Abstract
The wide application of nuclear resources in various fields has resulted in the production of radioactive waste, which poses a serious threat to lives and the environment. Nuclear waste contains long-lived radionuclides and, due to its mobility in environments, the proper management of generated waste is necessary. To impede the mobility of radionuclides in environments, various materials have been tested as suitable sorbents under different experimental conditions. In this review, we thoroughly discuss some key and recent contributions to the application of natural clays (NCs) and modified/functionalized clays (MCs) for the sorption of various radionuclides in their cationic and anion forms from (simulated) waste solutions under different experimental conditions. More specifically, we discuss the key developments toward the use of natural clays for the efficient sorption of various radioactive contaminates. Later, this review targets the modification/functionalization of natural clays using various organic moieties to improve their removal capacities for various radionuclides/hazardous ions present in waste solutions. Finally, we summarize the major aspects and highlight the key challenges to be addressed in future studies to further enhance the application of clays and clay-based materials for selective and effective removal of various radionuclides from waste solutions.
1. Introduction
The development of nuclear technology in various sectors, including weapons, power, aerospace and medicine, have generated a large amount of extremely hazardous radioactive waste (containing long half-life radionuclides) [1,2,3,4,5,6,7,8]. Besides the aforementioned anthropogenic activities, radionuclides can also be released and entered into the environment due to nuclear accidents/disasters, such as the Chernobyl and Fukushima Daiichi nuclear power plant incidents [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. The radiotoxicity output and exposure of the produced radioactive wastes/radionuclides are a tremendous threat to both humans as well as the wider geosphere/environment [27,28]. Once released, the radionuclides have the tendency to migrate in the environment [29,30]. After the Fukushima nuclear disaster, the radioactive levels of 239Pu, 234U and 238U suddenly increased, particularly in Hawaii, Alaska and California [31,32]. Most of these radionuclides were supposed to be migrated over the Pacific Ocean due to the wind orientation. In order to hinder the mobility of released radionuclides in various geological and environmental media, their efficient removal/sorption from aqueous waste solutions and proper management (solidification, immobilization and underground disposal) using various materials/matrices, are being investigated.
In the last few decades, numerous materials have been studied and tested for the sorption of radionuclides from (simulated) waste solutions under different experimental conditions [33,34,35,36,37,38,39,40,41,42,43,44,45]. Among various sorbents, clays are widely studied as natural sorbents for wastewater decontamination and as a potential barrier in landfills to hamper and stop the leaching of radioactive materials/radionuclides into the subsoil and groundwater [46,47,48,49,50,51,52,53,54]. Clays are a ubiquitous, key component of soil, sediment and bedrock, and are the most abundant and cheapest materials, which has attracted significant interest in the scientific community over the past few decades [55,56,57,58,59,60]. Fundamentally, clays are hydrous aluminum silicates commonly formed by prolonged chemical weathering of silicate-bearing rocks and are composed of aluminum and silicon ions bonded into tiny, thin plates by interconnecting oxygen and hydroxide ions. The main groups of clays include kaolinite, montmorillonite (bentonite) and illite [61]. The crystal structure of montmorillonite/bentonite is composed of two layers of silica tetrahedra with a central layer of alumina octahedron between them (TOT type). Bentonite clay has been widely applied as a potential buffer material in high-level radioactive waste (HLW) repositories due to its ability to absorb water molecules between the sheets, resulting in a significant expansion and the different exchangeable cations can replace (hazardous) ions in the structure [62,63].
Clays readily exchange cationic radionuclides in nuclear waste solutions due to the presence of alkali/alkaline earth metals in their crystalline aluminosilicate frameworks and they are known for their sorption of cationic contaminants due to their large surface area, high thermal stability, porous structure and cation exchange capacity (CEC) [64,65]. Additionally, significant developments have been made toward the modification of natural clays (NCs) to improve their affinity and selectivity as potential sorbents for the efficient removal of various contaminants from wastewater [66,67,68,69]. The NCs have been functionalized and modified via chemical alteration by incorporating organic moieties (such as quaternary amines and others) to form organoclays, which efficiently enhanced their affinity and sorption capacities for extremely hazardous anionic contaminants present in waste solutions [70,71].
This review presents the key developments in the design and application of various NCs and modified clay (MC) materials used for efficient and selective sorption of various hazardous radioactive cationic and anionic species from (simulated) waste solutions with a major emphasis on the pioneering examples and recent developments. Section 2 and Section 3 of this review present the core contents on the application of NCs and MCs respectively, for the removal of various radionuclides/ions under different experimental conditions. In Section 2, we discuss some of the key and recent progress on the application of NCs (bentonite/montmorillonite, etc.) for the effective removal of various cationic pollutants. Section 3 encompasses the design and modification of NCs to the organoclays using different organic moieties (in the optimized experimental conditions) and their use as potential sorbents for the efficient removal of hazardous species present in radioactive waste solutions. In Section 4, we offer the concluding remarks and highlight the key concerns that need to be addressed to meet the future challenges of the selection, design and application of suitable clay minerals/materials for the efficient removal of various cationic and anionic species present in various liquid nuclear waste streams under different experimental conditions.
2. Natural Clays (NCs) for Sorption of Radionuclides
Because of their numerous properties (thermal stability, high surface area, porous structure, cation exchange capacity, swelling behavior, etc.), NCs have been widely studied and applied in wastewater treatment/decontamination and in environmental waste management [72,73,74,75,76,77,78,79,80,81,82,83]. For decades, bentonite clay has been used as a potential buffer material in nuclear waste management in underground repositories globally [84,85,86,87,88,89,90,91,92]. Being ubiquitous in nature and one of the cheapest sorbent materials, NCs have attracted greater attention for their potential application in the sorption of hazardous/toxic metal ions as well as radionuclides from contaminated and waste water under different experimental conditions. More specifically, NCs have been highly useful for cationic hazardous/radionuclides/heavy metal removal due to the ion exchange between charge balancing cations (as alkali and alkaline earth metals) present in clays and cationic contaminant species (heavy metals, radionuclides, etc.) in targeted waste/solutions. The sorption behavior of various ions on bentonite clays can be determined by their specific surface area, chemical and mineralogical composition and cation exchange capacity [93,94,95,96]. This section encompasses the key and recent developments on the selection, characterization and application of potential NCs for the selective as well as effective removal of various radioactive metal cations and their surrogates under different physicochemical experimental conditions. The major outcomes/findings regarding the sorption of targeted metal cations under the optimized testing conditions have been highlighted and discussed throughout the selected studies.
Yu et al. investigated uranium (U) (VI) sorption on montmorillonite at different experimental conditions (pH, contact time, initial concentration of U(VI) solution, temperature and competing ions (NH4+, K+, Mg2+, SO42−, CO32−, NO3− and HCO3−)) via the batch method [97]. The chemical composition, the morphology before and after adsorption and the surface functional groups of montmorillonites were determined by x-ray fluorescence (XRF), scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FT-IR) analyses. The experimental results for the effect of contact time on the sorption of U(VI) on montmorillonite exhibited fast sorption kinetics as the sorption equilibrium was acquired within 30 min. The sorption data revealed that the removal of U(VI) from montmorillonite was altered by pH, temperature and competing ions. U(VI) sorption was enhanced at a pH between 4.0 and 7.0 and then lowered with further increased pH values. Moreover, the presence of competing cations (NH4+, K+ and Mg2+) in the testing solution impeded the sorption of U(VI) from montmorillonite, more specifically Mg2+, which decreased U(VI) sorption by approx. 40% (from approx. 70% to approx. 30%). The presence of CO32− in the solution affected the aqueous speciation of U(VI) and formed strong uranyl carbonate complexes, which, in turn, influenced the sorption by increasing the solubility of uranium. The U(VI) sorption from montmorillonite displayed the applicability of the second-order kinetic model and the Langmuir isotherm model, where the determined maximum adsorption capacity was close to the experimental data. The authors also calculated the thermodynamic parameters (the changes in free energy (ΔG), enthalpy (ΔH) and entropy (ΔS)) at different temperatures (298 K, 308 K and 318 K) and the results indicated that the adsorption of U(VI) on montmorillonite was an endothermic and spontaneous process.
Tran et al. reported the sorption behavior of uranium and cesium on bentonite in carbonate-rich environments as a function of ionic strength (both in low ionic strength (2.20 mM) artificial rainwater (ARW) and high ionic strength (169 mM) ARW), colloid concentration and initial metal concentration [98]. The sorption experiments were performed using salt concentrations to mimic those measured in the northern Negev Desert, Israel, so that the obtained data could be applied to interpret the U(VI) and Cs mobility under site-specific geochemical conditions. The presence and absence of U(VI) in ARW altered the Cs sorption on bentonite significantly (Figure 1). The Cs sorption was decreased by approx. 10%, containing 1–2 g/L bentonite colloids in the presence of U(VI) in ARW. Moreover, the sorption of U(VI) was decreased compared to Cs on bentonite under similar experimental conditions, which was attributed to the formation of stable ternary calcium-uranyl carbonate compounds in the solution (CaUO2(CO3)22−(aq) and Ca2UO2(CO3)3(aq)), rather than their interaction with bentonite. These ternary calcium-uranyl carbonate complexes can control U(VI) migration through a carbonate rock aquifer. Based on the results, the authors concluded that in brackish carbonate rock aquifers, both U(VI) and Cs can migrate as dissolved species rather than as colloid-associated solids. Furthermore, bentonite colloids in brackish solutions similar to AGW can be aggregated and deposited, thereby hindering the mobilization of both U(VI) and Cs.
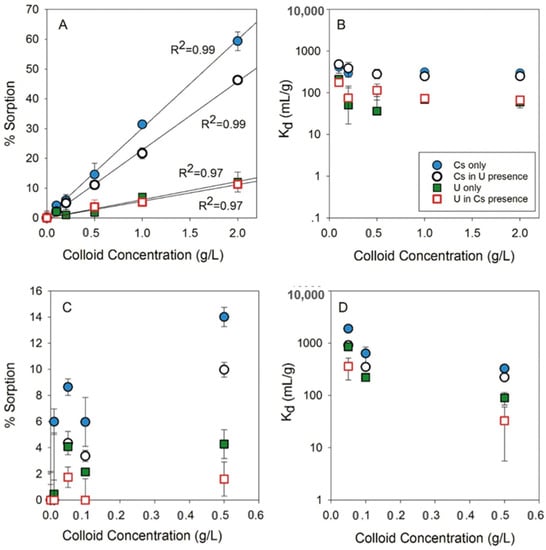
Figure 1.
Competitive metals’ sorption (in % and Kd)) in artificial rainwater (ARW) in a (A,B) high colloid concentration (0.1–2 g/L) and in a (C,D) low colloid concentration (0.01–0.5 g/L). Initial concentrations: Cs = 7.5 × 10−6 M, U(VI) = 3.6 × 10−6 M. Contact time: 14 days (adapted from reference [98]).
Philipp et al. very recently simulated the geochemical conditions (high pH and complex solution compositions) for radioactive waste in deep geological repositories and investigated the interactions of radionuclides with mineral surfaces under extreme conditions [99]. The authors reported the potential effect of Ca(II) on the retention capacity of Ca-bentonite for U(VI) and Np(VI) in hyper alkaline conditions using batch sorption experiments using time-resolved laser-induced luminescence spectroscopy (TRLFS). Zeta potential measurements as well as Ca2+ or Sr2+ sorption data on Ca-bentonite revealed that the alkaline earth metals sorbed efficiently between pH 8 and 13, which could be due to the partial compensation of the negative surface. Moreover, the uptake of U(VI) and Np(VI) in the absence and presence of Ca2+ or Sr2+ indicated that these cations considerably improved the radionuclide retention capacity of kaolinite and muscovite at pH ≥ 10. Site-selective TRLFS analysis data confirmed the presence of two U(VI) species on the alumosilicate surfaces. First, a ternary U(VI) complex, in which U(VI) is bound to the bentonite surface via bridging Ca2+ with the surface ≡ Ca − OH − U(VI)). Second, U(VI) uptake in the interlayer of calcium (aluminum) silicate hydrates (C-(A-)S-H). Finally, the authors concluded that the presence of alkaline earth elements could trigger the high retention capacity of hexavalent actinides on bentonite under hyper-alkaline repository conditions and, therefore, can be significant for the long-term retention of U(VI) and Np(VI) in underground repositories under the extremely alkaline geochemical conditions.
Seliman et al. investigated the uptake of mono-, di- and trivalent radionuclides from the contaminated solution using bentonite clay via the batch method [100]. In this study, the authors tested the sorption behavior of four radionuclides (134Cs(I), 90Sr(II), 133Ba(II) and 152Eu(III)) on Egyptian bentonite (Bent) and its Na+-modified form (Na-Bent) under different experimental conditions (contact time, pH of the solution and metal ion concentration) to identify the optimized parameters for their large scale application. The bentonite clay was obtained from the Alexandria governorate, Arab Republic of Egypt and was characterized by x-ray diffraction (XRD), XRF, SEM and ICP-OES techniques. The sorption of 134Cs, 90Sr, 133Ba and 152Eu on Bent and Na-Bent was observed to be highly dependent on the contact time, pH value and initial metal concentrations. The effect of contact time on the sorption of radionuclides on bentonite clay revealed fast sorption kinetics as the equilibrium was achieved within 10 min. The sorption data were applied well to the pseudo-second-order kinetic model. The distribution coefficient (Kd) values were calculated for the sorption of 134Cs, 90Sr, 133Ba and 152Eu o bentonite at different metal ion concentrations. The Kd values were determined in the order of 152Eu > 90Sr > 134Cs > 133Ba at lower metal ions’ concentrations for their sorption on both sorbents (Bent and Na-Bent). However, the Kd of 134Cs was found to be higher than 152Eu after 150 ppm for Bent and after 200 ppm for Na-Bent. 90Sr and 134Cs were preferably sequestered onto Na-Bent rather than Bent, especially at higher concentrations. The 134Cs, 133Ba, 90Sr and 152Eu adsorption data on Bent and Na-Bent were fitted by non-linear equations of Langmuir and Freundlich isotherm models and the linear equation of the Dubinin–Radushkevich (D-R) model. The applicability of the Langmuir isotherm model was observed for all metals adsorption on Bent and Na-Bent except for 133Ba the adsorption on Bent. The results for 133Ba adsorption on Bent followed the D-R model, whereas the Freundlich isotherm model was applied for 152Eu on Na-Bent. The adsorption energy values for the sorption of all metal ions were calculated from the D-R model and were found to be >8 kJ mol−1, which revealed that the sorption of these radionuclides on Bent and Na-Bent was mainly controlled by chemical reactions (by both cation exchange and surface complexation). Based on these results, the authors proposed that Egyptian bentonite with excellent adsorption capacity could be used as a potential sorbent for the treatment of aqueous waste as well as a buffer material in geological disposal sites.
Semenkova et al. reported Cs+ sorption on bentonite (obtained from the Kutch region of Gujarat, India) in the presence and absence of competing ions [101]. The major objective of the study was to develop a thermodynamic model to explore the possible interactions of Cs+ with different types of clay materials and to investigate the competition involving different cations in complex solutions. The obtained (raw) clay was crushed and sieved to size fractions < 75 μm, which the authors termed as original ‘Kutch clay’. Later, the original clay sample was chemically treated and converted into Na-form. To compare the performance of Kutch clay, the authors also used a well-known FEBEX bentonite from Spain in its sodium form (Na- FEBEX). All the solid samples were characterized using XRD, FT-IR, XRF and BET analyses. The two-site exchange mathematical thermodynamic model for the sorption of Cs+ on raw Kutch clays in different experimental conditions was developed. The study compared Cs+ sorption on raw Kutch clay and FEBEX clay over a wide range of pH and concentrations (Figure 2). The experimental results were fitted in non-linear isotherms for Cs+ sorption on both clays and the shapes of the isotherms encouraged the use of a two-site exchange thermodynamic model. The presence of different cations (Ca2+, Mg2+ and H+) in the solution considerably altered the Cs+ sorption on applied clay sorbents.
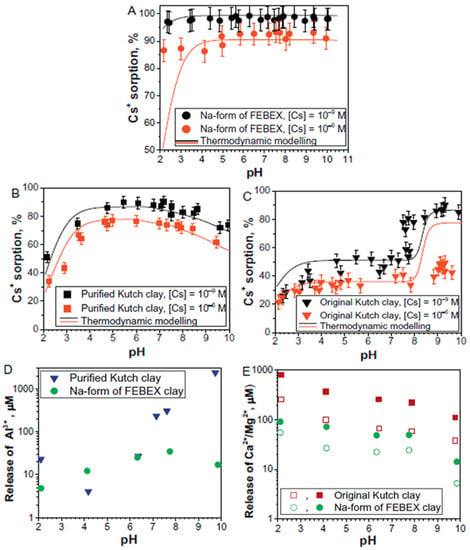
Figure 2.
Cs+ sorption on Na-form of FEBEX (A), purified (B) and original Kutch clays (C). Release of Al3+ from Na-form of FEBEX clay and purified Kutch clay (D) and Ca2+ and Mg2+ from original Kutch clay and Na-form of FEBEX clay (E). Clays concentration = 1 g/L, ionic strength (I) = 0.01 M (adapted from reference [101]).
Kim and Lee very recently simulated and interpreted the adsorption behavior of 22 elements/radionuclides (Am, Ac, Co, Cm, Cd, Cs, Cu, Na, Np, Ni, Nb, U, Sr, Sn, Pb, Pa, Pu, Po, I, Tc, Th and Zr) on bentonites using a machine-learning method [102]. The authors for the first time used the random forest (RF) method to predict the Kd values using a machine-learning model based on the Japan Atomic Energy Agency Sorption Database (JAEA-SDB). The study determined a database of ten input variables (pH, Kd, ionic strength, cation exchange capacity, radionuclide concentrations, solid–liquid ratio, oxidation number, surface area, ionic radius and electronegativity) for the RF model calculation. The study included two hyperparameters (the maximum number of variables to divide each node and the number of decision trees) together with the random seeds inside the RF model. The root mean square error values and correlation coefficient (R2) values were used to create the database for the Kd prediction and the performance of the RF on the defined systems. The R2 values were observed to be 0.8604 and 0.9175 for the nested cross-validation method and the general RF model, respectively, which indicated the meaningful applicability of the machine-learning method in predicting the Kd values. Additionally, the authors suggested that, apart from the investigated 22 radionuclides, this approach could be significantly applied to predict the Kd values for other radionuclides as well (based on their ionic radius, oxidation number and electronegativity). Finally, the authors proposed that their approach could be highly applicable to developing the Kd values prediction model for the interaction of various radionuclides on bentonites via a machine-learning algorithm and to simulate their retention behaviors in the deep geological repositories.
Izosimova et al. investigated the sorption of Cs(I) and Sr(II) on bentonites from the simulated aqueous waste solutions of cesium and strontium nitrates with different compositions and under various pH (3, 7 and 10) conditions [103]. Bentonite samples were collected from the deposits of Taganskoe (sample ‘T’ from the Republic of Kazakhstan), Dash-Salakhlinskoe (sample ‘DS’ from the Republic of Azerbaijan), Zyryanskoe (samples ‘Z’ from the Kurgan region, Russia) and 10th Khutor (‘10H’ from the Republic of Khakassia). The solid samples were characterized via different techniques (XRD, FT-IR and zeta potential measurements). The aqueous suspensions of bentonite T and DS attained pH values over nine and exhibited the presence of a considerable amount of non-silicate iron compounds as 1.0% and 0.5%, respectively. The uptake capacity of the clay materials for Cs(I) and Sr(II) from aqueous solutions varied from 50% to 90% and was displayed in the following order: T > DS > Z > 10 H. The sorption mechanism of Cs(I) and Sr(II) on bentonites was interpreted to be an outer-sphere complex formation on planar surfaces due to the ion exchange reaction. Moreover, at <0.5 mol/L Cs(I) and Sr(II) concentrations in the initial solutions, the Cs+ and Sr2+ sorption on bentonite was accompanied by competitive interactions with protons at pH < 6 and with mono- and divalent cations (Na+, K+, Ca2+ and Mg2+) at pH > 6.
Parisi reported the adsorption and separation behavior of cerium (III), lead (II) and crystal violet dye (CV) on K10-montmorillonite sorbent [104]. The selected montmorillonite clay was characterized by XRD and UV-visible spectroscopy. The study simulated the adsorption of Ce (III) and Pb (II) on montmorillonite mineral clay as the selective uptake of “model” contaminants from a ternary aqueous system. The authors tested various experimental conditions, including the solution pH, contact time, pollutant concentration and amount of montmorillonite clay. The obtained experimental data for the removal of all three contaminants on K10-montmorillonite were tested and analyzed via adsorption isotherms and the kinetic models. The results indicated considerable changes in the adsorption behavior of all three species under the defined experimental conditions. The Pb(II) and CV contaminants were adsorbed on K10-montmorillonite both in the acidic (pH 2) and near neutral (pH 6) conditions; however, the Ce(III) metal ions were adsorbed at pH 6 only, not in strongly acidic conditions (at pH 2). The authors proposed that the adsorption of CV dye on K10-montmorillonite attributed to the cation exchange as well as the surface complexation mechanism, involving the interlayers and surfaces/edges of montmorillonite. However, the removal of both metal cations (cerium (III) and lead (II)) from montmorillonite was achieved via cation exchange occurred at the clay surface. Finally, the authors concluded that the simultaneous decontamination of wastewaters containing multicomponent hazardous species could be achieved via rational experimental design and by optimizing the experimental conditions.
3. Modified/Functionalized Clays (MCs) for Sorption of Radionuclides
While NCs are widely used to sequester various cationic radionuclides, their modifications can further improve the removal capacity of hazardous contaminants from waste solutions [105,106,107,108,109]. The modification of NCs using organic cations (hexadecyl trimethyl ammonium (HDTMA), Benzethonium (BE) and hexadecyl pyridinium (HDPy), etc.) and inorganic additives can considerably alter their removal capacity of different anionic radionuclides [110,111,112,113]. During modification, the organic cations preferentially occupy negatively charged sites on the clay surface, changing the net charge on the minerals, which eventually triggers enhanced removal of anionic species by the ion exchange mechanism [114,115,116,117]. In this section, we discuss some key and recent contributions made toward the modification of NCs using different organic/inorganic surfactants/additives and their application for the removal of various radionuclide species from waste solutions under different experimental conditions.
Yang et al. very recently studied 99Tc removal from radioactive wastewater using modified bentonite using batch and column experiments [118]. The authors synthesized hexadecylpyridinium-chloride-monohydrate-modified bentonite (HDPy-bent) and applied it for the removal of perrhenate ReO4− (an analog for radioactive pertechnetate (99TcO4−)) under different testing conditions, such as the solution pH, contact time, initial concentration and competing anions. The solid material was characterized by FT-IR, x-ray photoelectron spectroscopy (XPS) and transmission electron microscopy combined with an energy-dispersive x-ray spectrometer (TEM-EDS). The obtained results revealed that the HDPy-bent sorbent exhibited outstanding ReO4− uptake capacity at a wide range of pH (2–10), fast adsorption kinetic and good adsorption capacity. Figure 3A–F exhibit the effects of pH and ionic strength on Re(VII) sorption on HDPy-bent, the zeta potential data of HDPy-bent sorbent prior to and after Re(VII) sorption, pseudo-second adsorption kinetic models at different temperatures and the UV–vis analysis results of Re(VII) sorption on HDPy-bent. The modified bentonite sorbent displayed high selectivity toward ReO4− in the presence of various competing anions (PO43−, SO42−, CO32−, Cl−, NO3− and HCO3−) as well as humic acid (HA). The uptake mechanism was interpreted as an anion exchange between ReO4− and Cl− and a precipitation reaction between HDPy+ and ReO4− in low concentration and high concentration, respectively. Moreover, based on the density functional theory (DFT) calculation, the authors suggested that ReO4− was adsorbed on the top of the pyridinium ring of HDPy+, establishing a face-to-face stacking with pyridinium ring and p-π interaction between O (of ReO4−) and pyridinium ring (Figure 4). The excellent selectivity of HDPy-bent toward ReO4− was attributed to the sturdier p-π interaction in HDPy+/ReO4− compared to the same interaction in HDPy+/other anions. Desorption experiments indicated that the HDPy-bent exhibited good durability and reusability. Finally, the authors concluded that their study demonstrates a low-cost adsorbent, which can potentially be used for the efficient removal of 99Tc from wastewater.
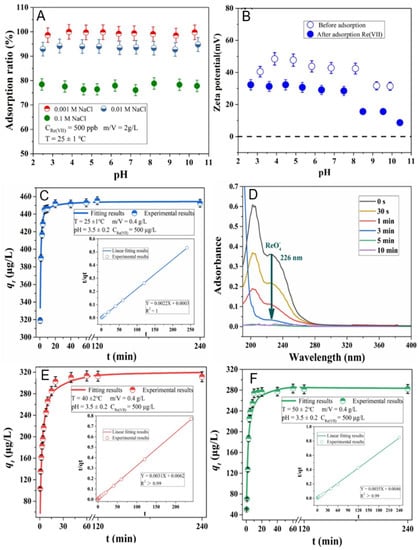
Figure 3.
The effects of ionic strength and pH on Re(VII) adsorption on HDPy-bent (A). The zeta potential measurement of HDPy-bent prior to and after Re(VII) adsorption (B). Pseudo-second adsorption kinetic models fitting at 25 ± 1 °C (C), 40 ± 2 °C (E) and 55 ± 2 °C (F) and UV–vis spectra of Re(VII) adsorption on HDPy-bent (D) (adapted from reference [118]).
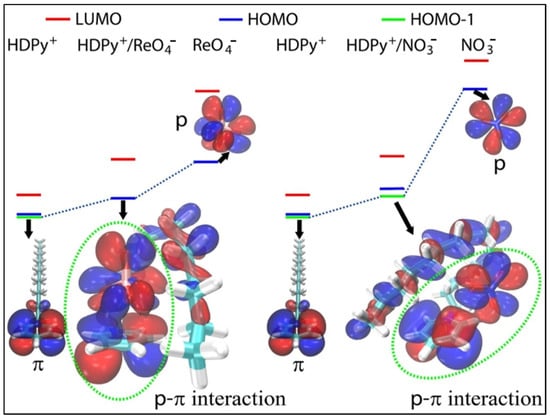
Figure 4.
Molecular orbital diagrams of HDPy+/ReO4− and HDPy+/NO3− presenting face-to-face stacking in HDPy+/ReO4− and side-to-side stacking in HDPy+/NO3− (adapted from reference [118]).
Very recently, Hong et al. investigated the application of surface-modified bentonite for the removal of Cs and Sr from (simulated) groundwater conditions [119]. The study proposed that the surface-modified bentonite could enhance the safety of radioactive waste in deep geological repositories by improving the radionuclide sorption capacity of bentonite. The authors applied an inorganic synthesis route to modify the bentonite surface to form NaP zeolite and to improve the radionuclides’ (Cs and Sr) removal efficiency on contaminated groundwater. They conducted a surface modification of bentonite (obtained from Yeonil, South Korea) by adding inorganic additives to form NaP zeolite on the surface of the bentonite. Sodium aluminate, sodium metasilicate and sodium hydroxide chemicals were used to synthesize NaP zeolite phases on the bentonite surface at 95 °C. XRD patterns and SEM analysis data confirmed the preparation of NaP/bentonite materials with spherical clusters of NaP zeolite covered on the surface of bentonite. FT-IR spectra and the Barrett-Joyner-Halenda (BJH) pore size distribution (PSD) analyses revealed that the water content and the macropores of NaP/bentonite composite materials were lowered compared to that of the original bentonite, which resulted in a smaller specific surface area of the NaP/bentonite composite compared to the original bentonite. The NaP-zeolite-modified bentonite surface enhanced the sorption efficiency of Cs and Sr. The original bentonite exhibited 72.8% and 70.5% of removal efficiencies of Cs and Sr, respectively, whereas, 10 wt% NaP/bentonite sorbed 95.4% Cs and 99.1% of Sr under the rest of the similar experimental conditions. Based on the results, the authors suggested that their approach could be significant for a facile surface modification and the application of bentonite for the efficient removal of radionuclides.
Soliman et al. applied thoron (TH, C16H11AsN2Na2O10S2)) modified montmorillonite for the sorption of cobalt radionuclide (Co(II)) from aqueous waste solutions [120]. The authors used FT-IR and XRD for qualitative analysis of the surface functional groups of the adsorbate and to confirm the organo-modification of the surface-modified montmorillonite. The characterization data revealed that TH was attached at the external sites of TH-modified montmorillonite (TMM) rather than the interlayer space. The authors performed batch adsorption experiments under various experimental conditions (contact time, pH, ionic strength, adsorbent dosage, adsorbate concentration and temperature). The obtained results suggested that the sorption of Co(II) was largely reliant on the pH as well as the quantity of the modifying agent loaded onto the montmorillonite. TMM achieved an enhanced Co(II) removal efficiency compared to its unmodified montmorillonite counterpart, which were calculated as 99% and 63%, respectively. Kinetic studies revealed 41.2%, 69.9% and 99.7% Co(II) sorption in 30, 20 and 10 min using 0.4, 0.2 and 0.1 mmol/L initial cobalt(II) concentration in solutions, respectively. The obtained results demonstrated the applicability of the pseudo-second-order kinetic model in the applied experimental conditions. Ionic strength played a significant role in the removal of Co(II) from waste solution, as Co(II) sorption was found to be 99% and 40% at 0.0001 and 0.1 M NaCl, respectively. The authors observed the applicability of both Langmuir and Freundlich isotherm models for Co(II) removal from aqueous waste. In the final experiments, the authors varied the reaction temperature to 30 °C, 45 °C, and 60 °C and calculated the thermodynamic parameters (changes in Gibbs free energy (ΔG) and enthalpy (ΔH)) for sorption of Co(II) on TMM sorbent. The results suggested that the cobalt(II) sorption on modified montmorillonite was spontaneous and endothermic in nature (ΔG = −2.687, −3.407 and −4.127 kJ/mol at 30 °C, 45 °C and 60 °C, respectively; ΔH° = 11.857 kJ/mol). Based on the results, the authors claimed that organo-modified montmorillonite (TMM) can be potentiality applied as an effective sorbent for the removal of Co(II) and other cationic radionuclides from aqueous radioactive waste under the optimized testing conditions.
Hu and Tan modified attapulgite clay for the sorption of Th(IV) ions from an aqueous solution at different contact times, sorbent contents, pH levels, ionic strengths and in the presence of fulvic acid (FA)/humic acid (HA) [121]. The authors investigated the sorption behavior and interaction mechanism of Th(IV) ions on the organo-modified attapulgite (OA). The study used ammonium citrate tribasic to modify the attapulgite clay and the OA was characterized by XRD and FT-IR techniques. The obtained sorption results suggested that the ammonium-citrate-tribasic-modified attapulgite clay exhibited an efficient Th(IV) sorption ability on aqueous waste solutions. Kinetic experiments revealed fast Th(IV) sorption on OA and the obtained data followed a pseudo-second-order kinetic model. Moreover, the Th(IV) sorption on OA was largely dependent on the pH, ionic strength and Th(IV) initial concentration. In the presence of HA/FA, the sorption of Th(IV) on OA sorbent was enhanced at pH < 4, whereas there was no considerable change in the sorption at pH > 4. Moreover, Th(IV) uptake on modified attapulgite was determined to be an endothermic process. The authors also varied the initial Th(IV) concentration in the sorption solution and tried to analyze the experimental data using three adsorption models (Langmuir isotherm, Freundlich isotherm and D-R isotherm). Among these, the Th(IV) adsorption data applied well in the Langmuir isotherm model compared to the Freundlich and D-R isotherm models. Finally, the authors concluded that the high Th(IV) sorption capacity of OA at fast reaction kinetics could make modified attapulgite a potential candidate for the removal of Th(IV) from waste solutions.
Ding et al. applied nickel MC for the Cs sorption on aqueous waste solutions [122]. The authors investigated the kinetics and sorption capacity of the pristine and modified akadama clay (AC) under various experimental conditions (solution pH, contact time, adsorbent dosage, Cs+ concentration, competitive cations and temperature). The study used lake water for the simulation of low-strength Cs+-contaminated water. Cs+ contamination in the lake in Japan is a typical water pollution (due to stormwater) related to radioactive accidents, and could result from the nuclear power stations due to some unavoidable natural disasters. The lake water was collected from the Matsumi Lake of the University of Tsukuba, Japan, which mainly receives stormwater. Prior to the experiments, the lake water was filtrated with a glass microfiber. Ni-modified AC clay (solid) samples were characterized via various techniques: XRD, FT-IR, N2 adsorption–desorption isotherms, SEM, EDS and thermo-gravimetric/differential thermal analyzer (TG/DTA). After modification, the AC was transformed into a typical mesoporous material and the pore diameter of the Ni-modified AC was reduced from >20 nm to <12 nm. Over 90% Cs+ removal from the modified AC sorbent was achieved from pH > 11 to pH ≥ 5 (alkaline to neutral conditions). The adsorption capacity of the Ni-modified AC was found to be 16.1 ± 0.9 mgg−1 (2.5 times higher than the pristine AC). The presence of competitive ions (Na+ and K+) in the waste solution resulted in reduced Cs+ adsorption, especially in the presence of K+, which was attributed to the similar characteristics of K+ and Cs+ (Figure 5). Moreover, 85% Cs+ removal efficiency on modified AC was achieved using lake water samples. Based on the results, the authors concluded that the modified AC was efficiently applied for Cs+ removal from low-strength Cs+-contaminated water and could be a suitable option analogous to other clays with respect to Cs+ adsorption capacity and selectivity.
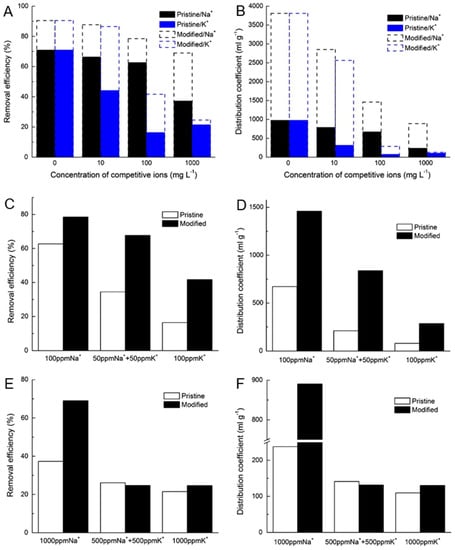
Figure 5.
Effect of competing ions (Na+ and K+) on the removal efficiency (A,C,E) and distribution coefficient (B,D,F) of Cs+ sorption on the pristine and modified akadama clay (AC). Initial Cs+ concentration: 1 ppm, dosage: 2.5 g L−1 (adapted from reference [122]).
Shakir et al. reported the simultaneous removal of monovalent (137Cs), divalent (60Co) and trivalent (152 + 154Eu) radionuclides, as well as chromotrope 2B (an anionic pollutant; C2B) from mixed radioactive process wastewater (MRPWW) using organo-bentonite (bentonite partially modified (PMB) with the cationic surfactant cetyltrimethylammonium bromide (CTAB)) [123]. The authors performed batch kinetics and isotherm studies to evaluate the adsorption efficiency of PMB. After modification, the organo-bentonite was characterized by XRD, XRF spectrophotometer and infrared spectroscopy. The modified PMB (78% of the cation exchange capacity) adsorbed all radionuclides and C2B from aqueous waste solutions. The study followed the pseudo-first-order kinetics and Langmuir adsorption isotherm model, which further indicated the sorption was mainly governed by intra-particle diffusion and external surface adsorption. Electrostatic and hydrophobic interactions were proposed to be the major sorption mechanism between C2B and PMB, whereas ion exchange appeared to be the main sorption mechanism for the removal of radionuclides from modified bentonite. The modified bentonite was capable of approx. 100% sorption of C2B and all radionuclides from the simulated MRPWW. The removal efficiency of PMB for 137Cs(I), 60Co(II) and 152 + 154Eu(III) were found to be 0.732 mmol/g, 0.644 mmol/g and 0.555 mmol/g, respectively. The thermodynamic parameters’ values (changes in free energy and enthalpy) at different sorption temperatures suggested that the sorption of C2B and radionuclides on PMB followed exothermic and spontaneous sorption.
Guerra et al. modified diquite (D) and bentonite (B) clay minerals (obtained from the Amazon region, Brazil) using 5-mercapto-1-methyltetrazole (MTTZ) and investigated the application of MCs for the adsorption of Th(IV) under different experimental conditions (solution pH, contact time and variation of Th(IV) concentration) [124]. After chemical treatment, the MC (DMTTZ and BMTTZ) materials were characterized by XRD, N2 adsorption/desorption isotherms, TEM and 13C NMR spectroscopy. The BET surface areas (SBET) of the chemically modified materials changed significantly due to the creation of additional microspores in the solid structure. The BET surface areas of the BMTTZ and DMTTZ samples were determined as 398.5 m2 g−1 and 178.8 m2 g−1, respectively, which were found to be quite higher than their natural counterparts, as the SBET of B and D samples were calculated as 41.42 m2 g−1 and 5.0 m2 g−1, respectively. The authors used a calorimetric titration procedure to determine the energetic effect (changes in enthalpy, free energy and entropy; ΔH°, ΔG° and ΔS°) at the solid–liquid interface caused by Th(IV) and phyllosilicate surfaces’ interaction and predicted that Th(IV) sorption on DMTTZ and BMTTZ sorbents were spontaneous and entropy-favorable. The maximum number of moles of Th(IV) adsorbed on DMTTZ and BMTTZ were determined as 10.45 × 10−2 mmol g−1 and 12.76 × 10−2 mmol g−1, respectively.
In another study, Guerra et al. reported the application of raw and chemically modified hectorite clays (obtained from the Amazon region, Brazil) for Th4+, U6+ and Eu3+ uptake from aqueous solutions [125]. Hectorite (H) clay was modified with 2-mercaptobenzimidazole (MBI) via heterogeneous and homogeneous treatment methods. The clay samples (both natural and modified materials) were analyzed using SEM and magic-angle-spinning nuclear magnetic resonance (MAS 29Si and 13C NMR) spectroscopy. After chemical modification, the peaks in the 112–129 ppm region in the 13C NMR spectra confirmed the attachment of organic functional groups onto the hectorite clay. The adsorption of Th4+, U6+ and Eu3+ on MC from aqueous waste solution followed the order of Eu3+ > U6+ > Th4+. The maximum number of moles adsorbed on modified hectorite clay was calculated as 14.01 mmolg−1, 12.85 mmolg−1 and 11.63 mmolg−1 for Eu3+, U6+ and Th4+ metal ions, respectively.
Makarov et al. investigated the application of activated carbon (additives) for the modification of bentonite and the role of composite/modified material in the removal of pertechnetate ions (TcO4−) from waste solution and its impact on the immobilization in bentonite-based engineered barriers [126]. The activated-carbon-modified bentonite clay composite materials were obtained by adding bentonite clays with 0.5, 1 and 5% mass ratios of milled activated carbon/graphite AG-3 (activated graphite derived from low-temperature coke dust/charcoal) and CAU (activated carbon of coconut shell). Synthesized sorbents were analyzed using XPS and X-ray absorption near-edge spectroscopy (XANES)/extended x-ray absorption fine structure (EXAFS) spectroscopy. The sorption tests were performed at a solid-to-liquid ratio of 1:20 in oxidizing conditions. The obtained sorption results suggested that the activated-carbon-modified bentonite clay (0.5–5% activated carbon (by weight) in the bentonite clay) significantly enhanced the TcO4- sorption capacity and achieved the Kd value up to 740 mLg−1. Finally, the authors suggested that the activated carbons (AG-3 and CAU) could be promising components for engineered clay-based barriers to mitigate the 99Tc mobility in the environment under oxidizing conditions.
4. Conclusions and Future Perspectives
In this review, we have highlighted and discussed some of the key as well as recent progress toward the design, characterization and applications of various clays for efficient sorption of both cationic and anionic radioactive contaminants from simulated waste solutions under different experimental conditions. We have particularly demonstrated the application of NCs (bentonite/montmorillonite) for the removal of different metal ions (surrogates of radionuclides) in the optimized experimental conditions. Moreover, this review has equally focused on the functionalization and modification of clay materials to improve the uptake capacity of the various radionuclides possessing variable chemical properties and high mobility behavior in the environment. Prior to the sorption experiments, the applied clays (both natural and functionalized clays) were efficiently characterized using various techniques (XRD, SEM, FT-IR, XPS, N2 adsorption/desorption isotherms, TGA, etc.) to analyze the key properties and their influences on the removal capacities of targeted ions/radionuclides. More specifically, the detailed characterization of modified clays with organic moieties has been widely discussed. The sorption efficiencies of various clays have been studied under different physicochemical conditions (pH, contact time, ionic strength, temperature, sorbent and sorbate doses, etc.) and these materials have displayed the potential sequestration of both anionic and cationic hazardous species at the optimized testing conditions (Table 1). The obtained results suggested that NCs have been very effective for the removal of metal cations, whereas modified/functionalized clay materials have exhibited excellent sorption capacities for both cationic and anionic specifies. The key aspects of this behavior of NCs and MCs can be attributed to their net/overall charges in aqueous media. It is noteworthy that NCs possess an overall negative charge in aqueous media and, therefore, are most suitable for the sequestration of metal cations/radionuclides. However, after functionalization/modification, the MCs can also be suitable for the efficient removal of anionic radionuclides/hazardous anions, as discussed and highlighted here. Therefore, being abundant and ubiquitous in nature, the clays can be effectively applied for the treatments of various radioactive contaminants (cations as well as anions) from waste solutions.

Table 1.
Sorption of radionuclides from (simulated) waste solutions using natural clays (NCs) and modified clays (MCs) at various experimental conditions.
Despite the significant developments in the application of clays as effective sorbent materials for the sorption of various cationic and anionic species from contaminated wastewater [127,128,129,130,131,132], a few challenges are required to be addressed to improve their application in future studies. It is noteworthy that after post-functionalization, the modified clays have exhibited efficient removal of various radionuclides’ species. However, clay modification using organics also has drawbacks in terms of cost efficiency, stability, environmental aspects and physicochemical characteristics of the modified organoclays on large-scale applications. Additionally, more detailed analytical approaches will be necessary to assess the overall performance of the functionalized clays for the treatment of radioactive waste in underground repositories. Future studies should focus on the application of theoretical and computational approaches (density functional theory calculations and molecular dynamics simulations) to assess further insights into the interaction mechanism of metal contaminants–radionuclides with various clays. A combined advancement (in experimental, analytical and theoretical approaches) to define the overall physicochemical properties of clays and clay-based sorbents under optimized conditions can be significant for their broader application in (a) the efficient and selective sorption of radioactive contaminants from waste solutions, (b) retarding their mobility in the environment and, finally, (c) in nuclear waste management. In addition, the management of the resulting waste after the sorption of radionuclides on clays and the fate of the final disposal via the immobilization of generated waste (or various waste form formulation) can be found in Singh et al. [27].
Overall, future studies should focus on resolving the aforementioned challenges and enhancing the application of clays/clay-based materials for the selective and effective removal of radionuclides from waste solutions. To meet these challenges, a detailed comprehensive database on the characterization and interaction behavior of natural and functionalized clays with various radioactive species (both cations and anions) using advanced analytical tools and theoretical as well as computational approaches are necessary. Additionally, the economic and environmental aspects should be equally considered during the selection, design and application of clay-based sorbents for treatments of radioactive waste in further studies.
Author Contributions
Conceptualization, B.K.S. and W.U.; writing: original draft preparation, B.K.S.; writing: review and editing, W.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Institute for Korea Spent Nuclear Fuel (iKSNF) and the National Research Foundation (NRF) of Korea grant funded by the Korean government (Ministry of Science and ICT, MSIT) (No. 2021M2E1A1085202).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosan, M.I. Radioactive waste classification, management and environment. Eng. Int. 2017, 5, 53–62. [Google Scholar] [CrossRef]
- Alexander, W.R.; Reijonen, H.M.; McKinley, I.G. Natural analogues: Studies of geological processes relevant to radioactive waste disposal in deep geological repositories. Swiss J. Geosci. 2015, 108, 75–100. [Google Scholar] [CrossRef]
- Fan, Q.; Li, P.; Pan, D. Radionuclides sorption on typical clay minerals: Modeling and spectroscopies. Interface Sci. Technol. 2019, 29, 1–38. [Google Scholar]
- Pearce, J.M. Limitations of nuclear power as a sustainable energy source. Sustainability 2012, 4, 1173–1187. [Google Scholar] [CrossRef]
- Petrescu, R.V.; Aversa, R.; Akash, B.; Berto, F.; Apicella, A.; Petrescu, F.I. Sustainable energy for aerospace vessels. J. Aircr. Spacecr. Technol. 2017, 1, 234–240. [Google Scholar] [CrossRef]
- Noyes, R. Nuclear Waste Cleanup Technologies and Opportunities; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Lee, W.E.; Ojovan, M.I. Fundamentals of radioactive waste (RAW): Science, sources, classification and management strategies. In Radioactive Waste Management and Contaminated Site Clean-Up; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–49, 50e. [Google Scholar]
- Corkhill, C.; Hyatt, N. Nuclear Waste Management; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Konoplev, A. Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment. Toxics 2022, 10, 578. [Google Scholar] [CrossRef]
- Hirose, K.; Povinec, P.P. Ten years of investigations of Fukushima radionuclides in the environment: A review on process studies in environmental compartments. J. Environ. Radioact. 2022, 251, 106929. [Google Scholar] [CrossRef]
- Tagami, K.; Hashimoto, S.; Kusakabe, M.; Onda, Y.; Howard, B.; Fesenko, S.; Pröhl, G.; Harbottle, A.R.; Ulanowski, A. Pre-and post-accident environmental transfer of radionuclides in Japan: Lessons learned in the IAEA MODARIA II programme. J. Radiol. Prot. 2022, 42, 020509. [Google Scholar] [CrossRef]
- Hashimoto, S.; Komatsu, M.; Miura, S. Radioactive Materials Released by the Fukushima Nuclear Accident. In Forest Radioecology in Fukushima; Springer: Singapore, 2022; pp. 1–10. [Google Scholar]
- Nanba, K. Behavior of Radionuclides in the Environment: Fukushima III; Springer Nature: London, UK, 2022. [Google Scholar]
- Onda, Y.; Taniguchi, K.; Yoshimura, K.; Kato, H.; Takahashi, J.; Wakiyama, Y.; Coppin, F.; Smith, H. Radionuclides from the Fukushima Daiichi nuclear power plant in terrestrial systems. Nat. Rev. Earth Environ. 2020, 1, 644–660. [Google Scholar] [CrossRef]
- Von Hippel, F.N. The radiological and psychological consequences of the Fukushima Daiichi accident. Bull. At. Sci. 2011, 67, 27–36. [Google Scholar] [CrossRef]
- Waddington, I.; Thomas, P.J.; Taylor, R.H.; Vaughan, G.J. J-value assessment of relocation measures following the nuclear power plant accidents at Chernobyl and Fukushima Daiichi. Process Saf. Environ. Prot. 2017, 112, 16–49. [Google Scholar] [CrossRef]
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima nuclear accidents: A review of the environmental impacts. Sci. Total Environ. 2014, 470, 800–817. [Google Scholar] [CrossRef]
- Povinec, P.P.; Gera, M.; Holý, K.; Hirose, K.; Lujaniené, G.; Nakano, M.; Plastino, W.; Sýkora, I.; Bartok, J.; Gažák, M. Dispersion of Fukushima radionuclides in the global atmosphere and the ocean. Appl. Radiat. Isot. 2013, 81, 383–392. [Google Scholar] [CrossRef]
- Beresford, N.A.; Copplestone, D. Effects of ionizing radiation on wildlife: What knowledge have we gained between the Chernobyl and Fukushima accidents. Integr. Environ. Assess. Manag. 2011, 7, 371–373. [Google Scholar] [CrossRef]
- Amano, H.; Akiyama, M.; Chunlei, B.; Kawamura, T.; Kishimoto, T.; Kuroda, T.; Muroi, T.; Odaira, T.; Ohta, Y.; Takeda, K.; et al. Radiation measurements in the Chiba Metropolitan Area and radiological aspects of fallout from the Fukushima Dai-ichi Nuclear Power Plants accident. J. Environ. Radioact. 2012, 111, 42–52. [Google Scholar] [CrossRef]
- Achim, P.; Monfort, M.; Le Petit, G.; Gross, P.; Douysset, G.; Taffary, T.; Blanchard, X.; Moulin, C. Analysis of radionuclide releases from the Fukushima Daiichi nuclear power plant accident part II. Pure Appl. Geophys. 2014, 171, 645–667. [Google Scholar] [CrossRef]
- Masson, O.; Baeza, A.; Bieringer, J.; Brudecki, K.; Bucci, S.; Cappai, M.; Carvalho, F.P.; Connan, O.; Cosma, C.; Dalheimer, A.; et al. Tracking of airborne radionuclides from the damaged Fukushima Daiichi nuclear reactors by European networks. Environ. Sci. Technol. 2011, 45, 7670–7677. [Google Scholar] [CrossRef]
- Koma, Y.; Shibata, A.; Ashida, T. Radioactive contamination of several materials following the Fukushima Daiichi Nuclear Power Station accident. Nucl. Mater. Energy 2017, 10, 35–41. [Google Scholar] [CrossRef]
- Imanaka, T.; Hayashi, G.; Endo, S. Comparison of the accident process, radioactivity release and ground contamination between Chernobyl and Fukushima-1. J. Radiat. Res. 2015, 56, i56–i61. [Google Scholar] [CrossRef]
- Querfeld, R.; Pasi, A.E.; Shozugawa, K.; Vockenhuber, C.; Synal, H.A.; Steier, P.; Steinhauser, G. Radionuclides in surface waters around the damaged Fukushima Daiichi NPP one month after the accident: Evidence of significant tritium release into the environment. Sci. Total Environ. 2019, 689, 451–456. [Google Scholar] [CrossRef]
- Kato, H.; Onda, Y.; Hisadome, K.; Loffredo, N.; Kawamori, A. Temporal changes in radiocesium deposition in various forest stands following the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2017, 166, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Hafeez, M.A.; Kim, H.; Hong, S.; Kang, J.; Um, W. Inorganic waste forms for efficient immobilization of radionuclides. ACS EST Eng. 2021, 1, 1149–1170. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, J.; Pak, D.; Kim, K.; Um, W. Technetium (Tc)/Rhenium (Re) Solubility and Leaching Behavior from Waste Forms: An Overview. Front. Nucl. Eng. 2023, 1, 1112080. [Google Scholar] [CrossRef]
- Kersting, A.B.; Efurd, D.W.; Finnegan, D.L.; Rokop, D.J.; Smith, D.K.; Thompson, J.L. Migration of plutonium in groundwater at the Nevada Test Site. Nature 1999, 397, 56–59. [Google Scholar] [CrossRef]
- Kato, K.; Konoplev, A.; Kalmykov, S.N. Behavior of Radionuclides in the Environment I; Springer: Singapore, 2020. [Google Scholar]
- Ochiai, E. Hiroshima to Fukushima—Biohazards of Radiation; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ochiai, E. Hiroshima to Fukushima; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Maamoun, I.; Eljamal, R.; Falyouna, O.; Bensaida, K.; Idham, M.F.; Sugihara, Y.; Eljamal, O. Radionuclides Removal from Aqueous Solutions: A Mini Review on Using Different Sorbents. Proc. Int. Exch. Innov. Conf. Eng. Sci. 2021, 7, 170–177. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Xi, W.; Zhao, Q.; Wang, S.; Qiu, M.; Wang, J.; Wang, X. Removal of radionuclides from aqueous solution by manganese dioxide-based nanomaterials and mechanism research: A review. ACS EST Eng. 2021, 1, 685–705. [Google Scholar] [CrossRef]
- Singh, B.K.; Tomar, R.; Kumar, S.; Jain, A.; Tomar, B.S.; Manchanda, V.K. Sorption of 137Cs, 133Ba and 154Eu by synthesized sodium aluminosilicate (Na-AS). J. Hazard. Mater. 2010, 178, 771–776. [Google Scholar] [CrossRef]
- Singh, B.K.; Tomar, R.; Tomar, R.; Tomar, S.S. Sorption of homologues of radionuclides by synthetic ion exchanger. Micropor. Mesopor. Mat. 2011, 142, 629–640. [Google Scholar] [CrossRef]
- Singh, B.K.; Jain, A.; Kumar, S.; Tomar, B.S.; Tomar, R.; Manchanda, V.K.; Ramanathan, S. Role of magnetite and humic acid in radionuclide migration in the environment. J. Contam. Hydrol. 2009, 106, 144–149. [Google Scholar] [CrossRef]
- Maskalchuk, L.N.; Baklai, A.A.; Leont’eva, T.G.; Makovskaya, N.A. Removal of Cesium Radionuclides from Aqueous Media with an Aluminosilicate Sorbent Prepared from Belaruskalii Production Waste. Radiochemistry 2019, 61, 459–463. [Google Scholar] [CrossRef]
- Bhadoria, R.; Singh, B.K.; Tomar, R. Sorption of toxic metals on sodium aluminosilicate (NAS). Desalination 2010, 254, 192–200. [Google Scholar] [CrossRef]
- Singh, B.K.; Tomar, R.; Kumar, S.; Kar, A.S.; Tomar, B.S.; Ramanathan, S.; Manchanda, V.K. Role of the humic acid for sorption of radionuclides by synthesized titania. Radiochim. Acta 2014, 102, 255–261. [Google Scholar] [CrossRef]
- Singh, B.K.; Bhadauria, J.; Tomar, R.; Tomar, B.S. Effect of humic acid on sorption of trace metal ions by sodium aluminosilicate. Desalination 2011, 268, 189–194. [Google Scholar] [CrossRef]
- Singh, B.K.; Tomar, R.; Jain, A.; Kumar, S.; Tomar, B.S.; Manchanda, V.K.; Ramanathan, S. Synthesis, characterization and role of magnetite in Cs migration in environment: Effect of humic acid. J. Appl. Geochem. 2008, 10, 507–513. [Google Scholar]
- Singh, B.K.; Mercier-Bion, F.; Lefevre, G.; Simoni, E. Effect of short chain aliphatic carboxylic acids for sorption of uranyl on rutile Zeta potential and in situ ATR-FTIR studies. J. Ind. Eng. Chem. 2016, 35, 325–331. [Google Scholar] [CrossRef]
- Wani, A.A.; Shahadat, M.; Khan, A.M.; Ali, S.W.; Ahmad, S.Z.; Uddin, M.K. Recent advances and future perspectives of polymer-based magnetic nanomaterials for detection and removal of radionuclides: A review. J. Mol. Liq. 2022, 365, 119976. [Google Scholar]
- Wang, J.; Zhuang, S. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Biotechnol. 2019, 18, 231–269. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.; Xu, X.; Wu, J. Coupled thermal-hydro model tests of double-layered buffer for nuclear waste repository. Prog. Nucl. Energy 2023, 156, 104541. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.G.; Ye, W.M. A systematic review of bentonite/concrete interaction system in HLW disposal repositories: Theoretical, experimental and geochemical modelling analysis. Constr. Build. Mater. 2022, 353, 129075. [Google Scholar] [CrossRef]
- Oladoye, P.O. Natural, low-cost adsorbents for toxic Pb (II) ion sequestration from (waste) water: A state-of-the-art review. Chemosphere 2022, 287, 132130. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, T.T.; Peng, H.K.; Ren, H.T.; Lin, J.H.; Lou, C.W. Natural-clay-reinforced hydrogel adsorbent: Rapid adsorption of heavy-metal ions and dyes from textile wastewater. Water Environ. Res. 2022, 94, e10698. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, A.A. A critical review on clay-based nanocomposite particles for application of wastewater treatment. Water Sci. Technol. 2022, 85, 3002–3022. [Google Scholar] [CrossRef] [PubMed]
- Ibigbami, T.B.; Adeola, A.O.; Olawade, D.B.; Ore, O.T.; Isaac, B.O.; Sunkanmi, A.A. Pristine and activated bentonite for toxic metal removal from wastewater. Water Pract. Technol. 2022, 17, 784–797. [Google Scholar] [CrossRef]
- Vieira, Y.; Netto, M.S.; Lima, É.C.; Anastopoulos, I.; Oliveira, M.L.; Dotto, G.L. An overview of geological originated materials as a trend for adsorption in wastewater treatment. Geosci. Front. 2022, 13, 101150. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, X.; Sun, D.A.; Zeng, Z. Thermal properties of GMZ bentonite pellet mixtures subjected to different temperatures for high-level radioactive waste repository. Acta Geotech. 2022, 17, 981–992. [Google Scholar] [CrossRef]
- Wu, T.; Feng, Z.; Geng, Z.; Xu, M.; Shen, Q. Restriction of Re (VII) and Se (IV) diffusion by barite precipitation in compacted bentonite. Appl. Clay Sci. 2023, 232, 106803. [Google Scholar] [CrossRef]
- Churchman, G.J.; Gates, W.P.; Theng, B.K.G.; Yuan, G. Clays and clay minerals for pollution control. Dev. Clay Sci. 2006, 1, 625–675. [Google Scholar]
- Murray, H.H. Overview—Clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The use of some clay minerals as natural resources for drug carrier applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef]
- Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Biomedical applications of cationic clay minerals. RSC Adv. 2015, 5, 29467–29481. [Google Scholar] [CrossRef]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General Introduction: Clays, clay minerals and clay science. Dev. Clay Sci. 2006, 1, 1–18. [Google Scholar]
- Zheng, X.; Li, X.; Xu, Y. Study on the shear strength and microstructure of Gaomiaozi bentonite under chemical conditions in a repository. Environ. Earth Sci. 2022, 81, 352. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, J.Y.; Cho, D.K. Re-evaluation of the bentonite buffer dimension in a geological repository for spent nuclear fuel under the KURT conditions. Prog. Nucl. Energy 2022, 145, 104138. [Google Scholar] [CrossRef]
- Claverie, M.; Garcia, J.; Prevost, T.; Brendlé, J.; Limousy, L. Inorganic and hybrid (organic–inorganic) lamellar materials for heavy metals and radionuclides capture in energy wastes management—A review. Materials 2019, 12, 1399. [Google Scholar] [CrossRef]
- Thiebault, T.; Brendle, J.; Auge, G.; Limousy, L. Zwitterionic-surfactant modified LAPONITE® s for removal of ions (Cs+, Sr2+ and Co2+) from aqueous solutions as a sustainable recovery method for radionuclides from aqueous wastes. Green Chem. 2019, 21, 5118–5127. [Google Scholar] [CrossRef]
- Beall, G.W. The use of organo-clays in water treatment. Appl. Clay Sci. 2003, 24, 11–20. [Google Scholar] [CrossRef]
- Lee, S.M.; Tiwari, D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: An overview. Appl. Clay Sci. 2012, 59, 84–102. [Google Scholar] [CrossRef]
- Groisman, L.; Rav-Acha, C.; Gerstl, Z.; Mingelgrin, U. Sorption of organic compounds of varying hydrophobicities from water and industrial wastewater by long-and short-chain organoclays. Appl. Clay Sci. 2004, 24, 159–166. [Google Scholar] [CrossRef]
- Rytwo, G.; Rettig, A.; Gonen, Y. Organo-sepiolite particles for efficient pretreatment of organic wastewater: Application to winery effluents. Appl. Clay Sci. 2011, 51, 390–394. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Hirotsu, T.; Sonoda, A. Montmorillonite modified with hexadecylpyridinium chloride as highly efficient anion exchanger for perchlorate ion. Chem. Eng. J. 2012, 191, 141–146. [Google Scholar] [CrossRef]
- Milutinović-Nikolić, A.; Maksin, D.; Jović-Jovičić, N.; Mirković, M.; Stanković, D.; Mojović, Z.; Banković, P. Removal of 99Tc (VII) by organo-modified bentonite. Appl. Clay Sci. 2014, 95, 294–302. [Google Scholar] [CrossRef]
- Sultana, S.; Karmaker, B.; Saifullah, A.S.M.; Galal Uddin, M.; Moniruzzaman, M. Environment-friendly clay coagulant aid for wastewater treatment. Appl. Water Sci. 2022, 12, 6. [Google Scholar] [CrossRef]
- Yusof, M.Y.M.; Idris, M.I.; Mohamed, F.; Nor, M.M. Adsorption of radioactive element by clay: A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 785, p. 012020. [Google Scholar]
- Es-sahbany, H.; El Yacoubi, A.; El Hachimi, M.L.; Boulouiz, A.; El Idrissi, B.C.; El Youbi, M.S. Low-cost and eco-friendly Moroccan natural clay to remove many bivalent heavy metal ions: Cu2+, Co2+, Pb2+ and Ni2+. Mater. Today Proc. 2022, 58, 1162–1168. [Google Scholar] [CrossRef]
- Matveenko, A.V.; Varlakov, A.P.; Zherebtsov, A.A.; Germanov, A.V.; Mikheev, I.V.; Kalmykov, S.N.; Petrov, V.G. Natural Clay Minerals as a Starting Material for Matrices for the Immobilization of Radioactive Waste from Pyrochemical Processing of SNF. Sustainability 2021, 13, 10780. [Google Scholar] [CrossRef]
- Kale, R.C.; Ravi, K. A review on the impact of thermal history on compacted bentonite in the context of nuclear waste management. Environ. Technol. Innov. 2021, 23, 101728. [Google Scholar] [CrossRef]
- Chikkamath, S.; Patel, M.A.; Kar, A.S.; Tomar, B.S.; Manjanna, J. Sorption and surface complexation modeling of 137Cs on Fe (II)-montmorillonite clay mineral relevant to nuclear waste disposal. Radiochim. Acta 2021, 109, 73–83. [Google Scholar] [CrossRef]
- Vereš, J.; Orolínová, Z. Study of the treated and magnetically modified bentonite as possible sorbents of heavy metals. Acta Montan. Slovaca 2009, 14, 152–155. [Google Scholar]
- Deepthi Rani, R.; Sasidhar, P. Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ. Earth Sci. 2012, 65, 1265–1274. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Rocha, F.; Janotka, I.; Komadel, P. An investigation into the use of blends of two bentonites for geosynthetic clay liners. Geotext. Geomembr. 2008, 26, 436–445. [Google Scholar] [CrossRef]
- Vejsada, J. The uncertainties associated with the application of batch technique for distribution coefficients determination—A case study of cesium adsorption on four different bentonites. Appl. Radiat. Isot. 2006, 64, 1538–1548. [Google Scholar] [CrossRef]
- Lee, J.O.; Kang, I.M.; Cho, W.J. Smectite alteration and its influence on the barrier properties of smectite clay for a repository. Appl. Clay Sci. 2010, 47, 99–104. [Google Scholar] [CrossRef]
- Ye, W.M.; Chen, Y.G.; Chen, B.; Wang, Q.; Wang, J. Advances on the knowledge of the buffer/backfill properties of heavily-compacted GMZ bentonite. Eng. Geol. 2010, 116, 12–20. [Google Scholar] [CrossRef]
- Liu, X.; Congress, S.S.C.; Cai, G.; Liu, L.; Puppala, A.J. Evaluating the thermal performance of unsaturated bentonite-sand-graphite as buffer material for waste repository using an improved prediction model. Can. Geotech. J. 2022. [Google Scholar] [CrossRef]
- Da, T.X.; Chen, T.; He, W.K.; Elshaikh, T.; Ma, Y.; Tong, Z.F. Applying machine learning methods to estimate the thermal conductivity of bentonite for a high-level radioactive waste repository. Nucl. Eng. Des. 2022, 392, 111765. [Google Scholar] [CrossRef]
- Alzamel, M.; Haruna, S.; Fall, M. Saturated hydraulic conductivity of bentonite–sand barrier material for nuclear waste repository: Effects of physical, mechanical thermal and chemical factors. Environ. Earth Sci. 2022, 81, 223. [Google Scholar] [CrossRef]
- Ito, S.; Tachibana, S.; Takeyama, T.; Iizuka, A. Constitutive model for swelling properties of unsaturated bentonite buffer materials during saturation. Soils Found. 2022, 62, 101161. [Google Scholar] [CrossRef]
- Zeng, Z.; Shao, J.; Sun, D.A.; Lyu, H.; Xu, Y.; Yang, C. Effect of thermal ageing on physical properties of MX80 bentonite under high-temperature conditions. Eng. Geol. 2022, 308, 106822. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, X.; Ming, H. Analysis of double-layered buffer in high-level waste repository. Ann. Nucl. Energy 2022, 165, 108660. [Google Scholar] [CrossRef]
- Zheng, W.; Li, T.; Gao, Y.; Liu, Y.; Zhu, X. Analysis of the Long-Term-Coupled Thermo-Hydro-Mechanical Performances and the Retardation Capacities of Composite Bentonite Buffers Using a Numerical Simulation Method. Arab. J. Sci. Eng. 2022, 47, 4785–4800. [Google Scholar] [CrossRef]
- Shao, J.; Zeng, Z.; Sun, D.A. Effect of heating time on hydro-mechanical behavior of MX80 bentonite. Environ. Earth Sci. 2022, 81, 483. [Google Scholar] [CrossRef]
- Haynes, H.M.; Bailey, M.T.; Lloyd, J.R. Bentonite barrier materials and the control of microbial processes: Safety case implications for the geological disposal of radioactive waste. Chem. Geol. 2021, 581, 120353. [Google Scholar] [CrossRef]
- Yıldız, B.; Erten, H.; Kış, M. The sorption behavior of Cs+ ion on clay minerals and zeolite in radioactive waste management: Sorption kinetics and thermodynamics. J. Radioanal. Nucl. Chem. 2011, 288, 475–483. [Google Scholar] [CrossRef]
- Rajec, P.; Mátel, L.; Orechovská, J.; Šúcha, J.; Novák, I. Sorption of radionuclides on inorganic sorbents. J. Radioanal. Nucl. Chem. 1996, 208, 477–486. [Google Scholar] [CrossRef]
- Brtáňová, A.N.N.A.; Melichová, Z.U.Z.A.N.A.; Komadel, P.E.T.E.R. Sorption of Cu2+ from aqueous solutions by Slovak bentonites. Ceram Silik 2012, 56, 55–60. [Google Scholar]
- Chen, Y.; Wang, J. Removal of radionuclide Sr2+ ions from aqueous solution using synthesized magnetic chitosan beads. Nucl. Eng. Des. 2012, 242, 445–451. [Google Scholar] [CrossRef]
- Yu, S.; Ma, J.; Shi, Y.; Du, Z.; Zhao, Y.; Tuo, X.; Leng, Y. Uranium (VI) adsorption on montmorillonite colloid. J. Radioanal. Nucl. Chem. 2020, 324, 541–549. [Google Scholar] [CrossRef]
- Tran, E.L.; Teutsch, N.; Klein-BenDavid, O.; Weisbrod, N. Uranium and cesium sorption to bentonite colloids under carbonate-rich environments: Implications for radionuclide transport. Sci. Total Environ. 2018, 643, 260–269. [Google Scholar] [CrossRef]
- Philipp, T.; Huittinen, N.; Azzam, S.S.A.; Stohr, R.; Stietz, J.; Reich, T.; Schmeide, K. Effect of Ca (II) on U (VI) and Np (VI) retention on Ca-bentonite and clay minerals at hyperalkaline conditions-New insights from batch sorption experiments and luminescence spectroscopy. Sci. Total Environ. 2022, 842, 156837. [Google Scholar] [CrossRef]
- Seliman, A.F.; Lasheen, Y.F.; Youssief, M.A.E.; Abo-Aly, M.M.; Shehata, F.A. Removal of some radionuclides from contaminated solution using natural clay: Bentonite. J. Radioanal. Nucl. Chem. 2014, 300, 969–979. [Google Scholar] [CrossRef]
- Semenkova, A.S.; Evsiunina, M.V.; Verma, P.K.; Mohapatra, P.K.; Petrov, V.G.; Seregina, I.F.; Bolshov, M.A.; Krupskaya, V.V.; Romanchuk, A.Y.; Kalmykov, S.N. Cs+ sorption onto Kutch clays: Influence of competing ions. Appl. Clay Sci. 2018, 166, 88–93. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.Y. Prediction of the Adsorption Behaviors of Radionuclides onto Bentonites Using a Machine Learning Method. Minerals 2022, 12, 1207. [Google Scholar] [CrossRef]
- Izosimova, Y.; Gurova, I.; Tolpeshta, I.; Karpukhin, M.; Zakusin, S.; Zakusina, O.; Samburskiy, A.; Krupskaya, V. Adsorption of Cs (I) and Sr (II) on Bentonites with Different Compositions at Different pH. Minerals 2022, 12, 862. [Google Scholar] [CrossRef]
- Parisi, F. Adsorption and separation of crystal violet, cerium (III) and lead (II) by means of a multi-step strategy based on k10-montmorillonite. Minerals 2020, 10, 466. [Google Scholar] [CrossRef]
- Cheng, J.; Leng, Y.; Gu, R.; Yang, G.; Wang, Y.; Tuo, X. Adsorption of uranium (VI) from groundwater by amino-functionalized clay. J. Radioanal. Nucl. Chem. 2021, 327, 1365–1373. [Google Scholar] [CrossRef]
- Mao, S.; Gao, M. Functional organoclays for removal of heavy metal ions from water: A review. J. Mol. Liq. 2021, 334, 116143. [Google Scholar] [CrossRef]
- Ci, Z.; Yue, Y.; Xiao, J.; Huang, X.; Sun, Y. Spectroscopic and modeling investigation of U (VI) removal mechanism on nanoscale zero-valent iron/clay composites. J. Colloid Interface Sci. 2023, 630, 395–403. [Google Scholar] [CrossRef]
- Bao, L.; Guo, F.; Wang, H.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Zhang, Q.; Nie, J.; Celik, A.; Islam, S.M.; et al. Functionalization of clay surface for the removal of uranium from water. Methods X 2021, 8, 101275. [Google Scholar] [CrossRef]
- Harbi, H.M.; Al-Baqawi, A.H.; El-Shahawi, M.S. Clay Minerals Functionalized by Magnetite (Fe3O4) Nanoparticles as Solid Platform for Removal of Chromium (VI) from Water: Characterization, Kinetics, Thermodynamics, Analytical Utility and Reusability Studies. Anal. Chem. Lett. 2020, 10, 636–653. [Google Scholar] [CrossRef]
- Yang, J.; Shi, K.; Gao, X.; Hou, X.; Wu, W.; Shi, W. Hexadecylpyridinium (HDPy) modified bentonite for efficient and selective removal of 99Tc from wastewater. Chem. Eng. J. 2020, 382, 122894. [Google Scholar] [CrossRef]
- Yang, J.; Tai, W.; Wu, F.; Shi, K.; Jia, T.; Su, Y.; Liu, T.; Mocilac, P.; Hou, X.; Chen, X. Enhanced removal of radioactive iodine anions from wastewater using modified bentonite: Experimental and theoretical study. Chemosphere 2022, 292, 133401. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Lee, J.Y. Adsorption of Aqueous Iodide on Hexadecyl Pyridinium-Modified Bentonite Investigated Using an Iodine–Starch Complex. Chemosensors 2022, 10, 196. [Google Scholar] [CrossRef]
- Li, D.; Seaman, J.C.; Kaplan, D.I.; Heald, S.M.; Sun, C. Pertechnetate (TcO4−) sequestration from groundwater by cost-effective organoclays and granular activated carbon under oxic environmental conditions. Chem. Eng. J. 2019, 360, 1–9. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Sarwar, M.T.; Yang, H. Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl. Clay Sci. 2023, 232, 106796. [Google Scholar] [CrossRef]
- Wall, N.A.; Maulden, E.; Gager, E.J.; Ta, A.T.; Ullberg, R.S.; Zeng, G.; Nava-Farias, L.; Sims, A.P.; Nino, J.C.; Phillpot, S.R.; et al. Functionalized Clays for Radionuclide Sequestration: A Review. ACS Earth Space Chem. 2022, 6, 2552–2574. [Google Scholar] [CrossRef]
- Li, D.; Kaplan, D.I.; Knox, A.S.; Crapse, K.P.; Diprete, D.P. Aqueous 99Tc, 129I and 137Cs removal from contaminated groundwater and sediments using highly effective low-cost sorbents. J. Environ. Radioact. 2014, 136, 56–63. [Google Scholar] [CrossRef]
- Yaming, L.; Mingliang, B.; Zhipeng, W.; Run, L.; Keliang, S.; Wangsuo, W. Organic modification of bentonite and its application for perrhenate (an analogue of pertechnetate) removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2016, 62, 104–111. [Google Scholar] [CrossRef]
- Yang, J.; Shi, K.; Wu, F.; Tong, J.; Su, Y.; Liu, T.; He, J.; Mocilac, P.; Hou, X.; Wu, W.; et al. Technetium-99 decontamination from radioactive wastewater by modified bentonite: Batch, column experiment and mechanism investigation. Chem. Eng. J. 2022, 428, 131333. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.; Um, W. Surface Modification of Bentonite for the Improvement of Radionuclide Sorption. J. Nucl. Fuel Cycle Waste Technol. 2022, 20, 1–12. [Google Scholar] [CrossRef]
- Soliman, M.A.; Rashad, G.M.; Mahmoud, M.R. Organo-modification of montmorillonite for enhancing the adsorption efficiency of cobalt radionuclides from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 10398–10413. [Google Scholar] [CrossRef]
- Hu, T.; Tan, L. Modifying attapulgite clay by ammonium citrate tribasic for the removal of radionuclide Th (IV) from aqueous solution. J. Radioanal. Nucl. Chem. 2012, 292, 819–827. [Google Scholar] [CrossRef]
- Ding, D.; Lei, Z.; Yang, Y.; Zhang, Z. Efficiency of transition metal modified akadama clay on cesium removal from aqueous solutions. Chem. Eng. J. 2014, 236, 17–28. [Google Scholar] [CrossRef]
- Shakir, K.; Ghoneimy, H.F.; Hennawy, I.T.; Elkafrawy, A.F.; Beheir, S.G.E.; Refaat, M. Simultaneous removal of chromotrope 2B and radionuclides from mixed radioactive process wastewater using organo-bentonite. Eur. J. Chem. 2011, 2, 83–93. [Google Scholar] [CrossRef]
- Guerra, D.L.; Viana, R.R.; Airoldi, C. Adsorption of thorium cation on modified clays MTTZ derivative. J. Hazard. Mater. 2009, 168, 1504–1511. [Google Scholar] [CrossRef]
- Guerra, D.L.; Viana, R.R.; Airoldi, C. Use of raw and chemically modified hectorites as adsorbents for Th (IV), U (VI) and Eu (III) uptake from aqueous solutions. Desalination 2010, 260, 161–171. [Google Scholar] [CrossRef]
- Makarov, A.V.; Safonov, A.V.; Konevnik, Y.V.; Teterin, Y.A.; Maslakov, K.I.; Teterin, A.Y.; Karaseva, Y.Y.; German, K.E.; Zakharova, E.V. Activated carbon additives for technetium immobilization in bentonite-based engineered barriers for radioactive waste repositories. J. Hazard. Mater. 2021, 401, 123436. [Google Scholar] [CrossRef]
- Klinkenberg, M.; Brandt, F.; Baeyens, B.; Bosbach, D.; Fernandes, M.M. Adsorption of barium and radium on montmorillonite: A comparative experimental and modelling study. Appl. Geochem. 2021, 135, 105117. [Google Scholar] [CrossRef]
- Stockmann, M.; Fritsch, K.; Bok, F.; Fernandes, M.M.; Baeyens, B.; Steudtner, R.; Müller, K.; Nebelung, C.; Brendler, V.; Stumpf, T.; et al. New insights into U (VI) sorption onto montmorillonite from batch sorption and spectroscopic studies at increased ionic strength. Sci. Total Environ. 2022, 806, 150653. [Google Scholar] [CrossRef]
- Glaus, M.A.; Frick, S.; Van Loon, L.R. A coherent approach for cation surface diffusion in clay minerals and cation sorption models: Diffusion of Cs+ and Eu3+ in compacted illite as case examples. Geochim. Cosmochim. Acta 2020, 274, 79–96. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Baeyens, B. Cation exchange and surface complexation of lead on montmorillonite and illite including competitive adsorption effects. Appl. Geochem. 2019, 100, 190–202. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Scheinost, A.C.; Baeyens, B. Sorption of trivalent lanthanides and actinides onto montmorillonite: Macroscopic, thermodynamic and structural evidence for ternary hydroxo and carbonato surface complexes on multiple sorption sites. Water Res. 2016, 99, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.M.; Baeyens, B.; Dähn, R.; Scheinost, A.C.; Bradbury, M.H. U (VI) sorption on montmorillonite in the absence and presence of carbonate: A macroscopic and microscopic study. Geochim. Cosmochim. Acta 2012, 93, 262–277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).