In Situ High-Temperature X-ray Powder Diffraction and Infrared Spectroscopic Study of Melanterite, FeSO4·7H2O
Abstract
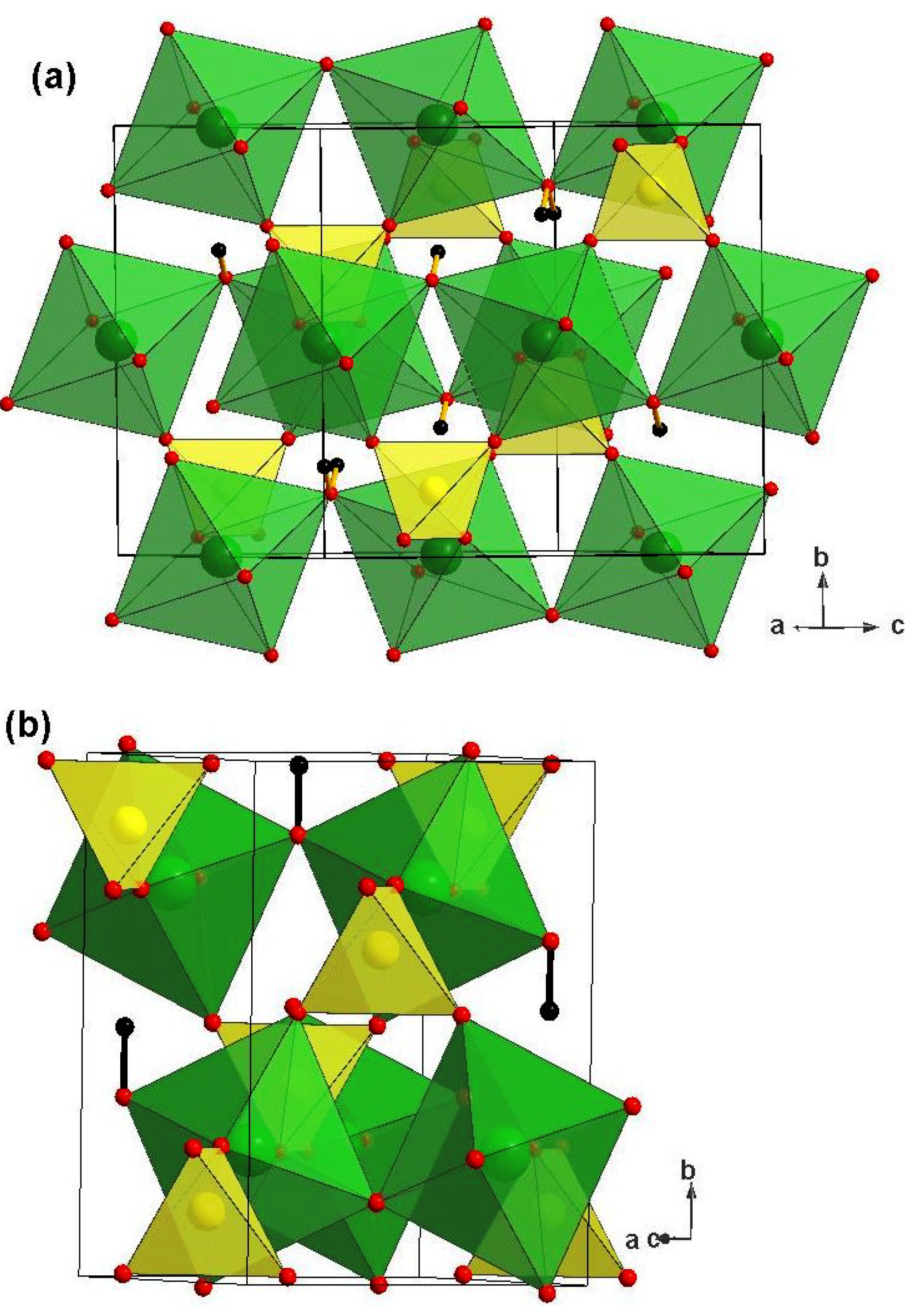
1. Introduction
2. Materials and Methods
3. Results
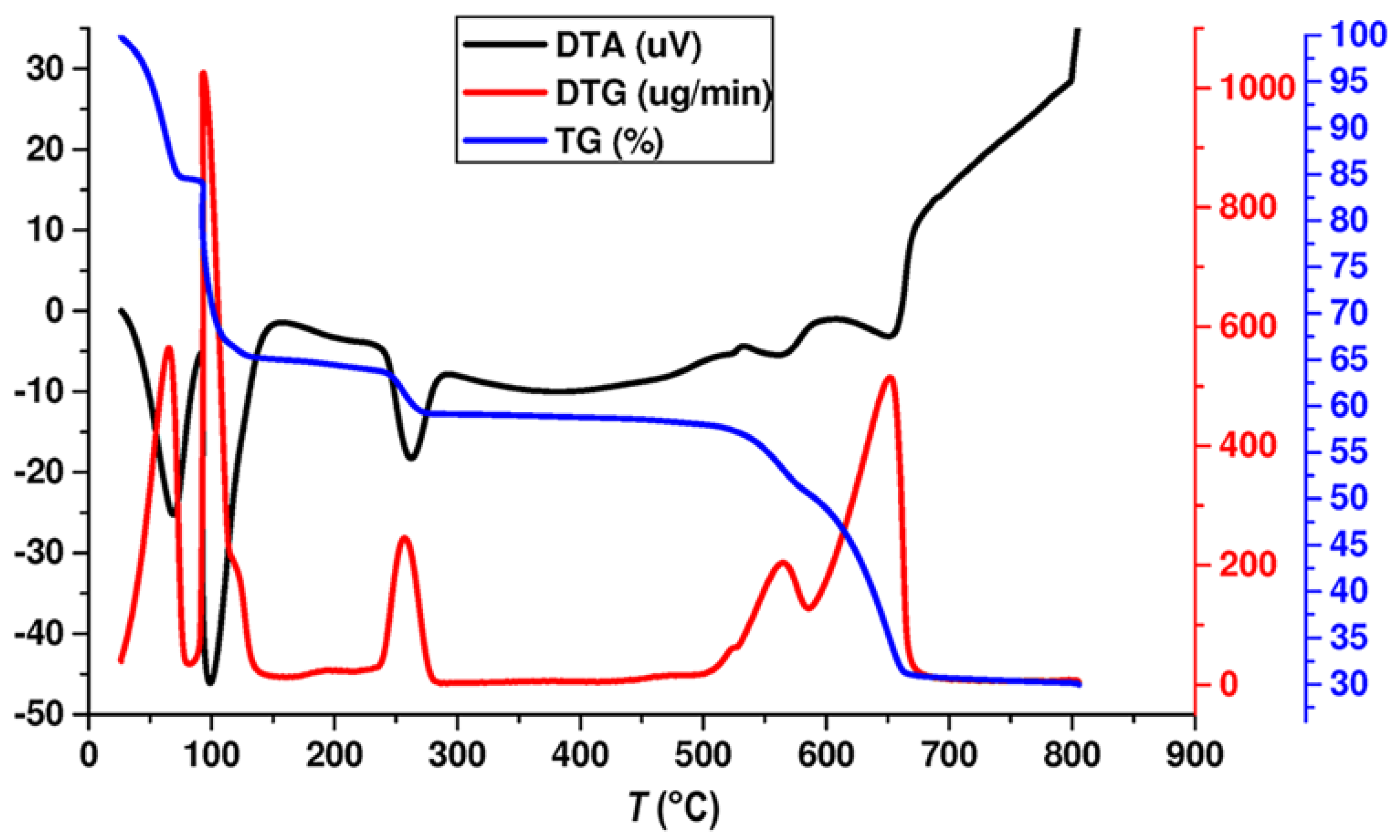
3.1. Thermal Analysis
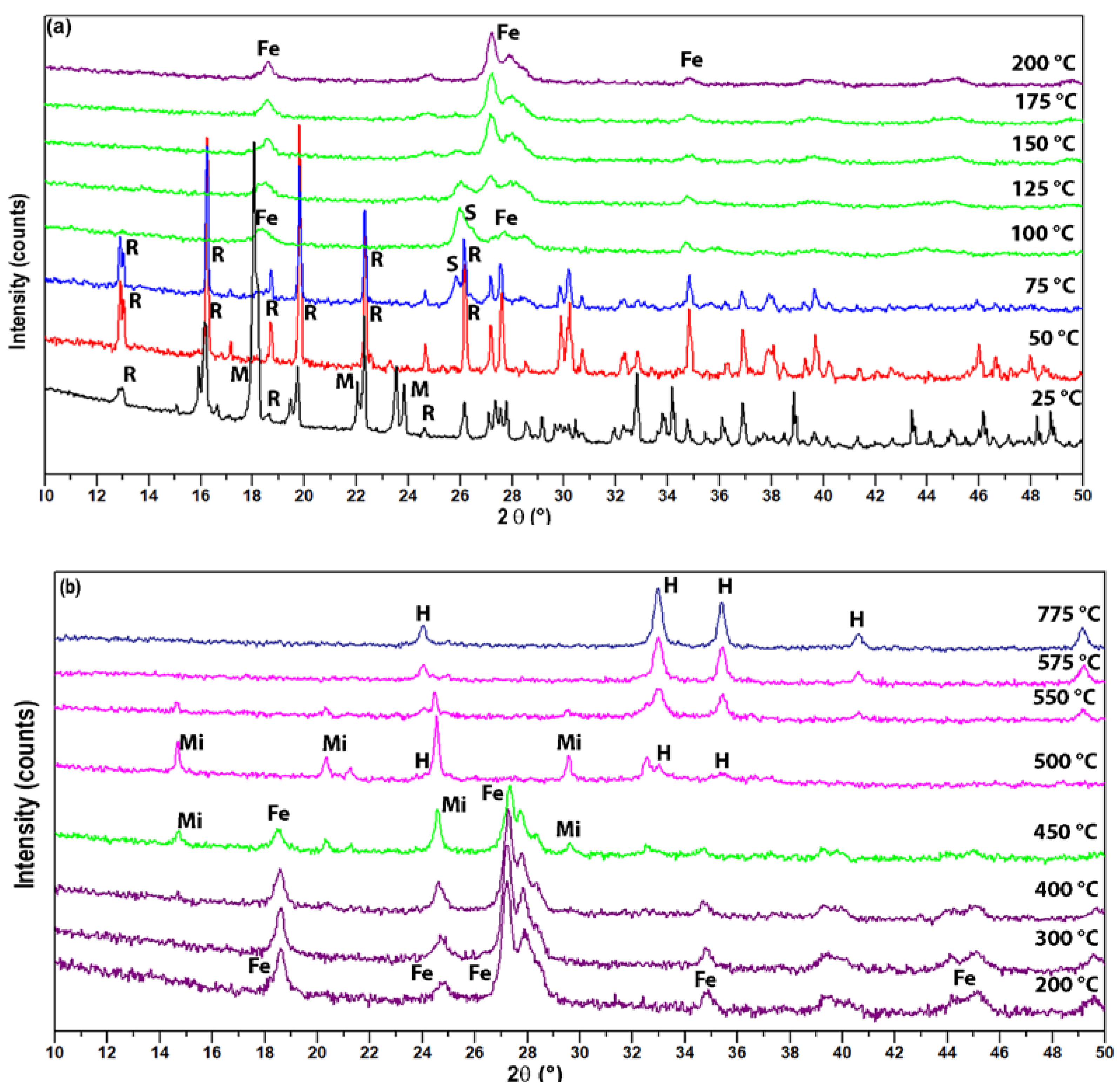
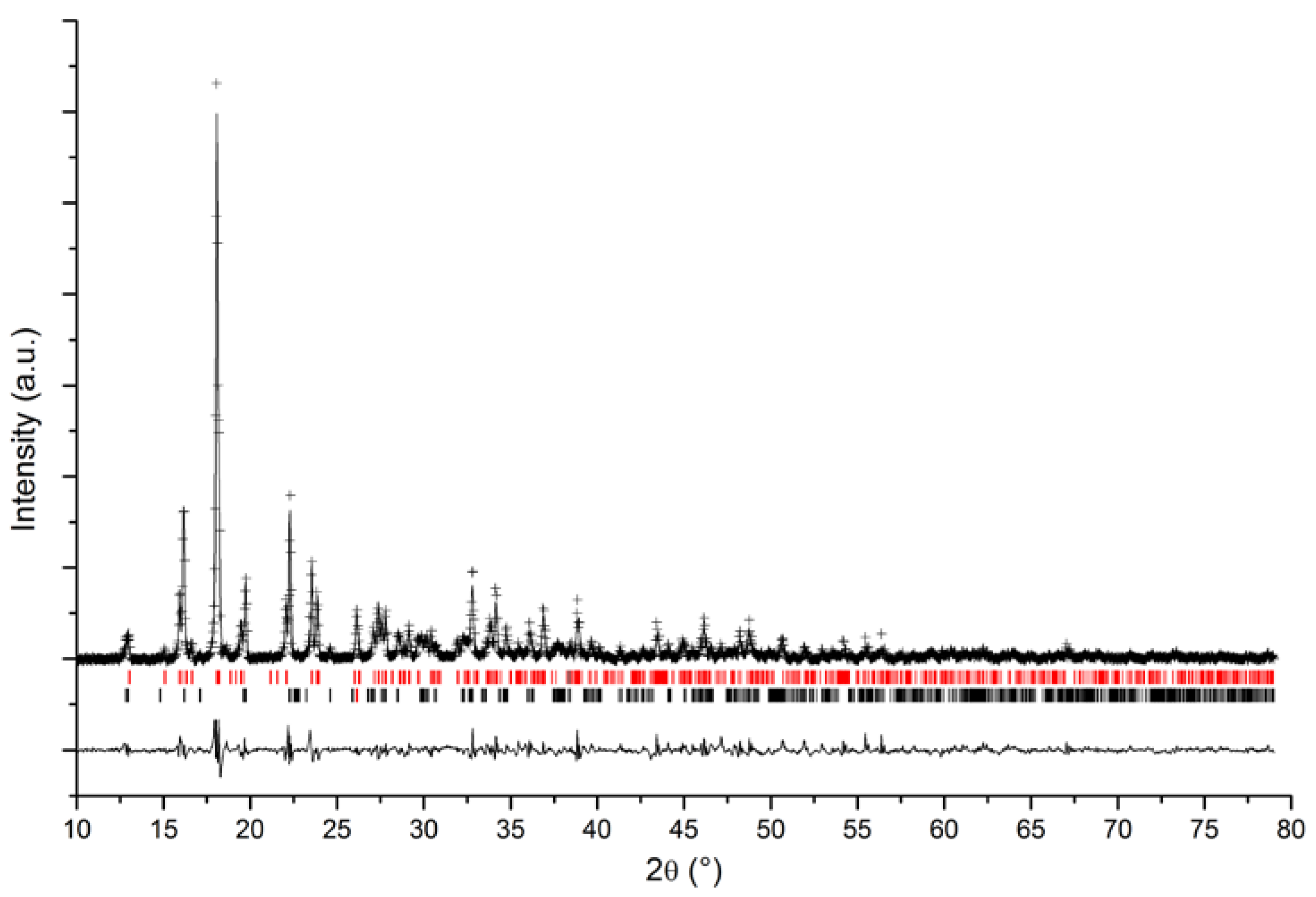
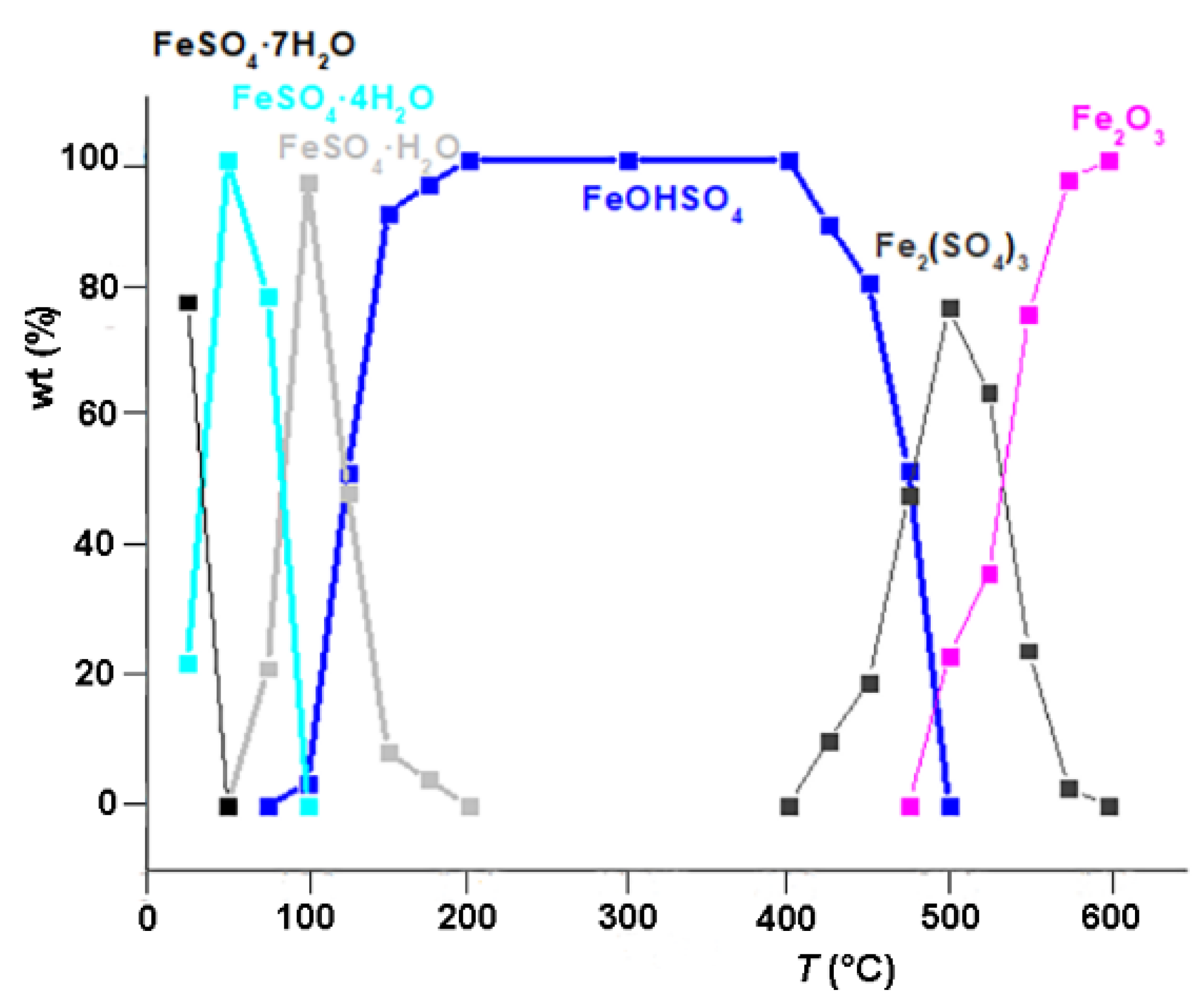
3.2. Quantitative Phase Analysis
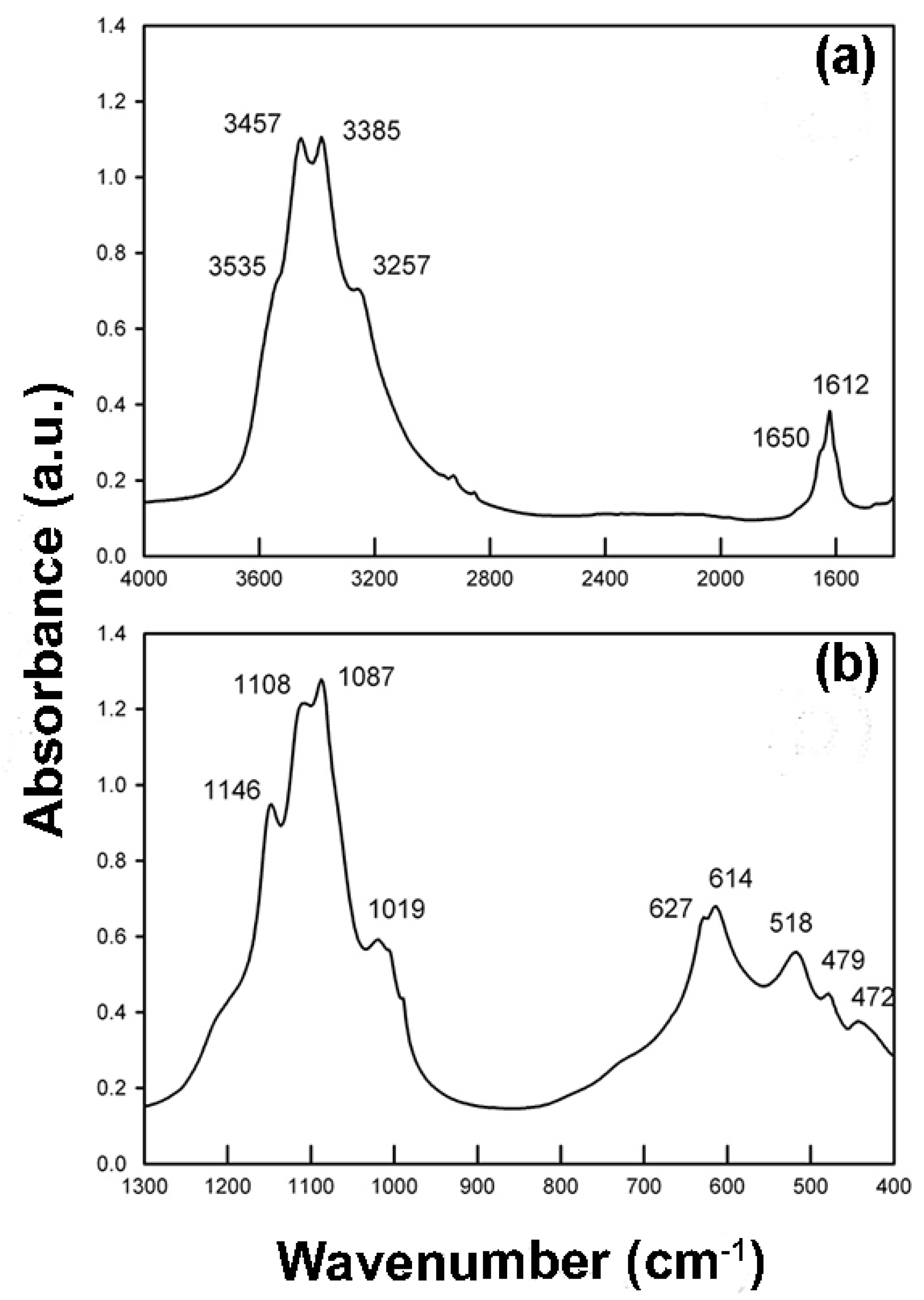
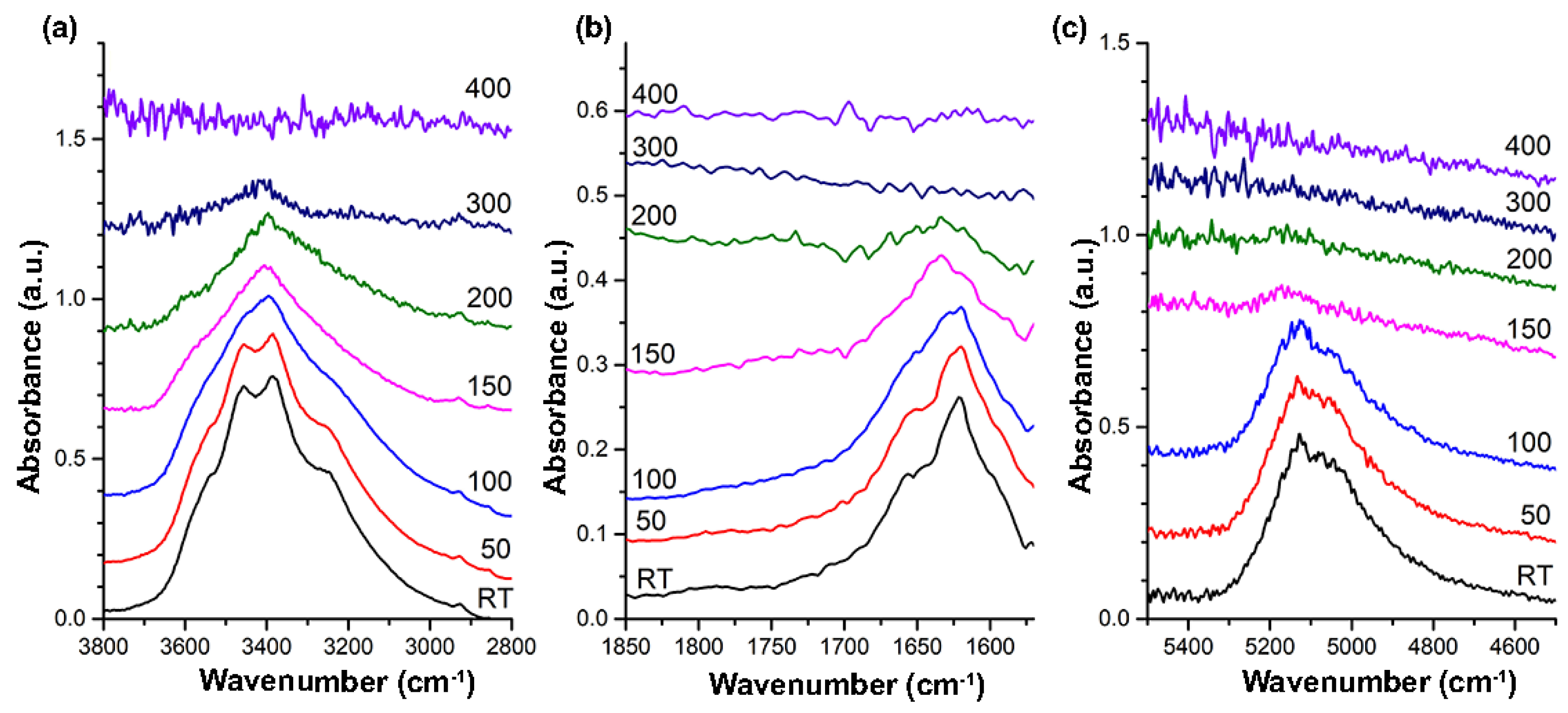
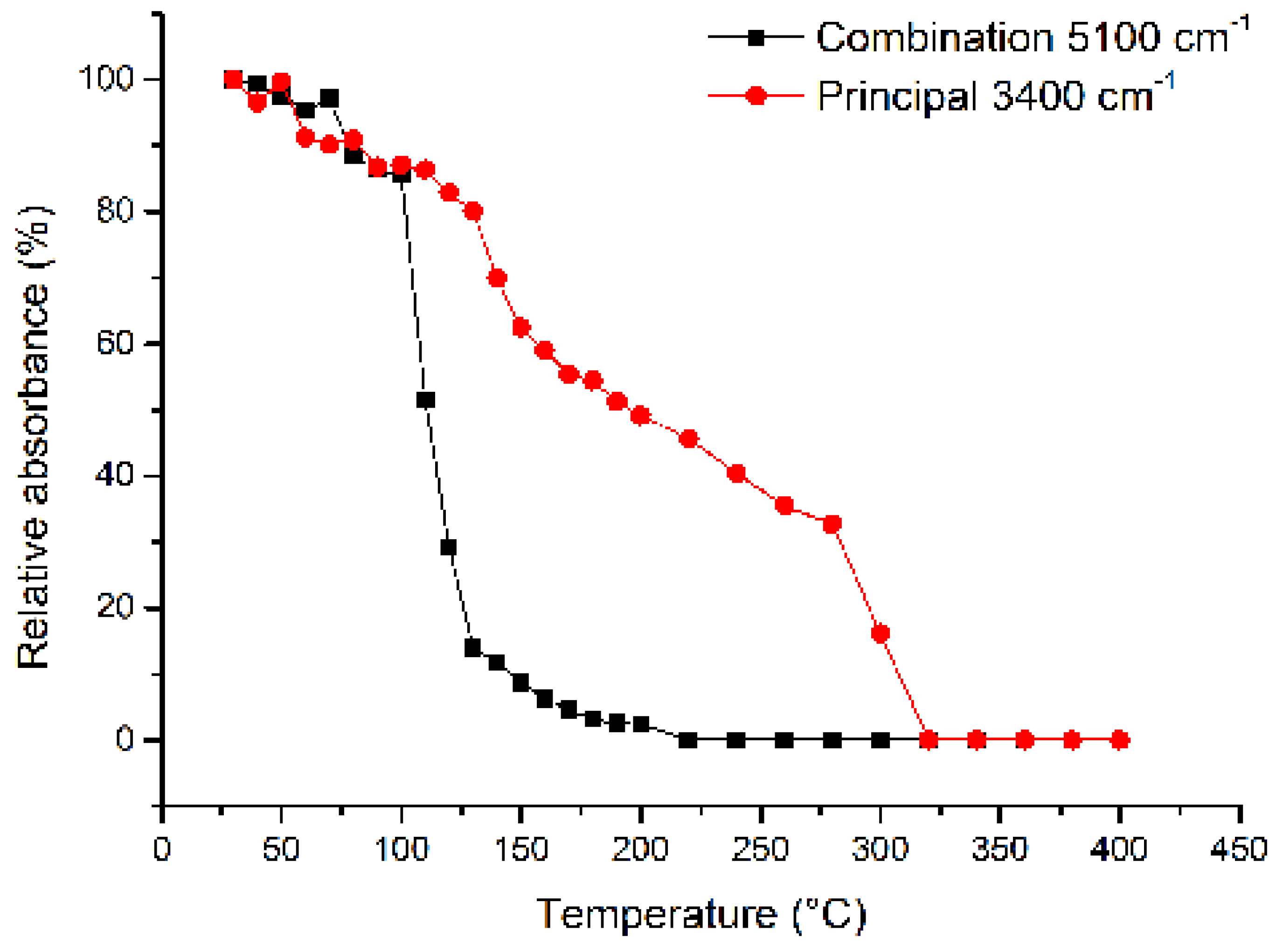
3.3. HT-FTIR Spectroscopy
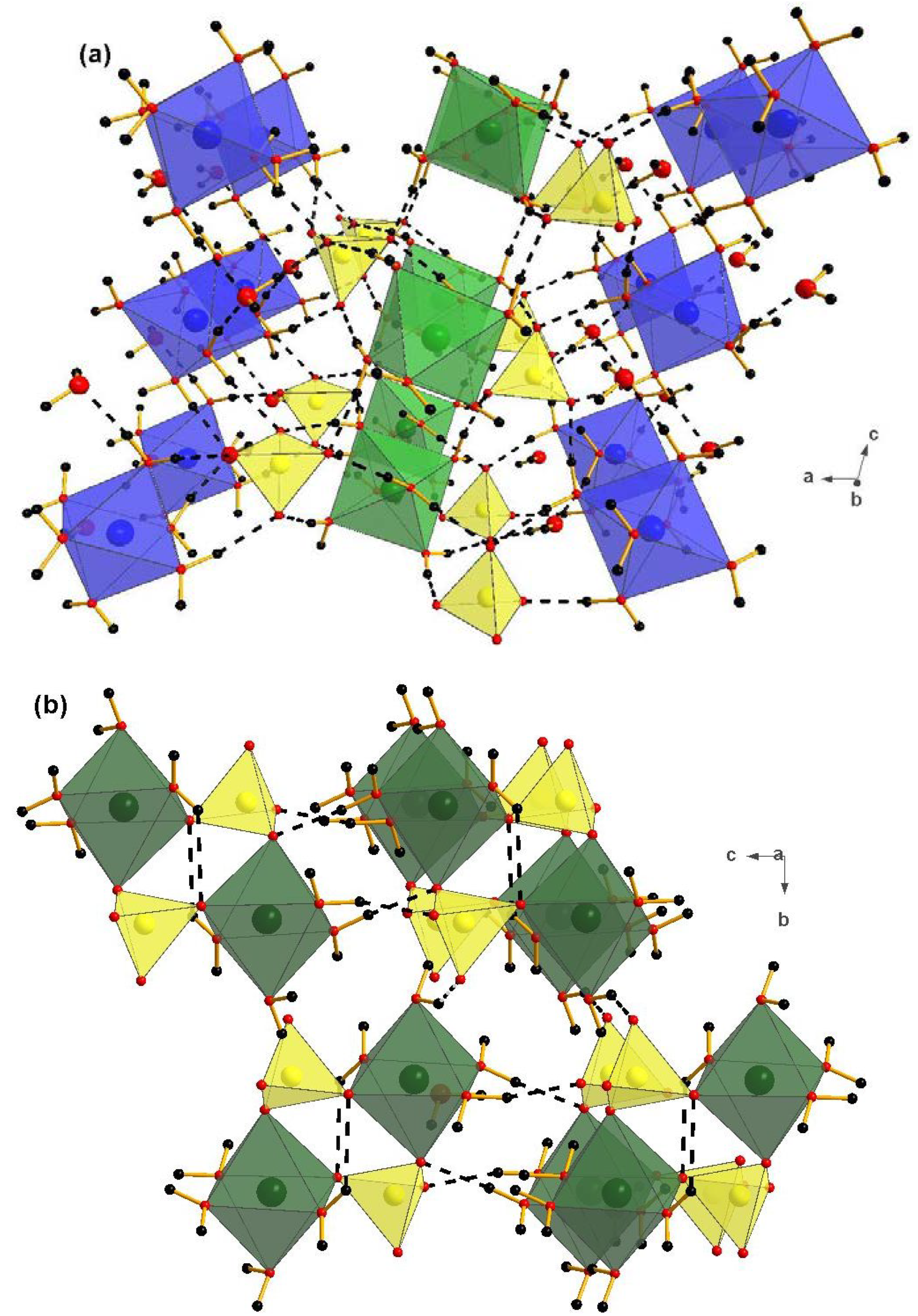
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frau, F. The formation-dissolution-precipitation cycle of melanterite at the abandoned pyrite mine of Genna Luas in Sardinia, Italy: Environmental implications. Mineral. Mag. 2000, 64, 995–1006. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Anderson, J.L.; Peterson, R.C. Determination of sulfate mineral phase equilibria as a function of relative humidity. Intermediate compositions in the (Mg,Fe,Zn)SO4–H2O system at 22 °C. Geol. Assoc. Can. Mineral. Assoc. Can. Program. Abstr. 2005, 30, 4. [Google Scholar]
- Buckby, T.; Black, S.; Coleman, M.L.; Hodson, M.E. Fe-sulphate-rich evaporative mineral precipitates from the Río Tinto, southwest Spain. Mineral. Mag. 2003, 67, 263–278. [Google Scholar] [CrossRef]
- Kanari, N.; Menad, N.; Ostrosi, E.; Shallari, S.; Diot, F.; Allain, E.; Yvon, J. Thermal behavior of hydrated iron sulfate in various atmospheres. Metals 2018, 8, 1084. [Google Scholar] [CrossRef]
- Lane, M.D.; Bishop, J.L.; Dyar, M.D.; King, P.L.; Parente, M.; Hyde, B.C. Mineralogy of the Paso Robles soils on Mars. Am. Mineral. 2008, 93, 728–739. [Google Scholar] [CrossRef]
- Baur, W.H. On the crystal chemistry of salt hydrates. III. The determination of the crystal structure of FeSO4·7H2O (melanterite). Acta Crystallogr. 1964, 17, 1167–1174. [Google Scholar] [CrossRef]
- Fronczek, F.R.; Collins, S.N.; Chan, J.Y. Refinement of ferrous sulfate heptahydrate (melanterite) with low-temperature CCD data. Acta Crystallogr. 2001, 57, i26–i27. [Google Scholar] [CrossRef]
- Peterson, R.C. The relationship between Cu content and distortion in the atomic structure of melanterite from the Richmond mine, Iron Mountain, California. Can. Mineral. 2003, 41, 937–949. [Google Scholar] [CrossRef]
- Anderson, J.L.; Peterson, R.C.; Swainson, I.P. The atomic structure and hydrogen bonding of deuterated melanterite, FeSO4·7D2O. Can. Mineral. 2007, 45, 457–469. [Google Scholar] [CrossRef]
- Mauro, D.; Biagioni, C.; Pasero, M. Crystal-chemistry of sulfates from Apuan Alps (Tuscany, Italy). I. Crystal structure and hydrogen bond system of melanterite, Fe(H2O)6(SO4)·H2O. Period. Mineral. 2018, 87, 85–92. [Google Scholar]
- Mitchell, A.G. The preparation and characterization of ferrous sulphate hydrates. J. Pharm. Pharmacol. 1984, 36, 506–510. [Google Scholar] [CrossRef]
- Cardoso Maia, L.; Rocha dos Santos, G.; Vinícius Alves Gurgel, L.; de Freitas Carvalho, C. Iron recovery from the coarse fraction of basic oxygen furnace sludge. Part I: Optimization of acid leaching conditions. Environ. Sci. Pollut. R. 2020, 27, 40135–40147. [Google Scholar] [CrossRef] [PubMed]
- Kanari, N.; Filippova, I.; Diot, F.; Mochón, J.; Ruiz-Bustinza, I.; Allaina, E.; Yvon, J. Utilization of a waste from titanium oxide industry for the synthesis of sodium ferrate by gas-solid reactions. Thermochim. Acta 2014, 575, 219–225. [Google Scholar] [CrossRef]
- Wang, T.; Debelak, K.A.; Roth, J.A. Dehydration of iron(II) sulfate heptahydrate. Thermochim. Acta 2007, 462, 89–93. [Google Scholar] [CrossRef]
- Prasad, T.P. Kinetics of thermal decomposition of intermediate hydrates ad basic salts of iron (II) sulphate heptahydrate. J. Therm. Anal. 1986, 31, 553–557. [Google Scholar] [CrossRef]
- Swamy, M.S.R.; Prasad, T.P. Kinetic of the thermal decomposition of iron (II) sulphate heptahydrate in air. Thermochim. Acta 1983, 62, 229–236. [Google Scholar] [CrossRef]
- Swamy, M.S.R.; Prasad, T.P.; Sant, B.R. Thermal analysis of ferrous sulphate heptahydrate in air II. The oxidation-decomposition path. J. Therm. Anal. 1979, 16, 471–478. [Google Scholar] [CrossRef]
- Gallagher, P.K.; Johnson, D.W.; Schrey, F. Thermal Decomposition of Iron(II) Sulfates. J. Am. Ceram. Soc. 1970, 53, 666–670. [Google Scholar] [CrossRef]
- Duval, C. Inorganic Thermogravimetric Analysis, 2nd ed.; Elsevier: New York, NY, USA, 1963; p. 722. [Google Scholar]
- Kamel, A.H.; Sawires, Z.; Khalifa, H.; Saleh, S.A.; Abdallah, A.M. The thermal decomposition of ferrous sulphate heptahydrate I. Dehydration and oxidation. J. Appl. Chem. Biotechnol. 1972, 22, 591–598. [Google Scholar] [CrossRef]
- Safiullin, N.S.; Gitis, E.B.; Panesenko, N.M. Termochemical transformations of FeSO4·7H2O during heating in oxidizing and inert media. Russ. J. Inorg. Chem. 1968, 13, 1493. [Google Scholar]
- Mahapatra, S.; Prasad, T.P. Solid-state hydrolysis of iron (II) sulphate heptahydrate. Thermichim. Acta 1988, 128, 305–309. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.; Gomez, M.A.; Captani, G.; Sbroscia, M.; Sodo, A. High-temperature study of basic ferric sulfate, FeOHSO4. Phys. Chem. Miner. 2020, 47, 43. [Google Scholar] [CrossRef]
- Jani, M.; Hodson, M.E.; Chippindale, A.M. In-situ Powder X-Ray Diffraction study on hydrous ferrous sulfate minerals. Adv. Mat. Res. 2015, 1087, 257–261. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.; Lacalamita, M.; Sbroscia, M.; Sodo, A.; Plaisier, J.R.; Cinque, G.; Schingaro, E. Crystal-chemistry and vibrational spectroscopy of ferrinatrite, Na3[Fe(SO4)3]·3H2O, and its high-temperature decomposition. Phys. Chem. Miner. 2019, 46, 119–131. [Google Scholar] [CrossRef]
- Biagioni, C.; Bonaccorsi, E.; Orlandi, P. Volaschioite, Fe3+4(SO4)O2(OH)6·2H2O, a new mineral species from Fornovolasco, Apuan Alps, Tuscany, Italy. Can. Mineral. 2011, 49, 605–614. [Google Scholar] [CrossRef]
- D’Orazio, M.; Mauro, D.; Valerio, M.; Biagioni, C. Secondary Sulfates from the Monte Arsiccio Mine (Apuan Alps, Tuscany, Italy): Trace-element budget and role in the formation of acid mine drainage. Minerals 2021, 11, 206. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos Reports: Los Alamos, New Mexico, 2000; pp. 86–748. [Google Scholar]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011; p. 180. [Google Scholar]
- Baur, W.H. Zur kristallchemie der salzhydrate. Die kristallstrukturen von MgSO4·4H2O (leonhardtit) und FeSO4·4H2O (rozenit). Acta Crystallogr. 1962, 15, 815–826. [Google Scholar] [CrossRef]
- Wildner, M.; Giester, G. The crystal structures of kieserite-type compounds. I. Crystal structures of Me(II)SO4·H2O (Me = Mn, Fe, Co, Ni, Zn). Neues Jb Miner. Monat. 1991, 7, 296–306. [Google Scholar]
- Miura, H.; Niida, K.; Hirama, T. Mikasaite, (Fe3+, Al)2(SO4)3, a new ferric sulphate mineral from Mikasa city, Hokkaido, Japan. Mineral. Mag. 1994, 58, 649–653. [Google Scholar] [CrossRef]
- Blake, R.L.; Hessevick, R.E.; Zoltai, T.; Finger, L.W. Refinement of the hematite structure. Am. Mineral. 1966, 51, 123–129. [Google Scholar]
- Brown, A.J.; Bish, D.L.; Bishop, J.L. Dehydration of ferrous sulfates monitored by XRD-Implications for CHEMIN. Lun. Planet. Sci. 2008, 39, 1008. [Google Scholar]
- Libowitzky, E. Correlation of O–H stretching frequencies and O–H…O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Reddy, S.N.; Rao, P.S.; Ravikumar, R.V.; Reddy, B.J.; Reddy, Y.P. Spectral investigations on melanterite mineral from France. Spec. Acta 2001, 57, 1283–1287. [Google Scholar] [CrossRef]
- Frost, R.L.; Wills, R.-A.; Martens, W.; Weier, M.; Reddy, B.J. NIR spectroscopy of selected iron(II) and iron(III) sulphates. Spec. Acta 2005, 62, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.H.; Kerr, P.F. Variations in infrared spectra, molecular symmetry and site symmetry of sulfate minerals. Am. Mineral. 1965, 50, 132–147. [Google Scholar]
- Ross, S.D. Sulphates and other oxy-anions of group VI. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of Great Britain and Ireland: Middlesex, UK, 1974; p. 4. [Google Scholar]
- Gomez, M.A.; Ventruti, G.; Celikin, M.; Assaaoudi, H.; Putz, H.; Becze, L.; Leea, K.E.; Demopoulos, G.P. The nature of synthetic basic ferric arsenate sulfate (Fe(AsO4)1–x(SO4)x(OH)x) and basic ferric sulfate (FeOHSO4): Their crystallographic, molecular and electronic structure with applications in the environment and energy. RSC Adv. 2013, 37, 16840–16849. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.; Bellatreccia, F.; Lacalamita, M.; Schingaro, E. Hydrogen bond system and vibrational spectroscopy of the iron sulfate fibroferrite, FeOH(SO4)·5H2O. Eur. J. Mineral. 2016, 5, 943–952. [Google Scholar] [CrossRef]
- Pannetier, G.; Bregeault, J.M.; Djega-Maria-Dassou, G. Thermal dissociation of ferrous sulfate heptahydrate. C.R. Acad. Sci. USA 1964, 258, 2832–2835. [Google Scholar]
- Ventruti, G.; Scordari, F.; Schingaro, E.; Gualtieri, A.F.; Meneghini, C. The order-disorder character of FeOHSO4 obtained from the thermal decomposition of metahohmannite, Fe23+(H2O)4[O(SO4)2]. Am. Mineral. 2005, 90, 679–686. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.; Scordari, F.; Susta, U.; Gualtieri, A.F. In situ high-temperature XRD and FTIR investigation of hohmannite, a water-rich Fe-sulfate, and its decomposition products. J. Therm. Anal. Calorim. 2015, 119, 1793–1802. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.D.; Corriero, N.; Malferrari, D.; Gualtieri, A.F.; Susta, U.; Lacalamita, M.; Schingaro, E. In situ high-temperature X-ray diffraction and spectroscopic study of fibroferrite, FeOH(SO4)·5H2O. Phys. Chem. Miner. 2016, 43, 587–595. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H.; Carpenter, M.A.; Wang, J.Y.; Groat, L.A.; Lager, G.A.; Wang, L.; Beran, A.; Bismayer, U. Temperature dependence of IR absorption of hydrous/hydroxyl species in minerals and synthetic materials. Am. Mineral. 2007, 92, 1502–1517. [Google Scholar] [CrossRef]
- Radica, F.; Della Ventura, G.; Bellatreccia, F.; Cestelli Guidi, M. HT-FTIR micro-spectroscopy of cordierite: The CO2 absorbance from in situ and quenched experiments. Phys. Chem. Miner. 2015, 43, 69–81. [Google Scholar] [CrossRef]
| Process | Swamy et al. [18] | Swamy and Prasad [17] | Mitchell [12] | Földvári [30] | Kanari et al. [5] | Cardoso Maia et al. [13] | This Study | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) | (b) | (c) | (d) | (e) | ||||||
| Formation of FeSO4·6H2O or FeSO4·5H2O | 52–65 | |||||||||
| Formation of FeSO4·4H2O | 90 | 53–69 | 70 | 80 | 98 | 54 | 70 | |||
| Formation of FeSO4·H2O | 190 | 150 | 130–200 | 106–120 | 100–200 | 86 | 133 | 159 | 94 | 100 |
| Decomposition of FeSO4·H2O to FeSO4 | 330–370 | 305–345 | 300 | 227 | 250 | 283 | 253 | |||
| Oxidation of FeSO4·H2O to FeOHSO4 | 370 | 260 | ||||||||
| Decomposition of FeOHSO4 to Fe2O(SO4)2 | 550; 590 eso | 580 | ||||||||
| Formation of Fe2(SO4)3 | 400–600 (f) | 560 | ||||||||
| Decomposition of Fe2O(SO4)2 to Fe2O3 | 810 | 700 | 680–800 | 617 | ||||||
| Decomposition of FeSO4 to Fe2O3 | 680–830 | 653 | 687 | 716 | ||||||
| Decomposition of FeOHSO4 and Fe2(SO4)3 to Fe2O3 | 660 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacalamita, M.; Ventruti, G.; Della Ventura, G.; Radica, F.; Mauro, D.; Schingaro, E. In Situ High-Temperature X-ray Powder Diffraction and Infrared Spectroscopic Study of Melanterite, FeSO4·7H2O. Minerals 2021, 11, 392. https://doi.org/10.3390/min11040392
Lacalamita M, Ventruti G, Della Ventura G, Radica F, Mauro D, Schingaro E. In Situ High-Temperature X-ray Powder Diffraction and Infrared Spectroscopic Study of Melanterite, FeSO4·7H2O. Minerals. 2021; 11(4):392. https://doi.org/10.3390/min11040392
Chicago/Turabian StyleLacalamita, Maria, Gennaro Ventruti, Giancarlo Della Ventura, Francesco Radica, Daniela Mauro, and Emanuela Schingaro. 2021. "In Situ High-Temperature X-ray Powder Diffraction and Infrared Spectroscopic Study of Melanterite, FeSO4·7H2O" Minerals 11, no. 4: 392. https://doi.org/10.3390/min11040392
APA StyleLacalamita, M., Ventruti, G., Della Ventura, G., Radica, F., Mauro, D., & Schingaro, E. (2021). In Situ High-Temperature X-ray Powder Diffraction and Infrared Spectroscopic Study of Melanterite, FeSO4·7H2O. Minerals, 11(4), 392. https://doi.org/10.3390/min11040392








