Abstract
Bacterial isolates assigned to the species Alicyclobacillus tolerans, which occupies an intermediate position between an organotrophic genus Alicyclobacillus and mixotrophic genus Sulfobacillus, were revealed as members of the acidophilic chemolithotrophic community during stirred-tank bioleaching of violarite–pentlandite–chalcopyrite concentrate at 40 °C. Surprisingly, this species succeeded more common iron-oxidizing community members after a series of bioleaching processes in bioreactors. The possibility of mixotrophic and organoheterotrophic growth of Al. tolerans, tolerance to low pH values (1.0–1.15), as well as preservation of cells via sporulation under unfavorable conditions, may explain its key role in the bioleaching of the copper–nickel bulk concentrate. Isolation of two other sulfur-oxidizing pure cultures dominating the microbial community, together with their phylogenetic characterization, allowed the assignment of these isolates to the species Acidithiobacillus caldus. This and other studies of acidophilic microbial communities are important for the development and intensification of the bioleaching processes, including a biobeneficiation approach previously proposed by us.
1. Introduction
Biohydrometallurgy is one of the most promising fields of the modern mining industry. For decades, different bio-approaches have been proposed and successfully applied at the laboratory and industrial scale [1]. Among these approaches, biotreatment of sulfide raw materials containing precious and nonferrous metals is widely applied. It is based on the activity of acidophilic microbial communities oxidizing iron and sulfur components and contributing to the dissolution of valuable metals or their selective accumulation in solids [2,3,4]. Biooxidation/bioleaching of sulfide ores, concentrates, and industrial wastes can be carried out by environmentally sound and cost-effective technologies that are based on chemical and biological principles [1,5,6,7].
In particular, bioleaching has been shown to be a promising method for processing bulk concentrates of nonferrous metals to obtain selective concentrates [4,8,9]. Bioleaching techniques may allow the obtainment of marketable high-grade products [4,9], contributing to the resource-efficient management of raw materials. Leaching the violarite–pentlandite–chalcopyrite concentrates is determined by the properties of refractory and readily oxidized minerals composing them. While nickel is gradually leached from pentlandite and violarite, chalcopyrite is very stable in ferric sulfate solutions [10]. During bioleaching of copper–nickel concentrates, elemental sulfur and ferrous iron are formed among the reaction products of the oxidation of chalcopyrite (CuFeS2), pentlandite ((Ni,Fe)9S8), and violarite (FeNi2S4). Further, S0 and Fe2+ are oxidized by acidophilic microorganisms to sulfate and ferric iron as final products, respectively [11,12]. The use of these patterns allows the production of selective high-grade copper concentrates, with very low nickel content. Acidophilic communities analyzed during the processing of nickel-containing concentrates at commercial or pilot plants were composed of chemolithotrophic microorganisms [13]. Members of the communities belonged to several functional and phylogenetic microbial groups. The structure of microbial communities depended on the composition of the processed material and conditions of the process, such as temperature, aeration, pH, the ratio of sulfide minerals, and other factors. For instance, during the processing of the nickel-containing concentrate at 49 °C, Acidithiobacillus (At.) caldus (63%), Acidimicrobium sp. (32%), and Sulfobacillus (S.) sp. (4%) prevailed, while at 55 °C, Sulfobacillus sp. became the predominant member of the community (93%), and the shares of At. caldus and Acidimicrobium sp. decreased to 5% and 2%, respectively [13]. During thermophilic bioleaching of a low-grade nickel-copper concentrate at 65, 70, 72, 73, 74, and 75 °C, thermophilic sulfur-oxidizing archaea of the genera Sulfolobus and Metallosphaera dominated the process. At the same time, another archaeon, Metallosphaera sp., was a minor component of the bioleaching community [14].
In our previous studies, bioleaching of the violarite–pentlandite–chalcopyrite concentrates at different temperatures (30, 35, 40, and 50 °C) indicated that nickel was almost completely removed at 40 °C. Thus, a high-grade copper concentrate containing 15.6% copper and only 0.54% nickel was obtained after 22 days of bioleaching [4]. Based on the 16S rRNA gene metabarcoding analysis, the microbial communities were dominated by bacteria of the genera Leptospirillum (L.) and Acidithiobacillus at the beginning of the process; Sulfobacillus spp. and archaea of the genera Cuniculiplasma and Ferroplasma (F.) were minor components. At the end of the process, communities formed at 35 and 40 °C included Acidithiobacillus spp., Sulfobacillus spp., and Alicyclobacillus (Al.) spp. [4]. The genus Alicyclobacillus belongs to the family Alicyclobacillaceae and consists of Gram-positive or Gram-variable aerobic, heterotrophic/mixotrophic, thermophilic or moderately thermophilic, acidophilic, spore-forming rods, including several iron- and sulfur-oxidizing species [15,16]. The fact that representatives of Alicyclobacillus turned out to be among prevailing microorganisms in the bioleaching microbial communities attracted our attention. To our knowledge, no studies have reported any significant contribution of these bacteria to the treatment of nickel-containing raw materials, although we have previously identified Alicyclobacillus spp. in the structure of acidophilic bioleaching communities [4,9].
Therefore, in this research, we aimed to isolate pure cultures of prevailing sulfur- and iron-oxidizing microorganisms (including Alicyclobacillus strains) from the microbial community formed by the end of the copper–nickel concentrate bioleaching at 40 °C, to identify the exact phylogenetic position of the isolates, as well as to determine their role in the concentrate bioleaching. Since the results obtained in this study concern microorganisms involved in the biotreatment of violarite–pentlandite–chalcopyrite concentrates, they can be used for the development of industrial approaches to processing sulfide raw materials.
2. Materials and Methods
2.1. Substrate and Conditions for Bioleaching in Bioreactor
This study was carried out with the bulk sulfidic concentrate obtained by froth flotation of the copper–nickel sulfide ore (Kamchatka territory, Russia). The mineral composition of the concentrate was as follows: pyrrhotite, pyrite, chalcopyrite, pentlandite, and violarite. Quartz, chabazite, jarosite, plagioclase, chlorite, and albite were gangue minerals. Table 1 shows the contents of the main elements and sulfide minerals in this copper–nickel concentrate. The particle size of the concentrate was d80 < 44 µm.

Table 1.
Content of main elements and sulfide minerals (wt%) in bulk copper–nickel concentrate.
Biobeneficiation (via bioleaching) of the concentrate in a 2.0 L bioreactor, with a working volume of 1.0 L, was carried out for 22 days at 40 °C. The solid sample of the concentrate of the aforementioned composition (10 g, 1%(w/v) pulp density) was added to 1.0 L of the inoculum containing 8.0 × 108 cells/mL (see Section 2.2). The initial pH value was adjusted to 1.15. The experiment was carried out while stirring (500 rpm) with an overhead 8-blade Rushton turbine impeller with intense aeration (4 v/(v·min)).
2.2. Microbial Community Used as Inoculum for Bioleaching
An acidophilic microbial consortium was formed for subsequent bioleaching experiments. The members of the consortium were obtained from the stirred-tank bioprocessing of zinc-containing wastes [17], as well as copper–zinc and copper–nickel concentrates [4,9]. Mixed and pure cultures of chemolithotrophic mixotrophs, autotrophs, and heterotrophs were also added to the consortium. Iron- and sulfur-oxidizing species composing the original consortium were as follows: S. thermosulfidooxidans, S. thermotolerans, At. ferrooxidans, At. caldus, At. thiooxidans, L. ferriphilum, F. acidiphilum, Cuniculiplasma sp., Acidiplasma (Ad.) sp., Acidibacillus sp., Ferrimicrobium sp., Alicyclobacillus spp., and Acidiphilium multivorum. This original community, rich in different microbial cultures, was subsequently grown in a flask containing 0.9 L of the culture medium at 40 °C to obtain inoculum for the tests in bioreactors. The culture medium contained the reduced salt base of the 9K medium [18] of the following composition (g/L): (NH4)2SO4, 0.75; KCl, 0.025; K2HPO4·3H2O, 0.125; MgSO4·7H2O, 0.125; Ca(NO3)2·4H2O, 0.0025; FeSO4·7H2O, 30.0; S0, 10.0; and yeast extract, 0.1. The microbial consortium of the abovementioned structure (0.1 L) was added to 0.9 L of the culture medium, pH was adjusted to 1.6 (using 5 M H2SO4), and the flask was incubated for 48 h at 40 °C in a Redline RI53 thermostat (Binder, Germany). For aeration, the air was supplied at a flow rate of 1 v/(v·min). At the end of cultivation, the obtained solution had a pH value of 1.3 and contained 8.0 × 108 cells/mL and Fe3+ (5.0 g/L). Unoxidized elemental sulfur was separated from the solution by filtration on paper filter. The solution was used for subsequent stirred-tank bioleaching tests.
2.3. Isolation and Characterization of Predominant Microorganisms during Bioleaching
To determine microorganisms dominating the acidophilic community formed after 22 days of biobeneficiation of the bulk violarite–pentlandite–chalcopyrite concentrate at 40 °C, the following research was carried out. To obtain the community formed by the end of bioleaching, the suspension was centrifuged to precipitate solids (100× g, 2 min), and the obtained supernatant was used for subsequent inoculation of the liquid media selective for iron and sulfur oxidizers.
Pure cultures of the predominant sulfur- and iron-oxidizing strains were isolated by serial tenfold dilutions (10−1–10−10) in the selective media containing the reduced salt base of the 9K medium of the composition shown in Section 2.2 and ferrous iron (50 mM Fe2+; pH 1.7) or elemental sulfur (1%, w/v; pH 2.2), with or without yeast extract (0.02%, w/v). The amount of inoculum was 10% (v/v). The strains were cultured in 250 mL Erlenmeyer flasks (100 mL of the liquid medium) on Unimax-1010 rotor shakers (Heidolph Instruments, Schwabach, Germany; 180× rpm) in Inkubator-1000 thermostats (Heidolph Instruments, Schwabach, Germany) at 40 °C.
To determine the characteristics of the growth of the isolated Alicyclobacillus strain and substrate oxidation, the bacterium was cultivated in flasks in the medium containing Fe2+ (75 mM) or S0 (1%, w/v) in the presence or absence of yeast extract (0.02%, w/v) (see initial pH values and salt base composition of the medium in Section 2.2). Yeast extract (0.04%) was used to test the growth of the strain AMF (pH 2.2) under obligately heterotrophic conditions.
Two series of experiments in each variant of the bacterial growth included three parallels (flasks) and two replicates for measured parameters. Statistical processing was performed using Microsoft Excel 2013. The standard deviation (SD) of the arithmetic mean was calculated, and the significance of the results was assessed using the Student’s t-test at the significance level p ≤ 0.05.
2.4. Taxonomic Research and Phylogenetic Analysis
Cells that grew in the last dilution were visually checked for purity of the culture using light microscopy, and reinoculated in flasks containing fresh media. Late-exponential cells were collected by centrifugation (8000× g, 20 min, 4 °C) and sequentially washed with the iron-free 9K medium salt base (pH 1.7) for subsequent determination of the phylogenetic position of the strains. The extraction and purification of DNA were carried out using the DNAEKSPRESS kit (NPF Litekh, Russia) according to the manufacturer’s recommendations. The concentrations of the DNA preparations were 30–50 µg/mL. PCR amplification of the 16S rRNA gene fragment with standard primers for the bacterial 16S rRNA gene fragment, amplicon purification and subsequent sequencing, as well as phylogenetic identification of the pure microbial cultures, were carried out as previously described [17,19]. To identify microorganisms at the species level, analysis of the similarity of nucleotide sequences of the 16S rRNA gene fragments was carried out using the Nucleotide Basic Local Alignment Search Tool (BLAST; Bethesda, MD, USA, https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 25 January 2022). The 16S rRNA gene sequences of Acidithiobacillus and Alicyclobacillus strains were deposited in the GenBank databases under accession numbers OM407403 and OM407404.
The phylogenetic tree was constructed with the MEGA 11 (version 11.0.10) software package [20] using the sequences of the type strains and some other isolates identified as the closest Alicyclobacillus strains with the BlastN algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi; accessed on 27 January 2022). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches [21]. The evolutionary history was inferred using the Neighbor-Joining method [22]. The evolutionary distances were computed using the Maximum Composite Likelihood method [23] and were in the units of the number of base substitutions per site.
2.5. Analytical Methods
The number of cells in 1 mL was determined by tenfold dilutions and subsequent direct counting in the Goryaev chamber using a Mikmed-2 microscope equipped with a phase-contrast device (LOMO, Moscow, Russia). The cell morphology was also observed using light microscopy. The values of the pH and redox potential were measured with a pH-150MI pH meter–millivoltmeter (Izmeritelnaya tekhnika, Moscow, Russia). The concentration of the Fe3+ ions in the liquid phase was determined by complexometric titration [24]. The same method was used to determine the total concentration of iron after the reaction with ammonium peroxydisulfate. The concentration of ferrous iron ions was calculated as the difference between the total and ferric iron. Flame atomic absorption spectroscopy (FAAS) (Perkin Elmer 3100, PerkinElmer, Waltham, MA, USA) was used to determine the concentrations of copper and nickel. All experiments were carried out in duplicate. Statistical processing was performed using Microsoft Excel 2013. The standard deviation (SD) of the arithmetic mean was calculated, and the significance of the results was assessed using Student’s t-test at the significance level p ≤ 0.1.
3. Results and Discussion
3.1. Parameters of Liquid Phase of Pulp during Bioleaching
Characteristics of the liquid phase of the pulp during the bioleaching (biobeneficiation) of the violarite–pentlandite–chalcopyrite concentrate at 40 °C are shown in Figure 1 and Table 2. Ferrous iron ions were determined in the liquid phase only in the first two days of the process. This could be associated with the fast chemical dissolution of pyrrhotite. The presence of all iron in the oxidized form after four days of the process indicated the immediate and complete oxidation of ferrous iron released from sulfide minerals by acidophilic microorganisms. Simultaneously, part of ferric iron was precipitated in the form of jarosite, as previously described [4]. The nickel and copper concentration increased gradually for 19 days. We have previously shown that nickel sulfides are dissolved relatively slowly (for more than 21 days) [4]. At the end of the process in this research, concentrations of dissolved nickel and copper reached 11.9 and 1.33 mM, respectively. During the experiment, the pH values of the liquid phase remained almost unchanged in the range of 1.10–1.20. The redox potential value decreased at the beginning of the process from 910 to 780 mV due to the consumption of ferric iron for the oxidation of sulfide minerals. After four days of the process, the redox potential value increased and stabilized at the level of 930–943 mV. According to the metabarcoding analysis (V3–V4 hypervariable region of the 16S rRNA gene) carried out in our previous research [4], the microbial community formed at the end of the process at 40 °C was phylogenetically diverse, containing members of the following genera: Acidithiobacillus, Sulfobacillus, Alicyclobacillus, Leptospirillum, Acidibacillus, and Cuniculiplasma. We have previously suggested that the crucial role in bioleaching of the copper–nickel concentrates belongs to Sulfobacillus spp. and Alicyclobacillus spp. since they have been identified in all communities formed at moderate and higher temperatures of the process (35, 40, and 50 °C). In this study, we aimed to determine the predominant sulfur and iron oxidizers in the community formed at 40 °C and to determine the role of Alicyclobacillus sp. Previously, 40 °C has been shown to be an optimum temperature for the biobeneficiation of copper–nickel concentrates [4,9]. Therefore, our present study concerned this temperature mode.
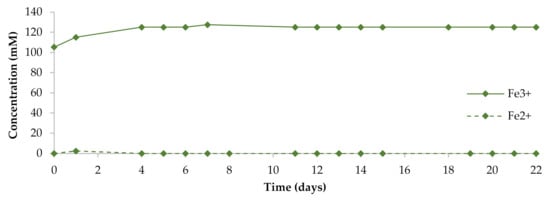
Figure 1.
Iron concentration in the liquid phase of the pulp during bioleaching of the concentrate.

Table 2.
Parameters of the pulp liquid phase (pulp density, 1 wt%) at the beginning and after 22 days of bioleaching of copper–nickel concentrate in a bioreactor at 40 °C.
3.2. Isolation and Identification of Strains Dominating Bioleaching Microbial Community
To determine microorganisms dominating the microbial community at the end of the bioleaching process, pure cultures of iron and sulfur oxidizers were isolated under autotrophic and mixotrophic conditions by a method of tenfold serial dilutions. Four selective nutrient media were used. Two media contained ferrous iron (50 mM) or elemental sulfur (1% (w/v)) (autotrophic conditions), while two other media were supplemented with yeast extract (0.02%) in addition to inorganic energy substrates (mixotrophic growth). The growth of microbial isolates was shown until the 10−8 dilution in the presence of ferrous iron and 10−9 dilution in the media containing S0. These results indicated that the abundance of the predominant strains in the liquid phase of the pulp in the bioreactor reached 108–109 cells/mL.
The similarity of the nucleotide sequences of the 16S rRNA gene fragments of two pure cultures isolated from the auto- and mixotrophic media containing S0 was 100%. These cultures were designated AMS-3 and AMS-4 and showed the closest similarity (99.2% similarity; 100% coverage) to At. caldus strains, including strains MP3 and MP4. These strains have been previously isolated by us from the process of complete biological oxidation of the product of ferric leaching of the zinc concentrate, which contained 9% of sphalerite, 5% of chalcopyrite, and 29.7% of elemental sulfur [17]. The pure cultures AMS-3 and AMS-4 were considered the same strain (designated AMS) that could actively grow in the medium containing sulfur in the presence or absence of organic substances. The partial nucleotide sequence of the 16S rRNA gene (1267 bp) of the strain AMS was deposited in the GenBank databases under accession number OM407403.
While the prevalence of the sulfur oxidizer At. caldus in the bioleaching of copper–nickel and zinc-containing raw materials has been repeatedly shown, the predominant iron oxidizers in microbial communities are usually Ferroplasma spp. and Leptospirillum spp., although their numbers tend to decrease at the end of the process if the availability of ferrous iron is low [2,4,9,13]. Bacteria Sulfobacillus spp. that are capable of both sulfur and iron oxidation in the presence of small amounts of organic substances are also often detected as predominant cultures in such processes [13,25]. At the end of the bioleaching of the copper–nickel concentrate, the microbial communities were represented by the species S. thermosulfidooxidans at 40 and 50 °C, while S. thermotolerans was determined at 35, 40, and 50 °C.
Growth in the media containing ferrous iron in the presence or absence of yeast extract allowed the obtainment of two pure cultures of rods, which were designated AMF-1 and AMF-2. Nucleotide sequences of their 16S rRNA genes showed 100% similarity. Therefore, these cultures were regarded as the same strain designated AMF, and the nucleotide sequence of the 16S rRNA gene (1414 bp) of this strain was deposited in the GenBank databases under accession number OM407404.
The closest phylogenetic relatives of the isolates AMF-1 and AMF-2 belonged to the species Al. tolerans, with the closest similarity to the strain PCG-3 (99.65% similarity; 99% coverage). Thus, unexpectedly, Al. tolerans AMF proved to be a predominant iron oxidizer in the microbial community during bioleaching of the violarite–pentlandite–chalcopyrite concentrate. At the same time, the strain Al. tolerans PCG-3 was isolated as a member of the indigenous microbial community from the sample of the pyrite–arsenopyrite gold-bearing flotation concentrate at 45–47 °C. The strain PCG-3 was a minor component of the microbial community and was physiologically similar to the type strain Al. tolerans K1T [19].
Based on the partial 16S rRNA gene sequences of the pure culture of Al. tolerans AMF obtained by Sanger sequencing and the 16S rRNA gene sequences of other Alicyclobacillus spp. available in the GenBank databases, a phylogenetic tree was constructed (Figure 2). The analyzed 16S rRNA gene sequence clustered in the same subgroup with other Al. tolerans strains, including the strain PCG-3 and the type strain K1.

Figure 2.
Dendrogram of Alicyclobacillus strains showing the phylogenetic position of Al. tolerans AMF, according to the 16S rRNA gene sequences. The phylogenetic tree was constructed using MEGA 11 [20]. The evolutionary history was inferred using the Neighbor-Joining method [21]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches [22]. The bootstrap values of ≥80 are shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method [23] and are in the units of the number of base substitutions per site. This analysis involved 14 nucleotide sequences. There were a total of 1558 positions in the final dataset. Bacillus subtilis IAM 12118 was used as an outgroup.
3.3. Characterization of Al. tolerans AMF Growth and Substrate Oxidation
Specific characteristics of growth and substrate oxidation of the strain Al. tolerans AMF are summarized in Table 3. The strain AMF was compared to the type strain K1, which is known by the limited requirements for Fe2+, S−/S0, or sulfide minerals as electron donors under mixotrophic growth conditions. In general, Al. tolerans has been shown to occupy an intermediate position between the organotrophic genus Alicyclobacillus and mixotrophic genus Sulfobacillus [26].

Table 3.
Main characteristics of growth and substrate oxidation of Al. tolerans AMF isolated from a bioreactor during bioleaching of the copper–nickel concentrate in comparison with phenotypic features of Al. tolerans K1T.
Under mixotrophic conditions, the pure culture of the strain AMF isolated from the pulp of the reactor actively oxidized ferrous iron: 60 mM Fe3+ after three days of cultivation, reaching the maximum cell abundance of 2.7 × 108 cells/mL after 20 h of growth. Therefore, the strain was capable of only incomplete oxidation of iron (15 mM Fe2+ remained unoxidized). The strain was also able to grow chemolithoautotrophically in the medium containing ferrous iron during two culture transfers (0.5–0.8 × 108 cells/mL after 48 h of growth), without additional organic substrates, showing weak oxidation of iron (10–15 mM of Fe3+). It is noteworthy that the strain was previously detected in the communities bioleaching copper–nickel concentrates within a wide temperature range of 35–50 °C. Moreover, in contrast to the type strain K1, the lower pH limit of which is 1.5, the strain AMF was resistant to very low pH values and predominated in the community when the pH of the aqueous phase of the pulp was 1.0.
However, although the bacterial isolate was identified as a predominant iron oxidizer in the community, it also showed active and stable growth (maximum cell yield, 2.6 × 108 cells/mL after 24 h of growth) under heterotrophic conditions in the medium containing only organic compounds (0.04% yeast extract). Within subsequent five days of cultivation, active growth of the strain gradually turned to sporulation. The spores were oval endospores located terminally or subterminally within cells.
Under autotrophic conditions, no considerable growth in the medium containing elemental sulfur was shown. In the medium containing S0 and yeast extract, the growth occurred due to the consumption of organic substrates (no decrease in the pH of the liquid medium was recorded, which is evident of no sulfur oxidation to sulfuric acid). Light microscopy allowed the determination of forespores after one day of cultivation and subsequent formation of endospores: up to 80% of the cell population after six days of culturing. The phylogenetically close strain Al. tolerans PCG-3 also grew well in the media with different organic compounds and was capable of weak oxidation of both sulfur and iron in the 9K medium containing yeast extract [19].
Thus, ferrous iron oxidation, heterotrophic growth, low pH values and wide range of temperatures for growth, as well as the ability of the strain AMF to sporulate during prolonged cultivation, could be important characteristics that might help it to become an active and predominant member of the microbial community during bioleaching of the violarite–pentlandite–chalcopyrite concentrate.
3.4. Succession of Microorganisms during Violarite–Pentlandite–Chalcopyrite Concentrate Bioleaching and Role of Al. tolerans AMF in Microbial Community
To date, the microbial communities formed during bioleaching of nickel-containing sulfide raw materials have not been widely studied. Few papers have been devoted to this issue [4,9,11,13,14,28]. During the past two years, we have carried out several processes of bioleaching of chalcopyrite–sphalerite and chalcopyrite–violarite–pentlandite concentrates [4,9,17]. Regarding the present research, the proportions of microbial community members in these processes were of interest since these communities were subsequently used as inoculum for bioleaching. Particularly, the proportion of Alicyclobacillus spp. (most probably related to Al. tolerans, according to metabarcoding analysis) was only 0.1–0.4% of the total cell number of the communities [9]. Nevertheless, after this community was used as part of the inoculum for the biobeneficiation process of the copper–nickel concentrate at different temperatures (35, 40, and 50 °C), Al. tolerans has been determined in the communities at all temperatures at the end of the bioleaching. Moreover, at 40 °C, it was shown to be a predominant (~10% of all cells in the community) iron oxidizer (this study). Therefore, more common Sulfobacillus, Ferroplasma/Acidiplasma, and Leptospirillum strains were succeeded by the mixotrophic/organotrophic strain Al. tolerans AMF. Interestingly, Al. tolerans AMF dominated the community, although this strain (as well as the type strain Al. tolerans K1), was characterized by the lowest rate of iron oxidation in comparison with other thermotolerant and moderately thermophilic iron oxidizers that most commonly prevailed in bioleaching processes (Table 4).

Table 4.
Iron oxidation rate of some thermotolerant and moderately thermophilic acidophiles.
The fact that at the end of the biobeneficiation of the chalcopyrite–pentlandite–violarite concentrate, a heterotrophic microorganism capable of iron oxidation was one of the predominant cultures is possibly associated with the complete oxidation state of iron within the whole process, as well as the lysis of some cells of the community and the presence of organic substances released in the liquid phase of the pulp. Since the crucial role in bioleaching belonged to a mixotrophic/heterotrophic acidophile, this study underlined the underestimated contribution of organoheterotrophic microorganisms. Heterotrophs facilitate the growth of chemolithoautotrophic community members (by eliminating organic compounds toxic to obligate autotrophs) and, therefore, oxidation of the concentrate components by them [17]. We propose that the strain Al. tolerans AMF also adapted readily to nickel in the medium (11.9 mM at the end of the process), in addition to 1.33 mM of dissolved copper, as well as to low pH values, which could also give this strain certain advantages in the microbial communities under these specific conditions. At the same time, the ability of the strain AMF to form endospores is a very important mechanism that allows the strain to be preserved in the community under unfavorable conditions, for instance, when the energy substrates are not available or limited.
Interestingly, the role of the strain PCG-3 in the oxidation of the pyrite–arsenopyrite gold-bearing concentrate can also be the utilization of organic substances released from lysed cells, since it has also been determined at the end of bioleaching (3–5% of the total cell number) [19]. Nevertheless, Al. tolerans PCG-3 has not been isolated as a predominant iron oxidizer, and other microorganisms (S. thermotolerans and two strains of F. acidiphilum) have also been determined in the community at the end of the process [19]. According to another research article, two other species of the genus Alicyclobacillus (Al. ferrooxydans and Al. ferripilum) have been detected as the dominant heterotrophs capable of iron oxidation, which enhanced the efficiency of simultaneous sludge digestion and metal leaching process with respect to sludge stabilization and metal leaching [35]. The frequent occurrence of the Alicyclobacillus 16S rRNA gene sequences, together with the sequences belonging to Acidithiobacillus and Sulfobacillus spp., was reported in the marine shore sulfidic mine tailings dump at the Chañaral Bay in the Atacama Desert, northern Chile [36].
4. Conclusions
Summarizing the results of this research, several important conclusions can be drawn. First, Alicyclobacillus isolates that were present at the end of the process in the bioreactor were assigned to the heterotrophic/mixotrophic species Al. tolerans, and the strain Al. tolerans AMF turned out to be a predominant iron oxidizer in the acidophilic microbial community bioleaching the violarite–pentlandite–chalcopyrite concentrate. Second, this strain succeeded more common iron oxidizers and showed efficient growth under heterotrophic conditions, which underlines its contribution as an organoheterotroph to the process of bioleaching of the copper–nickel concentrate. Third, the participation of Al. tolerans in the process, together with the predominant sulfur-oxidizing At. caldus AMS, made it possible to carry out an efficient biobeneficiation process of the violarite–pentlandite–chalcopyrite concentrate. The characteristics of the strain AMF, such as the adaptation to increased concentrations of nickel and copper in the aqueous phase of the pulp and low pH, ability to use organic substrates and ferrous iron in its metabolism, as well as the formation of spores, could explain the prevalence of the strain AMF under the bioleaching process conditions. Our results underlined the role of mixotrophic/heterotrophic microorganisms in effective bioprocesses. These studies, as well as further research of acidophilic microbial communities, contribute to the development of the field of bioleaching/biobeneficiation of the chalcopyrite–violarite–pentlandite bulk concentrates and copper–nickel raw materials.
Author Contributions
Conceptualization, A.P. and M.M.; methodology, A.P. and M.M.; validation, A.P., M.M. and N.F.; investigation, A.P., M.M. and N.F.; formal analysis and resources, A.P., M.M. and N.F.; data curation, A.P. and M.M.; writing—original draft preparation, A.P. and M.M.; writing—review and editing, A.P., M.M. and N.F.; visualization, A.P. and M.M.; supervision and project administration, A.P.; funding acquisition, M.M., A.P. and N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 21-14-00077. Microbiological experiments on the growth of Al. tolerans under autotrophic and heterotrophic conditions were supported by the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The 16S rRNA gene sequences of the strains Acidithiobacillus caldus AMS and Alicyclobacillus tolerans AMF were deposited in the GenBank databases under accession numbers OM407403 and OM407404.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Boxall, N.J.; Morris, C.; Cheng, K.Y. Prospective directions for biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Kondrat’eva, T.F.; Pivovarova, T.A.; Tsaplina, I.A.; Fomchenko, N.V.; Zhuravleva, A.E.; Murav’ev, M.I.; Melamud, V.S.; Bulayev, A.G. Diversity of the communities of acidophilic chemolithotrophic microorganisms in natural and technogenic ecosystems. Microbiology 2012, 81, 1–24. [Google Scholar] [CrossRef]
- Mahmoud, A.; Cezac, P.; Hoadley, A.F.A.; Contamine, F.; D’Hugues, P. A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Panyushkina, A.; Fomchenko, N.; Babenko, V.; Muravyov, M. Effect of temperature on biobeneficiation of bulk copper–nickel concentrate with thermoacidophilic microbial communities. Metals 2021, 11, 1969. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lakaniemi, A.-M.; Tuovinen, O.H. Acid and ferric sulfate bioleaching of uranium ores: A review. J. Clean. Prod. 2020, 264, 121586. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Tong, L.; Sand, W. Some aspects of industrial heap bioleaching technology: From basics to practice. Miner. Process. Extr. Metall. Rev. 2021. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Muravyov, M.I. Two-step biohydrometallurgical technology of copper-zinc concentrate processing as an opportunity to reduce negative impacts on the environment. J. Environ. Manag. 2018, 226, 270–277. [Google Scholar] [CrossRef]
- Harvey, T.J.; Yen, W.T.; Paterson, J.G. Selective zinc extraction from complex copper/zinc sulphide concentrates by pressure oxidation. Miner. Eng. 1992, 5, 975–992. [Google Scholar] [CrossRef]
- Muravyov, M.; Panyushkina, A.; Bulaev, A.; Fomchenko, N. Biobeneficiation of bulk copper-zinc and copper–nickel concentrates at different temperatures. Miner. Eng. 2021, 170, 107040. [Google Scholar] [CrossRef]
- Arpalahti, A.; Lundström, M. The leaching behavior of minerals from a pyrrhotite-rich pentlandite ore during heap leaching. Miner. Eng. 2018, 119, 116–125. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of nickel-copper sulfides. Hydrometallurgy 2008, 91, 70–88. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides: A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Norris, P.R. Acidophile diversity in mineral sulfide oxidation. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin, Germany, 2007; pp. 199–216. [Google Scholar]
- Gericke, M.; Govender, Y. Bioleaching strategies for the treatment of nickel-copper sulphide concentrates. Miner. Eng. 2011, 24, 1106–1112. [Google Scholar] [CrossRef]
- Wisotzkey, J.D.; Jurtshuk, P.; Fox, G.E.; Deinhart, G.; Poralla, K. Comparative sequence analyses on the 16S rRNA (rRNA) of Bacillus acidocaldarius, Bacillus acidoterrestris, and Bacillus cycloheptanicus and proposal for creation of a new genus, Alicyclobacillus gen. nov. Int. J. Syst. Bacteriol. 1992, 42, 263–269. [Google Scholar] [CrossRef]
- Ciuffreda, E.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Alicyclobacillus spp.: New insights on ecology and preserving food quality through new approaches. Microorganisms 2015, 3, 625–640. [Google Scholar] [CrossRef] [Green Version]
- Muravyov, M.; Panyushkina, A. Distinct roles of acidophiles in complete oxidation of high-sulfur ferric leach product of zinc sulfide concentrate. Microorganisms 2020, 8, 386. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Panyushkina, A.E.; Tsaplina, I.A.; Grigor’eva, N.V.; Kondrat’eva, T.F. Thermoacidophilic microbial community oxidizing the gold-bearing flotation concentrate of a pyrite-arsenopyrite ore. Microbiology 2014, 83, 539–549. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.G.; Jacobsen, W.R. Determination of iron and iron-aluminum mixtures by titration with EDTA. Anal. Chem. 1960, 32, 215–217. [Google Scholar] [CrossRef]
- Panyushkina, A.E.; Babenko, V.V.; Nikitina, A.S.; Selezneva, O.V.; Tsaplina, I.A.; Letarova, M.A.; Kostryukova, E.S.; Letarov, A.V. Sulfobacillus thermotolerans: New insights into resistance and metabolic capacities of acidophilic chemolithotrophs. Sci. Rep. 2019, 9, 15069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavaiko, G.I.; Bogdanova, T.I.; Tourova, T.P.; Kondrat’eva, T.F.; Tsaplina, I.A.; Egorova, M.A.; Krasil’nikova, E.N.; Zakharchuk, L.M. Reclassification of ‘Sulfobacillus thermosulfidooxidans subsp. thermotolerans’ strain K1 as Alicyclobacillus tolerans sp. nov. and Sulfobacillus disulfidooxidans Dufresne et al. 1996 as Alicyclobacillus disulfidooxidans comb. nov., and emended description of the genus Alicyclobacillus. Int. J. Syst. Evol. Microbiol. 2005, 55, 941–947. [Google Scholar]
- Duda, V.I.; Suzina, N.E.; Severina, L.O.; Bogdanova, T.I.; Tsaplina, I.A.; Karavaiko, G.I. Ultrastructural organization of Alicyclobacillus tolerans strain K1T cells. Arch. Microbiol. 2006, 185, 63–68. [Google Scholar] [CrossRef]
- Behera, S.K.; Manjaiah, M.; Sekar, S.; Panda, S.K.; Mavumengwana, V.; Mulaba-Bafubiandi, A.F. Optimization of microbial leaching of base metals from a South African sulfidic nickel ore concentrate by Acidithiobacillus ferrooxidans. Geomicrobiol. J. 2018, 35, 447–459. [Google Scholar] [CrossRef]
- Watling, H.R.; Perrot, F.A.; Shiers, D.W. Comparison of selected characteristics of Sulfobacillus species and review of their occurrence in acidic and bioleaching environments. Hydrometallurgy 2008, 93, 57–65. [Google Scholar] [CrossRef]
- Huynh, D.; Giebner, F.; Kaschabek, S.R.; Rivera-Araya, J.; Levican, G.; Sand, W.; Schlömann, M. Effect of sodium chloride on Leptospirillum ferriphilum DSM 14647T and Sulfobacillus thermosulfidooxidans DSM 9293T: Growth, iron oxidation activity and bioleaching of sulfidic metal ores. Miner. Eng. 2019, 138, 52–59. [Google Scholar] [CrossRef]
- Tsaplina, I.A.; Zhuravleva, A.E.; Ismailov, A.D.; Zakharchuk, L.M.; Krasil’nikova, E.N.; Bogdanova, T.I.; Karavaiko, G.I. The dependence of intracellular ATP level on the nutrition mode of the acidophilic bacteria Sulfobacillus thermotolerans and Alicyclobacillus tolerans. Microbiology 2007, 76, 654–662. [Google Scholar] [CrossRef]
- Panyushkina, A.E.; Tsaplina, I.A.; Kondrat’eva, T.F.; Belyi, A.V.; Bulaev, A.G. Physiological and morphological characteristics of acidophilic bacteria Leptospirillum ferriphilum and Acidithiobacillus thiooxidans, members of a chemolithotrophic microbial consortium. Microbiology 2018, 87, 326–338. [Google Scholar] [CrossRef]
- Bulaev, A.G.; Erofeeva, T.V.; Labyrich, M.V.; Mel’nikova, E.A. Resistance of Acidiplasma archaea to heavy metal ions. Microbiology 2017, 86, 583–589. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Pivovarova, T.A.; Karavaiko, G.I.; Kondrat’eva, T.F.; Moore, E.R.B.; Abraham, W.-R.; Lünsdorf, H.; Timmis, K.N.; Yakimov, M.M.; Golyshin, P.N. Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea. Int. J. Syst. Evol. Microbiol. 2000, 50, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, A.; Sreekrishnan, T.R. Heavy metal bioleaching and sludge stabilization in a single-stage reactor using indigenous acidophilic heterotrophs. Environ. Technol. 2017, 38, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Korehi, H.; Blöthe, M.; Sitnikova, M.A.; Dold, B.; Schippers, A. Metal mobilization by iron- and sulfur-oxidizing bacteria in a multiple extreme mine tailings in the Atacama Desert, Chile. Environ. Sci. Technol. 2013, 47, 2189–2196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).