at one end the peptide; up to three PTMs allowed per peptide; allowed fixed PTMs included carbamidomethylation [C]; allowed variable PTMs included oxidation [M], oxidation or hydroxylation [RYFPNKD], [G]@C-term, carboxymethylation [KW, X@N-term], and deamidation [NQ]. PEAKS PTM [6] and SPIDER algorithms were enabled. PSM FDR was set at ≤1%. Proteins were accepted as detected if they met a protein score threshold of −10logP ≥ 20, included at least 1 unique peptide, and were identified by at least 2 distinct peptides. Proteins of common lab contaminants (e.g., keratin) or those that were fungal or bacterial in origin were excluded from further analyses.
2.3. Validation of Post-Translational Modifications
For each protein, PTMs were accepted as detected on a given amino acid residue if it met the following criteria: (1) it was detected in a PSM that met the above criteria for protein/peptide identification, (2) the mass shift of the PTM was isolated to that residue, with fragmentation ions supporting its localization on either side, in at least one PSM, and (3) the first PSM was supported by at least one additional PSM, which showed equally confident localization of the PTM or, at most, allowed for placement on only one alternative residue. For example, deamidation of a glutamine residue (Q) would be accepted as real in the sequence GLPGqPGAP, if two PSMs were detected with fully localizing fragmentation (GL|PG|q|PG|AP and G|LPG|q|P|GA|P) or if one fully localized fragment (GL|PG|q|PG|AP) and one nearly localized fragment (GL|P|Gq|PG|AP) were identified. This approach undoubtedly excludes from consideration preserved or diagenetic PTMs that were successfully identified by MS analyses but could not meet the rigorous threshold of acceptance. For example, a PTM that was localized on a residue in a fully fragmented peptide of a confidently identified protein would be excluded from acceptance if a second PSM bearing it was not recovered, or if it was identified but its fragmentation was poor. Although this conservative approach eliminates potentially informative data, it was used to ensure that any inferences drawn about trends in PTM preservation and diagenetic alternation were based solely on those most robustly identified. Additionally, PTMs identified by the software at a broken terminus of a semi-specific peptide were excluded from consideration (see Section 3.2.3).
3. Results and Discussion
3.1. Proteins Identified
Initial MS analyses of this specimen originally yielded three bone proteins: collagen I, collagen II, and collagen V [1]. Reanalysis of the specimen using an extraction workflow with a non-demineralized reagent and a modified FASP sample preparation to remove humics yielded 4 additional collagen chains and 12 non-collagenous proteins (NCPs) when the data were searched using Mascot [2], though collagen II was not reidentified. In the present analyses, the mass spectral data from Schroeter et al. [2] were re-evaluated using PEAKS [9], a bioinformatics program with algorithms that allow for the identification of unspecified PTMs (i.e., PeaksPTM [6]) and mutations (i.e., SPIDER [10]). Analyses with PEAKS yielded a total of 41 proteins, including 12 collagens (17 collagen chains) and 29 NCPs (Table 1). Among these were 22 proteins newly identified in this study (5 collagens, 8 collagen chains, and 17 NCPs). The results included collagen II, which escaped detection in Mascot, and osteocalcin. Osteocalcin was previously identified in this specimen by immunofluorescence [8] but had gone undetected by multiple subsequent MS analyses [1,2]. The latter findings highlight the fact that immunological assays may successfully identify the presence of low-abundance proteins in samples that are difficult to detect in MS, and that immunology can be a useful tool in identifying preserved proteins that are present in a tissue but may require a more optimized approach for MS characterization.

Table 1.
A comparison of protein identifications across three studies. Reanalysis of the same moa specimen, first with improved protein extraction methodology [2], then with bioinformatics software that allows for unspecified post-translational modifications [6] (this study), has resulted in a more than 7-fold increase in identifications compared to the initial study [1], including a wide variety of non-collagenous proteins.
3.2. Post-Translational Modifications (PTMs)
Prior analyses of this specimen using different extraction and bioinformatics methodologies identified several PTMs, both biological and diagenetic [1]. Reported biological PTMs included hydroxylated proline, alkylation, methylation, dimethylation, and fucosylation, and reported diagenetic PTMs included backbone cleavage/hydrolysis, deamidation, loss of dihydroxylation (via glutamic semialdehyde), and carboxymethylation. While the biological PTMs were variably reidentified in the current study (e.g., hydroxylated proline was recovered, alkylation was not), all of the previously identified diagenetic PTMs were detected, and additional modifications of both varieties were identified for the first time from this taxon (Table 2), including in NCPs.

Table 2.
Post-translational modifications that were detected in all proteins identified in this study. The presence or absence of select modifications are listed for each protein (see abbreviations below). In the right-hand column, a list of all additional PTMs detected for each protein, the residue or terminal end to which they were localized, and their associated mass shift are listed (notated mass shifts have been rounded to the nearest whole number). Abbreviations: hydroxylation of proline [HYP(P)]; glutamic semialdehyde from hydroxylated proline [GS(HYP)]; oxidation of methionine [OX(M)]; deamidation of asparagine or glutamine [DM(NQ)]; carboxymethylation of lysine (i.e., carboxymethyllysine) [CML(K)]; hydrolysis [HYD]; variable mass shift at the terminal end of the peptide [T-VMS].
3.2.1. Biological PTMs
Hydroxylation[+16]: Hydroxylated proline (Hyp), a biological PTM that is crucial for the formation of the triple helix in collagen I [11] and routinely found in preserved collagen sequences (e.g., [12,13]), was identified in 14 collagenous α-chains and 1 NCP (collagen + calcium-binding EGF domain-containing protein). The majority of these proteins simultaneously displayed instances of oxidative damage to Hyp residues (see Section 3.2.3). A few occurrences of doubly hydroxylated proline[+32] were also identified.
Methylation[+14] and Acetylation[+42]: Methylation of lysine, a PTM that profoundly affects cellular functions, [14] was previously reported in collagen I from this specimen [1]. Although methylated lysines were detected in peptides of collagen I in this data set, these instances did not meet the stringent criteria for acceptance employed in this analysis (see Section 2.3). Methylated lysine was, however, robustly identified in apolipoprotein, an NCP that participates in the transport and cellular homeostasis of cholesterol [15]. The current study also replicated [1] the identification of acetylation[+42] of lysine in both α-chains of collagen I in this fossil. Acetylation of lysine, a PTM long recognized as a regulatory mechanism of histones, is now known to exist in thousands of non-histone proteins [16].
Amidation[−1]: Amidation of valine was identified in α2-antiplasmin. α2-antiplasmin is an NCP that plays a crucial role in blood clotting [17]. Given that amidation is known to both occur in valine and to prolong the life of proteins in the bloodstream by making them less susceptible to proteolysis [18], it is likely that this detected modification is endogenous to the original bone tissue.
Glycosylation: Previous analyses of this specimen reported fucosylation, the enzymatic glycosylation of residues with a fucose sugar [1]. Although the current analysis did not identify fucosylation specifically, it did identify a different type of glycosylation, HexNacylation[203+] of asparagine. HexNacylation has been reported on Asn residues of proteins extracted from extant bone tissues using a solid digestion protocol [19], supporting its identification here as a preserved, endogenous PTM. It has also been reported in archeological eggshell ranging ~325–8775 years in age [20].
Ubiquitylation[114+]: This study also identified ubiquitylation (also known as ubiquitination) of threonine in a peptide of thrombospondin-1 (TSP1; Figure 1). Ubiquitylation is a PTM that is crucial for eukaryotic intercellular processes [21]. This includes a central role in regulating angiogenesis [22] (i.e., the formation of new blood vessels), as does TSP1 [23]. Although ubiquitylation of lysine residues has been heavily focused on in molecular research, it has been established [24] that ubiquitylation is a much more versatile PTM, occurring on cysteine, serine, and threonine residues. Thus, the repeated detection of this modification with strict localization to threonine strongly supports the preservation of this PTM in this fossil specimen. To the author’s knowledge, this is the first report of ubiquitylation in archeological bone.
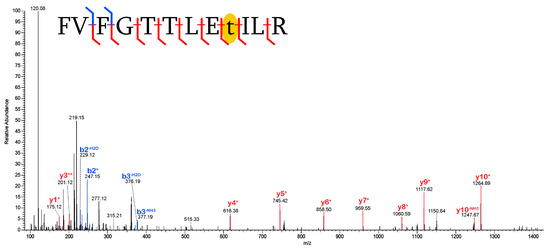
Figure 1.
Ubiquitylation of threonine in a peptide of thrombonspondin-1 (TSP1). The placement of the ubiquitylation mass shift [+114], which is isolated to a single residue by robust identification of the y3+ and y4+ fragment ions, is highlighted in yellow. Blue denotes b- fragment ions and red denotes y- fragment ions.
Phosphorylation[+80]: Phosphorylation was detected on threonine (Figure 2) and serine (Figure S1) residues of collagen I, α2. Phosphorylation is a PTM extensively observed to affect the structure and/or function of proteins across a range of biological mechanisms which most commonly affect serine, threonine, and tyrosine residues [25,26]. Phosphorylation of both serine and threonine has been robustly identified in extant collagen I [27], though characterizing the kinase and process of this phosphorylation is still an area of investigation [28]. The phosphoester bonds of phosphorylated serine (pSer) and threonine (pThr) are labile and susceptible to loss during CID or HCD fragmentation [28,29]. As a result, PSMs of peptides containing pSer and pThr produced by MS/MS typically contain fragment ions consistent with a [+80] mass shift associated with the modification, as well as additional ions with a [−98] mass shift where the ion underwent a neutral loss of phosphoric acid during fragmentation [29]. That we see this characteristic pattern in the collagen I PSMs where phosphorylation is identified (Figure 2 and Figure S1) strongly supports that this PTM has been preserved in these tissues. Phosphorylation of serine residues were robustly identified in the enamel of the early Pleistocene rhinocerotid Stephanorhinus [30] and hominid Gigantopithecus [31] and in ostrich eggshell ~151 Ka [20]. The current data set adds phosphorylation of collagen I to the known preservation of this PTM in degraded tissue.
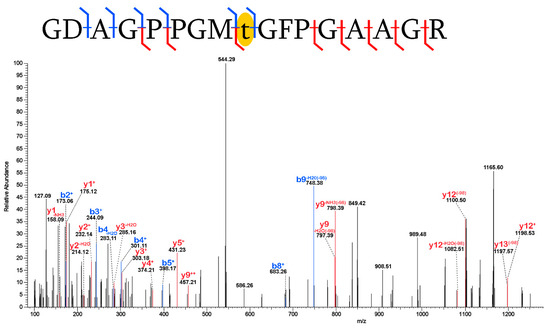
Figure 2.
Phosphorylation of threonine in a peptide of collagen I (α2). The placement of the phosphorylation mass shift [+80] (highlighted in yellow) was isolated to a single residue by detection of the y9++, y9−H20, y9−NH3, b8+, and b9−H20 fragment ions. As is typical for PSMs of peptides bearing phosphorylation [29], we see some ions with a [−98] mass shift, consistent with the neutral loss of phosphoric acid during the fragmentation process. Blue denotes b- fragment ions and red denotes y- fragment ions.
3.2.2. Tentative Biological PTMs
Diglutamylation[+258]: Diglutamylation of glutamic acid was detected in PSMs of collagen XI (α2), a protein that regulates collagen fibrillogenesis [32]. Although the polyglutamylation of glutamate residues is a biological PTM well characterized in tubulins [33], it is unclear whether it can occur in collagen. Thus, while a substantial mass shift consistent with the addition of two glutamates was isolated to glutamic acid residues multiple times in multiple PSMs of collagen XI, its interpretation as a preserved biological PTM is tentative.
Carboxylation[+44]: Carboxylation of glutamic acid and aspartic acid was identified in PSMs of collagen I. Carboxyglutamic acid (Gla) has a long history of recovery from fossil tissues, including after hydrolysis of fossil bone to extract Gla for 14C dating [34] and in osteocalcin sequences from a 55 Ka bison [35]. In these instances, this PTM was inferred to be a preserved biological modification, as Gla is known to occur in the NCP osteocalcin [34,35]. However, because Gla was identified here in collagen I rather than osteocalcin, its assignment as a biological PTM is tentative.
3.2.3. Diagenetic PTMs
Deamidation[+1] and hydrolysis: The most common diagenetic sequence alterations observed in this data set were deamidation of asparagine or glutamine residues, which was detected in 32 proteins (~69.6%), and hydrolytic backbone cleavage (Figure 3 and Figure S2), which was detected on 30 proteins (~65.2%). Both of these PTMs have been previously observed in this fossil [1] as well as in other fossil tissues (e.g., [36,37,38,39,40]). Interestingly, these diagenetic modifications occurred (or did not occur) in tandem for most of the proteins detected in this study. Of the 46 proteins detected, 27 (58.7%) possessed both, 11 (23.9%) possessed neither, and only 8 (17.4%) possessed only one or the other. The fact that these PTMs have a similar pattern of both occurrence and absence across proteins may point to a relationship between deamidation and susceptibility to structural degradation, rather than linear age [41]. However, a more rigorous and in-depth statistical analyses of the co-occurrence of these PTMs is needed to investigate this possibility.
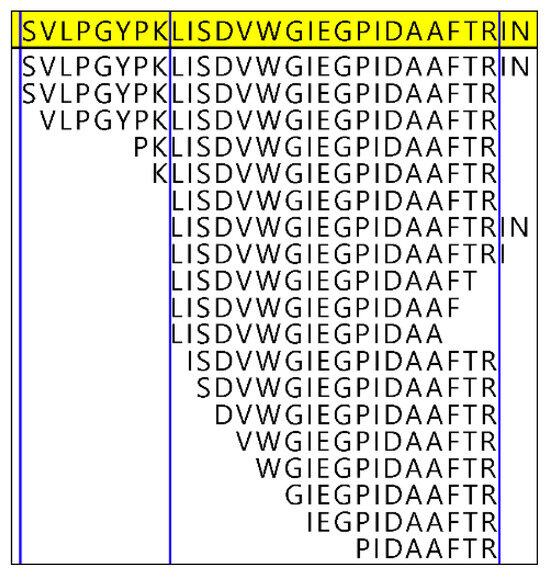
Figure 3.
Hydrolysis observed across peptides of vitronectin, a non-collagenous bone protein. This region of the vitronectin sequence, which is 28 amino acid residues long in the non-degraded protein, was represented by 19 different iterative combinations of hydrolysis and “missed” enzymatic cleavage (i.e., R and K residues where the enzyme did not successfully cut the sequences, as marked by the blue vertical lines). Diagenetic backbone is observed at both the N- and C-termini of these peptides.
Although no peptides are reported here that show simultaneous diagenetic cleavage at both the C- and N-termini of the peptides, it should be noted this is a result of the specified search parameters. Because the bioinformatics search only permitted the identification of semi-specific peptides (see methods above), only peptides that were successfully cleaved by trypsin (i.e., after Arg/R and Lys/K) residues on one (or both) sides could be detected. This means that “non-tryptic” preserved peptides—i.e., peptides that are diagenetically cleaved at both ends—are likely to be present and preserved in this specimen, yet remain undetected in the current search.
Dehydration[−18]: Dehydration of aspartic acid, serine, and/or threonine residues was detected in peptides of collagens I (α1; α2) and V (α1) and α2-antiplasmin (Figures S3 and S4). Dehydration is an effect of thermal degradation, which can be purposefully induced to cleave proteins into peptides for LC-MS/MS analyses (as an alternative or supplement to enzymatic digestion) and is expected at aspartic acid, serine, and threonine residues, as well as glutamic acid [42]. Dehydration has long been recognized in fossil specimens and has been proposed as a possible mechanism of preservation [20]. Thermal degradation is experimentally induced at a temperature of ~220 °C [42], and because the samples analyzed here were never heated beyond a maximum of 60 °C [2], it is unlikely that the observed dehydration is an experimental artifact. However, given that this specimen is only ~1000 years old and found in cave deposits [7], further investigation is needed to determine whether this diagenetic alteration is linked solely to thermal conditions experienced by a fossil or can also be induced by other geochemical conditions.
Oxidation: The most striking diagenetic pattern observed broadly in this specimen is oxidative damage, which is expressed through a wide diversity of oxidative PTMs across many different proteins and amino acids. These include (1) oxidation of threonine to 2-amino-3-oxo-butanoic acid[−2] (Figure 4 and Figure S5); (2) oxidation of arginine to glutamic semialdehyde[−43]; (3) dihydroxidation of tryptophan[+32]; (4) dihydroxylation of phenylalanine[+32]; (5) oxidation of lysine to aminoadipic semialdehyde[−1]; (6) oxidation of histidine, tryptophan, and aspartic acid[+16]; (7) oxidation of proline to pyroglutamic acid[+14]; (8) formation of pyrrolidone from proline[−28] (Figure 5); (9) sulphone formation on methionine[+32] (Figure S6); (10) oxidation of tryptophan to kynurenin[+4] (Figure S7A); (11) oxidation of tryptophan to oxolactone[+14] (Figure S7B).
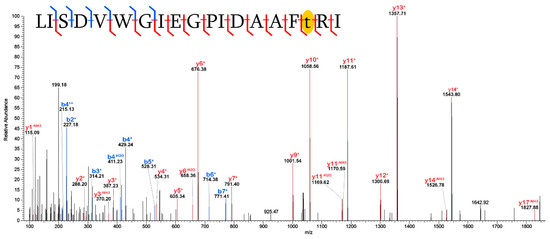
Figure 4.
Oxidation of threonine to 2-amino-3-oxo-butanoic acid in a peptide of vitronectin. The placement of the [−2] mass shift is isolated to a single threonine residue by identification of the y2+, y3+, and y3−NH3 fragment ions and is highlighted in yellow. Blue denotes b- fragment ions and red denotes y- fragment ions.
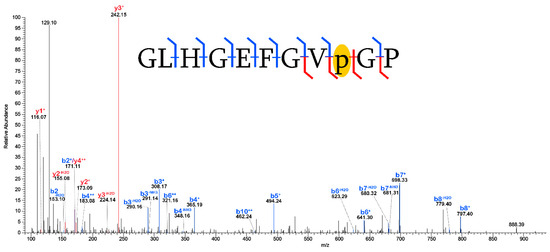
Figure 5.
Formation of pyrrolidone from proline in a peptide of collagen I (α2). The placement of the [−28] mass shift is robustly isolated to a single proline residue by the identification of the b8+, b8−H2O, y2+, y2−H2O, y3+, and y3−H2O fragment ions and is highlighted in yellow. Blue denotes b- fragment ions and red denotes y- fragment ions.
Additionally, both the current data and previous reports from this specimen [1] show large variations in the number of hydroxylated prolines in different PSMs of the same peptide. For example, PSMs of a given collagen peptide sequence may variably show anywhere between three hydroxylated prolines and none. This is likely also the result of oxidative damage. Oxidation of proline to glutamic semialdehyde results in a [+16] mass shift that makes the residue isobaric to (and therefore indistinguishable from) a hydroxylated proline. Thus, wide ranges of variability in the amount of Hyp within a given sequence should be interpreted as diagenetic oxidative damage. For these specific diagenetiforms to be identified, many instances of the same sequence must be detected to allow for comparison. In this data set, high variability in Hyp was observed in many of the proteins that contain biologically induced Hyp, including the NCP collagen + calcium-binding EGF domain-containing protein.
The pattern and extensive nature of the oxidative damage in these proteins is consistent with oxidation described for other archeological remains. The mono-, di-, or tri- oxidation of chromophoric residues (e.g., tryptophan, histidine, methionine, and phenylalanine) has been frequently recognized in archeological bones [43] and materials [44], as well as Pleistocene dentition [30,31,45]. Tryptophan in particular has been shown to be especially susceptible to the formation of multiple oxidative degradation products [30], a pattern that is also seen here, where four forms of oxidative damage to Trp was observed on the NCP vitronectin (Figure S7). Similarly, the oxidation of lysine to aminoadipic semialdehyde has also been reported in paleoproteomic studies [12,44], as has the oxidation of proline to pyroglutamic acid [20].
To this wide array of previously known oxidation, the current data adds two additional modifications: the oxidation of threonine to 2-amino-3-oxo-butanoic acid[−2], which was detected in collagen V (α2) (Figure S5) and the NCP vitronectin (Figure 4), and the oxidation of proline to pyrrolidone[−28], which was observed on collagen I (α2) (Figure 5). To the author’s knowledge, this is the first report of these diagenetic PTMs in paleoproteomic data.
Advanced Glycation End products (AGEs): Carboxymethylation[+58] of lysine, an advanced glycation end product (AGE) [46], had previously been identified on collagen sequences in this specimen [1] as well as other archaeological and paleontological remains (e.g., [43,47]). Here, carboxymethylation of lysine was detected in collagens I (α1; α2), II (α1) (Figure S8), and XI (α1), as well as in the NCP thrombospondin 1 (TSP1; Figure 6).
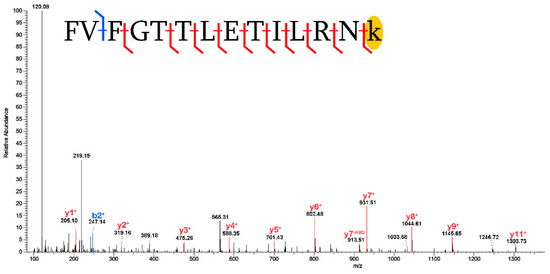
Figure 6.
Carboxymethylation of lysine in a peptide of thrombonspondin-1 (TSP1). The placement of the carboxymethylation mass shift [+58], which is isolated to a single residue by identification of the y1+ fragment ion, is highlighted in yellow. Blue denotes b- fragment ions and red denotes y- fragment ions.
In addition, a second type of AGE [48,49], dihydroxy methylglyoxal adduction[+72], was detected on arginine residues in collagen I (α2) (Figure 7). Methylglyoxal adducts are known to form on arginine, lysine, and cysteine residues, and can undergo secondary modifications that produce cross-linked macromolecules [48]. AGEs on arginine residues, specifically imidazolone[+40] and methylimidazolone[+54], have been recognized on archeological paintings [44] and bones [43]. These, as well as carboxymethylation of lysine, have been interpreted as the end result of reactions with glyoxal and methylglyoxal products from carbohydrate degradation [44]. While the dihydroxy methylglyoxal adduct detected on this specimen may potentially be associated with this process, the larger mass shift [+72] of this AGE distinguishes it from the imidazolones that have previously been reported in degraded tissues. Just as carboxymethylated lysine has become more frequently recognized in fossil tissues analyzed by LC-MS/MS since its first report (e.g., [43]), proactive searching for this variant of methylglyoxal adduction may reveal more instances of the AGE in paleoproteomic samples.
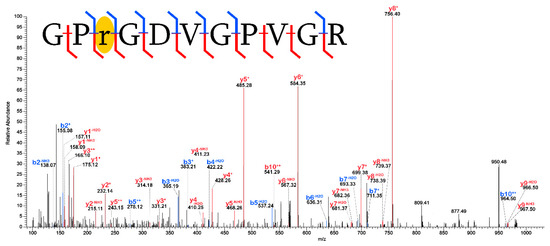
Figure 7.
Dihydroxy methylgloxal adduction on an arginine residue in a peptide of collagen I (α2). The placement of the [+72] mass shift is robustly isolated to a single residue by identification of the b2+, b2−NH3, b3+, b3−H2O, y8+, y8−H2O, y8−NH3, y9−H2O, and y9−NH3 fragment ions and is highlighted in yellow. Blue denotes b- fragment ions and red denotes y- fragment ions.
Variable Terminal Mass Shift: Another distinct pattern of diagenetic alteration observed extensively in this specimen is the presence of highly variable, non-specific mass shifts at the broken terminus of peptides that experienced hydrolysis (Figure 8 and Figure S9). Although PEAKS “identified” a multitude of diverse PTMs on broken peptide ends, these identifications are inconsistent from peptide to peptide, and the specific modifications observed on terminal residues do not appear in more complete instances of the peptide (i.e., they are never detected in peptides where the residue is more internal) (Figure 8). This pattern suggests that the inconsistent mass shifts observed at these broken ends do not represent specific PTMs but the presence of a variable mass for which the software cannot otherwise account.
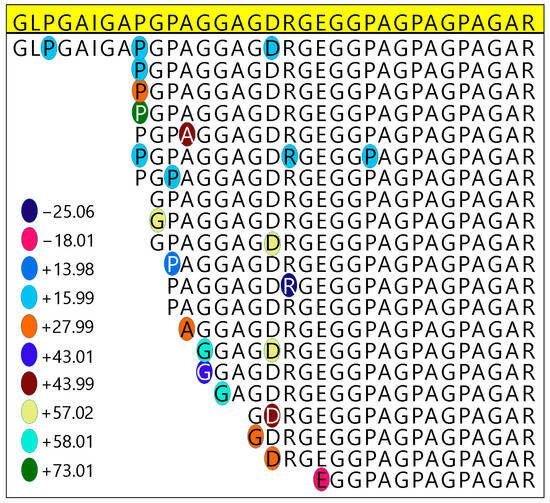
Figure 8.
Progressive hydrolysis observed in a peptide of collagen I, with an inconsistent mass shift at the broken N-terminal. The collagen I sequence shown represents 33 amino acid residues that in a typical enzymatic digestion with trypsin should result in 2 “tryptic” peptides (i.e., peptides cut after all R and K residues, and only R and K residues). MS analyses detected 10 different portions of this sequence, none of which are fully tryptic. Additionally, the broken N-termini of these peptides appear to incorporate mass shifts highly variable in size which are not present on the same residues in more intact peptides.
Whether these masses are partial pieces of broken amino acid residues left behind from backbone cleavage, or the addition of some other diagenetic substance (e.g., humic substance remnants [50,51]), needs further investigation. Regardless, the pattern that this diagenetic alteration causes has implications for how we search and interpret paleoproteomic data. For example, in addition to semi-specific searches to accommodate the identification of diagenetically broken peptides, software/algorithms that are tolerant of unspecified (and unusually placed) PTMs is needed to detect peptides with an anomalous terminal mass shift. Otherwise, these preserved peptides will go entirely undetected. Furthermore, because proteomic search software that can detect these peptides interpret these masses as a wide variety of (seemingly random) PTMs, it is important that researchers do not assume that summaries of PTM identification provided by the software for their data set are accurate. Such summaries will include the software’s “best guess” of what these unidentified mass shifts may represent, making them inaccurate in terms of both the PTMs identified and their frequency.
3.2.4. Potential Experimental Artifacts
Several modifications were identified that potentially represent an artifact of the experimental design rather than preserved biological modifications or alterations from the geochemical environment of burial. For example, the formation of the sodium adducts[+22], which were detected on aspartic and glutamic acid residues in collagen I and vitronectin, is a commonly observed effect of using electrospray ionization (ESI) to introduce the digested peptides to the mass spectrometer [52].
Formylation[+28]: Formylation was observed on valine and lysine in collagens I and II and α2-antiplasmin (Table 2). This modification can be induced by the use of formic acid in sample preparation, to which lysine residues are particularly susceptible [53]. Given that a high concentration of formic acid was used to precipitateany remaining humic substances out of the final solution of digested peptides [2], it is likely that these modifications are artifacts. Although this modification is not biologically or geologically informative, methods using this technique on samples abundant in humic substances should account for potential formylation to prevent peptides bearing this artifact from going undetected.
Lactone formation[−2] from hydroxylated lysine: A (−2 Da) mass shift was identified on lysine resides in collagen I. Although this mass shift was identified by PEAKS (version 8.0) software as “2-amino-3-oxo-butanoic acid[−2] from lysine”, lysine is not a typical residue for this modification. Rather, this mass shift more likely represents the formation of lactone from hydroxylated lysine. Hydroxylysine, which occurs in collagen I (though to a lesser degree than hydroxylated proline), readily undergoes interconversion to lactone [54]. This conversion would result in a −18 Da mass loss from a hydroxylated proline residue, which is isobaric with a −2 Da mass loss from a non-hydroxylated lysine residue. As discussed for oxidative damage to Hyp above, because the search software can only assess the end state of the residue, it interprets the lactone end product as an anomalous −2 Da loss on a lysine residue. Because hydroxylated lysine is susceptible to lactone formation in acidic conditions [55], this alteration is conservatively interpreted as a result of formic acid during sample preparation [2] rather than diagenesis.
Methylation[+14]/Ethylation[+28]: The detection of methylation on aspartic acid and glutamic acid may result from the usage of methanol during the stage-tipping procedure [2]. Methanol has been shown to cause methylation at these sites in samples where a mixture of methanol and water has been used in sample preparation [56]. Additionally, previously reported methylation of serine in histones using tandem MS has been shown to in fact be a peculiar artifact of fragmentation when interrogated using MS/MS/MS, wherein the methylation was actually on the neighboring lysine [57]. Taken together, methylation of residues other than lysine has been excluded from consideration of biological or diagenetic relevance. The ethylation of aspartic acid detected in peptides of collagen I (α2) could also potentially be to the use of ethanol during the same stage-tipping procedure [2].
3.2.5. Summation of PTM Recovery
A total of 34 post-translational modifications were identified. These have been interpreted to represent 8–10 biological PTMs that have been retained in the degraded tissue, 18 PTMs that were geochemically induced via diagenetic processes (including 2 AGEs and 12 oxidative alterations), and 6 modifications that were experimentally induced. A summary of the modifications identified and their categorization is provided in Table 3.

Table 3.
Categorization of PTMs identified in this study. Ten potential biological PTMs, eighteen diagenetic PTMs, and five modifications attributable to experimental artifact were detected. “?” denotes a PTM that is tentatively identified as biological. “AGE” denotes an advanced glycation end product diagenetic PTM. “OX” denotes a diagenetic PTM interpreted to have been caused by oxidation. For the modifications identified as experimental artifacts, the hypothesized source of the artifact is listed.
4. Conclusions
The reanalysis of this moa specimen with optimized extraction methodology and higher resolution MS instrumentation has ultimately resulted in a more than 11-fold increase in the known proteome for this taxon. This increase in proteomic information has also allowed for the broader characterization of PTMs in this specimen across many collagens and NCPs. In the future, in-depth characterization of where these PTMs localize within the molecule, particularly for larger bone proteins (e.g., collagen I), may reveal additional insights into how preservation and/or degradation of proteinaceous material occurs at the molecular level. Additionally, although the specimen analyzed for this study was heavily inundated with diagenetic humic substances [2], it is relatively young (~800–1000 years). Comparable characterization of older archaeological and Pleistocene specimens, as well as specimens from a variety of ages with different macroscopic degradation profiles (e.g., poor histology; no humics) and depositional environments (e.g., lacustrine; marine), is needed to explore the relationship between the taphonomic history of a fossil and the type and abundance of PTMs that are detected in its tissues.
Importantly, although this specimen was subjected to a multi-stage extraction procedure designed for ‘problematic’ specimens with high amounts of humic substances [2], this analysis shows that informative, typically labile biological PTMs (e.g., phosphorylation; ubiquitylation) are capable of surviving the process. Therefore, although a shorter, more gentle extraction methodology (e.g., single-pot solid-phase-enhanced sample preparation, or SP3) [58] may be ideal for specimens that do not require post-digestion humic removal, the modified FASP method can be used for heavily inundated samples without the sacrifice of all labile modifications. These data also further highlight the fact that specimens once presumed to hold very little proteomic material beyond collagen may in fact possess proteomic and PTM data far beyond initial appearances, limited more by the applied methodology than geochemistry. With additional optimization for the unique needs of different biological tissue types (e.g., for the hydrophobicity of β-keratin in feathers), it is possible that workflows such as the modified FASP method [2] can be used to probe a more diverse range of fossilized tissues.
These results also show that paleoproteomic data are rife with mass anomalies that, while not necessarily biologically or geochemically informative, must be allowed for in database search parameters to detect the maximum amount of preserved peptides. Further characterization of the variable mass shifts observed at the broken termini of peptides, such as those observed in these data, may reveal further information about the iterative progression of protein degradation in ancient tissues and the factors that can arrest it.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min14020137/s1, Figure S1. Phosphorylation of serine in a peptide of collagen I (α2); Figure S2. Backbone cleavage observed across peptides of thrombospondin-1 (TSP1), a non-collagenous bone protein; Figure S3. Dehydration of serine in peptide of α2-antiplasmin; Figure S4. Dehydration of threonine in collagen I (α2); Figure S5. Oxidation of threonine to 2-amino-3-oxo-butanoic acid in a peptide of collagen V (α2); Figure S6. Sulphone formation on methionine on a peptide of collagen I (α1); Figure S7. Two different forms of tryptophan oxidation in a vitronectin peptide; Figure S8. Carboxymethylation of lysine in a peptide of collagen II (α1); Figure S9. Progressive hydrolysis observed in a peptide of osteomodulin, with an inconsistent mass shift at the broken N-terminal; Table S1. PEAKS protein-peptide identifications (1% FDR) from moa samples, searched against the SwissProt database; Table S2. PEAKS protein-peptide identifications (1% FDR) from moa samples, searched against the NCBI Archosauria database; Table S3. PEAKS protein-peptide identifications (1% FDR) from moa samples, searched against the NCBI Aves database; Table S4. PEAKS protein-peptide identifications (1% FDR) from control samples, searched against the SwissProt database; Table S5. PEAKS protein-peptide identifications (1% FDR) from control samples, searched against the NCBI Archosauria database; Table S6. PEAKS protein-peptide identifications (1% FDR) from control samples, searched against the NCBI Aves data.
Funding
Funding for this research was provided by an Arnold O. Beckman Post-doctoral Fellowship and the Biological Sciences Department of North Carolina State University.
Data Availability Statement
All raw spectrometry data that were reanalyzed in this manuscript are available at Dryad: http://dx.doi.org/10.5061/dryad.3tv1523.
Acknowledgments
The author would like to thank K. Blackburn, M. Goshe, M. Schweitzer, and W. Zheng. The author also thanks three reviewers whose comments and insights improved this manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Cleland, T.P.; Schroeter, E.R.; Schweitzer, M.H. Biologically and diagenetically derived peptide modifications in moa collagens. Proc. R. Soc. B 2015, 282, 20150015. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, E.R.; Blackburn, K.; Goshe, M.B.; Schweitzer, M.H. Proteomic method to extract, concentrate, digest and enrich peptides from fossils with coloured (humic) substances for mass spectrometry analyses. R. Soc. Open Sci. 2019, 6, 181433. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, E.R.; Cleland, T.P.; Schweitzer, M.H. Deep Time Paleoproteomics: Looking Forward. J. Proteome Res. 2022, 21, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P.; Schroeter, E.R. A Comparison of Common Mass Spectrometry Approaches for Paleoproteomics. J. Proteome Res. 2018, 17, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Schroeter, E.R.; Goshe, M.B. Protein molecular data from ancient (>1 million years old) fossil material: Pitfalls, possibilities and grand challenges. Anal. Chem. 2014, 86, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; He, L.; Xin, L.; Shan, B.; Ma, B. PeaksPTM: Mass spectrometry-based identification of peptides with unspecified modifications. J. Proteome Res. 2011, 10, 2930–2936. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R. Soft tissue and cellular preservation in vertebrate skeletal elements from the Cretaceous to the present. Proc. R. Soc. Lond. B. 2007, 274, 183–197. [Google Scholar] [CrossRef]
- Cleland, T.P.; Voegele, K.; Schweitzer, M.H. Empirical evaluation of bone extraction protocols. PLoS ONE 2012, 7, e31443. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef]
- Han, Y.; Ma, B.; Zhang, K. Spider: Software for protein identification from sequence tags with de novo sequencing error. J. Bioinform. Comput. Biol. 2005, 3, 697–716. [Google Scholar] [CrossRef]
- Kotch, F.W.; Guzei, I.A.; Raines, R.T. Stabilization of the collagen triple helix by O-methylation of hydroxyproline residues. J. Am. Chem. Soc. 2008, 130, 2952–2953. [Google Scholar] [CrossRef]
- Cappellini, E.; Jensen, L.J.; Szklarczyk, D.; Ginolhac, A.; Da Fonseca, R.A.R.; Stafford, T.W.; Holen, S.R.; Collins, M.J.; Orlando, L.; Willerslev, E.; et al. Proteomic analysis of a pleistocene mammoth femur reveals more than one hundred ancient bone proteins. J. Proteome Res. 2012, 11, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Heiden, M.G.V.; Neveu, J.M.; et al. Biomolecular characterization and protein sequences of the campanian hadrosaur b. canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef]
- Luo, M. Chemical and Biochemical Perspectives of Protein Lysine Methylation. Chem. Rev. 2018, 118, 6656–6705. [Google Scholar] [CrossRef]
- Mangaraj, M.; Nanda, R.; Panda, S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian J. Clin. Biochem. 2016, 31, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Conrad, R.J.; Verdin, E.; Ott, M. Lysine Acetylation Goes Global: From Epigenetics to Metabolism and Therapeutics. Chem. Rev. 2018, 118, 1216–1252. [Google Scholar] [CrossRef]
- Carpenter, S.L.; Mathew, P. a2-antiplasmin and its deficiency: Fibrinolysis out of balance. Haemophilia 2008, 14, 1250–1254. [Google Scholar] [CrossRef]
- Naseer, S.; Ali, R.F.; Muneer, A.; Fati, S.M. iAmideV-Deep: Valine amidation site prediction in proteins using deep learning and pseudo amino acid compositions. Symmetry 2021, 13, 560. [Google Scholar] [CrossRef]
- Cleland, T.P. Solid Digestion of Demineralized Bone as a Method to Access Potentially Insoluble Proteins and Post-Translational Modifications. J. Proteome Res. 2018, 17, 536–542. [Google Scholar] [CrossRef]
- Demarchi, B.; Hall, S.; Roncal-Herrero, T.; Freeman, C.L.; Woolley, J.; Crisp, M.K.; Wilson, J.; Fotakis, A.; Fischer, R.; Kessler, B.M.; et al. Protein sequences bound to mineral surfaces persist into deep time. eLife 2016, 5, e17092. [Google Scholar] [CrossRef]
- Weissman, A.M.; Shabek, N.; Ciechanover, A. The predator becomes the prey: Regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 2011, 12, 605–620. [Google Scholar] [CrossRef]
- Rahimi, N. The ubiquitin-proteasome system meets angiogenesis. Mol. Cancer Ther. 2012, 11, 538–548. [Google Scholar] [CrossRef]
- Lawler, P.R.; Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef]
- Kelsall, I.R. Non-lysine ubiquitylation: Doing things differently. Front. Mol. Biosci. 2022, 9, 1008175. [Google Scholar] [CrossRef] [PubMed]
- Jagannadham, M.V.; Nagaraj, R. Detecting the site of phosphorylation in phosphopeptides without loss of phosphate group using MALDI TOF mass spectrometry. Anal. Chem. Insights 2008, 2008, 21–29. [Google Scholar] [CrossRef]
- Yu, L.-R.; Veenstra, T.D. Characterization of Phosphorylated Proteins Using Mass Spectrometry. Curr. Protein Pept. Sci. 2020, 22, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yalak, G.; Olsen, B.R. Proteomic database mining opens up avenues utilizing extracellular protein phosphorylation for novel therapeutic applications. J. Transl. Med. 2015, 13, 125. [Google Scholar] [CrossRef]
- Qiu, Y.; Poppleton, E.; Mekkat, A.; Yu, H.; Banerjee, S.; Wiley, S.E.; Dixon, J.E.; Kaplan, D.L.; Lin, Y.S.; Brodsky, B. Enzymatic Phosphorylation of Ser in a Type I Collagen Peptide. Biophys. J. 2018, 115, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf, S.B.; Asara, J.M. Determining in vivo phosphorylation sites using mass spectrometry. Curr. Protoc. Mol. Biol. 2012, 1, 18.19.1–18.19.27. [Google Scholar] [CrossRef]
- Cappellini, E.; Welker, F.; Pandolfi, L.; Ramos-Madrigal, J.; Samodova, D.; Rüther, P.L.; Fotakis, A.K.; Lyon, D.; Moreno-Mayar, J.V.; Bukhsianidze, M.; et al. Early Pleistocene enamel proteome from Dmanisi resolves Stephanorhinus phylogeny. Nature 2019, 574, 103–107. [Google Scholar] [CrossRef]
- Welker, F.; Ramos-Madrigal, J.; Kuhlwilm, M.; Liao, W.; Gutenbrunner, P.; de Manuel, M.; Samodova, D.; Mackie, M.; Allentoft, M.E.; Bacon, A.M.; et al. Enamel proteome shows that Gigantopithecus was an early diverging pongine. Nature 2019, 576, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Luo, E.Y.; Adams, S.M.; Adams, T.; Ye, Y.; Shetye, S.S.; Soslowsky, L.J.; Birk, D.E. Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon. Matrix Biol. 2020, 94, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Ruse, C.I.; Chin, H.G.; Pradhan, S. Polyglutamylation: Biology and analysis. Amino Acids 2022, 54, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Burky, R.R.; Kirner, D.L.; Taylor, R.E.; Hare, P.E.; Southon, J.R. 14C Dating of Bone Using y-Carboxyglutamic Acid and a-Carboxyglycine (Aminomalonate). Radiocarbon 1998, 40, 11–20. [Google Scholar] [CrossRef][Green Version]
- Nielsen-Marsh, C.M.; Ostrom, P.H.; Gandhi, H.; Shapiro, B.; Cooper, A.; Hauschka, P.V.; Collins, M.J. Sequence preservation of osteocalcin protein and mitochondrial DNA in bison bones older than 55 ka. Geology 2002, 30, 1099–1102. [Google Scholar] [CrossRef]
- Van Doorn, N.L.; Wilson, J.; Hollund, H.; Soressi, M.; Collins, M.J. Site-specific deamidation of glutamine: A new marker of bone collagen deterioration. Rapid Commun. Mass Spectrom. 2012, 26, 2319–2327. [Google Scholar] [CrossRef]
- Welker, F.; Collins, M.J.; Thomas, J.A.; Wadsley, M.; Brace, S.; Cappellini, E.; Turvey, S.T.; Reguero, M.; Gelfo, J.N.; Kramarz, A.; et al. Ancient proteins resolve the evolutionary history of Darwin’s South American ungulates. Nature 2015, 522, 81–84. [Google Scholar] [CrossRef]
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef]
- Hill, R.C.; Wither, M.J.; Nemkov, T.; Barrett, A.; D’Alessandro, A.; Dzieciatkowska, M.; Hansen, K.C. Preserved proteins from extinct bison latifrons identified by tandem mass spectrometry; Hydroxylysine glycosides are a common feature of ancient collagen. Mol. Cell. Proteom. 2015, 14, 1946–1958. [Google Scholar] [CrossRef]
- Warinner, C.; Korzow Richter, K.; Collins, M.J. Paleoproteomics. Chem. Rev. 2022, 122, 13401–13446. [Google Scholar] [CrossRef]
- Schroeter, E.R.; Cleland, T.P. Glutamine deamidation: An indicator of antiquity, or preservational quality? Rapid Commun. Mass Spectrom. 2016, 30, 251–255. [Google Scholar] [CrossRef]
- Liu, C.; Topchiy, E.; Lehmann, T.; Basile, F. Characterization of the dehydration products due to thermal decomposition of peptides by liquid chromatography-tandem mass spectrometry. J. Mass Spectrom. 2015, 50, 625–632. [Google Scholar] [CrossRef]
- Ntasi, G.; Palomo, I.R.; Marino, G.; Piaz, F.D.; Sirano, F.; Cappellini, E.; Birolo, L.; Petrone, P. Molecular signatures written in bone proteins of 79 AD victims from Herculaneum and Pompeii. Sci. Rep. 2022, 12, 8401. [Google Scholar] [CrossRef]
- Mackie, M.; Rüther, P.; Samodova, D.; Di Gianvincenzo, F.; Granzotto, C.; Lyon, D.; Peggie, D.A.; Howard, H.; Harrison, L.; Jensen, L.J.; et al. Palaeoproteomic Profiling of Conservation Layers on a 14th Century Italian Wall Painting. Angew. Chemie Int. Ed. 2018, 57, 7369–7374. [Google Scholar] [CrossRef]
- Welker, F.; Ramos-Madrigal, J.; Gutenbrunner, P.; Mackie, M.; Tiwary, S.; Rakownikow Jersie-Christensen, R.; Chiva, C.; Dickinson, M.R.; Kuhlwilm, M.; de Manuel, M.; et al. The dental proteome of Homo antecessor. Nature 2020, 580, 235–238. [Google Scholar] [CrossRef]
- Shapiro, B.P.; Owan, T.E.; Mohammed, S.F.; Meyer, D.M.; Mills, L.D.; Schalkwijk, C.G.; Redfield, M.M. Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation 2008, 118, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P.; Schroeter, E.R.; Feranec, R.S.; Vashishth, D. Peptide sequences from the first Castoroides ohioensis skull and the utility of old museum collections for palaeoproteomics. Proc. R. Soc. B 2016, 283, 20160593. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.T.; Lopez Gonzalez, E.D.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, L.; Young, C.; Simpson, B.S.; Acland, M.; Dhillon, V.S.; Costabile, M.; Fenech, M.; Hoffmann, P.; Deo, P. Proteomic Analysis of Methylglyoxal Modifications Reveals Susceptibility of Glycolytic Enzymes to Dicarbonyl Stress. Int. J. Mol. Sci. 2022, 23, 3689. [Google Scholar] [CrossRef] [PubMed]
- van Klinken, G.J.; Hedges, R.E.M. Experiments on Collagen-Humic Interactions: Speed of Humic Uptake, and Effects of Diverse Chemical Treatments. J. Archaeol. Sci. 1995, 22, 263–270. [Google Scholar] [CrossRef]
- Szpak, P.; Krippner, K.; Richards, M.P. Effects of Sodium Hydroxide Treatment and Ultrafiltration on the Removal of Humic Contaminants from Archaeological Bone. Int. J. Osteoarchaeol. 2017, 27, 1070–1077. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K. Adduct Formation in ESI/MS by Mobile Phase Additives. J. Am. Soc. Mass Spectrom. 2017, 28, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Lenčo, J.; Khalikova, M.A.; Švec, F. Dissolving Peptides in 0.1% Formic Acid Brings Risk of Artificial Formylation. J. Proteome Res. 2020, 19, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Cudic, M.; Lauer-Fields, J.L.; Fields, G.B. Improved synthesis of 5-hydroxylysine Hyl derivatives. J. Pept. Res. 2005, 65, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Grabarkiewicz, T.; Grobelny, P.; Hoffmann, M.; Mielcarek, J. DFT study on hydroxy acid-lactone interconversion of statins: The case of fluvastatin. Org. Biomol. Chem. 2006, 4, 4299–4306. [Google Scholar] [CrossRef]
- Atik, A.E.; Guray, M.Z.; Yalcin, T. Observation of the side chain O-methylation of glutamic acid or aspartic acid containing model peptides by electrospray ionization-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1047, 75–83. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Zhang, Z.; Xing, G.; Wysocka, J.; Zhao, Y. MS/MS/MS reveals false positive identification of histone serine methylation. J. Proteome Res. 2010, 9, 585–594. [Google Scholar] [CrossRef][Green Version]
- Cleland, T.P. Human Bone Paleoproteomics Utilizing the Single-Pot, Solid-Phase-Enhanced Sample Preparation Method to Maximize Detected Proteins and Reduce Humics. J. Proteome Res. 2018, 17, 3976–3983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).