Abstract
The role of biogenicity in the mineral world is larger than many might assume. Biological processes and physical and chemical processes interact both at the Earth’s surface and far underground, leading to the formation of banded iron and manganese deposits, among others. Microbial mats can form giant sedimentary ore deposits, which include enrichment of further elements. This article reviews the ways in which microbially-mediated processes contribute to mineralization, the importance of mineralized microbial textural features, and the methods that must be used to obtain high-resolution datasets. If the chosen methodology and/or the size dimension of investigation is not appropriate, then it is not possible to recognize that a system is microbially mediated, and the conclusion will be incomplete. We call attention to variable authigenic mineralization as the result of complex mineralization of cells and extracellular polymeric substances in the starving basins, which form giant ore deposits together with ore-forming minerals. Microbial mats and other biosignatures can serve as indicators of environmental reconstruction in ore formations. We suggest tests and analyses that will allow the potential role of biomineralization to be properly investigated for a more comprehensive view of formation processes and their implications.
1. Introduction
Twenty-two years ago, our attention turned to the focus of biomineralization as the main process in the low-temperature (T) sedimentary Mn ore formation of Jurassic Mn carbonate ore at Úrkút, Hungary, and it was only 10 years ago when the first papers were published [1,2]. The first 12 years were spent in contradictory discussions and a desire to reach consensus with national experts, but this was not successful. After publications in high-ranking journals, we hoped that international readers would find convincing aspects in our arguments. From that time, the international interest increased, and our research group was invited to cooperate in Mn and Fe ore research. Numerous papers have been published in the framework of these cooperative research projects [3,4,5,6,7,8,9,10,11].
In order to understand the content of sedimentary Fe-Mn—both main and rare elements—we have to take into consideration the role of microbial life in the geological context of mineralization, since the mechanisms driving mineralization often supersede purely inorganic reactions. Microbially mediated mineralization is a special metabolic process leading to the formation of particular minerals. Microbially mediated minerals are very fine-grained (µm scale), and in comparison to chemical processes, microbial processes are very effective. Details on the geomicrobiological background of the mineral assemblage of ore deposits can be found in Ref. [12].
A common topic of debate is the sources of metals and their accompanying elements as well as the determination of the selective enrichment processes that result in particular deposits, such as iron and manganese deposits, as well as other metals. These debates lead to disharmony and controversy in the interpretation of datasets. Hydrothermal models are commonly used, but unfortunately they overlook the basic role of microbial processes in the sequestration of metal ions from geofluids into solid forms as minerals and in the selective enrichments of particular elements. We can take as an example huge formations of extreme K-rich altered pyroclastic rock masses, which were originally classified as metasomatic occurrences [13,14]; more recent data [15] support microbial processes in their element enrichment. The important role of microbially mediated ore-forming processes and cell mineralization in general is summarized in Ref. [12] and supplemented by case studies such as those of Úrkút in Hungary [1,2], Datangpo in China [5], and Urucum in Brazil [6]. It has been a long journey from the first working hypothesis to the convincing complex interpretation of microbialite ores.
Deposits form through complex diagenetic processes—followed by syngenetic element enrichment—including the decomposition and mineralization of cell and extracellular polymeric substances (EPS) of Fe, Mn, and cyanobacteria. Bioessential elements are provided through these processes; the specific combination depends on factors such as the store of elements available, the type of metabolism, microbial species, and the mineralogical characteristics. Cell and EPS material mineralization forms a significant contribution to the material of ore deposits and other rock types, in terms of both quantity and mineral composition. In oxic environments, microbial Mn and Fe oxidation determines the global biogeochemical cycle of these elements.
On the 10th anniversary of publication, we are sharing our experiences and scientific results, because we think that this information will contribute to more comprehensive interpretations of such deposits.
Microtextural observations of samples of ore deposits as a first step clearly showed mineralized microbial biosignatures. Based on this, our working hypothesis was the research of microbially mediated sedimentary manganese and iron ore formations. To solve this, an adequate multi-methodology was chosen to offer a high-resolution mineral and organic matter dataset on mineral species and distribution in the samples on a scale that is in the dimension of microbial activity. The authors have worked on sedimentary manganese and iron mineralizations for decades, and this multi-methodological research and complex interpretation on the basis of the structural hierarchical system is the focus of their research. Until now, there have been no similar publications, which is why the cited papers are those of the authors on different deposits. One of the main purposes of this self-overview is to call for attention to ore microbialites and to the appropriate methodology and complex interpretation of their research.
In these publications, different aspects were discussed and interpreted based on published results, including laboratory experiments, which helped to understand the mosaic of knowledge on these deposits and to build up a more comprehensive interpretation that is in harmony with the datasets.
Some of these key points are as follows:
- (1)
- Why the enzymatic Mn oxidation under obligatory oxic conditions is the main primary process during formation of manganese ore deposits; why enzymatic activity requires obligatory oxic redox conditions. Details are given in Ref. [1].
- (2)
- Manganite (MnO(OH)), a so-called longstanding phase as noted by Ref. [16] and a characteristic mineral in all Mn carbonate deposits, is a concentration indicator of elevated Mn(II) contribution rather than a redox indicator; details are given in Ref. [1].
- (3)
- Redox conditions during formation are also a key point. Our results concluded that formation happened under an oxic water column, and anoxic conditions developed via diagenesis [5,9,10,17,18,19].
- (4)
- We call attention to the indefinite usage of the denomination of redox conditions, which is common in published materials; this is also based on important cited papers in Refs. [2,6].
- (5)
- We highlighted the selective element enrichments resulting from microbial mediation and pointed out that geochemical ratio studies must be used with caution (e.g., Mg selective enrichment on cell material, microbial oxidation of Ce and Co by Mn oxidizing bacteria) [1].
- (6)
- We noted the importance of the suboxic diagenetic zone, which is also the zone of the microbially mediated reduction of Mn(IV) oxides and Fe(III) oxides based on free energy aspects [1,2,5,6,20,21].
- (7)
- We pointed out the role of interpretation of mineral assemblages in the deposits, which also represent aspects of environmental conditions. The fact is that the primary ore formation processes are similar, but the output can be very different in terms of the local effects and the mass balance conditions (e.g., concentration of buried organic matter, its reactivity, concentration of metal oxides and other minerals, accumulation rate).
- (8)
- These Fe and Mn ore systems are dual microbial systems, as the Fe microbial system is present together with the Mn microbial system (Table 1). The strength of each can differ, but they occur together in an intimate connection (Raman profiles are offered in the papers). Diagenesis results in variable mineralogy of the original Fe and Mn biomats.
 Table 1. Mineral assemblage of Mn and Fe deposits and environmental mineralogical considerations.
Table 1. Mineral assemblage of Mn and Fe deposits and environmental mineralogical considerations.
Another obstacle that we continue to encounter as we share our proposals and findings with the research community is the complexity of the topic of microbially mediated ores. This is a multidisciplinary topic, with ties to so many different areas of learning; another feature is that it requires large datasets from a variety of methods and instruments. This broad reach makes it difficult to discuss all processes involved in much detail in a single manuscript. At the same time, in-depth explanation means pulling in knowledge from a number of related fields. There are still very few specialists in this area of study. All of these factors make it difficult to find reviewers who are equipped and willing to review manuscripts in this area. We are truly grateful to those reviewers who have taken an open-minded approach to this new field and helped us begin to communicate it to a wider audience.
This paper aims to give a comprehensive self-overview of the experiences of the research group, of the background, and of the research results for variable types of sedimentary Mn and Fe deposits. These deposits represent important economic value and can provide valuable information on the role and development of biomineralization. Last but not least, we offer suggestions and key points concerning this field.
2. Studied Deposits
The Jurassic black shale-hosted deposit at Úrkút was the locality where the two-step microbially mediated ore formation model was elaborated [1]. The model included enzymatic Mn oxidation, which now we label Cycle I. Microbial (enzymatic) Mn(II) oxidation can take place only in oxic conditions (>2 mL/L dissolved oxygen). Cycle II is Mn reduction (MnR) and reactive organic matter decomposition via heterotrophic microbial mediation; rhodochrosite (kutnohorite) is a common (dominant) component in this process.
Based on this experience, researchers recognized similar microbially mediated formations in the Triassic, sandstone-hosted Fe-carbonate (siderite, ankerite) iron deposit at Rudabánya, NE Hungary [67]. This was followed by the small-size hydrothermal Cretaceous chert-hosted Mn and Fe-Mn deposits associated with the Neyriz ophiolite complex in the Abadeh-Tashk area, SE Fars Province, SW Iran [4]. This was the first case when FTIR (124 spectra) and Raman spectroscopy (1200 spectra) were used together in addition to optical rock microscopy, to support microbial mediation in ore formation based on textural and mineralogical evidence. The methodology developed in the direction of high resolution, using smaller step size dimensions in Raman and finally using a 10 µm step size. Without going into detail, the nine deposits (Table 1, legend) offered experience that we think can be useful in the investigation of ore microbialites. From the nine deposits, seven are black shale-hosted Mn carbonate of variable geological ages from Neoproterozoic Cryogenian (Datangpo, Guizhou Province, South China) via Carboniferous (Masi—Central Guangxi, South China), Tianshan and Kunlun (China) (with some oxide) and Permian (Xinglong—N Guizhou, South China) to Jurassic Molango (Mexico) and Úrkút (Hungary). Most of these occurrences are macroscopically laminated; the sections were prepared perpendicularly to lamination. In some cases, lamination occurred on the microscopic level. Two Neoproterozoic deposits, banded iron formations (BIFs) found at Urucum, Brazil, contain BIF-hosted Mn oxide and BIF Fe oxide giant deposits at Urucum, Brazil. The studied deposits are not metamorphosed, or only slightly, which reveals information on syngenetic conditions. Based on the basic character that these deposits are dual Fe-Mn microbial systems, interpretation was made on mineral assemblages and micro-textural features. The investigations were made both in the host countries and in Hungary, and our research group collected a total of approximately 1900 OM photos, 1300 CL photos, 1500 FTIR spectra, 53,500 Raman spectra, and 3000 SEM-EDS spectra.
3. General Framework
3.1. Sketch of Syngenetic and Diagenetic Network
The following is a short summary on microbial Mn and Fe oxidation. We were able to establish that the syngenetic situation is similar in both cases: it is a double microbial ore forming system that is suboxic in the case of Fe and oxic in the case of Mn. However, this results in a wide variety of deposits in terms of their mineralogy. The geodynamic situation gives evidence of a rifting (or failed rifting) zone, and distal hydrothermal discharge appears to have been present. Differences in the mineralogy seem to be caused by two main factors: (i) accumulation ratio—if accumulation is slow, part of the organic matter will be oxidized by atmospheric oxygen so a smaller amount of reactive organic matter will be buried; and (ii) mass balance—differences in the accumulating organic matter (type, mass), metal oxide concentration and type, and other forming minerals. So far we have found evidence for this from the Mesoproterozoic to the Quarternary. Oxygen supply can be affected by currents and/or cyanobacterial ventilation [5]. These situations are starving basins; if a considerable contribution of debris occurs, the microbial biomat system will be destroyed. We often find complex situations when the macroscopic occurrence suggests debris-based accumulation, but ultimately we find that the minerals are the result of authigenic formation, as confirmed by cathodoluminescence microscopy [5,6].
3.2. Fe Accumulations
Giant Fe ore geological reservoirs of global importance and major economic value occur around the world. Banded ironstone formations (BIFs) are sedimentary rock types mainly of Precambrian age [68]. The most characteristic features of such formations are their alternating laminae (bands), whose width ranges from mm to a few cm in scale. The laminae are comprised of black iron oxides—generally magnetite (Fe3O4) or hematite (Fe2O3)—and red, iron-poor shales and cherts [69]. Ferrihydrite, goethite, and siderite are also commonly occurring syngenetic and diagenetic minerals.
One hypothesis for the formation of BIFs is microbial origin [70]: Fe-rich biomats formed by Fe-oxidizing bacteria play a fundamental role in the genesis of BIFs. This is supported by micro-textural evidence in the form of mineralized filaments encrusted by Fe oxide minerals, which form an important biosignature. Four types of microbial metabolisms that can oxidize Fe2+ forming Fe-oxide minerals have been identified [32,35,37,40,71]:
- -
- acidophilic and oxic;
- -
- photoferrotroph, driven by light, occurring in anoxic/anaerobic and neutrophilic conditions;
- -
- suboxic/anaerobic, where neutrophilic NO3− reducers coupled with Fe(II) oxidizers contribute to the biochemical milieu;
- -
- suboxic and neutrophilic, e.g., Gallionella (Mariprofundus)-like Fe-oxidizing microbes, which are common in many environments.
All cases can be characterized and determined by considering environmental conditions based on mineral assemblages. In addition, bacteria are also thought to play an indirect role in the oxidation of Fe, as the geochemical conditions of the environment can be modified by microbial activity, potentially leading to the chemical oxidation of Fe.
Metabolic processes are determined by environmental proxies (Eh, pH, light), providing paleoenvironmental considerations based on microbial mineralization, which are also known as paleoenvironmental indicators. Formation time duration can be estimated by microbial growth population cycles in fine, cyclic mineral lamination in rocks [2,57].
The most important microbially mediated minerals are ferrihydrite and lepidocrocite, poorly crystallized minerals that transform—via silica segregation—into more stable minerals like goethite and hematite (in its reduced form as magnetite) over months to years by dehydration–dissolution [37,49,57]. The mineralized microbial cycles are realized in more variable forms, as summarized in Table 1. Later, during diagenesis, Fe oxide and the segregated silica react with cations and anions released via cell and EPS material decomposition, leading to complex mineralization. Examples include aegirine, celadonite, chamosite, and phlogopite [6]. These processes are often overprinted by diagenetic microbially mediated mineralization, which results in the mineralization of organic carbon in the form of metal carbonates. For Fe, this typically occurs in the form of siderite or mixed carbonates like ankerite, while pyrite, marcasite, and Fe-anatase also occur.
3.3. Mn Accumulations
Giant Mn ore geological reservoirs—of global importance and high economic value today—formed when the conditions became more oxic. Before going into the detail regarding enzymatic Mn oxidation, we must address why Mn is a microbial element. In low T aquatic systems (T < 150 °C, sedimentary environment), manganese precipitation is microbial, and obligatory oxic conditions are needed for first sequestration of the dissolved Mn(II), because the multicopper oxidaze enzymatic process is active only under these conditions. Mn(II) precipitation is microbial, for atomic structure (electron configuration) reasons, because the transmission of the two electrons to form Mn4+ has different energy content. The third electron transmission is (Mn3+/Mn2+): ΔGo: 67 kJ/mol (energy demand); the fourth electron transmission is (MnO2/Mn3+): ΔGo: −137 kJ/mol [44,46,72].
Mn oxide reduction via heterotrophic microbial mediation has also been proven by laboratory experiments. As we see from this example, in the case of Mn, we used the results on the behavior of the Mn element and also on laboratory experiments using natural microbes, concerning syngenetic mineralogy (vernadite, todorokite, birnessite), and microbiological research on the demand of conditions (oxygen supply), concerning enzymatic activity. Multicopper oxidase needs obligatory oxic conditions; this is a basic demand of starting the enzymatic engine to realize a giant mass of the given element. This itself convincingly verifies the microbial formation of Mn ores.
A biologically formed Mn oxide (e.g., vernadite, birnessite, buserite) is the first product of microbial enzymatic Mn(II) oxidation, as was reported in Refs. [43,44,46,47]. We can refer to this enzymatic Mn oxidation as Cycle I in Mn ore formation. During this cycle, chemolihoauthotrophic microbes sequestrate and precipitate Mn(II) from geofluids in the form of variable Mn oxide-hydroxides. For microbial (enzymatic) Mn(II) oxidation, oxic conditions (>2 mL/L dissolved oxygen) are obligatory. This means that Mn deposits are the indicators of oxic conditions even in the case of Mn carbonate deposits, where this oxic redox condition is the demand of the enzymatic engine to form ore deposits. The forming bio-oxide is poorly crystallized, thermodynamically unstable 7-Å-vernadite (hexagonal phyllomanganate) [43]. Surplus Mn(II) in the system serves as a reductant and contributes to the stabilization of secondary abiotic mineral products (manganite, see [1] on components in minerals of Úrkút, and [6] for evidence from Urucum). Cation binding like Mg and Ni supports phyllomanganate transformation to stable tectomanganate [47]. Mg adsorption on the cells is caused by reaction with EPS; this mechanism is supported by experimental evidence [34]. This sheds light on the complex processes, which include not only direct microbial oxidation of Mn(II) to Mn(IV), but also determine the cation composition of the forming Mn oxide minerals. Variable Mn minerals thus form (Mn, Fe, Mg, Ca, K, Na)2·(Mn5O12)·3H2O as todorokite, the general fine-grained poorly crystallized biomineral found in marine Fe- and Mn deposits.
Stabilization of the syngenetic Mn oxide hydroxides occurs via diagenesis, forming pure forms such as pyrolusite, ramsdellite, nsutite, hausmannite, or manganite as well as variable cation-bound forms (e.g., Na, K, Ca, Mg, Ba, Fe) such as cryptomelane, jacobsite, romanèchite, and manjiorite [26,34,43,44,47,56,73]. In accordance with findings in Refs. [1,56,74], rhodochrosite is the result of early diagenetic sporadic heterotrophic, sub-oxic microbial activity (representing Cycle II in Mn ore formation in black shale-hosted Mn carbonate deposits). Diagenetic interaction of Mn oxide with segregated silica forms braunite and serandite [6].
3.4. Consequences of Very Small Grain Size
Very small grain size is a basic feature of low T biominerals, expressed in µm or a hundreds of nanometers. This is caused by the quick nucleation of oversaturation, and the small core size remains because of the low solubility of ions, which limits diffusion-based crystal growth. Material scientists have found that the small grain size results in peculiar material features, and unusual optical electric behavior and modified surface structure and surface activity are characteristic. This is important in environmental science, since nano-scale microbial material is very frequent in fluids, sediments, and soils. These features raise major research challenges, since physical separation is impossible and high-resolution in situ methods (SEM, TEM, LA-ICP-MS, FTIR, Raman spectroscopy) are needed to investigate these materials.
3.5. Preservation of Metastable Minerals
Turning back to minerals, there are two things that should be mentioned. Some may question how it is possible that metastable, poorly crystallized minerals like ferrihydrite survive billions of years. There are two factors to consider here: (i) Cell conservation is provided by the minerals around the cells and EPS. EPS plays a similar role in cell fossilization, providing a protective function through its peptidoglycans and polymers (compounds that can withstand degradation, possibly explaining the metastable state of these minerals) [12,75]. (ii) It is often raised that the occurrence of ferrihydrite is scarce or even missing, and the answer to this debate is the problem of usage of an inappropriate methodology [60].
So, as a summary (of deposit size and metal enrichment) we concluded that:
A. Syngenetic formation—source of elements—is similar
- -
- The geodynamic situation refers to a rifting (or failed rifting) zone, and distal hydrothermal discharge is the metal source;
- -
- Oxygen supply is important: it is suboxic in the case of Fe and obligatory oxic in the case of Mn (to form a deposit, a very effective enzymatic enrichment engine is needed). The oxygen supply can be due to currents or cyanobacterial ventilation, and even small variations can determine what kind of deposit will be formed or not formed (suboxic: Fe, obligatory oxic: Mn);
- -
- Starving basin conditions are required. If a considerable contribution of debris takes place, the microbial biomat system will be destroyed;
- -
- Enrichment process: enzymatic selective element enrichment is very effective (enzymatic engine and its redox demand).
B. Diagenesis (variable outlook of deposits concerning mineralogy)
- -
- What cause differences? We can mention accumulation ratio differences and mass balance differences (accumulating organic matter (type, mass), metal oxide concentration and type, other forming minerals and clay minerals). These factors result in differences among deposits both in ore and other mineral content (Table 1).
3.6. Geochemical Conditions
Thus, while not an abiogenic redox system, redox is the demand of enzymatic activity, which is the main factor of metal enrichment. The Mn and Fe deposits are so-called redox deposits, but we point out that this fact is not in the abiogenic meaning. The enzymatic activity has redox demand, which is oxic in the case of Mn oxidizing bacteria and suboxic in the case of Fe oxidizing bacteria. In the case of Fe oxidizing bacteria, we note the above-detailed four types (see Section 3.2).
To shed light on the oxygen demand of Mn and Fe bacteria and supply the environmental and biofacies terms, we present Table 2 and Figure 1.

Table 2.
Environmental oxygen levels.
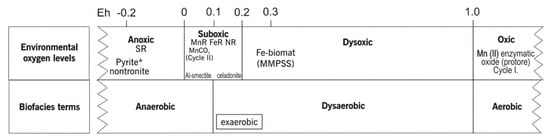
Figure 1.
Terminology of oxygen-restricted environments and facies, mineralogy, and Fe-rich biomat formation conditions [1,33]. * Mat pyritization is seen in thin sections of the Úrkút Mn deposit; Eh values are in volts. MMPSS—mineralized microbially produced sedimentary structures; NR—nitrate reduction zone; FeR—Fe3+ reduction zone; MnR—Mn4+ reduction zone; SR—sulfate reduction zone. Cycles I and II are the two-step microbially mediated Mn-carbonate formation model. Cited from [2], with permission of GSA Publishing House.
Remnant syngenetic minerals are reported to be microbially mediated minerals forming under obligatory oxic (Mn) and suboxic (Fe) conditions with neutral and semi-neutral pH. Microbially mediated Mn and Fe oxidation have different oxygen demands, and the diagenetic zones represent different oxygen conditions. The nomination “suboxic” has a double meaning, which can cause discrepancies. To avoid misunderstanding, definitions are listed in Table 2.
In general, Eh > 0 represents oxic conditions, but oxygen concentrations can differ, as shown in Table 2 and Figure 1, and the microbially mediated processes occur at a given oxygen content. Diagenetic zonation also separates the oxic, suboxic, and anoxic zones, and oxidizing agents vary: O2 in the oxic zone, NO3−, MnO2, and Fe2O3 in the suboxic zone, and SO42− in the anoxic zone [1,2,6,20,21,33].
Ferrihydrite and lepidocrocite on the Fe side, and vernadite, todorokite, birnessite, and manganite on the Mn side, are regarded as syngenetic minerals [40]. Accordingly, it is obvious that ore formation started with microbial Fe oxidation. That is why interpretation always starts with a description of the Fe system. The geochemical conditions of Fe and Mn, including the demand of microbial Fe oxidizing microbes and diagenetic zones, are given in Figure 2 [6].
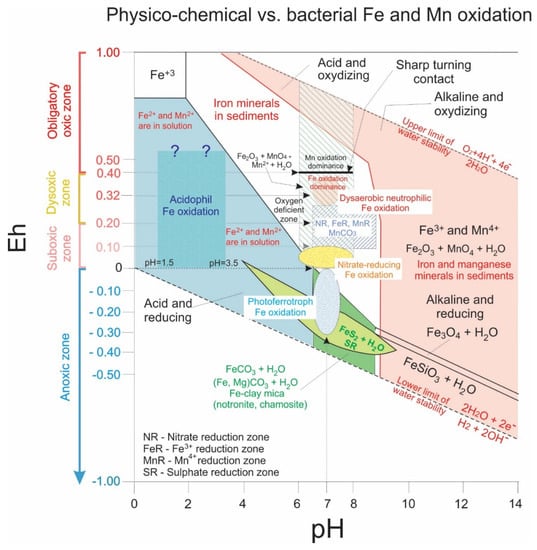
Figure 2.
Physico-chemical vs. bacterial Fe and Mn oxidation (modified in accordance with [6,30,76] as cited from [6], with permission of Elsevier Publishing House). Eh is in volts. Concerning NR, FeR, MnR, and SR, see Table 2 and Figure 1 and note that “suboxic” zone is used for these diagenetic zones in the sense of an oxidant agent. Sharp-turning contact between Fe and Mn mineralization as transformation to obligatory oxic conditions is estimated at Eh = 0.4 V (DO > 2 mL/L).
The diagenetic conditions of the celadonite-bearing Úrkút Mn carbonate deposit are summarized in Figure 3 [17]. We give the conditions of the syngenetic microbial activity as well. Based on environmental mineralogy, changing diagenetic conditions were calculated. In Úrkút, a slight increase in pH and decrease in Eh occurred. In other deposits, other processes resulted in a slight decrease in pH, forming kaolinite/dickite clay minerals [6], but Eh remained in the zone of oxic (suboxic) conditions [1,2,17,60]. In other cases, slightly basic conditions formed via diagenesis (Figure 3).
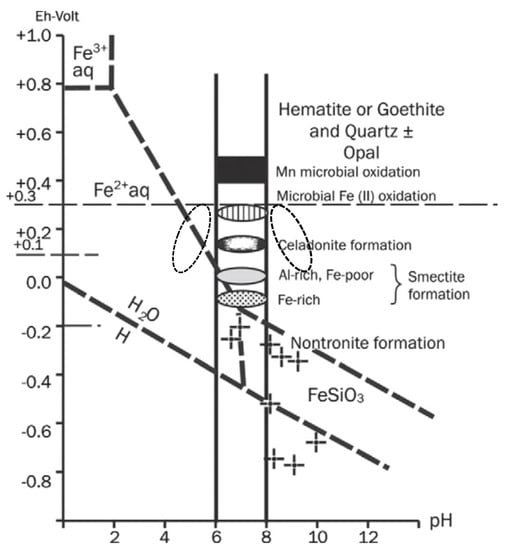
Figure 3.
Estimated formation conditions of the clay minerals in the black shale-hosted Mn carbonate ore deposit (Úrkút) zone of microbial Fe(II) oxidation (+0.3 V Eh using recent analogs) [37,77,78], and the results of nontronite synthesis laboratory experiments (by dashed lines) [23,24]. Note: In general, oxidizing conditions mean high oxygen concentration (high Eh values up to +0.4 V), while reduced conditions reveal a lack of oxygen (low Eh down to −0.2 V). Eh estimation is based on [33,79]. Changing diagenetic conditions are signed by black dotted circle. Modified after [17] (with permission of Elsevier Publishing House).
3.7. Aspects of Biogenicity
From the 1990s, technical development offered the opportunity to investigate microbial systems in laboratory experiments and also in situ natural investigations (among them biomineralization); further, natural systems like stromatolites and biomats began to be intensively studied in situ, which resulted in thousands of publications. Research into ore microbialites applied this information. Well-founded statements about the biogenic origin require a system-based approach and comprehensive, complex, high-resolution studies [71].
Tracking this, we find reports on 2.6–3.2-billion-year-old biomats and microbially produced structures, like the Mozaan Group, South Africa [80], and the Moodies Group, South Africa [81]. Concerning BIFs, variable types of microbial contribution in element (metal) enrichment and precipitation have been raised and interpreted [37,70]. In the case of organic rich Mn carbonate deposits, the convincing microbially mediated formation is less debated than suboxic–oxic formations like Neoproterozoic Urucum Mn [6] and Fe ore deposits [11].
The time series of sedimentary layers of biogenic microbial origin can be extended in time to older rocks. It is therefore worth reviewing the search process, principles, and methodology in detail.
3.7.1. Biogenic Origin
It has been studied whether only complex hierarchical system–based interpretation can be successful [12,60,71,82]. The importance of methodological peculiarities was summarized in Ref. [60].
The aspects of complex interpretation are also summarized in Refs. [12,60]; however, we summarize them here for geological formations, which are increasingly well established. The aspects of complex interpretation in biogenicity as cited in Ref. [82] include:
- (1)
- Microbial microtexture: biomat, filamentous (often sequented), coccoid like, vermiform; brain-like and stromatolite-like macrotexture—fine lamination multiple mineral cyclicity;
- (2)
- Bioindicator minerals that can be modified by diagenetic and other processes, mineralized cycles, and the importance of mineral assemblages; cyclicity, as population growth cycles of microbes are a very important feature, originating from the fundamental behavior of microbial life, and if there is a mineralizing microbe type it will result in ore lamination on the micrometer scale. In the case of non-mineralizing types, the cyclic character will also occur, as during diagenesis the cell and EPS material will also mineralize;
- (3)
- Presence of organic matter embedded in minerals; even in the oxic Urucum Fe formation, there is variable embedded organic matter;
- (4)
- Biosignatures like isotope signals (“vital effect”) (C, S, N, Fe), shape of minerals, and selective enrichment of bioessential elements (Fe, Mn, Si, Zn, Co, As, Be, U, P, Ce, Mg, Ca, K, Na, S), the biological character of Mn and Fe and their atomic structural causes and consequences [46], and mechanisms in the background of microbial element enrichments [60];
- (5)
- Recent analogies of biomineralization and biomarker organic matter;
- (6)
- Paleoenvironmental analogies (sedimentary);
- (7)
- Preservation.We can add an 8th and 9th aspect based on our experience:
- (8)
- The extremely large horizontal and vertical extension and the giant mass in the case of sedimentary ore deposits (e.g., areal extension of the Urucum Fe-Mn deposit is around 800 km2, and its thickness is 400 m; it contains hundreds of millions of tonnes of ore);
- (9)
- The complexity of natural systems can also be a new aspect; natural systems are complex systems with variable microbial systems (e.g., dual Fe and Mn and also cyanobacterial), several mineral types, as direct microbial ones occur with accompanying mineral assemblage, all in a coherent way (texture, minerals, embedded organic matter, isotopic composition of given elements). Investigation of ore deposits aims at as many of the above aspects as possible.
3.7.2. Overview of the Recent State of the Art in Biogenic Thinking
What do we see these days? Results of thousands of laboratory experiments are being published using microbes collected in a variety of natural environments (marine, hydrothermal, black and white smokers, lacustrine, desert, sabkha, soil, ice), and the fact of biomineralization is fundamental, offering data on microtextural features, mineralogy (type, grain size), isotope signals, enrichment of bioessential elements, geochemical conditions of mineralization, features of the forming minerals, preservation of metastable mineral forms, stabilization processes of minerals in time, and metabolism of microbes. This information represents robust evidence on biomineralization, and ancient ore deposits show strong similarities with these results, as analogies (points 1–5), the size dimension of recent natural occurrences (biomats) (point 8) ([12] and references therein), and also complexity (point 9) on many hierarchical levels (from atomic level to deposit size).
Environmental similarities (point 6) as sedimentary basins can also be mentioned, as in situ natural occurrences also led to thousands of publications.
The geological samples represent a special group because of the age horizon and diagenetic processes, overprinting, modifying syngenetic textural features, and mineral composition (point 7). Syngenetic minerals are poorly crystallized, with very small grain size. During diagenesis, stabilization occurs. Decomposition of cell and EPS material offering the element pool also influences complex mineralization. Diagenesis can modify the textural outlook and the position of newly formed minerals. However, our experience with ore deposits showed that basic microbial characteristics are preserved, and a coherent interpretation can be made [1,2,4,5,6,9,10,67,82]. Thus, diagenesis causes differences in the rock record, which makes comparison with laboratory observations difficult and challenging.
3.7.3. Mechanisms in the Background of Element Enrichments
The question of what mechanisms affect element enrichments can be discussed from the side of microbes or from the side of the ore-forming system. Various mechanisms can be found in the background of microbial element enrichment. These include
- (1)
- Direct oxidation of main ore-forming metals to sequestrate giant masses of biominerals (Fe-, Mn-oxidizing microbial activity); direct oxidation can include trace elements as well (e.g., Co and Ce are oxidized by Mn-oxidizing bacteria);
- (2)
- Structure (mineral) stabilizing role, such as Mg bound from seawater (sinking process) by EPS [34];
- (3)
- Adsorption, as in Ni adsorption from seawater or hydrothermal fluids (biogenic signature–decay of organic matter–plankton) by sheet MnOOH (birnessite), resulting in Ni–Mn(II)–sheet MnOOH (birnessite–buserite) [47]. In another example, it is common to find U, Th, Ra, and Rh adsorption on active Fe-biomats [83].
- (4)
- Detoxication, for instance Ce(III) to Ce(IV) by MnOB on MnOOH, which is a microbial oxidation path with P.
- (5)
- Vitamin demand, such as the need of Co for vitamin B12 [71].
- (6)
- Enzymatic demand, metalloproteins [84].
- (7)
- Protection against UV radiation and high Fe concentration (such as Si); FeOB consumes silica against stress, and it is also used for protection by microbes [58].
- (8)
- Energy aspects. The group of enzymatic redox reactions that supply energy is an important category of biomineralization [37]. Fe, Mn, S, and P are important elements of the energy and electron fluxes of living systems [31].
The element content of deposits is of great economic importance: not only for the main element(s) as mineable materials, but also the accompanying main and minor or trace elements can make a positive contribution, increasing the economic value of the sedimentary ores. On the other hand, minor or trace elements may occur that make processing difficult and thus more expensive or that have harmful effects that can entirely exclude the processing of ores (e.g., high P content in Mn ores).
3.7.4. Mineralogical Interpretation—Source of Elements
Ore microbialites contain ore minerals (syngenetic and simply stabilized) along with a variety of other minerals. The nine deposits studied are summarized in Table 1. The mineral species were grouped as Mn, Fe, and other mineral assemblages, and in the groups we followed the traditional mineral classes. A total of 76 mineral species were detected: 22 in Mn, 21 in Fe, and 33 in other mineral assemblages. The distribution of these minerals in the deposits differs: 23 frequently occurring minerals were identified, found in 6–9 deposits; 16 occurred moderately frequently (in 3–5 deposits); and 37 minerals occurred only rarely (in 1 or 2 deposits). From the table, it is obvious that variable syn- and diagenetic mineral assemblages occur in the deposits.
As shown in Table 1, both Mn and Fe ore microbialite systems occur. To better follow the processes, separated mineralogy is used. The formation of syngenetic Mn (S-Mn, vernadite, todorokite, birnessite, manganite) and Fe minerals (S-Fe, ferrihydrite, lepidocrocite) takes place via direct microbial enzymatic processes as different lines of mineralization, but they are intimately bound. After burial decomposition of cell and EPS material begins, cations and anions are released and form an element reservoir (pool) for complex diagenetic mineralization. Simultaneously poorly crystallized Mn and Fe minerals stabilize in the form of minerals such as pyrolusite, hematite, goethite, and segregated quartz: minerals that represent the clear (simple) diagenetic line of stabilization of Mn and Fe minerals. A highly variable cation-determined mineral group forms for diagenetic Mn minerals because of the favorable crystal structure of Mn oxide (Table 1, D-Mn), which is affected by the element pool of cell and EPS decomposition (designated by brackets). Furthermore, as organic matter mineralizes, carbonate minerals such as rhodochrosite, kutnohorite, and Mn-calcite form. However, the crystal structure does not allow such variability in the case of Fe, and thus Fe carbonate forms such as siderite, ankerite, and variable silicates (pyroxene-/aegirine, amphibol-/riebeckite, and Fe-bearing clay minerals: celadonite, nontronite, and chamosite) are found (Table 1, D-Fe). Since the two systems can also influence each other, combined diagenetic minerals like serandite and braunite also form where Mn oxide and segregated silica interact (in the stabilization process of ferrihydrite) (Table 1, D–C).
Aside from the diagenetic lines of Mn and Fe (and their combination), other minerals—not containing the ore metals (Mn, Fe)—also form as a result of complex mineralization. Previously, a debris origin was postulated for such minerals, but more recent studies support authigenic formation based on their CL features. Quartz, anatase, and silicates like feldspars, clay minerals, apatite, and mica can form in this way. The formation of microbialites took place in starving basins, meaning that the element pool of cell and EPS decomposition served as the element source. The highly variable mineral assemblage of Mn and Fe ore microbialites is explained by this interpretation, as is the fact that diagenetic ore minerals are highly varied; it also accounts for the numerous other mineral phases that occur in these giant deposits. It may also help explain the peculiar amoeboid textural features of some of these minerals [6].
Environmental mineralogy allows us to clarify the syngenetic formation conditions as oxic-suboxic and semineutral, and via diagenesis it either remained oxic or locally turned to anoxic, slightly acidic, or alkaline (Figure 3). The cyclic occurrence of minerals is very important; they refer to microbial mediation, so these minerals represent robust evidence. Representative mineral cycles determined by Raman spectroscopy are discussed in detail in the papers on deposits (see legend of Table 1).
The mineralized microbial cycles of the syngenetic phase in the case of Mn are vernadite, todorokite, birnessite, and manganite; in the case of Fe they are ferrihydrite and lepidocrocite.
In addition to the most common diagenetic cyclic minerals, like rhodochrosite and kutnohorite on the Mn side and hematite, goethite, pyrite, and siderite on the Fe side, the mineral assemblages offer special insight into peculiar diagenetic details (among them, microbially mediated ones) like Fe-anatase, kutnohorite-MnS (alabandite, rambergite), marcasite, and magnetite cycles.
In manganese carbonate ores of the Zunyi location, cyclic anatase is proposed as a fossil Fe-biomat system [10]. Similar anatase cycles have been observed in the Early Carboniferous manganese deposits in central Guangxi, South China [9]. Anatase biosignatures are reported by [85]. Ti is bound on organic matter, occurring via decomposition and release of cations, and with an ample supply of Fe3+ the Eh-pH conditions under low temperature were realized in the form of an anatase crystal structure. Although the Fe content of anatase is not documented in Ref. [9], EPMA and Raman analyses detected the occurrence of Fe and Ti in mineralized biomat-like microtextures. Based on these data, anatase cycles can be interpreted as the diagenetic product of an Fe-biomat system. The presence of pyrite in the mineral assemblage means anoxic, acidic conditions, which could also be favorable for Fe-bearing anatase mineralization.
The Mn sulfide–kutnohorite formation model established in Ref. [86] can explain rambergite and alabandite formation. After deposition of a Mn-oxide lamina, Mn-sulfide crystals grow; the precipitation of Mn-sulfide in preference to Mn-carbonate requires a high excess of free sulfide relative to alkalinity and an environment completely depleted in Fe. High in situ H2S and alkalinity concentrations should be present below the Mn-oxide lamina. Large amounts of Mn2+ can be produced rapidly by bacterial Mn reduction of the Mn-oxide lamina, providing a favorable environment for the formation of Mn sulfide and kutnohorite.
Marcasite (FeS2) is a cyclic mineral found in the Xinglong deposit [10], with an important role in the interpretation of diagenetic conditions. Marcasite occurs together with the mineral assembly of quartz + pyrite + anatase. As marcasite occurs in the form of cycles, this supports the claim that this mineral represents the diagenetic product of Fe-biomat. Formation of pyrite occurs in the condition of pH = ~6, but the formation of marcasite demands pH < 5. Thus, the relative abundance of pyrite versus marcasite in sediments should be a function of pH. Because marine waters are slightly alkaline (pH = ~8), it has been presumed that marcasite cannot form in marine sediments during early diagenesis, but the truth is that it is common [30]. During early diagenesis, pre-existing sulfide grains are wholly or partially destroyed in order to supply dissolved Fe2+ or readily soluble iron (e.g., Fe(OH)3) for the growth of new iron sulfides. Iron sulfide destruction to sulfate results in acidity (SO42− + H+) and dissolved ferrous ion (Fe2+), causing the pH to decrease and the Fe2+ concentration in the pore water to increase [30], conditions that favor marcasite reprecipitation in the presence of an H2S influx from underlying sediments [87,88]. The re-oxidation of reducing, organic, and iron sulfide-rich sediments (“burndown”) has been extensively investigated [89], and marcasite can act as a mineral-based indicator of burndown events. Marcasite occurs in intimate association with calcite dissolution and precipitation of diagenetic quartz. This makes chemical sense, because low pH conditions are required for marcasite formation, calcite dissolution, and precipitation of dissolved silica. Amorphous silica segregation occurs through the destruction of opaline components, organic complexes, or the transformation of ferrihydrite [90].
Magnetite forming opposite cycles with organic matter supports the role of its decomposition and heterotrophic microbial magnetite mineralization, where ferrihydrite is proposed as precursor mineral. In low T sedimentary systems, magnetite forms via heterotrophic microbial mediation, in anaerobic sedimentary environments [39,52]. In these conditions, most Fe(III) reduction is due to the enzymatic reduction of Fe(III) by microorganisms. Dissimilatory Fe(III)-reducing microorganisms have been reported to effectively couple the oxidation of organic compounds to the reduction of Fe(III). Initial studies of these Fe(III)-reducing organisms revealed that ultrafine-grained magnetite is an end-product of dissimilatory Fe(III) reduction [39,52].
There are other minerals that do not show cyclic occurrence but can form via microbial mediation; here we mention magnesite [59] and talc [66] as interesting examples.
Carbonate formation needs special attention in these microbialites. The traditional way is the early diagenetic Mn (Ca-rhodochrosite–kutnohorite) and Fe carbonate (siderite) formation in the suboxic zone via microbially mediated heterotrophic Mn and Fe reduction and organic matter oxidation and mineralization in the form of carbonates characterized by a light C isotope signal (Cycle II). However, there are cases where fine-grained mixed carbonate of very variable composition occurs (Ca-rhodochrosite—kutnohorite, siderite, ankerite), and the bulk isotope signal does not support Cycle II. Carbonate composition is well characterized by triangle diagrams. In these cases, other carbonate formation models can be proposed.
Three models can be discussed for carbonate sources and processes [11]:
- (1)
- Abiogenic mixed syngenetic carbonate formation. Based on the geological environment, crystallization was syn-diagenetic and would not have caused significant changes in the carbonate compositions. Therefore, the water composition of the primary sedimentary system affects the Fe-Mn enrichment of the carbonates, depending on the particular metal-endowed fluid-rock system in which carbonate was precipitated or recrystallized.
- (2)
- Cyanobacterially mediated syngenetic calcite formation and Mn- and Fe-replacement via early diagenesis. Fe- and Mn diagenetic replacement of Ca in carbonates resulted in mixed forms of highly variable composition.
- (3)
- Diagenetic mixed carbonate formation via organic matter decomposition. The most likely formation of carbonates is diagenetic mixed carbonate formation via organic matter decomposition. This scenario is supported by mineral assemblages, micro-textural features, and negative δ13CPDB-carb values.
Recent investigations in preparation also support our previous results in the case of Mesoproterozoic Chinese and Postmasburg-Kalahari samples concerning mineralized microbially produced texture and mineral assemblage as well as variable embedded organic matter (Figure 4). Younger deposits like Carboniferous Chinese deposits and Jurassic Mexican deposits show similar results.
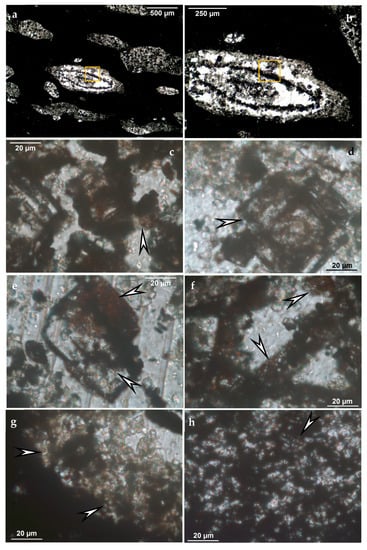
Figure 4.
Mineralized microbial biosignatures shown by arrows, in Mn carbonate ore, Postmasburg-Kalahari Mesoproterozoic, drill core sample code: M15-30, provided by J. Gutzmer. (a) Representative texture; (b) enlarged area on (a); (c) representative microbial microtexture; (d) enlarged area on (b); (e–h) representative microbial microtexture; (photos made by optical rock microscope, transmitted light, 1 Nicol, NIKON ECLIPSE 600 rock microscope, Institute for Geology and Geochemistry, Research Centre for Astronomy and Earth Sciences, ELKH, Budapest, Hungary).
Because this section focuses on element composition and mineralogy, we do not discuss other biogenicity indicators like isotope datasets here, but we wish to note that such datasets are also important.
4. Methodology
To verify microbial contribution, methods must be chosen that are appropriate for poorly crystallized minerals and structural hierarchical interpretation. A complete interpretation of a geological site can be made only if sufficient data is available, and this should include investigation of evidence for potential microbial mediation.
The following methods for collecting samples and data are recommended in order to carry out an analysis on microbially mediated formation to inform comprehensive analysis and interpretation. In the description of ore deposits, the main areas of investigation are structure (texture), material (mineralogy, organic matter, chemistry, main and trace elements, isotopes), formation process, and metal source [60].
- (1)
- The dimension of investigations must fall into the microbial size dimension (i.e., there is a need for high magnification microtextural observations as a first step to investigate the possible occurrence of microbial mediation). To solve this, high resolution in situ measurements and optical rock microscopy observations are needed.
- (2)
- Besides bulk analyses, in situ determination and distribution of mineral assemblage and embedded organic matter is needed to detect mineralized microbial cycles (mineralized biomats) and is also important to distinguish authigenic and allothigenic minerals (FTIR, Raman spectroscopy, cathodoluminescence microscopy).
- (3)
- If the possibility of microbial mediation in a formation is suspected (through microtextural evidence), the choice of appropriate methods is essential. For instance, XRD does not detect X-ray amorphous poorly crystallized microbially mediated minerals (e.g., ferrihydrite); furthermore, attention must be paid to levels of excitation energy, which is capable of converting minerals like ferrihydrite to more stable hematite. Thus, along with Raman spectroscopy, FTIR spectroscopy at lower excitation energy is suggested.
- (4)
- Debris character can be a "virtual" outlook, showing large minerals of authigenic processes and influencing textural features; this can be clarified easily using cathodoluminescence microscopy.
- (5)
- The authigenic formation of minerals that are well known as being of a particular origin may actually appear as authigenic materials. One example is magmatic or metamorphic conditions (quartz, feldspar—earlier known), but also pyroxene as aegirine, amphibol as riebeckite, and others, like dickite as high T and p minerals also common as low T authigenic minerals. Thus, a more detailed investigation of mineral type may be needed, and a complex approach is proposed to avoid a false interpretation.
- (6)
- Due to selective element enrichment (isotopes) resulting from microbial processes, we must use geochemical ratio methods with caution. This enrichment can indicate a microbially influenced element source, which often is not recognized as a possible interpretation. It also demonstrates the need for a multi-methodology approach and complex interpretation.
- (7)
- It is very important to distinguish whether clay-size dimensions or clay minerals occur. Certain data must be gathered: size dimension, the minerals in the fraction, and the origin of clay minerals (detrital, hydrothermal, or microbial). Often, microbial origin is indeed the case. This must be investigated in the case of marls or claystones (other clay-bearing rocks or ores), since the general assumption with clay-rich rocks is increased wet weathering and run off from terrestrial parts. In many cases, that may be a false interpretation.
- (8)
- It is extremely important to distinguish between syngenetic and diagenetic anoxia, since this determines original formation conditions. Environmental mineralogy based on mineral assemblage can provide clarification.
- (9)
- Mass balance considerations must be taken into consideration; the ratio of accumulating metal oxides, organic matter, and other components influences the diagenetic processes and the final character of the deposits (oxide, carbonate).
- (10)
- Identifying suboxic zones is important due to the consummation of organic matter by metal oxides and hydroxides, and the forming of diagenetic minerals.
If any of these factors are overlooked, and therefore it is not recognized that a system is microbially mediated, then the conclusions of the study will be incomplete.
Comparison of Observation by Optical Rock Microscopy and Electron Optical Methodology
We have found that optical rock microscopy offers a more detailed picture of mineralized microbially mediated fine texture than electron optical observation (Figure 5). This is true, for example, for carbonate pore filling among quartz grains [67]. Another example is a quartz-hosted mineralized biosignature (Figure 6) [91].
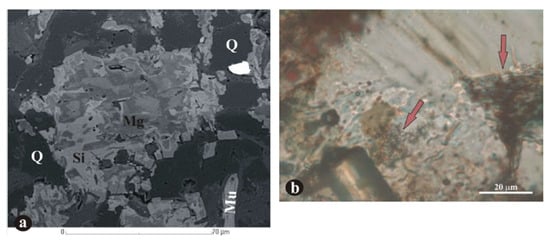
Figure 5.
Microtextural images. EPMA image (a), legend: Q: quartz grains; Si: siderite; Mg: magnesite; Mu: muscovite. Optical rock microscope image (b), microbial filamentous forms on a similar part, with different magnification. Though not exactly the same parts are shown on the photos, it is obvious that the optical rock microscopy (OM) photo shows more fine details of mineralized biosignatures (arrows) (Figure 2e,f, iron ore, [67], with permission of Elsevier Publishing House).
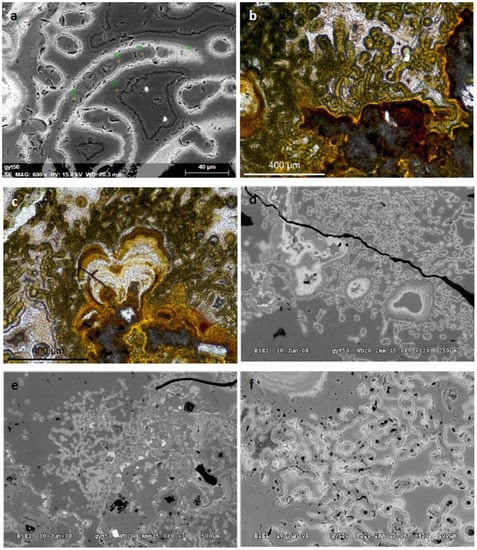
Figure 6.
Microbial filamentous forms on EPMA and OM: similar part, similar magnification; the EPMA magnification is increased from Ref. [91], with permission of copyright holder. The biomineralization occurred on altered andesite. (a) Microbial filamentous forms on EPMA BEI image; (b) Same part on OM, transmitted light, 1N; (c) OM image, transmitted light, 1N; (d–f) microbial filamentous forms on EPMA BEI image of the same part, magnification is increasing. The greenish-yellow haloes around the filaments are mineralized extracellular polymeric substances (EPSs), which are not visible on BEI images.
5. Deposits and indications: Sample Investigations
The aim is the identification of the geological rock sample, and sedimentary ore sample in the recent case. As a first step looking into the optical microscope, on higher magnification (400×, 1000×), we see a peculiar microbial-like microtexture in a general sense. The constituents are minerals and organic matter. The similarity with experimental or recent natural microbial microtexture is not absolute, as diagenetic processes influence the appearance and also the mineral content of the system via compaction, stabilization of minerals, newly formed minerals from the given element pool, and mineral distribution. In spite of this, conclusive features of microbial activity (biomat, mineralized microbially produced textures in the form of, e.g., filamentous forms with sequented (pearl-necklace-like) inner structure) are preserved, and we can identify them. The size dimension of textural features falls into the microbial size dimension. The mineral type also influences the preservation opportunity of these tiny forms: quartz (silica) offers the best results, while a carbonate matrix can partly destroy the tiny forms, because of the elevated crystallization energy of carbonate minerals. The microbially mediated minerals are fine grained, which does not allow physical separation of samples and makes it difficult to imagine 3D microtextural features; the section direction is random, and the orientation of the filaments is also random.
Microscopic observations are of great importance, as they raise the opportunity of observation of microbially mediated rock (ore) formation, and this determines the usage of appropriate methodology and size dimension of measurements. The importance of high resolution in situ investigations (mineral and organic matter identification) is fundamental.
We have ores, extreme Fe (Mn) enrichment, giant mass, and high metal concentration. We have to suppose an effective enrichment process. We must build this phenomenon onto mineral types based on experimental results using natural microbes and observations in nature [6]. Detailed information on mineral assemblages is offered for a Neoproterozoic oxic Fe deposit (Urucum) and Permian and Carboniferous Mn carbonate deposits in Refs. [9,10,11].
In the sample investigation, Fe and Mn minerals are identified—syngenetic and diagenetic stabilized and also newly formed ones on the Fe side and Mn side—and combined. Besides ore minerals, other minerals also formed as the result of complex mineralization. The distribution of ore minerals is ordered into cycles of microbial size dimension. Population growth cycles represent this distribution as robust evidence. Embedded variable organic matter was also determined. We use high resolution in situ FTIR and Raman spectroscopic investigation on microbial size dimension, which results in a huge dataset of thousands of spectra. It is commonly questioned why we need such an extensive dataset; microbial size dimension and cyclicity are needed for conclusive recognition and interpretation.
Concerning the stable isotope signal on routinely used C, isotope determination was made on bulk samples; this is why the result of a given mixed carbonate background is measured. In the case of Mn carbonate formations, it is obvious that diagenetic processes occur.
The case is different if we have an oxide type deposit, like Urucum Fe. The question remains as to why. In this case, it is not the metal oxide (Fe oxide) that decomposes organic matter in a bond—also a microbially mediated reduction process; other processes can play an important role in the decomposition of organic matter, which we have to take into consideration. For example, if the sedimentation rate is slow, atmospheric oxygen can play an important role in its decomposition. Though some lower amount of embedded organic matter can still be determined, the carbonate carbon will miss a considerable organic carbon supply, and a light C isotope signal will not occur or will not be conclusive. In this case, other carbonate independent element isotope signals, such as Fe, can give supporting evidence on microbial mediation, as is the case in the Urucum Fe deposit [92].
It is important to underline that we found double Fe-Mn (Mn-Fe) systems based on the occurrence of these minerals, which raise the possibility of dual microbial ore forming systems.
6. Summary
In this article, we offered a self-overview of our own research experiences covering two decades on Fe-Mn ore microbialites. We interpreted the datasets based on strong analogies in a coherent way, using (i) the literature on experiments using natural microbes, including textural features and mineralogy, mineral cycles, behavior of microbes, metabolic types, features of elements on atomic level; (ii) natural recent biomat occurrences of a large size and mineralogy based on recent observations; (iii) the mineral assemblage, which is very important robust evidence; (iv) isotope signals. We call attention to the fact that if the methodology and/or the size dimension of investigation is not appropriate, then these four factors will be overlooked. In this case, it will not be recognized that the system is microbially mediated (or evidence is insufficient to exclude microbial mediation), and the conclusions will be incomplete.
Let us share one last comment. The understanding of papers concerning microbially mediated mineralization (ore microbialites) needs basic knowledge on the following subjects (though this list is not exhaustive):
- (1)
- The atomic scale features of elements, which determine microbial behavior and processes (e.g., Mn and Fe are “microbial” elements);
- (2)
- The connections between the Periodic Table and microbial life;
- (3)
- The background of selective microbial element enrichments;
- (4)
- Time duration differences on biological and geological scales;
- (5)
- Biomineralization, such as microbial metabolism, the metabolic types of given microbes (e.g., Fe-oxidizing microbes, Fe-reducing microbes), the role of prokaryots, autotrophy and heterotrophy;
- (6)
- Microbial systems in the frame of population growth cycles;
- (7)
- The main natural environments of microbial activity.
Taking into consideration the above comprehensive and comparative discussion, we prefer interpretation of biogenic origin as the most convincing for Fe and Mn deposits of variable geological ages belonging to this microbially mediated ore group. Fundamental roles are played by similar syngenetic and diagenetic variables and the complex mineralization of cell and EPS decomposition and the element pool, and these produce peculiar authigenic mineral formations.
Author Contributions
Conceptualization, M.P. and I.G.; writing—original draft preparation, M.P. and I.G.; writing—review and editing, M.P. and I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Correspondence and requests for materials should be addressed to M.P. (rodokrozit@gmail.com).
Acknowledgments
The authors thank the support of the Hungarian National Research, Development and Innovation Office, National Scientific Research Fund No. 125060.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Polgári, M.; Hein, J.R.; Vigh, T.; Szabó-Drubina, M.; Fórizs, I.; Bíró, L.; Müller, A.; Tóth, A.L. Microbial processes and the origin of the Úrkút manganese deposit, Hungary. Ore Geol. Rev. 2012, 47, 87–109. [Google Scholar] [CrossRef]
- Polgári, M.; Hein, J.R.; Tóth, A.L.; Pál-Molnár, E.; Vigh, T.; Bíró, L.; Fintor, K. Microbial action formed Jurassic Mn-carbonate ore deposit in only a few hundred years (Úrkút, Hungary). Geology 2012, 40, 903–906. [Google Scholar] [CrossRef]
- Gyollai, I.; Polgári, M.; Fintor, K.; Pál-Molnár, E.; Popp, F.; Koeberl, C. Microbial activity records in Marinoan Snowball Earth postglacial transition layers connecting diamictite with cap carbonate (Otavi Group, NW-Namibia). Austrian J. Earth Sci. 2017, 110, 2–18. [Google Scholar] [CrossRef]
- Rajabzadeh, M.A.; Haddad, F.; Polgári, M.; Fintor, K.; Walter, H.; Molnár, Z.; Gyollai, I. Investigation on the role of microorganisms in manganese mineralization from Abadeh-Tashk area, Fars Province, southwestern Iran by using petrographic and geochemical data. Ore Geol. Rev. 2017, 80, 229–249. [Google Scholar] [CrossRef]
- Yu, W.; Polgári, M.; Gyollai, I.; Fintor, K.; Szabó, M.; Kovács, I.; Fekete, J.; Du, Y.; Zhou, Q. Microbial metallogenesis of Cryogenian manganese ore deposits in South China. Precambrian Res. 2019, 322, 122–135. [Google Scholar] [CrossRef]
- Biondi, J.C.; Polgári, M.; Gyollai, I.; Fintor, K.; Kovács, I.; Fekete, J.; Mojzsis, S.J. Biogenesis of the Neoproterozoic kremydilite manganese ores from Urucum (Brazil)–A new manganese ore type. Precambrian Res. 2020, 340, 105624. [Google Scholar] [CrossRef]
- Gracheva, M.; Homonnay, Z.; Kovács, K.; Béres, K.A.; Biondi, J.C.; Wenchao, Y.; Kovács Kis, V.; Gyollai, I.; Polgári, M. Mössbauer characterization of microbially mediated iron and manganese ores of variable geological ages. Ore Geol. Rev. 2021, 134, 104124. [Google Scholar] [CrossRef]
- Karlik, M.; Gyollai, I.; Vancsik, A.; Fintor, K.; Szalai, Z.; Mindrescu, M.; Grădinaru, I.; Vágási, I.; Bozsó, G.; Pál-Molnár, E.; et al. High resolution mineralogical characterization of sediments-lake Bolatau-Feredeu (Romania). Carpathian J. Earth Environ. Sci. 2021, 16, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Polgári, M.; Fintor, K.; Gyollai, I.; Szabó, M.; Velledits, F.; Liu, Z.; Du, Y. Contribution of microbial processes to the enrichment of Middle Permian manganese deposits in northern Guizhou, South China. Ore Geol. Rev. 2021, 136, 104259. [Google Scholar] [CrossRef]
- Yu, W.; Polgári, M.; Gyollai, I.; Fintor, K.; Huang, H.; Szabó, M.; Du, Y. Microbial metallogenesis of early carboniferous manganese deposit in central Guangxi, South China. Ore Geol. Rev. 2021, 136, 104251. [Google Scholar] [CrossRef]
- Polgári, M.; Biondi, J.C.; Gyollai, I.; Fintor, K.; Szabó, M. Origin of the Urucum iron formations (Neoproterozoic, Brazil): Textural and mineralogical evidence (Mato Grosso do Sul–Brazil). Ore Geol. Rev. 2021, 139, 104456. [Google Scholar] [CrossRef]
- Polgári, M.; Gyollai, I.; Fintor, K.; Horváth, H.; Pál-Molnár, E.; Biondi, J.C. Microbially mediated ore-forming processes and cell mineralization. Front. Microbiol. 2019, 10, 2731. [Google Scholar] [CrossRef]
- Varga, G. Kálitrachit és káliumdús kőzetek a Mátrában (Potassium trachyte and potassium rich rocks in the Mátra Mts.). Annu. Rep. Hung. Geol. Inst. 1990, 1992, 241–276. [Google Scholar]
- Nagy, B. K-rich rocks and their relation to mineralization in the Mátra Mountains (North Hungary). Acta Geol. Hung. 2006, 49, 33–41. [Google Scholar] [CrossRef]
- Polgári, M.; Nagy, B.; Fintor, K.; Gyollai, I.; Kovács, I.; Szabó, M.; Mojzsis, S. Contribution to the origin of K-rich rocks in the Mátra Mountains (North Hungary) 2022. (Research Report, National Scientific Research No. 125060, manuscript in Hungarian).
- Roy, S. Manganese Deposits; Academic Press: London, UK, 1981; p. 458. [Google Scholar]
- Polgári, M.; Hein, J.R.; Németh, T.; Pál-Molnár, E.; Vigh, T. Celadonite and smectite formation in the Úrkút Mn-carbonate ore deposit (Hungary). Sediment. Geol. 2013, 294, 157–163. [Google Scholar] [CrossRef][Green Version]
- Polgári, M.; Hein, J.R.; Bíró, L.; Gyollai, I.; Németh, T.; Sajgó, C.; Fekete, J.; Schwark, L.; Pál-Molnár, E.; Vigh, T.; et al. Mineral and chemostratigraphy of a Toarcian black shale hosting Mn-carbonate microbialites (Úrkút, Hungary). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 459, 99–120. [Google Scholar] [CrossRef]
- Polgári, M.; Németh, T.; Pál-Molnár, E.; Futó, I.; Vigh, T.; Mojzsis, S.J. Correlated chemostratigraphy of Mn-carbonate microbialites (Úrkút, Hungary). Gondwana Res. 2016, 29, 278–289. [Google Scholar] [CrossRef]
- Berner, R.A. Early Diagenesis: A Theoretical Approach; Princeton University Press: Princeton, NJ, USA, 1980; p. 250. [Google Scholar]
- Coleman, M.L. Geochemistry of diagenetic non-silicate minerals: Kinetic considerations. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1985, 315, 39–56. [Google Scholar]
- Listova, L.P. Experimental Studies of Physic-Chemical Conditions of Sedimentation of Manganese Oxides and Carbonate. Ocherki Metallogen. Osadochn. Porod.; Publication of the AN USSR: Moscow, Russia, 1961; pp. 319–351. [Google Scholar]
- Harder, H. Nontronite synthesis at low temperatures. Chem. Geol. 1976, 18, 169–180. [Google Scholar] [CrossRef]
- Harder, H. Synthesis of iron layer silicate minerals under natural conditions. Clays Clay Miner. 1978, 26, 65–72. [Google Scholar] [CrossRef]
- Trudinger, P.A.; Swaine, D.J. Biogeochemical Cycling of Mineral-Forming Elements; Elsevier: Amsterdam, The Netherlands, 1979; p. 608. [Google Scholar]
- Giovanoli, R. On natural and synthetic manganese nodules. In Geology and Geochemistry of Manganese, 1st ed.; Varentsov, I.M., Grasselly, G., Eds.; Akadémiai Publishing House: Budapest, Hungary, 1980; pp. 159–203. [Google Scholar]
- Sung, W.; Morgan, J.J. Oxidative removal of Mn (II) from solution catalysed by the γ-FeOOH (lepidocrocite) surface. Geochim. Cosmochim. Acta 1981, 45, 2377–2383. [Google Scholar] [CrossRef]
- Cole, T.G.; Shaw, H.F. The nature and origin of authigenic smectites in some recent marine sediments. Clay Miner. 1983, 18, 239–252. [Google Scholar] [CrossRef]
- Ewers, W.E. Chemical Factors in the Deposition and Diagenesis of Banded Iron-Formation. In Developments in Precambrian Geology 6, Iron Formation: Facts and Problems; Trendall, A.F., Morris, R.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 491–512. [Google Scholar]
- Maynard, J.B. Geochemistry of Sedimentary Ore Deposits; Springer: New York, NY, USA, 1983. [Google Scholar]
- Skinner, H.C.W. A review of apatites, iron and manganese minerals and their roles as indicators of biological activity in black shales. Precambrian Res. 1993, 61, 209–229. [Google Scholar] [CrossRef]
- Ehrenreich, A.; Widdel, F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 1994, 60, 4517–4526. [Google Scholar] [CrossRef]
- Wignall, P.B. Black Shales; Oxford Clarendon Press: Oxford, UK, 1994; p. 124. [Google Scholar]
- Mandernack, K.W.; Post, J.; Tebo, B.M. Manganese mineral formation by bacterial spores of the marine Bacillus, strain SG-1: Evidence for the direct oxidation of Mn (II) to Mn (IV). Geochim. Cosmochim. Acta 1995, 59, 4393–4408. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B.; Widdel, F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 1996, 62, 1458–1460. [Google Scholar] [CrossRef]
- Banfield, J.F.; Nealson, K.H. Geomicrobiology: Interactions between Microbes and Minerals. In Reviews in Mineralogy 35; Mineralogical Society of America: Washington, DC, USA, 1997; p. 448. [Google Scholar]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lack, J.G.; Coates, J.D. Biogenic magnetite formation through anaerobic biooxidation of Fe (II). Appl. Environ. Microbiol. 2001, 67, 2844–2848. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology, 4th ed.; Marcell Dekker Inc.: New York, NY, USA, 2002; pp. 183–274. [Google Scholar]
- Bazylinski, D.A.; Frankel, R.B. Biologically controlled mineralization in prokaryotes. Rev. Mineral. Geochem. 2003, 54, 217–247. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, I.; Roh, Y. Biomineralization of a poorly crystalline Fe (III) oxide, akaganeite, by an anaerobic Fe (III)-reducing bacterium (Shewanella alga) isolated from marine environment. Geosci. J. 2003, 7, 217–226. [Google Scholar] [CrossRef]
- Villalobos, M.; Toner, B.; Bargar, J.; Sposito, G. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochim. Cosmochim. Acta 2003, 67, 2649–2662. [Google Scholar] [CrossRef]
- Bargar, J.R.; Tebo, B.M.; Bergmann, U.; Webb, S.M.; Glatzel, P.; Chiu, V.Q.; Villalobos, M. Biotic and abiotic products of Mn (II) oxidation by spores of the marine Bacillus sp. strain SG-1. Am. Mineral. 2005, 90, 143–154. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T. Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.J. Kinetics of reaction between O2 and Mn (II) species in aqueous solutions. Geochim. Cosmochim. Acta 2005, 69, 35–48. [Google Scholar] [CrossRef]
- Bodeï, S.; Manceau, A.; Geoffroy, N.; Baronnet, A.; Buatier, M. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments. Geochim. Cosmochim. Acta 2007, 71, 5698–5716. [Google Scholar] [CrossRef]
- Lemos, V.P.; Lima da Costa, M.; Lemos, R.L. Vivianite and siderite in lateritic iron crust: An example of bioreduction. Quim. Nova 2007, 30, 36–40. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron oxides in the laboratory: Preparation and characterization; John Wiley & Sons: Hoboken, NJ, USA, 2007; p. 188. [Google Scholar]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic iron oxidizing bacteria produce organic stalks to control mineral growth: Implications for biosignature formation. Multidiscip. J. Int. Soc. Microb. Ecol. 2011, 5, 717–727. [Google Scholar] [CrossRef]
- Piepenbrock, A.; Dippon, U.; Porsch, K.; Appel, E.; Kappler, A. Dependence of microbial magnetite formation on humic substance and ferrihydrite concentrations. Geochim. Cosmochim. Acta 2011, 75, 6844–6858. [Google Scholar] [CrossRef]
- Biagioni, C.; Capalbo, C.; Lezzerini, M.; Pasero, M. Ferrihollandite, BaMn4+ 6Fe3+ 2O16, from Apuan Alps, Tuscany, Italy: Description and crystal structure. Eur. J. Mineral. 2014, 26, 171–178. [Google Scholar] [CrossRef]
- Zeyen, N.; Benzerara, K.; Li, J.; Groleau, A.; Balan, E.; Robert, J.-L.; Estève, I.; Tavera, R.; Moreira, D.; López-García, P. Formation of low-T hydrated silicates in modern microbialites from Mexico and implications for microbial fossilization. Front. Earth Sci. 2015, 3, 64. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Wu, L.; Zeng, Q.; Dong, H.; Bishop, M.E.; Wang, H. Humic acid-enhanced illite and talc formation associated with microbial reduction of Fe(III) in nontronite. Chem. Geol. 2016, 447, 199–207. [Google Scholar] [CrossRef]
- Johnson, J.E.; Webb, S.M.; Ma, C.; Fischer, W.W. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim. Cosmochim. Acta 2016, 173, 210–231. [Google Scholar] [CrossRef]
- Gyollai, I.; Polgári, M.P.; Fintor, K.; Popp, F.; Mader, D.; Pál-Molnár, E.; Nagy, S.; Koeberl, C. Microbially mediated deposition of postglacial transition layers from the Neoproterozoic Otavi Group, Namibia: Evidence of rapid deglaciation after the Sturtian cryogenic period. Carpathian J. Earth Environ. Sci. 2015, 10, 63–76. [Google Scholar]
- Młoszewska, A.M.; Cole, D.B.; Planavsky, N.J.; Kappler, A.; Whitford, D.S.; Owttrim, G.W.; Konhauser, K. UV radiation limited the expansion of cyanobacteria in early marine photic environments. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Sanz Montero, M.E.; Rodríguez Aranda, J.P. Magnesite formation by microbial activity: Evidence from a Miocene hypersaline lake. Sediment. Geol. 2020, 12, 51. [Google Scholar] [CrossRef]
- Polgári, M.; Gyollai, I. Geochemical constraints on the element enrichments of microbially mediated manganese and iron ores–An overview. Ore Geol. Rev. 2021, 136, 104203. [Google Scholar] [CrossRef]
- Fortin, D.; Ferris, F.G.; Beveridge, T.J. Surface-mediated mineral development by bacteria. In Geomicrobiology: Interactions between Microbes and Minerals; Banfield, J., Nealson, K.H., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1997; Volume 35, pp. 162–180. [Google Scholar]
- Polgári, M.; Gyollai, I.; Bérczi, S. Terraforming on Early Mars. In Terraforming Mars; Beech, M., Seckbach, J., Gordon, R., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 161–279. [Google Scholar]
- Madondo, J.; Polgári, M.; Gyollai, I.; Fintor, K.; Kovács, I.; Szabó, M. Contribution to the origin of Molango manganese deposit, Mexico. 2022. (Research Report, National Scientific Research No. 125060, manuscript in Hungarian).
- Dong, Z.; Polgári, M.; Gyollai, I.; Fintor, K.; Kovács, I.; Szabó, M. Contribution to the origin of manganese deposit at Kunlun Mts., China 2022. (Research Report, National Scientific Research No. 125060, manuscript in Hungarian).
- Dong, Z.; Polgári, M.; Gyollai, I.; Fintor, K.; Kovács, I.; Szabó, M. Contribution to the origin of manganese deposit at Tianshan Mts., China 2022. (Research Report, National Scientific Research No. 125060, manuscript in Hungarian).
- Tosca, N.J.; Macdonald, F.A.; Strauss, J.V.; Johnston, D.T.; Knoll, A.H. Sedimentary talc in Neoproterozoic carbonate successions. Earth Planet. Sci. Lett. 2011, 306, 11–22. [Google Scholar] [CrossRef]
- Bodor, S.; Polgári, M.; Szentpétery, I.; Földessy, J. Microbially mediated iron ore formation, Silicic Superunit, Rudabánya, Hungary. Ore Geol. Rev. 2016, 72, 391–401. [Google Scholar] [CrossRef]
- Rosing, M.T.; Rose, N.M.; Bridgwater, D.; Thomsen, H.S. Earliest part of Earth’s stratigraphic record: A reappraisal of the >3.7 Ga Isua (Greenland) supracrustal sequence. Geology 1996, 24, 43–46. [Google Scholar] [CrossRef]
- Katsuta, N.; Shimizu, I.; Helmstaedt, H.; Takano, M.; Kawakami, S.; Kumazawa, M. Major element distribution in Archean banded iron formation (BIF): Influence of metamorphic differentiation. J. Metamorph. Geol. 2012, 30, 457–472. [Google Scholar] [CrossRef]
- Gutzmer, J.; Beukes, N.J. Origin and paleoenvironmental significance of major iron formations at the Archean-Paleoproterozoic boundary. In Banded Iron Formation-Related High-Grade Iron Ore.—Reviews in Economic Geology; Hagemann, S., Rosière, C.A., Gutzmer, J., Beukes, N.J., Eds.; Society of Economic Geologists (SEG): Littleton, CO, USA, 2008; Volume 15. [Google Scholar] [CrossRef]
- Knoll, A.H.; Canfield, D.E.; Konhauser, K.O. Fundamentals of Geobiology; Wiley-Blackwell: Oxford, UK, 2012; p. 456. [Google Scholar]
- Webb, S.; Dick, G.J.; Bargar, J.R.; Tebo, B.M. Evidence for the presence of Mn (III) intermediates in the bacterial oxidation of Mn(II). Microbiology 2005, 102, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Mandernack, K.W.; Tebo, B.M. Manganese scavenging and oxidation at hydrothermal vents and in vent plumes. Geochim. Cosmochim. Acta 1993, 57, 3907–3923. [Google Scholar] [CrossRef]
- Maynard, J.B. Manganiferous sediments, rocks, and ores. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 327–349, 407. [Google Scholar]
- Konhauser, K. Biomineralization. In Introduction to Geomicrobiology; Kohnhauser, K., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 139–191. [Google Scholar]
- Garrels, R.M.; MacKenzie, F.T. Evolution of Sedimentary Rocks; Nortom: New York, NY, USA, 1971. [Google Scholar]
- Hallbeck, L.; Pedersen, K. Culture parameters regulating stalk formation and growth rate of Gallionella ferruginea. Microbiology 1990, 136, 1675–1680. [Google Scholar] [CrossRef]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-oxidizing bacteria: An environmental and genomic perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef]
- Tyson, R.V.; Pearson, T.H. Modern and ancient continental shelf anoxia: An overview. Geol. Soc. Lond. Spec. Publ. 1991, 58, 1–24. [Google Scholar] [CrossRef]
- Noffke, N.; Hazen, R.; Nhleko, N. Earth’s earliest microbial mats in a siliciclastic marine environment (2.9 Ga Mozaan Group, South Africa). Geology 2003, 31, 673–676. [Google Scholar] [CrossRef]
- Noffke, N.; Eriksson, K.A.; Hazen, R.M.; Simpson, E.L. A new window into Early Archean life: Microbial mats in Earth’s oldest siliciclastic tidal deposits (3.2 Ga Moodies Group, South Africa). Geology 2006, 34, 253–256. [Google Scholar] [CrossRef]
- Cady, S.L.; Farmer, J.D.; Grotzinger, J.P.; Schopf, J.W.; Steele, A. Morphological Biosignatures and the Search for Life on Mars. Astrobiology 2003, 3, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Tsezos, M.; Baird, M.H.I.; Shemilt, L.W. The elution of radium adsorbed by microbial mats. Chem. Eng. J. 1987, 34, B57–B64. [Google Scholar] [CrossRef]
- Dupont, C.L.; Butcher, A.; Ruben, E.; Valas, R.E.; Bourne, P.E.; Caetano-Anollés, G. History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc. Natl. Acad. Sci. USA 2010, 107, 10567–10572. [Google Scholar] [CrossRef] [PubMed]
- Glamoclija, M.; Steele, A.; Fries, M.; Schieber, J.; Voytek, M.; Cockell, C. Association of anatase (TiO2) and microbes: Unusual fossilization effect or a potential biosignature? Geol. Soc. Am. Spec. Pap. 2009, 458, 965–975. [Google Scholar]
- Burke, I.T.; Kemp, A.E.S. Microfabric analysis of Mn-carbonate laminae deposition and Mn-sulfide formation in the Gotland Deep, Baltic Sea. Geochim. Cosmochim. Acta 2002, 66, 1589–1600. [Google Scholar] [CrossRef]
- Murowchick, J.B.; Barnes, H.L. Marcasite precipitation from hydrothermal solutions. Geochim. Cosmochim. Acta 1986, 50, 2615–2629. [Google Scholar] [CrossRef]
- Schoonen, M.A.A.; Barnes, H.L. Reactions forming pyrite and marcasite from solution: I. Nucleation of FeS2 below 100 °C. Geochim. Cosmochim. Acta 1991, 55, 1495–1504. [Google Scholar] [CrossRef]
- Egger, M.; Hagens, M.; Sapart, C.J.; Dijkstra, N.; van Helmond, N.A.G.M.; Mogoll´on, J.M.; Risgaard-Petersen, N.; van der Veen, C.; Kasten, S.; Riedinger, N.; et al. Iron oxide reduction in methane-rich deep Baltic Sea sediments. Geochim. Cosmochim. Acta 2017, 207, 256–276. [Google Scholar] [CrossRef]
- Baele, J.M.; Bouvain, F.; De Jong, J.; Matielli, N.; Papier, S.; Préat, A. Iron microbial mats in Modern and Phanerozoic environments. In Instruments, Methods, and Missions for Astrobiology XI; International Society for Optics and Photonics: Washington, DC, USA, 2008; Volume 7097, p. 70970N12. [Google Scholar]
- Müller, A. Morphology and genesis of chalcedony and opal in S-Mátra Mts. Dél-mátrai kalcedon és opál mintázatok morfológiája és genetikája. 2009. Research Report, National Scientific Research No. 68992, manuscript in Hungarian.
- Angerer, T.; Hagemann, S.G.; Walde, D.H.; Halverson, G.P.; Boyce, A.J. Multiple metal sources in the glaciomarine facies of the Neoproterozoic Jacadigo iron formation in the “Santa Cruz deposit”, Corumbá, Brazil. Precambrian Res. 2016, 275, 369–393. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).