Zun-Kholba Orogenic Gold Deposit, Eastern Sayan, Russia: Geology and Genesis
Abstract
:1. Introduction
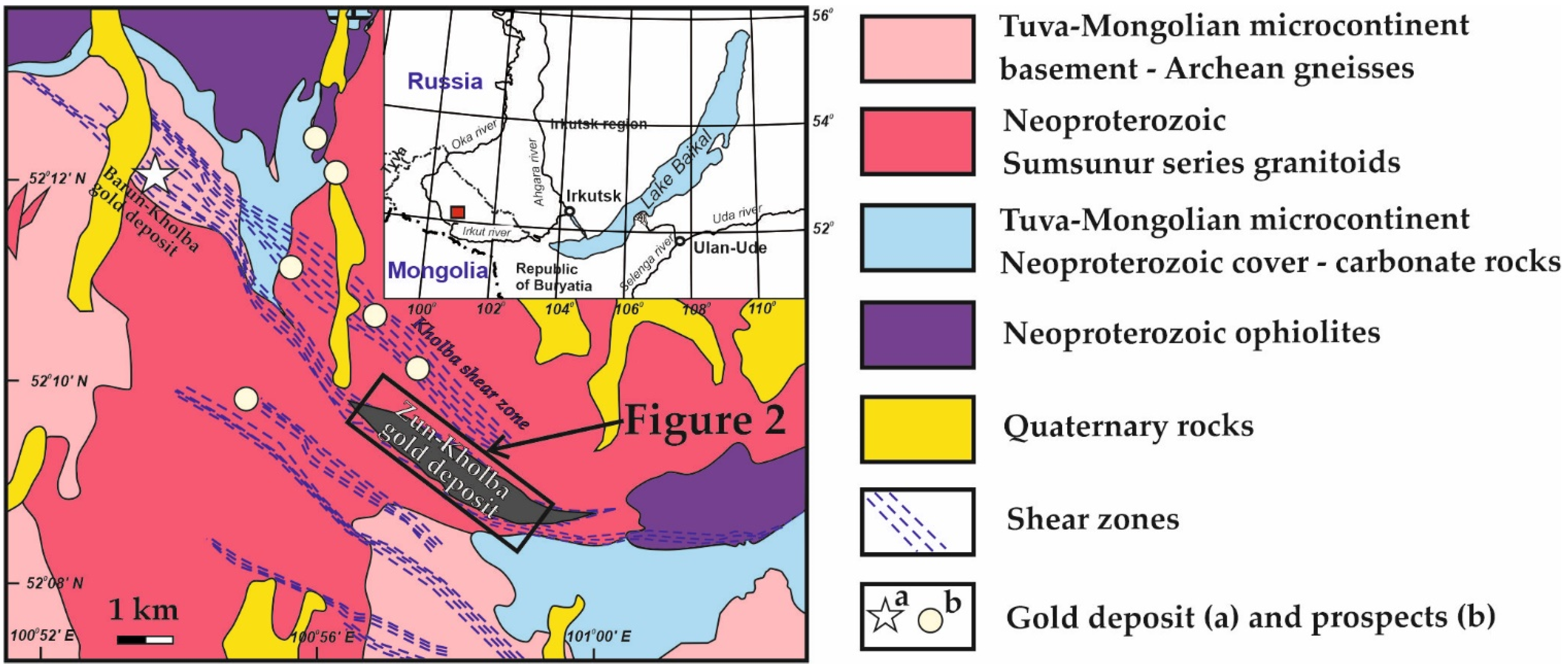
2. Regional Geology and Tectonics
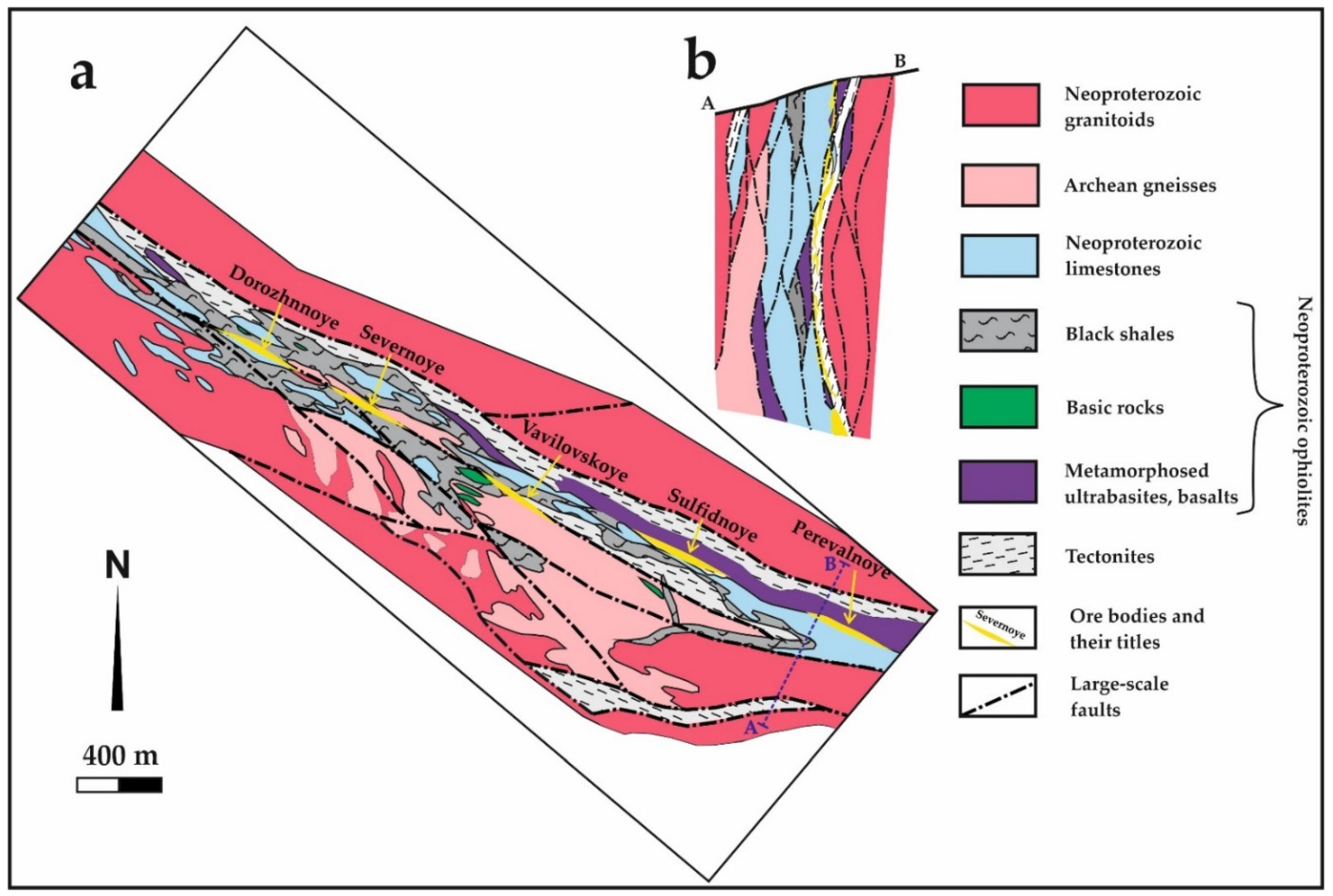
3. Deposit Geology
4. Sampling and Analytical Methods
5. Results
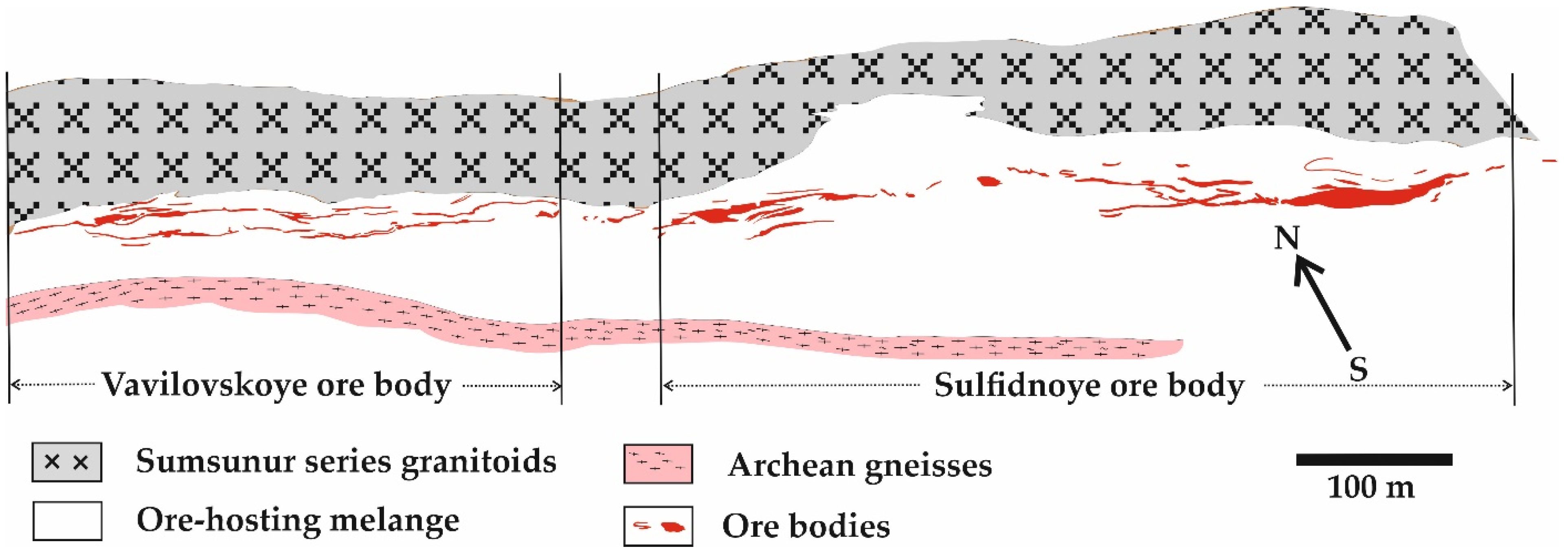
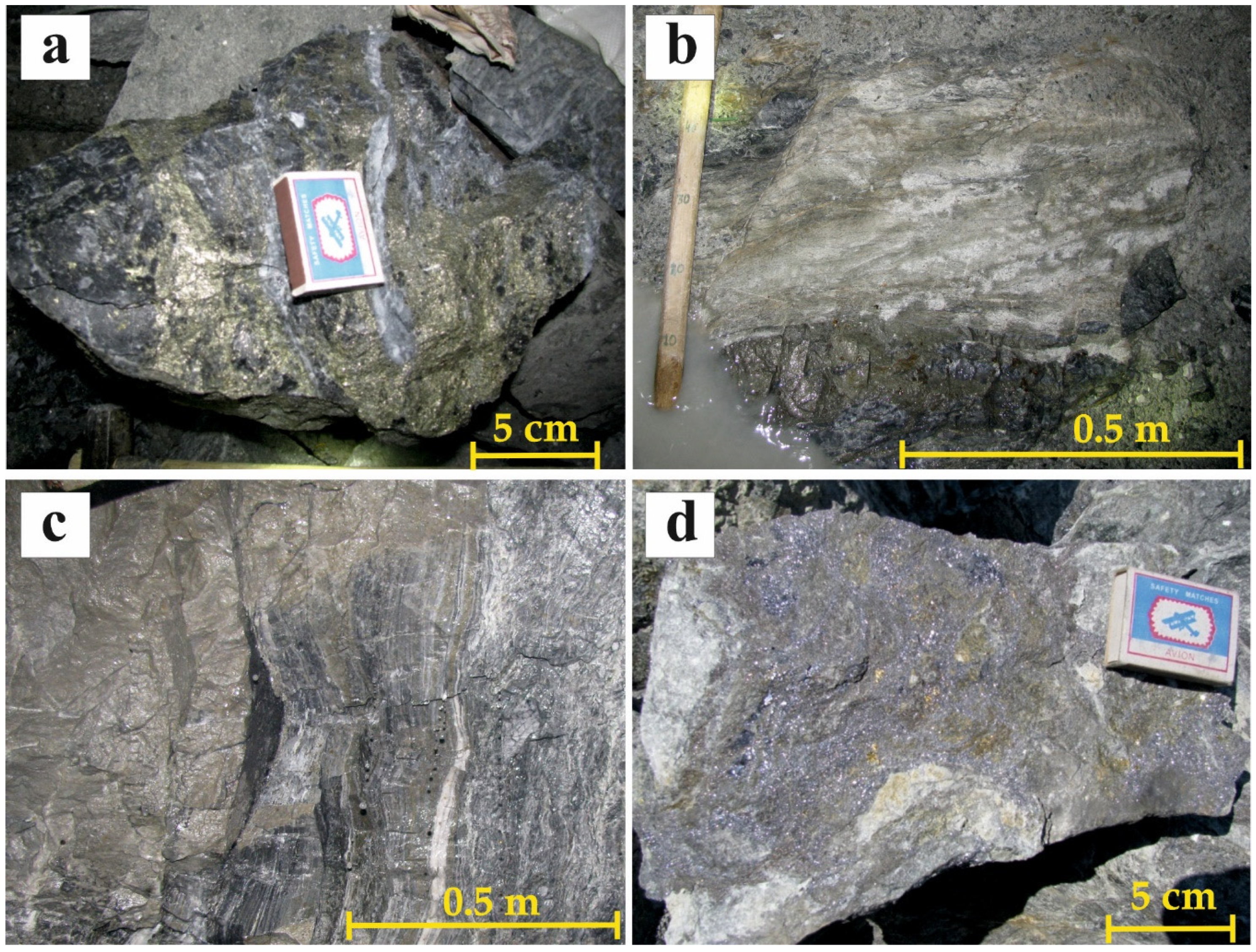
5.1. Sturcture of the Ore Bodies
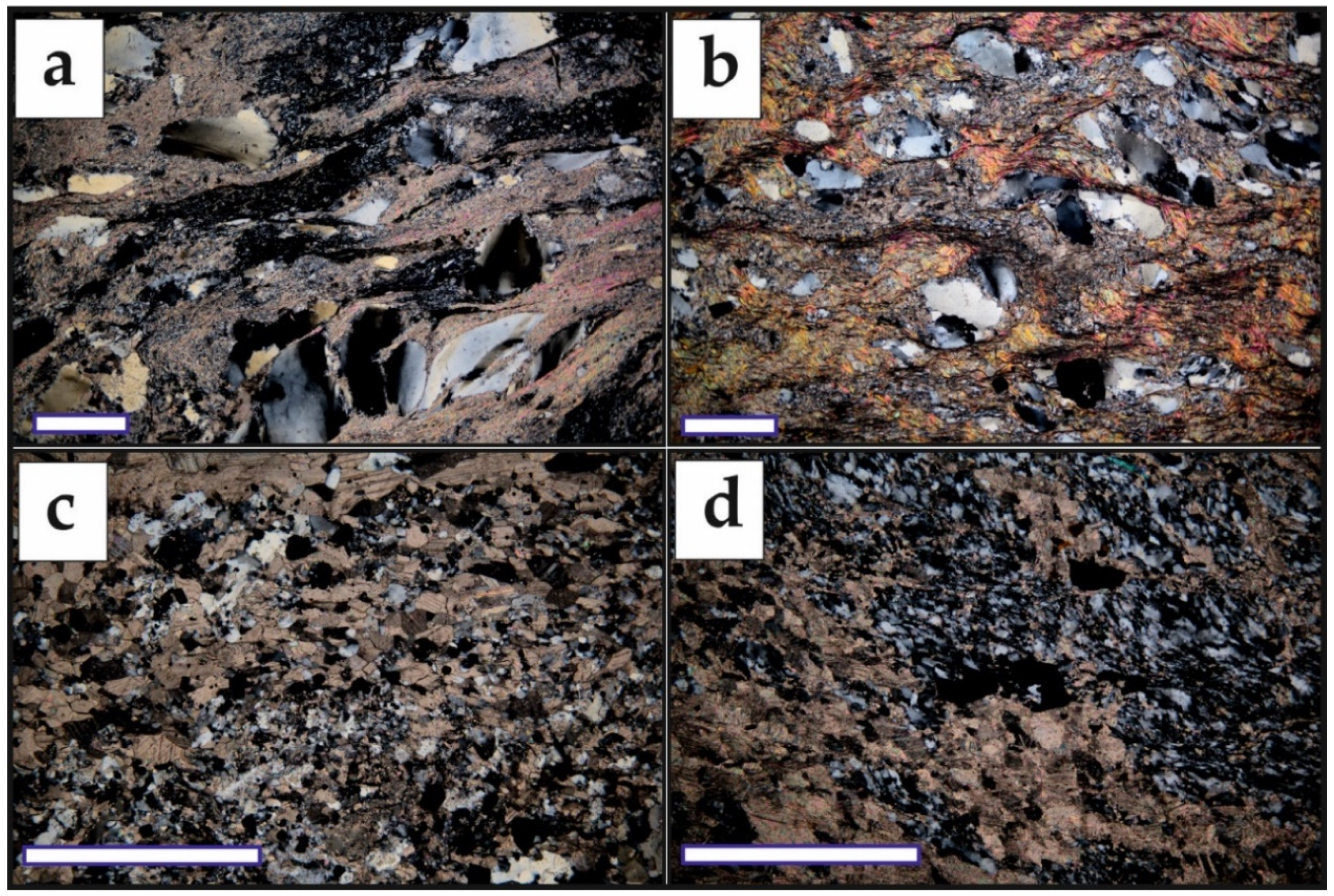
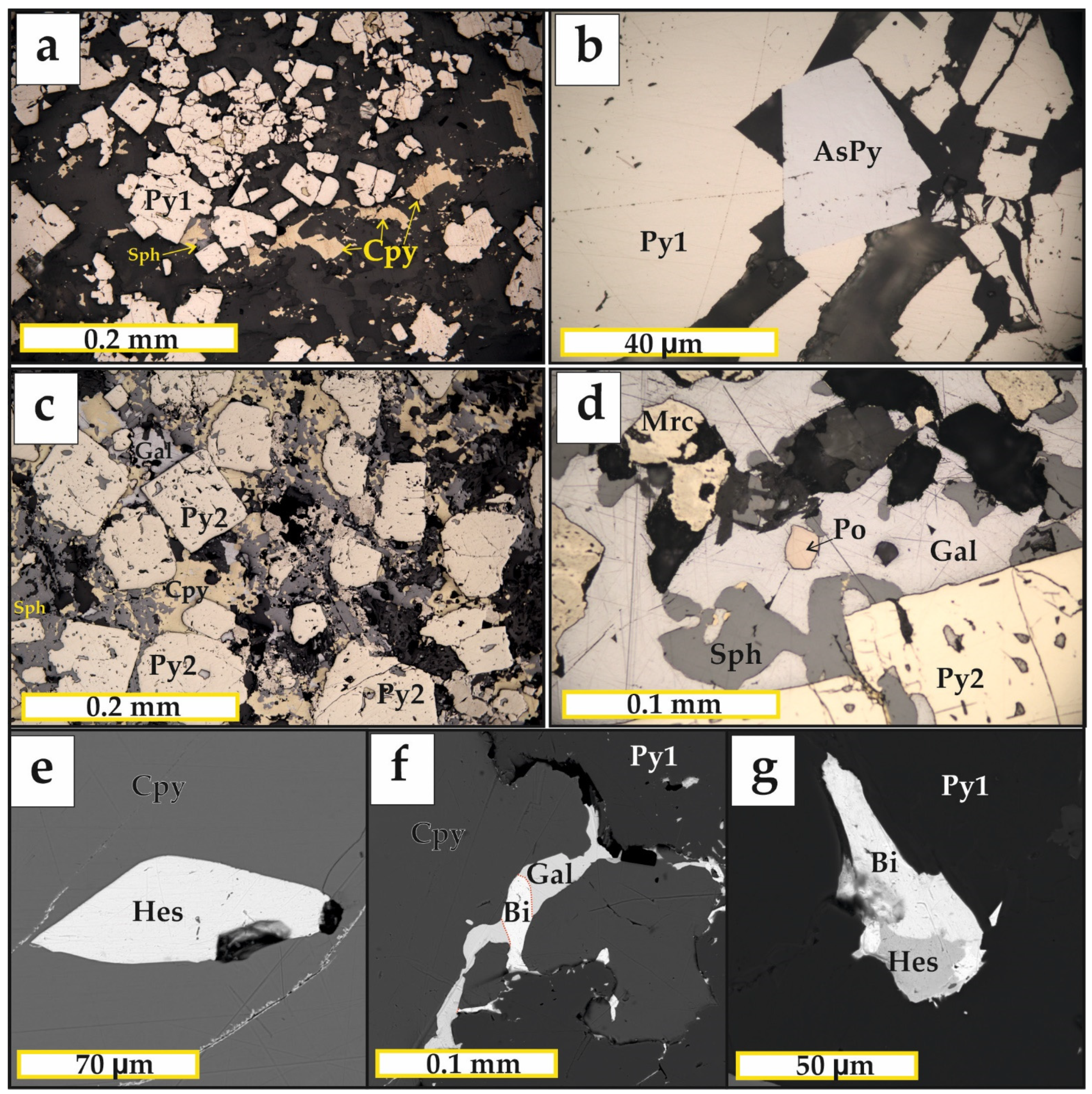
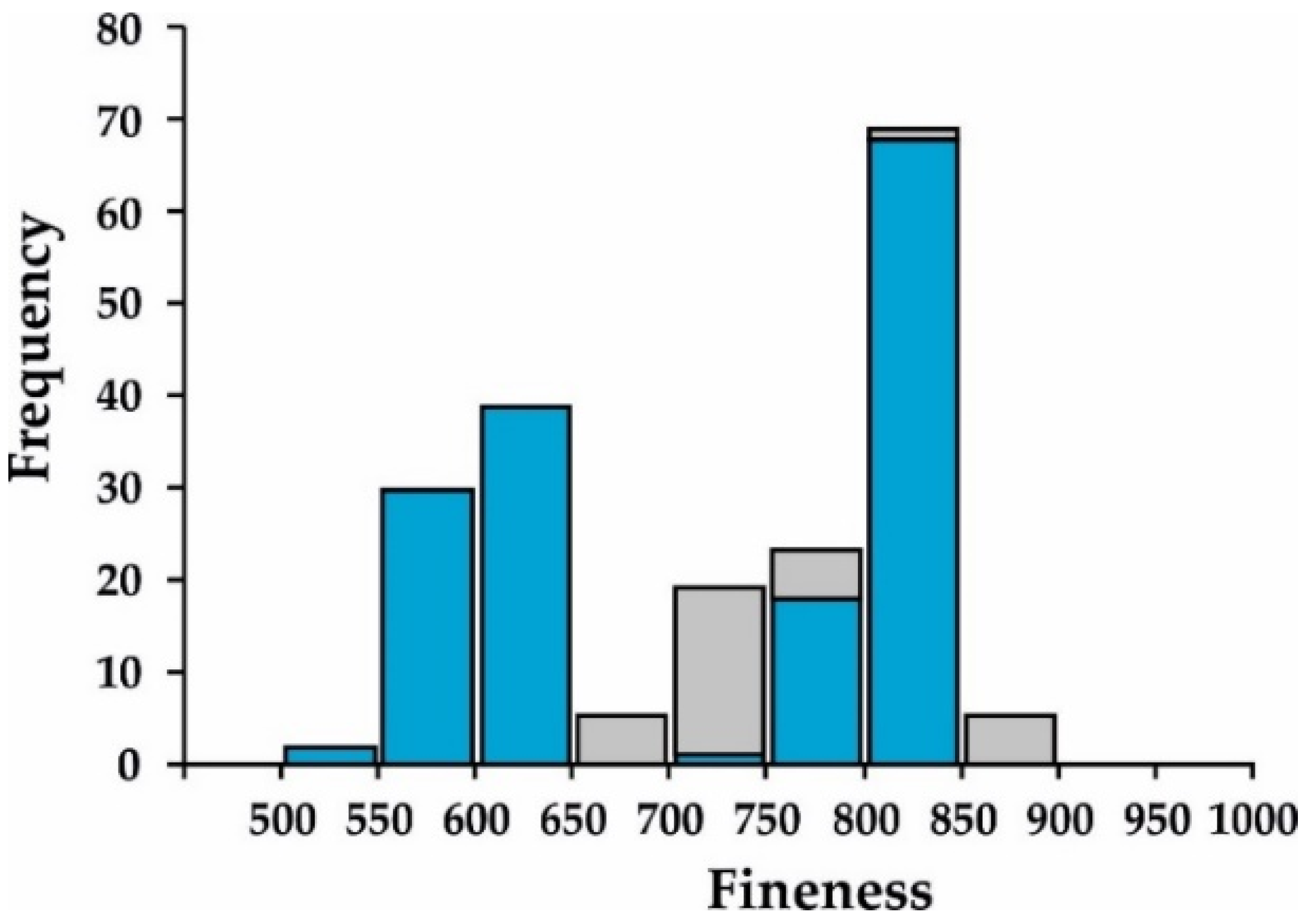
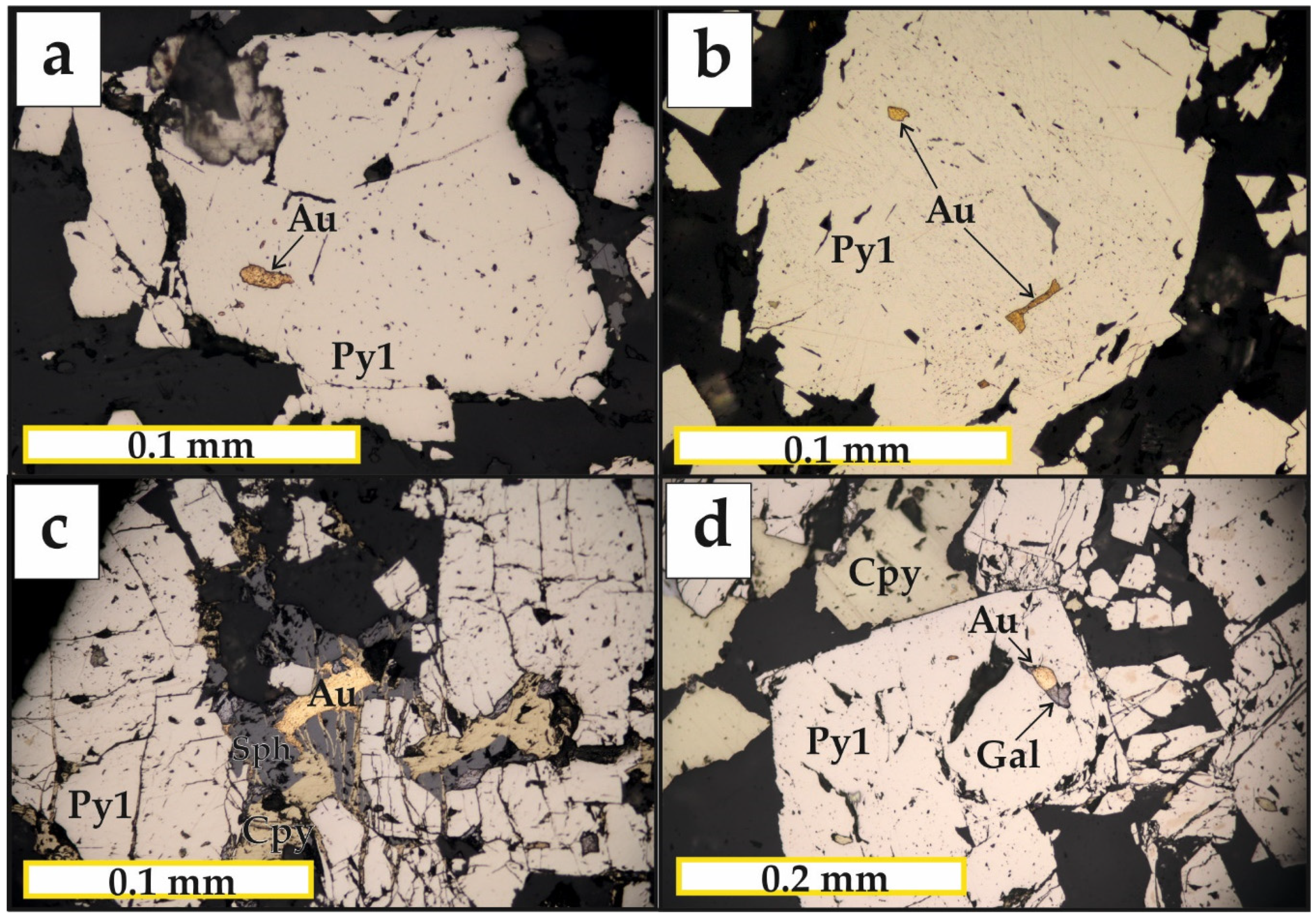
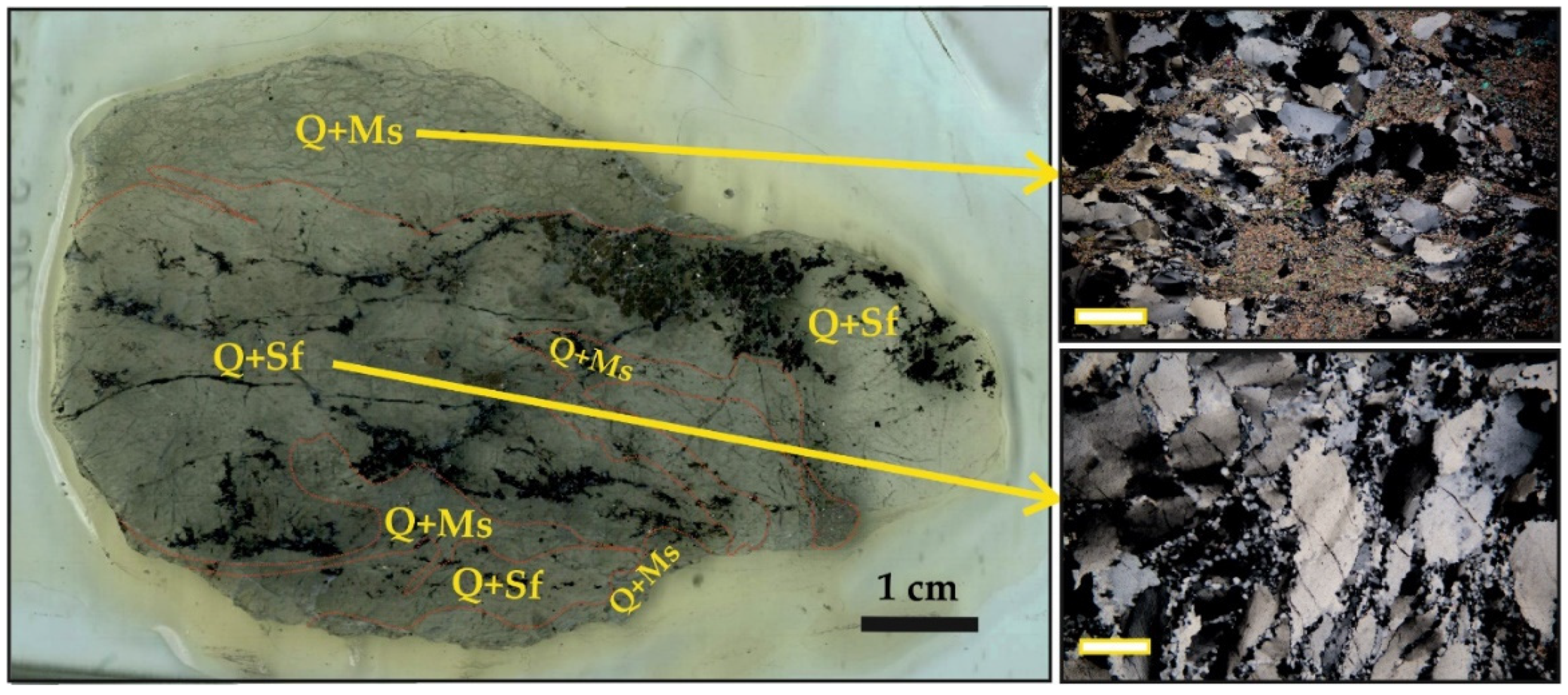
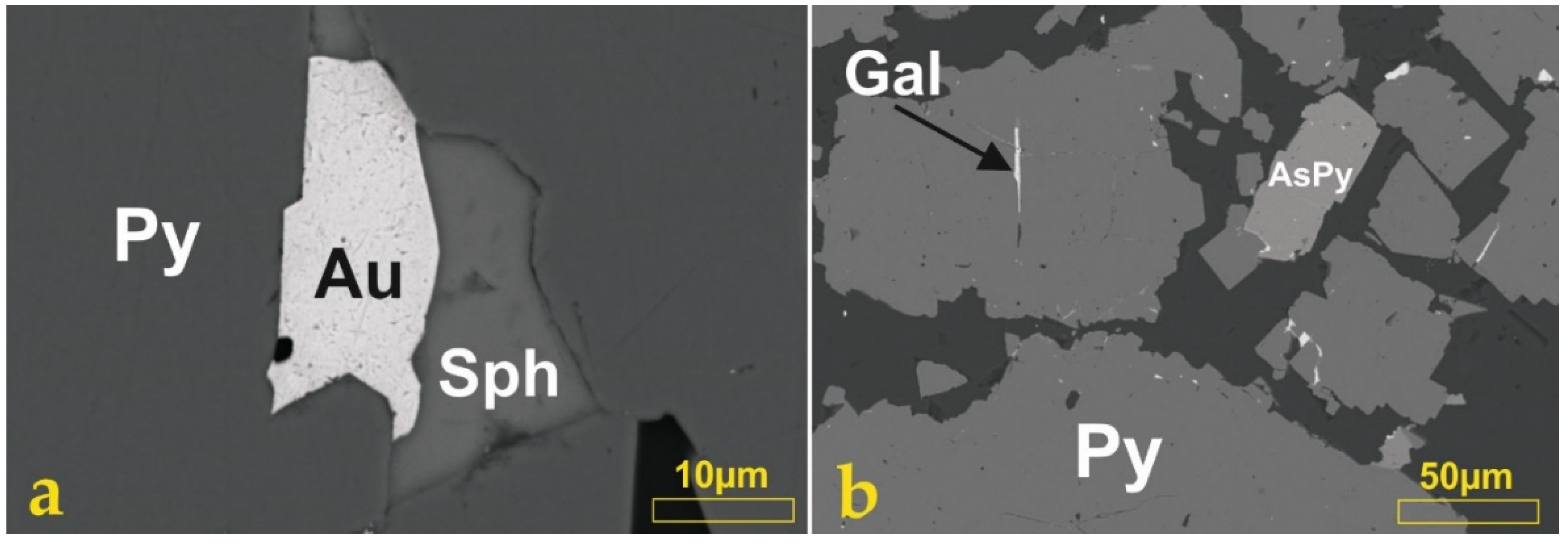
5.2. Mineralogy of the Ores
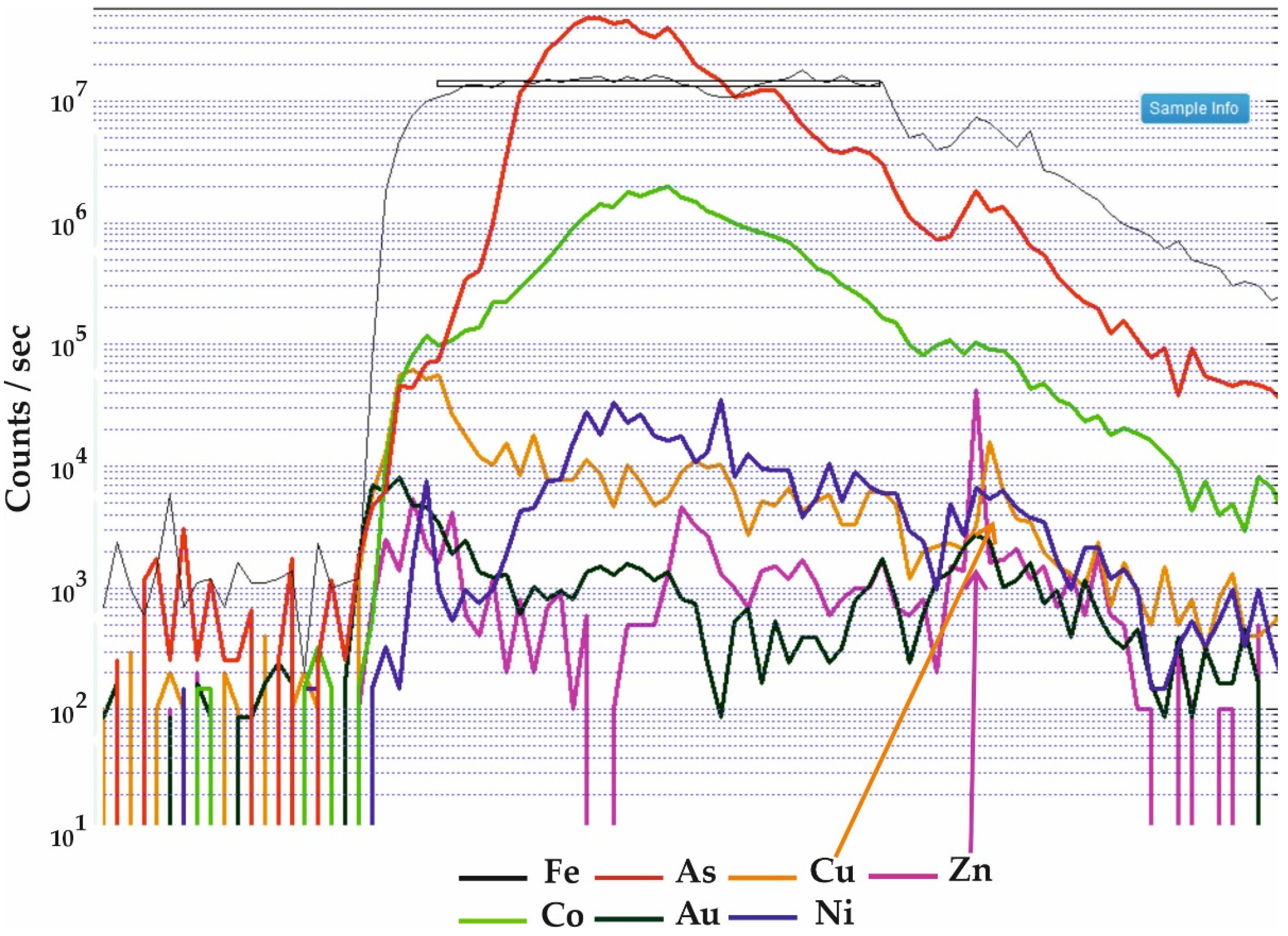
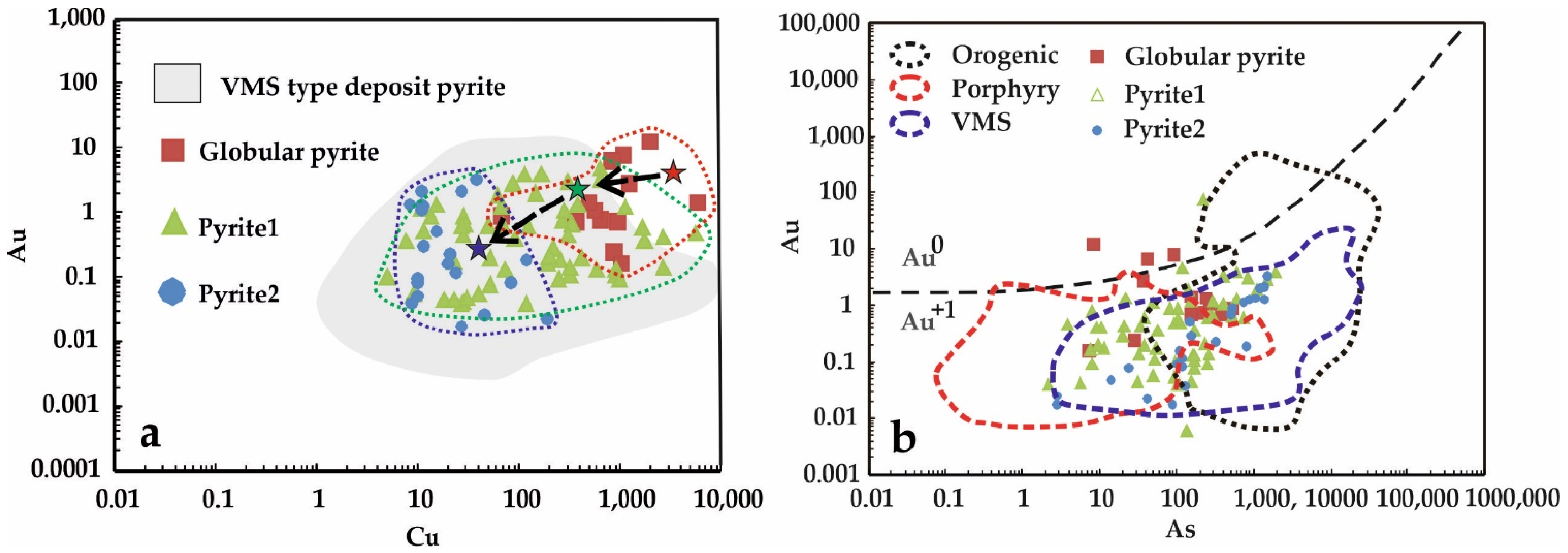
5.3. Pyrite and Pyrrhotite Geochemistry
5.4. Stable Isotope Compositions
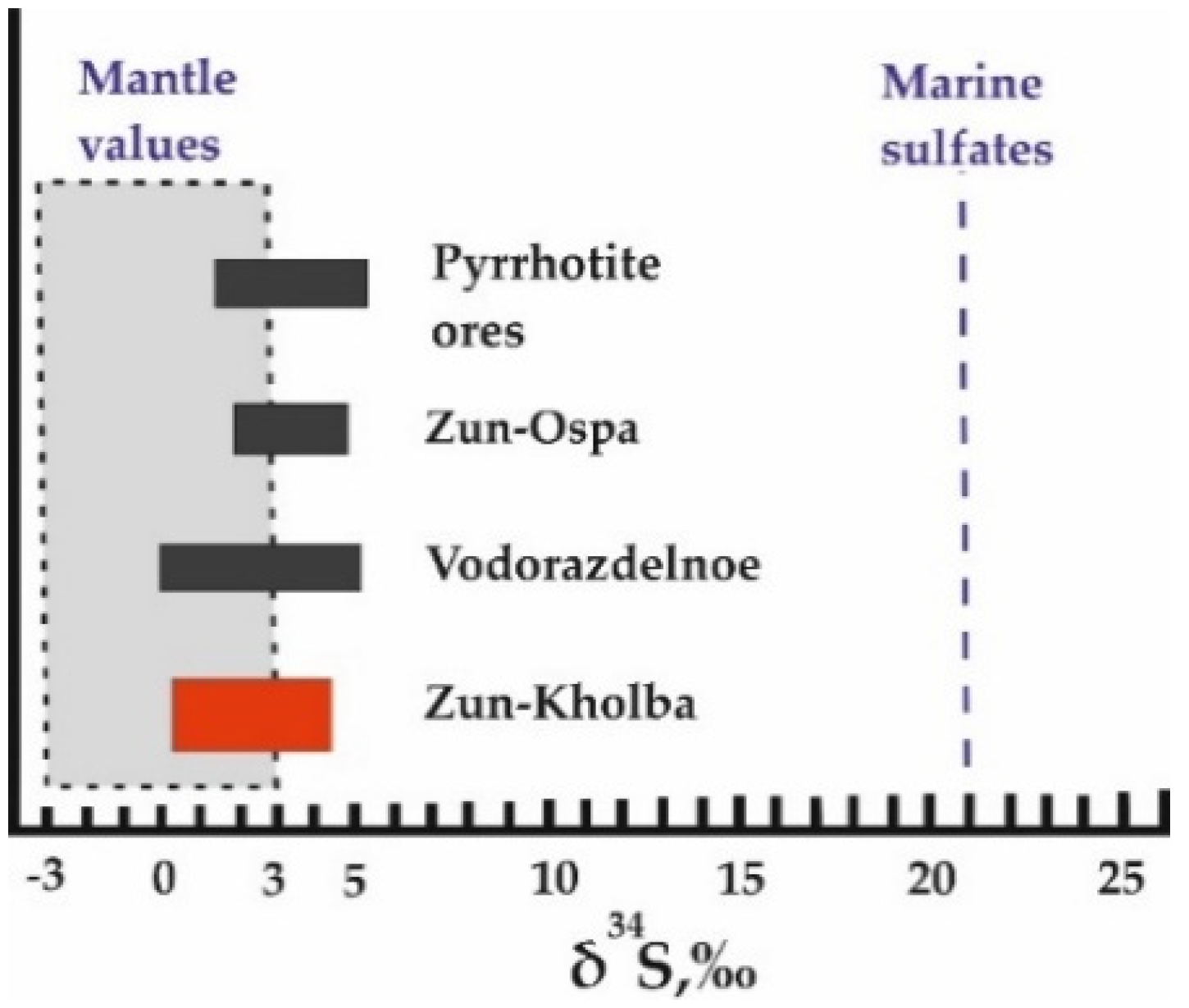
5.4.1. Sulfur Isotopes
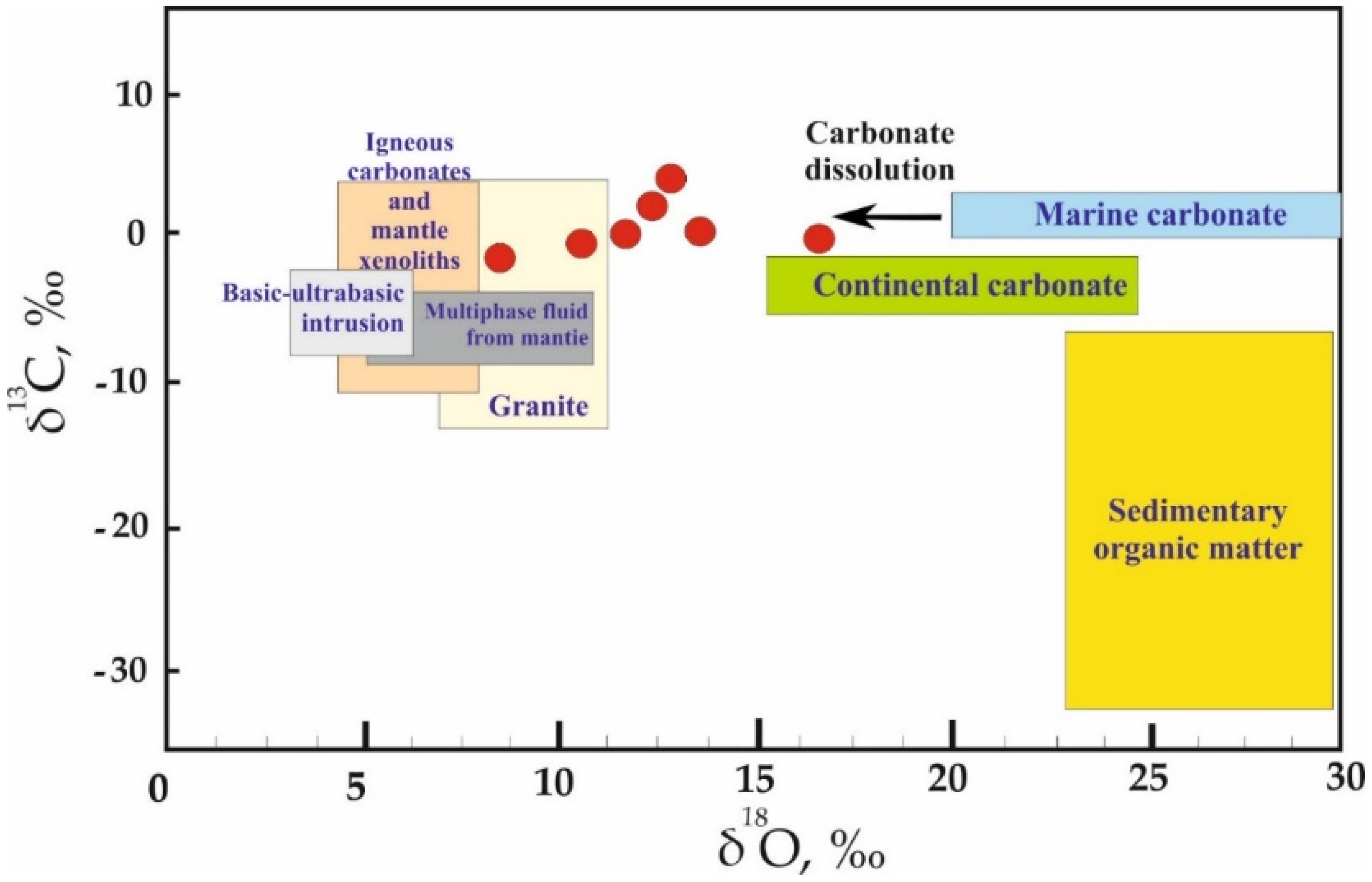
5.4.2. Oxygen and Carbon Isotopes
5.5. Fluid Inclusion Study
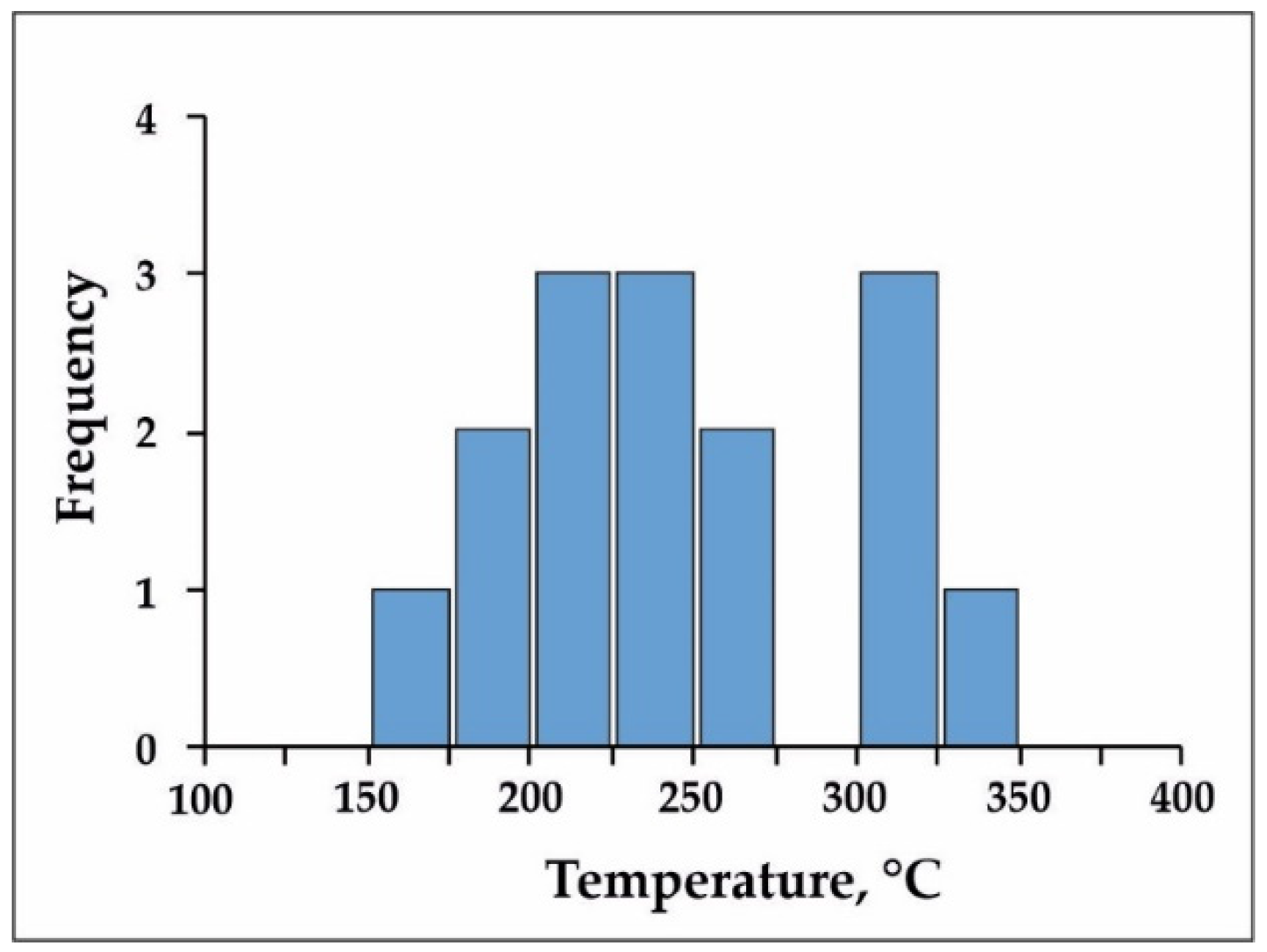
5.6. Mineral Geothermometry
5.7. Pressure Estimations
6. Discussion
6.1. P–T Conditions of Ore-Formig Mineral Deposition
6.2. Deposit Genesis
7. Conclusions
- Using different independent thermobarometric methods, it has been found that the formation of quartz–sulfide replacement ores in the Zun-Kholba gold deposit occurred at temperatures from 420–433 °C in the deepest part to 316 °C in the shallowest part of the deposit. Pressures during ore formation in the middle part of the deposit were 570–950 bar. The pressure and temperature of the ore formation decreased upwards.
- The determined P–T parameters of ore formation allow us to classify the Zun-Kholba deposit as a mesozonal to epizonal orogenic gold deposit.
- The ores were formed from homogeneous fluids of low to moderate salinity (2.4 to 7.9 wt.% eq. NaCl), where Fe chlorides were the dominant salt.
- The sulfur isotope compositions, as well as pyrite geochemistry, show that the source of some ore-forming elements is ancient fragments of “black smokers” being hosted by ophiolitic blocks. These pre-ore VMS occurrences are scattered in the Zun-Kholba deposit area.
- Oxygen isotope data prove the metamorphic origin of the ore-forming fluids with some mixing with meteoric waters at the shallow depths.
- Ore formation at the Zun-Kholba deposit is consistent with the metamorphic devolatilization model, where the ophiolites containing sulfide ore fragments and partly host rocks were a source of gold, sulfur, and related ore-forming components.
- The Zun-Kholba deposit was formed during the late Paleozoic deformation caused by the appearance of a regional transpressional shear zone.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groves, D.I.; Goldfarb, R.J.; Gebre-Mariam, M.; Hagemann, S.G.; Robert, F. Orogenic gold deposits: A proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol. Rev. 1998, 13, 7–27. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos 2015, 233, 2–26. [Google Scholar] [CrossRef]
- Ridley, J.R.; Diamond, L.W. Fluid chemistry of orogenic lode gold deposits and implications for genetic models. SEG Rev. 2000, 13, 141–162. [Google Scholar]
- Goldfarb, R.J.; Baker, T.; Dube, B.; Groves, D.I.; Hart, C.J.; Gosselin, P. Distribution, character and genesis of Gold Deposits in Metamorphic terranes. Econ. Geol. 100th Anniv. Vol. 2005, 407–450. [Google Scholar]
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Deng, J.; Wang, Q.; Yang, L.; Zhang, L. A holistic model for the origin of orogenic gold deposits and its implications for exploration. Miner. Depos. 2020, 55, 275–292. [Google Scholar] [CrossRef]
- Kouhestani, H.; Pashidnejad-Omran, N.; Rastad, E.; Mohajjol, M.; Goldfarb, R.; Ghaderi, M. Orogenic gold mineralization at the Chah Bagh deposit, Muteh gold district, Iran. J. Asian Earth Sci. 2014, 91, 89–106. [Google Scholar] [CrossRef]
- Wang Ch Shao, Y.-J.; Evans, N.J.; Li, H.; Zhou, H.-D.; Huang, K.-X.; Liu, Z.-F.; Chen, Y.; Lai Ch Liu, Q.-Q. Genesis of Zixi gold deposit in Xuefengshan, Jiangnan Orogen (South China): Age, geology and isotopic constraints. Ore Geol. Rev. 2020, 117, 103301. [Google Scholar] [CrossRef]
- Izvekova, A.D.; Damdinov, B.B.; Damdinova, L.B.; Moskvitina, M.L. Gold-telluride mineralization in ore of Pionerskoe gold-quartz deposit (Eastern Sayan, Russia). Geol. Ore Depos. 2021, 63, 579–598. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Taylor, R.D.; Collins, G.S.; Goryachev, N.A.; Orlandini, O.F. Phanerozoic continental growth and gold metallogeny of Asia. Gondwana Res. 2014, 25, 48–102. [Google Scholar] [CrossRef]
- Feofilaktov, G.A. About the genetic relationship of gold mineralization wuth granitoid massifs of the Kytoi-Urik ore cluster (Eastern Sayan). In Ore-bearing capacity and structure of ore deposits of the Buryat Autonomous Soviet Socialist Republic. Proc. Geol. Dep. Buryat Branch Sib. Branch Acad. Sci. USSR Ulan-Ude 1970, 2, 10. (In Russian) [Google Scholar]
- Brazhnik, A.V. Zun-Kholba gold deposit. Ores Met. 1993, 3–6, 80–90. (In Russian) [Google Scholar]
- Zhmodik, S.M.; Dobretsov, N.L.; Mironov, A.G.; Roshchektaev, P.A.; Karmanov, N.S.; Kulikov, A.A.; Nemirovskaya, N.A.; Ochirov Yu, C. Mineralogical and geochemical signatures of hydrothermal-sedimentary origin of gold ore formation of the Kholba deposits, Eastern Sayan, Russia. Resour. Geol. Spec. Issue 1993, 17, 287–313. [Google Scholar]
- Kuzmichev, A.B. Neoproterozoic accretion of the Tuva-Mongolian massif, one of the Precambrian terranes in the Central Asian Orogenic Belt. In Composition and evolution of Central Asian Orogenic Belt: Geology, Evolution, Tectonics, and Models; Kroner, A., Ed.; Borntraeger Science Publishers: Stuttgart, Germany, 2015; pp. 66–92. [Google Scholar]
- Khain, E.V.; Bibikova, E.V.; Kroner, A.; Zhuravlev, D.Z.; Sklyarov, E.V.; Fedotova, A.A.; Kravchenko-Berezhnoy, I.R. The most ancient ophiolite of the Central Asian fold belt: U-Pb and Pb-Pb zircon ages for the Dunzhugur Complex, Eastern Sayan, Siberia, and geodynamic implications. Earth Planet. Sci. Lett. 2002, 199, 311–325. [Google Scholar] [CrossRef]
- Fedotova, A.A.; Khain, E.V. Tectonics of the South of the Eastern Sayan and Its Position in the Ural-Mongolian Belt; Scientific World: Moscow, Russia, 2002. (In Russian) [Google Scholar]
- Kuzmichev, A.B.; Larionov, A.N. Neoproterozoic island arcs in East Sayan: Duration of magmatism (from U-Pb zircon dating of volcanic clastics). Russ. Geol. Geophys. 2013, 54, 34–43. [Google Scholar] [CrossRef]
- Damdinov, B.B.; Zhmodik, S.M.; Travin, A.V.; Yudin, D.S.; Goryachev, N.A. New data on the age of gold mineralization in the southeastern part of Eastern Sayan. Dokl. Earth Sci. 2018, 479, 429–432. [Google Scholar] [CrossRef]
- Buslov, M.M. Tectonics and geodynamics of the Central Asian Fold belt: The role of Late Paleozoic large-amplitude strike-slip faults. Russ. Geol. Geophys. 2011, 52, 52–71. [Google Scholar] [CrossRef]
- Gordienko, I.V.; Roshtekhaev, P.A.; Gorokhovskiy, D.V. Oka ore district of the Eastern Sayan: Geology, structural-metallogenic zonation, genetic types of ore deposits, their geodynamic formation conditions, and outlook for development. Geol. Ore Depos. 2016, 58, 361–382. [Google Scholar] [CrossRef]
- Damdinov, B.B. Mineral types of gold deposits and regularities of their localization in Southeastern East Sayan. Geol. Ore Depos. 2019, 61, 118–132. [Google Scholar] [CrossRef]
- Mironov, A.G.; Zhmodik, S.M. Gold deposits of the Urik-Kitoi metallogenic zone (Eastern Sayan, Russia). Geol. Ore Depos. 1999, 41, 46–60. [Google Scholar]
- Zhmodik, S.M.; Postnikov, A.A.; Buslov, M.M.; Mironov, A.G. Geodynamics of the Sayan-Baikal-Muya accretion-collision belt in the Neoproterozoic-Early Paleozoic and regularities of the formation and localization of precious-metal mineralization. Russ. Geol. Geophys. 2006, 47, 183–197. [Google Scholar]
- Dewey, J.F.; Holdsworth, R.E.; Strachan, R.A. Transpression and transtension zones. In Continental Transpressional and Transtensional Tectonics; Geological Society; Holdsworth, R.E., Strachan, R.A., Dewey, J.F., Eds.; Special Publications: London, UK, 1998; pp. 1–14. [Google Scholar]
- Damdinov, B.B.; Damdinova, L.B.; Zhmodik, S.M.; Mironov, A.G. Gold-bearing pyrrhotite ores in East Sayan: Composition and Formation Conditions (by the Example of the Ol’ginskoe Ore Occurrence). Russ. Geol. Geophys. 2019, 60, 514–531. [Google Scholar] [CrossRef]
- Coplen, T.B. Normalization of oxygen and hydrogen data. Chem. Geol. 1988, 72, 293–297. [Google Scholar]
- Friedman, I.; O’Neil, J.; Cebula, G. Two new carbonate stable isotope standards. Geostand. Newsl. 1982, 6, 11–12. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O-NaCl fluid inclusions. In Fluid Inclusions in Minerals, Methods and Applications; De Vivo, B., Frezzotti, M.L., Eds.; Virginia Tech: Blacksburg, VA, USA, 1994; pp. 117–130. [Google Scholar]
- Borisenko, A.S. Study of the salt composition of solutions of gas–liquid inclusions in minerals by the cryometric method. Russ. Geol. Geophys. 1977, 18, 16–27. [Google Scholar]
- Stipp, M.; Stunitz, H.; Heilbronner, R.; Schmid, S.M. The eastern Tonale fault zone: A ‘natural laboratory’ for crystal plastic deformation of quartz over a temperature range from 250 to 700 °C. J. Struct. Geol. 2002, 24, 1861–1884. [Google Scholar] [CrossRef]
- Tauson, V.; Lipko, S.; Smagunov, N.; Kravtsova, R.; Arsent’ev, K.Y. Distribution and segregation of trace elements during the growth of ore mineral crystals in hydrothermal systems: Geochemical and mineralogical implications. Russ. Geol. Geophys. 2018, 59, 1718–1732. [Google Scholar] [CrossRef]
- Dehnavi, A.S.; McFarlane, C.R.M.; Lentz, D.R.; Walker, J.A. Assessment of pyrite composition by LA-ICP-MS techniques from massive sulfide deposits of the Bathurst Mining Camp, Canada: From textural and chemical evolution to its application as a vectoring tool for the exploration of VMS deposits. Ore Geol. Rev. 2018, 92, 656–671. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.T.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Xie, J.; Tang, D.; Qian, L.; Wang, Y.; Sun, W. Geochemistry of sulfide minerals from skarn Cu (Au) deposits in the Fenghuangshan ore field, Tongling, Eastern China: Insights into ore-forming process. Ore Geol. Rev. 2020, 122, 103537. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotope of sulfur and carbon. In Geochemistry of Hydrothermal Deposits. Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Airiyants, E.V.; Zhmodik, S.M.; Mironov, A.G.; Borovikov, A.A. Gold mineralization in siliceous-carbonate rocks of southeastern East Sayan. Russ. Geol. Geophys. 2007, 48, 389–399. [Google Scholar] [CrossRef]
- Damdinov, B.B.; Damdinova, L.B. Zun-Ospa Gold Deposit, Eastern Sayan: Geology, Ore Composition, and Genesis. Geol. Ore Depos. 2018, 60, 241–264. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur isotope geochemistry of sulfide minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- O’Hara, K.; Sharp, Z.; Moecher, D.; Jenkin, G.T. The effect of deformation on oxygen isotope exchange in quartz and feldspar and the significance of isotopic temperatures in mylonites. J. Geol. 1997, 105, 193–204. [Google Scholar] [CrossRef]
- Schulz, B.; Audren, C.; Triboulet, C. Oxygen isotope record on fluid-rock-SiO2 interaction during Variscan progressive deformation and quartz veining in the meta-volcanosefdiments of Belle-Ile (Southern Brittany). J. Struct. Geol. 2002, 24, 1281–1297. [Google Scholar] [CrossRef]
- Bottinga, Y.; Javoy, M. Comments on oxygen isotope geothermometry. Earth Planet. Sci. Lett. 1973, 20, 250–265. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Gibbons, J.A.; Maltsev, O.; Atudorei, V.; Pack, A.; Sengupta, S.; Shock, E.L.; Knauth, L.P. A calibration of the triple oxygen isotope fractionation in the SiO2-H2O system and applications to natural samples. Geochim. Cosmochim. Acta 2016, 186, 105–119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, D.; Wu, G.; Di, Y.; Li, X.; Bu, X.; Liu, J. Origin of the Daping gold deposit in the Ailaoshan metallogenic belt, SW China: Insights from geology, isotope geochemistry and geochronology. Ore Geol. Rev. 2018, 96, 1–12. [Google Scholar] [CrossRef]
- Moloshag, V.P. Using of the mineral composition to the physical-chemical formation conditions of the sulfide ores from Ural. Lithosphere 2009, 2, 28–40. (In Russian) [Google Scholar]
- Shikazono, N. A comparison of temperatures estimated from the electrum–sphalerite–pyrite–argentite assemblage and filling temperatures of fluid implications from epithermal Au-Ag vein-type deposits in Japan. Econ. Geol. 1985, 80, 1415–1424. [Google Scholar] [CrossRef]
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe-As-S and their application. Can. Mineral. 1976, 14, 364–386. [Google Scholar]
- Steele-MacInnis MLecumberri-Sanchez, P.; Bodnar, R.J. HokieFlincs_H2O-NaCl: A Microsoft excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O–NaCl. Comput. Geosci. 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. Synthetic fluid inclusions XX. Critical PTx properties of H2O–FeCl2 fluids. Geochim. Cosmochim. Acta 2015, 148, 50–61. [Google Scholar] [CrossRef]
- Simmons, S.F.; White, N.C.; John, D.A. Geological characteristics of epithermal precious and base metal deposits. Soc. Econ. Geol. 100th Anniv. Vol. 2005, 485–522. [Google Scholar]
- Tuba, G.; Kontak, D.J.; Choquette, B.G.; Pfister, J.; Hastie, E.C.; van Hees, E.H. Fluid diversity in the gold-endowed Archean orogenic systems of the Abitibi greenstone belt (Canada) I: Constraining the PTX of prolonged hydrothermal systems. Ore Geol. Rev. 2021, 135, 104221. [Google Scholar] [CrossRef]
- Prokofiev VYu Pek, A.A. Problems in estimation of the formation depth of hydrothermal deposits by data on pressure of mineralizing fluids. Geol. Ore Depos. 2015, 57, 1–20. [Google Scholar] [CrossRef]
- Lewis, J.C.; Byrne, T.B. History of metamorphic fluids along outcrop-scale faults in a Paleogene accretionary prism, SW Japan: Implications for prismscale hydrology. Geochem. Geophys. Geosyst. 2003, 4, 9007. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, H.A.; Ord, A. Evolution of porosity, permeability and fluid pressure in dilatant fault post-failure: Implications for fluid flow and mineralization. Geofluids 2005, 5, 272–288. [Google Scholar] [CrossRef]
- Vilor, N.V.; Volkova, M.G.; Budyak, A.E.; Goryachev, N.A.; Pavlova, L.A.; Spiridonov, A.M.; Bryanskiy, N.V.; Danilov, B.S. Sulfoarsenide mineralization with gold in the crumpling zone on the East Trans-Baikal branch of the Mongol-Okhotsk Sutura (Pogromnoye deposit, Eastern Transbaikalia, Russia). Pac. Geol. 2021, 40, 33–50. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2009; 285p. [Google Scholar]
- Grebenschikova, V.I.; Shmotov, A.P. Stages of formation of the Zun-Kholba gold-ore deposit (East Sayan). Geol. Geophys. 1997, 28, 756–764. [Google Scholar]
- Gongalves, P.; Poivlet, J.-C.; Oliot, E.; Trap, P.; Marquer, D. How does shear zone nucleate? An example from the Suretta nappe (Swiss Eastern Alps). J. Struct. Geol. 2016, 86, 166–180. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Craw, D.; Teagle, D.A. Metabasalts as sources of metals in orogenic gold deposits. Miner. Depos. 2015, 50, 373–390. [Google Scholar] [CrossRef]
- Budyak, A.E.; Bryukhanova, N.N. Selenium, bismuth, and mercury in black shale-hosted gold deposits of different genetic types. Geochem. Int. 2012, 50, 791–797. [Google Scholar] [CrossRef]
- Tarasova, Y.I.; Budyak, A.; Chugaev, A.; Goryachev, N.; Tauson, V.; Skuzovatov, S.Y.; Reutsky, V.; Abramova, V.; Gareev, B.; Bryukhanova, N. Mineralogical and isotope-geochemical (δ13C, δ34S and Pb-Pb) characteristics of the Krasniy gold mine (Baikal-Patom Highlands): Constraining ore-forming mechanisms and the model for Sukhoi Log-type deposits. Ore Geol. Rev. 2020, 119, 118–136. [Google Scholar] [CrossRef]
- Wilson, C.J.; Osborne, D.J.; Robinson JA Miller, J.M. Structural Constraints and Localization of Gold Mineralization in Leather Jacket Lodes, Ballarat, Victoria, Australia. Econ. Geol. 2016, 111, 1073–1098. [Google Scholar] [CrossRef] [Green Version]
- Zoheir, B.; El-Wahed, M.A.; Pour, A.B.; Abdelnasser, A. Orogenic Gold in Transpression and Transtension Zones: Field and Remote Sensing Studies of the Barramiya–Mueilha Sector, Egypt. Remote Sens. 2019, 11, 2122. [Google Scholar] [CrossRef] [Green Version]
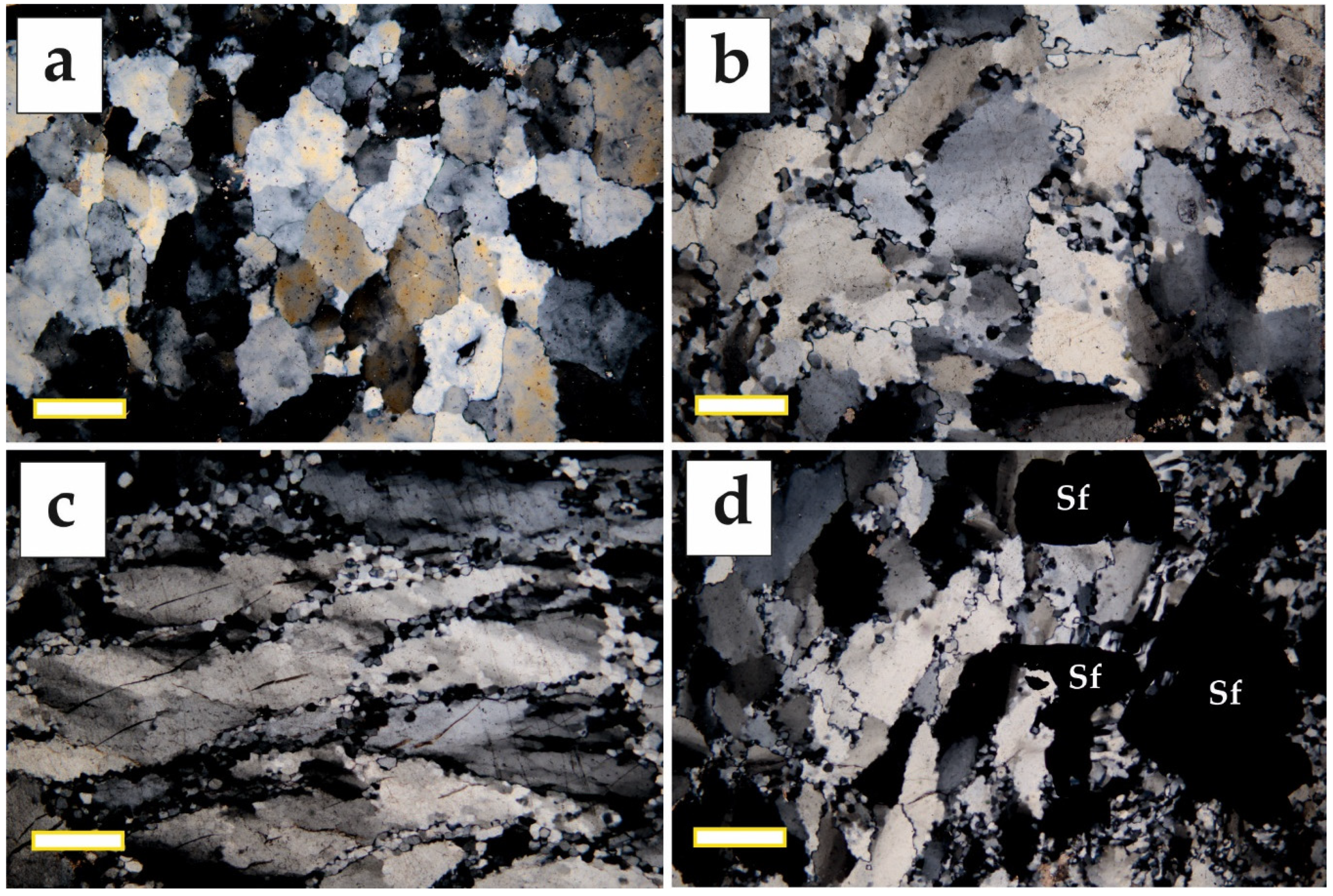
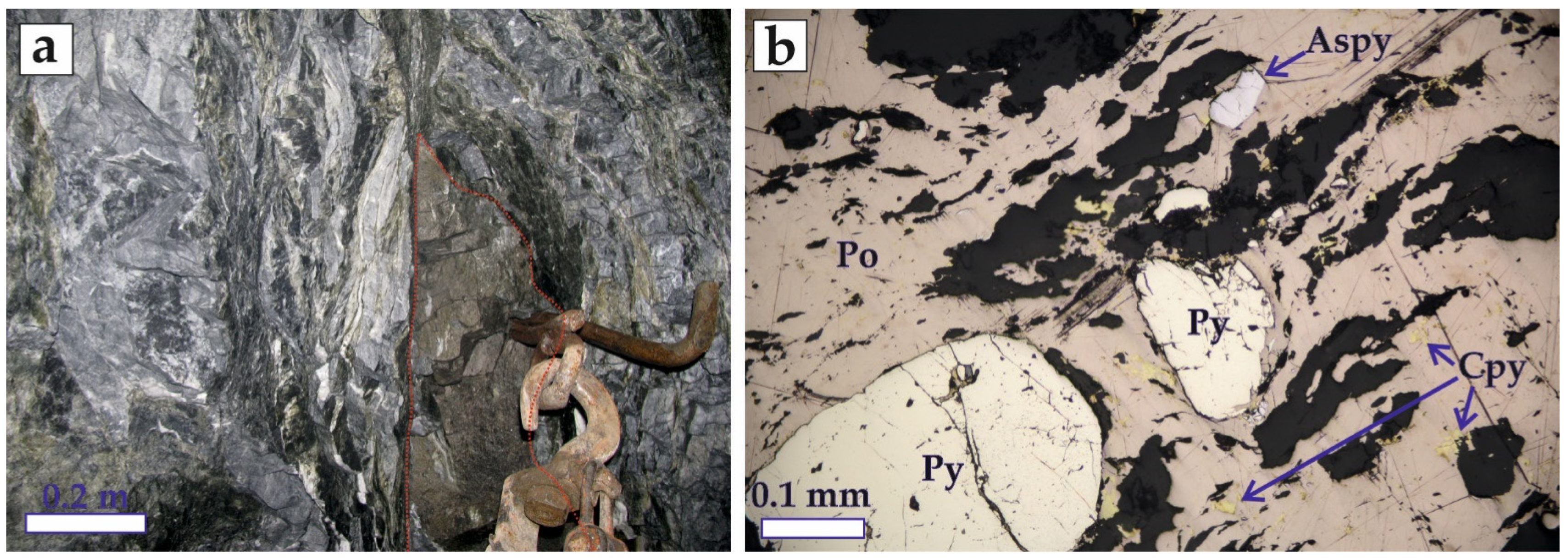

| Quartz–Pyrite Ores | Quartz–Base Metal–Sulfide Ores | Pre-Ore Massive Pyrite–Pyrrhotite Bodies (Boudins) | |
|---|---|---|---|
| Main minerals | Pyrite | Pyrite, galena, sphalerite | Pyrite, pyrrhotite |
| Minor minerals | Chalcopyrite, galena, sphalerite, arsenopyrite, native gold | Chalcopyrite, pyrrhotite, tetrahedrite, native gold | Sphalerite, galena, arsenopyrite, native gold |
| Rare minerals | Native bismuth, hessite (AgTe2), scheelite, tetradymite (Bi2Te2S), vikingite (Ag5Pb8Bi13S30) | Cubanite, hessite | Tetradymite, hessite |
| Mineral | Pre-Ore Stage | Ore-Forming Minerals Deposition Sequence |
|---|---|---|
| Quartz |  | |
| Carbonate |  | |
| Globular pyrite |  | |
| Pyrite1 |  | |
| Pyrite2 |
 | |
| Arsenopyrite |  |  |
| Chalcopyrite |  |
 |
| Scheelite |  | |
| Mиллepит |  | |
| Marcasite |  | |
| Sphalerite |  |
 |
| Galena |  | |
| Ag-tetrahedrite |
 | |
| Pyrrhotite |  |  |
| Tetradymite |  | |
| Native bismuth |  | |
| Hessite |  | |
| Vikingite |
 | |
| Native gold |  |
| Mineral | Sample | V | Cr | Mn | Co | Ni | Cu | Ga | Zn |
|---|---|---|---|---|---|---|---|---|---|
| Globular pyrite, n = 13 | Mean | 0.20 | 15.16 | 6.79 | 1390.10 | 46.75 | 1287.67 | 0.31 | 756.98 |
| Min | 0.10 | 2.80 | 0.28 | 24.37 | 2.50 | 70.70 | 0.00 | 2.48 | |
| Max | 0.50 | 57.00 | 24.10 | 6730.00 | 418.00 | 6200.00 | 1.88 | 2890.00 | |
| SD | 0.12 | 17.55 | 7.75 | 1867.66 | 112.06 | 1552.28 | 0.55 | 1008.40 | |
| Pyrite from the quartz–pyrite ores (Pyrite1), n = 61 | Mean | 0.21 | 0.73 | 7.95 | 201.70 | 6.01 | 443.52 | 0.11 | 642.62 |
| Min | 0.01 | 0.01 | 0.10 | 0.10 | 0.10 | 4.91 | 0.00 | 1.61 | |
| Max | 5.40 | 5.00 | 165.00 | 1910.00 | 30.60 | 5500.00 | 1.12 | 8570.00 | |
| SD | 0.09 | 0.14 | 2.94 | 46.55 | 0.86 | 110.61 | 0.03 | 210.37 | |
| Pyrite from the quartz-base metal-sulfide ores (Pyrite2), n = 21 | Mean | 0.03 | 0.27 | 1.61 | 9.54 | 67.90 | 34.94 | 0.16 | 267.92 |
| Min | 0.01 | 0.01 | 0.05 | 0.31 | 0.53 | 8.39 | 0.00 | 1.48 | |
| Max | 0.07 | 1.47 | 21.30 | 74.00 | 520.00 | 192.00 | 0.50 | 1550.00 | |
| SD | 0.02 | 0.36 | 4.68 | 17.59 | 129.95 | 43.94 | 0.21 | 514.35 | |
| Pyrrhotite, n = 3 | Mean | 0.19 | 5.47 | 1.60 | 0.62 | 11.67 | 1250.67 | 0.13 | 5.53 |
| Min | 0.08 | 2.60 | 0.00 | 0.42 | 7.20 | 172.00 | 0.03 | 3.80 | |
| Max | 0.37 | 8.60 | 4.80 | 0.80 | 15.00 | 2620.00 | 0.26 | 8.30 | |
| Mineral | Sample | Ge | As | Se | Mo | Ag | In | Sn | Cd |
| Globular pyrite, n = 13 | Mean | 11.83 | 172.64 | 2.24 | 0.40 | 26.73 | 0.52 | 0.48 | 30.98 |
| Min | 10.84 | 7.85 | 0.14 | 0.01 | 2.40 | 0.02 | 0.08 | 0.08 | |
| Max | 12.53 | 559.00 | 5.70 | 4.00 | 88.00 | 3.09 | 3.00 | 124.00 | |
| SD | 0.61 | 166.30 | 1.51 | 1.09 | 27.28 | 0.88 | 0.79 | 40.50 | |
| Pyrite from the quartz–pyrite ores (Pyrite1), n = 61 | Mean | 7.76 | 227.07 | 5.47 | 1.01 | 51.26 | 0.23 | 0.78 | 24.09 |
| Min | 6.20 | 2.18 | 0.32 | 0.00 | 0.31 | 0.05 | |||
| Max | 8.77 | 1914 | 19.70 | 21.60 | 1100 | 4.99 | 12.60 | 345.00 | |
| SD | 0.08 | 44.75 | 0.63 | 0.49 | 23.69 | 0.10 | 0.25 | 8.69 | |
| Pyrite from the quartz-base metal-sulfide ores (Pyrite2), n = 21 | Mean | 6.99 | 474.09 | 0.69 | 0.02 | 3.78 | 0.01 | 0.24 | 26.65 |
| Min | 6.48 | 2.86 | 0.03 | 0.00 | 0.22 | 0.00 | 0.08 | 0.10 | |
| Max | 7.50 | 1496 | 1.90 | 0.03 | 38.00 | 0.05 | 1.62 | 260.00 | |
| SD | 0.22 | 521.94 | 0.55 | 0.01 | 7.61 | 0.02 | 0.33 | 70.63 | |
| Pyrrhotite, n = 3 | Mean | 14.77 | 0.35 | 3.13 | 1.58 | 8.47 | 0.03 | 0.19 | 0.53 |
| Min | 14.60 | 0.24 | 0.50 | 0.02 | 6.44 | 0.01 | 0.12 | 0.28 | |
| Max | 14.90 | 0.47 | 6.20 | 3.70 | 10.70 | 0.06 | 0.30 | 0.90 | |
| Mineral | Sample | Sb | Te | Au | Hg | Tl | Pb | Bi | |
| Globular pyrite, n = 13 | Mean | 6.51 | 22.44 | 2.66 | 0.29 | 0.20 | 1209.15 | 18.32 | |
| Min | 1.30 | 1.51 | 0.15 | 0.07 | 0.03 | 18.50 | 2.58 | ||
| Max | 35.20 | 69.00 | 11.70 | 1.44 | 0.92 | 8000.00 | 91.00 | ||
| SD | 8.74 | 21.86 | 3.52 | 0.41 | 0.23 | 2460.08 | 23.46 | ||
| Pyrite from the quartz–pyrite ores (Pyrite1), n = 61 | Mean | 2.73 | 9.90 | 2.02 | 1.83 | 0.04 | 629.21 | 50.27 | |
| Min | 0.03 | 0.14 | 0.01 | 0.04 | 0.01 | 5.75 | 0.27 | ||
| Max | 15.17 | 105.00 | 78.00 | 80.00 | 0.33 | 8650.00 | 564.00 | ||
| SD | 0.43 | 2.14 | 1.25 | 1.33 | 0.01 | 181.28 | 10.91 | ||
| Pyrite from the quartz-base metal-sulfide ores (Pyrite2), n = 21 | Mean | 11.93 | 0.15 | 0.65 | 0.32 | 0.01 | 582.12 | 0.06 | |
| Min | 0.32 | 0.00 | 0.02 | 0.01 | 0.00 | 4.45 | 0.01 | ||
| Max | 212.00 | 0.49 | 3.22 | 2.10 | 0.05 | 6900.00 | 0.37 | ||
| SD | 43.82 | 0.17 | 0.86 | 0.43 | 0.01 | 1683.31 | 0.08 | ||
| Pyrrhotite, n = 3 | Mean | 0.61 | 2.87 | 0.06 | 0.00 | 0.05 | 623.33 | 3.06 | |
| Min | 0.43 | 0.73 | 0.00 | 0.00 | 0.02 | 430.00 | 2.24 | ||
| Max | 0.87 | 6.10 | 0.14 | 0.08 | 0.08 | 970.00 | 4.37 | ||
| № | Sample | δ34S, ‰ | δ34Sfl, ‰ | Mineral | Level, m |
|---|---|---|---|---|---|
| 1 | Zk-233-1 | 1.3 | 1.1 | Chalcopyrite | 2250 |
| 2 | Zk-233-2 | 1.1 | −0.1 | Pyrite2 | 2250 |
| 3 | Z-574 | 1.6 | 0.4 | Pyrite2 | 2134 |
| 4 | Z-532 | 1.3 | 0.1 | Pyrite2 | 2032 |
| 5 | Kh-5 | 4 | 2.8 | Pyrite1 | 1840 |
| 6 | Z-505 | 1.1 | −0.1 | Pyrite1 | 1740 |
| 7 | Z-508 | 1.5 | 0.3 | Pyrite1 | 1740 |
| 8 | Zkhl-4 | 1.8 | 0.6 | Pyrite1 | 1490 |
| 9 | Zkhl-6 | 2.5 | 1.3 | Pyrite1 | 1490 |
| 10 | Zkhl-10 | 1.8 | 0.6 | Pyrite1 | 1490 |
| 11 | Zkhl-30 | 1.7 | 0.5 | Pyrite1 | 1490 |
| 12 | Zkhl-31 | 1.2 | 0 | Pyrite1 | 1490 |
| 13 | Zkhl-32 | 2.6 | 1.4 | Pyrite1 | 1490 |
| 14 | Zkhl-33 | 2 | 0.8 | Pyrite1 | 1490 |
| 15 | Zk-60 | 1 | −0.2 | Pyrite1 | 1440 |
| 16 | Zk-68 | 0.4 | −0.8 | Pyrite1 | 1440 |
| 17 | Zk-72 | 0.3 | −0.9 | Pyrite1 | 1390 |
| 18 | Zk-94 | 1.5 | 0.3 | Pyrite1 | 1340 |
| 19 | Zk-97 | 0 | −0.6 | Pyrrhotite | 1290 * |
| 20 | Zk-99-1 | 0.6 | −0.6 | Globular pyrite | 1290 * |
| 21 | Zk-99-2 | 0.2 | −0.4 | Pyrrhotite | 1290 |
| 22 | Zkh-8 | 2.3 | 2.1 | Chalcopyrite | |
| 23 | Kh-899 | 2.8 | 1.6 | Pyrite | |
| 24 | Kh-907 | 3 | 1.8 | Pyrite | |
| 25 | Kh-910 | 2.5 | 1.3 | Pyrite | |
| 26 | Kh-1674 | 2.8 | 1.6 | Pyrite | |
| 27 | Kh-977 | 3.8 | 2.6 | Pyrite | |
| 28 | Kh-979 | 4 | 2.8 | Pyrite | |
| 29 | Kh-1007 | 4.6 | 3.4 | Pyrite |
| Num. | Sample | δ13C, ‰ | δ18O, ‰ | δ18Ofl, ‰ | Mineral | Level |
|---|---|---|---|---|---|---|
| 1. | Kh-41 | 0.42 | 11.9 | Carbonate | 1840 | |
| 2. | Kh-57 | 1.18 | 12.0 | Carbonate | 1840 | |
| 3. | Kh-70 | 0.2 | 13.3 | Carbonate | 1840 | |
| 4. | Kh-102 | −0.53 | 11.1 | Carbonate | 1840 | |
| 5. | Kh-106 | −1.72 | 8.0 | Carbonate | 1840 | |
| 6. | Kh-128 | −1.16 | 10.2 | Carbonate | 1740 | |
| 7. | Z-702 | 0.12 | 16.1 | Carbonate | ||
| 8. | Zk-229 | 12.4 | 6.0 | Quartz | 2250 | |
| 9. | Zk-322 | 15.7 | 9.3 | Quartz | 2165 | |
| 10. | Zk-323 | 16.1 | 9.7 | Quartz | 2165 | |
| 11. | Zk-326 | 14.8 | 8.4 | Quartz | 2165 | |
| 12. | З-546 | 13.3 | 9.4 | Quartz | 2032 | |
| 13. | Kh-21 | 13.3 | 9.4 | Quartz | 1840 | |
| 14. | Kh-97 | 15.8 | 11.9 | Quartz | 1840 | |
| 15. | Z-517 | 14.2 | 9.5 | Quartz | 1740 | |
| 16. | Sev-3 | 17.5 | 12.8 | Quartz | 1490 | |
| 17. | Zkhl-20 | 17.8 | 13.1 | Quartz | 1490 | |
| 18. | Zkhl-8 | 17.8 | 13.1 | Quartz | 1490 | |
| 19. | Zk-62 | 16.4 | 11.7 | Quartz | 1440 | |
| 20. | Zk-330 | 10.2 | 9.3 | Muscovite * | 1260 | |
| 21. | Zk-330-1 | 13.2 | 9.3 | Quartz * | 1260 | |
| 22. | Zk-310-2 | 15.4 | 11.5 | Quartz | 1260 |
| Num. | Sample | Teut | TIce melt. | Thom | Bulk Salinity, wt.% eq. NaCl | P, kbar |
|---|---|---|---|---|---|---|
| °C | ||||||
| High-Temperature FI | ||||||
| 1 | Zkhl-22 | −37 | −5 | 340 | 7.9 | 0.57 |
| 2 | Zkhl-22 | −37 | −3.8 | 325 | 6.2 | 0.71 |
| 3 | Zkhl-20 | −37 | −1.4 | 307 | 2.4 | 0.87 |
| 4 | Zkhl-22 | −38 | −3.6 | 305 | 5.9 | 0.95 |
| Low-Temperature FI | ||||||
| 5 | Zkhl-20 | −38 | −3.5 | 275 | 5.7 | |
| 6 | Zkhl-22 | −37 | −1.3 | 268 | 2.2 | |
| 7 | Zkhl-21 | −38 | −6 | 248 | 9.2 | |
| 8 | Zkhl-20 | −38 | −0.6 | 240 | 1.1 | |
| 9 | Zkhl-20 | −3.4 | 235 | 5.6 | ||
| 10 | Zkhl-22 | −38 | −3.4 | 225 | 5.6 | |
| 11 | Zkhl-20 | −37 | −0.5 | 208 | 0.9 | |
| 12 | Zkhl-20 | −37 | −2.8 | 207 | 4.7 | |
| 13 | Zkhl-20 | −37 | −1.5 | 185 | 2.6 | |
| 14 | Zkhl-20 | −37 | −3.5 | 182 | 5.7 | |
| 15 | Zkhl-22 | −37 | −1.5 | 168 | 2.6 | |
| Mineral | Fe | Zn | Cd | As | S |
|---|---|---|---|---|---|
| Sphalerite | 1.8–7.7 3.8 | 59.7–65.2 61.9 | 0–1.3 0.91 | 32.1–33.9 33.1 | |
| Arsenopyrite | 30.3–38.1 35.6 | 38.8–45.4 42.4 | 19.9–24.1 21.1 |
| Num. | Sample | NAgElect | XFeSSph | Asars, at% | T, °C | Level |
|---|---|---|---|---|---|---|
| 1 | Z-580 | 0.5198 | 0.0591 | 318 | 2134 | |
| 2 | Z-580 | 0.5293 | 0.0591 | 314 | 2134 | |
| 3 | Z-580 | 0.5266 | 0.0591 | 315 | 2134 | |
| Average | 0.5252 | 0.0591 | 316 | |||
| 4 | Z-506 | 0.3049 | 0.1126 | 464 | 1740 | |
| 5 | Zkhl-10 | 0.3468 | 0.0670 | 409 | 1490 | |
| 6 | Zkhl-10 | 0.4504 | 0.0670 | 354 | 1490 | |
| Average | 0.3986 | 0.0670 | 382 | |||
| 7 | ZK-335 | 0.2606 | 0.0389 | 437 | 1260 | |
| 8 | ZK-335 | 0.2462 | 0.0469 | 458 | 1260 | |
| 9 | ZK-335 | 0.2550 | 0.0469 | 451 | 1260 | |
| 10 | ZK-335 | 0.2541 | 0.0469 | 452 | 1260 | |
| 11 | ZK-335 | 0.2578 | 0.0469 | 449 | 1260 | |
| 12 | ZK-335 | 0.2580 | 0.0469 | 449 | 1260 | |
| 13 | ZK-335 | 0.2606 | 0.0469 | 447 | 1260 | |
| Average | 0.2894 | 0.0567 | 437 | |||
| 14 | Z-506 | 27.85 | 271 | 1740 | ||
| 15 | Z-506 | 30.84 | 399 | 1740 | ||
| 16 | Z-506 | 30.34 | 377 | 1740 | ||
| 17 | Z-506 | 29.14 | 326 | 1740 | ||
| 18 | Z-506 | 30.70 | 393 | 1740 | ||
| 19 | Z-506 | 29.70 | 350 | 1740 | ||
| 20 | Z-506 | 30.53 | 385 | 1740 | ||
| Average | 29.87 | 357 | ||||
| 21 | Zkhl-10 | 30.88 | 400 | 1490 | ||
| 22 | Zkhl-10 | 30.55 | 386 | 1490 | ||
| 23 | Zkhl-12 | 30.71 | 393 | 1490 | ||
| Average | 30.71 | 393 | ||||
| 24 | ZK-95 | 29.56 | 344 | 1290 | ||
| 25 | ZK-95 | 30.12 | 368 | 1290 | ||
| 26 | ZK-95 | 29.97 | 362 | 1290 | ||
| 27 | ZK-99 | 30.34 | 377 | 1290 | ||
| 28 | ZK-99 | 30.38 | 379 | 1290 | ||
| 29 | ZK-99 | 30.20 | 371 | 1290 | ||
| 30 | ZK-99 | 29.90 | 359 | 1290 | ||
| 31 | ZK-335 | 29.95 | 361 | 1260 | ||
| 32 | ZK-335 | 29.69 | 350 | 1260 | ||
| Average | 30.01 | 363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damdinov, B.B.; Goryachev, N.A.; Moskvitina, M.L.; Damdinova, L.B.; Izvekova, A.D.; Reutsky, V.N.; Posokhov, V.F.; Artemyev, D.A. Zun-Kholba Orogenic Gold Deposit, Eastern Sayan, Russia: Geology and Genesis. Minerals 2022, 12, 395. https://doi.org/10.3390/min12040395
Damdinov BB, Goryachev NA, Moskvitina ML, Damdinova LB, Izvekova AD, Reutsky VN, Posokhov VF, Artemyev DA. Zun-Kholba Orogenic Gold Deposit, Eastern Sayan, Russia: Geology and Genesis. Minerals. 2022; 12(4):395. https://doi.org/10.3390/min12040395
Chicago/Turabian StyleDamdinov, Bulat Batuevich, Nikolay Anatolyevich Goryachev, Maria Leonidovna Moskvitina, Ludmila Borisovna Damdinova, Alexandra Dmitrievna Izvekova, Vadim Nikolaevich Reutsky, Victor Fedorovich Posokhov, and Dmitry Alexandrovich Artemyev. 2022. "Zun-Kholba Orogenic Gold Deposit, Eastern Sayan, Russia: Geology and Genesis" Minerals 12, no. 4: 395. https://doi.org/10.3390/min12040395
APA StyleDamdinov, B. B., Goryachev, N. A., Moskvitina, M. L., Damdinova, L. B., Izvekova, A. D., Reutsky, V. N., Posokhov, V. F., & Artemyev, D. A. (2022). Zun-Kholba Orogenic Gold Deposit, Eastern Sayan, Russia: Geology and Genesis. Minerals, 12(4), 395. https://doi.org/10.3390/min12040395







