Abstract
Monohydrocalcite (MHC) is a metastable hydrous calcium carbonate that requires Mg in the mother solution during formation in the laboratory. MHC prepared by previously reported methods always contains a large amount of Mg (Mg/Ca ratio up to 0.4) because of the simultaneous formation of amorphous Mg carbonate during synthesis, which has hindered detailed elucidation of the mineralogical characteristics of MHC. Here, we synthesized MHC at low temperature (5 °C) and found that it contained little Mg (Mg/Ca ratio < 0.01). X-ray absorption near-edge structure analysis of synthesized MHC revealed that the Mg present was structurally incorporated within the MHC, and that the chemical speciation of this Mg was similar to that of Mg in aragonite. Thus, low-temperature synthesis is an effective means of producing MHC without also producing amorphous Mg carbonate.
1. Introduction
Monohydrocalcite (MHC, CaCO3·H2O) is a hydrous calcium carbonate, and it is metastable with respect to anhydrous phases (CaCO3), such as calcite and aragonite [1,2]. MHC most frequently occurs as an authigenic mineral in alkaline saline lakes [3,4]. Recent research has suggested that the water chemistries of alkaline saline lakes, such as pH and the concentrations of Ca and Mg, are controlled by the formation of MHC in association with amorphous Mg carbonate (AMC, MgCO3·nH2O) [5].
The metastable nature of MHC makes it a promising material for environmental remediation [1]. Previous studies have shown that MHC effectively sorbs contaminants, such as arsenic, lead, and phosphorous [6,7,8], although the presence of impurities, especially for Mg with MHC, suppresses the sorption ability of MHC for arsenic and prosperous [9,10]. Thus, a simple, reproducible method for the synthesis of MHC containing low levels of impurities would be useful.
Nishiyama et al. [11] systematically examined the formation of MHC from Na2CO3–CaCl2–MgCl2 solutions and found that MHC forms when the initial Na2CO3 concentration exceeds the total Ca concentration and a large amount of Mg is present. Although methodologies based on these conditions allow for reproducible synthesis of MHC, the MHC obtained contains a large amount of Mg as an impurity [12,13,14]. The dominant host of this Mg is AMC, which is formed together with MHC [14,15]. However, the co-existence of AMC with MHC has hindered detailed elucidation of the mineralogical and physicochemical characteristics of MHC [2,16]. Here, we report a simple, reproducible method for the synthesis of MHC with low Mg content.
2. Materials and Methods
All of the following experiments were conducted in a temperature-controlled room at 5 °C. A series of 50 mL solutions containing 0.05 mol/kg MgCl2 and 0.01 mol/kg CaCl2 were prepared in polypropylene bottles. Stock Na2CO3 solution was then added to the bottles to obtain initial Na2CO3 concentrations of 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.11, and 0.12 mol/kg. Immediately after the addition of the Na2CO3 solution, whitish precipitates began to form in the bottles. The bottles were covered with Kimwipe paper to allow interaction of the solutions with atmospheric CO2 during the reaction period [5]. After the bottles were left for 400 h to allow the reaction to proceed, the obtained suspensions were vacuum-filtered through 0.2 μm membranes. The filtrates were acidified with concentrated HNO3 to produce 0.6% HNO3 solutions, which were then examined by inductively coupled plasma optical emission spectroscopy (ICP-OES: ES-710S; Varian, Inc., Palo Alto, CA, USA). The solids on the filter papers were freeze-dried without washing and then used for mineralogical characterization.
X-ray diffraction was used to determine the mineralogical compositions of the solids (Ultima IV: Cu Kα, 40 kV, 30 mA; Rigaku Corp., Tokyo, Japan). The selected MHC samples were observed using scanning electron microscopy (SEM; S-3000N; Hitachi High-Technologies Corp., Tokyo, Japan) with energy dispersed X-ray spectroscopy (EDS; EMAX500; Horiba Ltd., Kyoto, Japan) to elucidate their mineralogy. ICP-OES was used to determine the concentrations of Ca and Mg in the filtrates. For the solids identified to contain MHC, the Mg/Ca ratio was determined by wet chemical analysis by first dissolving 100 mg of solid in 40 mL of 0.6% HNO3 solution and then measuring the Ca and Mg concentrations in the solution by ICP-OES. Triplicate measurements of the obtained solutions showed that the analytical errors with the ICP-OES are always < 2%. Our previous study showed that the uncertainties of the Mg/Ca ratio associated with the procedure of the wet chemical analyses of the MHC are < 4% [15]. The significant digits given in this study (Table 1) are based on the analytical errors and the uncertainties. The Mg K-edge X-ray absorption near-edge structure spectrum of the solid obtained with an initial Na2CO3 concentration of 0.10 mol/kg was measured using the BL-19B beamline at the Photon Factory, KEK, Japan. A powdered solid sample was loaded on conductive double-sided carbon tape attached to a stainless steel sample holder. The spectrum was obtained in fluorescence X-ray yield mode with a silicon drift detector under a high vacuum (approx. 1 × 10−6 Pa) and processed using the REX2000 data analysis software package (Rigaku Co. Ltd., Tokyo, Japan).

Table 1.
Summary of the synthesis experiments conducted in the present study and by Nishiyama et al. [11] and Fukushi et al. [15].
3. Results and Discussion
Figure 1 shows the X-ray diffraction patterns of the solids obtained with various initial Na2CO3 concentrations. When the initial Na2CO3 concentration was 0.06 mol/kg, the solid contained only magnesian calcite ((Ca,Mg)CO3), and when the initial Na2CO3 concentration was 0.05 mol/kg or less, the solid contained only calcite. However, when the initial Na2CO3 concentration was 0.07 mol/kg or greater, the solid contained MHC. When the initial Na2CO3 concentration was 0.07 mol/kg, small peaks attributed to aragonite were observed in the X-ray diffraction pattern, but when the initial Na2CO3 concentration was 0.08 or greater, the solid contained only MHC. These findings are consistent with those of Nishiyama et al. [11] who examined the formation of MHC using the same concentrations of CaCl2 and MgCl2 but a higher temperature (25 °C) than that used in the present study (5 °C) and found that the solids obtained when the initial Na2CO3 concentration was greater than 0.07 mol/kg contained only crystalline MHC (Table 1). Thus, the formation of MHC from Na2CO3–CaCl2–MgCl2 solutions at low temperature (5 °C) was comparable with that at 25 °C [5,11,15].
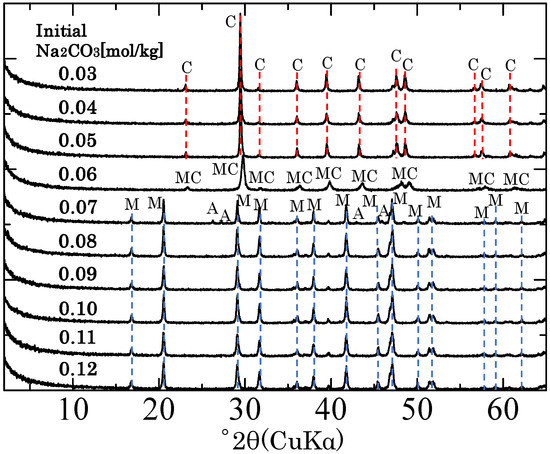
Figure 1.
X-ray diffraction patterns of the solids obtained with different initial concentrations of Na2CO3. A: aragonite (CaCO3), C: calcite (CaCO3), MC: magnesian calcite ((Ca,Mg)CO3), M: monohydrocalcite (CaCO3·nH2O).
In the present study, the initial concentration of Mg was 0.01 mol/kg for each synthesis experiment. After reaction, the concentration of Mg in the filtrates removed from the solids that contained MHC only was in the range 0.0095 to 0.010 mol/kg, and no relationship between Mg concentration in the filtrate and initial Na2CO3 concentration was observed. The Mg/Ca ratio of the obtained MHC was 0.01 or lower, as estimated by wet chemical analysis (Table 1). In contrast to our syntheses at low temperature, the syntheses of Nishiyama et al. [9] at 25 °C revealed filtrate Mg concentration in the range 0.0075 to 0.0092 and Mg/Ca ratios in the range of 0.02 to 0.06 from synthesis experiments conducted under the comparable initial solution compositions at 25 °C.
It is reported that there are two types of Mg speciation associated with MHC formation, where Mg can be structurally coordinated either with MHC or with the discrete phase AMC [15]. Fukushi et al. [15] suggested that when Mg is structurally coordinated with MHC, the coordination environment of Mg resembles that in aragonite. Figure 2 shows Mg K-edge spectra obtained for the MHC obtained in the present study with an initial Na2CO3 concentration of 0.1 mol/kg (#9 in Table 1); AMC; MHC with high (#F3 in Table 1) or low (#F1 in Table 1) Mg content synthesized at 25 °C; and Mg-doped aragonite [15]. The shape of the spectra for the MHCs with high and low Mg content can be attributed to contributions by the two endmembers, i.e., Mg in aragonite and AMC [15]. The shape of the spectrum for the MHC obtained in the present study was well-matched with that of Mg-doped aragonite, suggesting that AMC formation was almost negligible during MHC synthesis at low temperature.
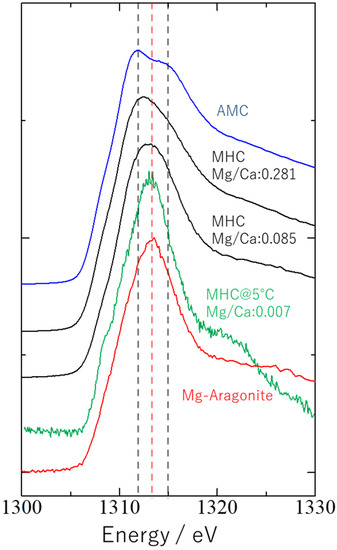
Figure 2.
Mg K-edge X-ray absorption near-edge structure spectra of the monohydrocalcite (MHC) synthesized in the present study at 5 °C and an initial Na2CO3 concentration of 0.1 mol/kg and four reference materials: amorphous Mg carbonate (AMC; Fukushi et al. [15]), MHC with high or low Mg content synthesized at 25 °C (Fukushi et al., [15]), and Mg-doped aragonite [15]. The spectrum for AMC showed two peaks, at 1312 and 1315 eV (black dotted lines), and that for aragonite showed a sharp peak at around 1313 eV (red dotted line).
The Mg partitioning behavior was also examined from the solution phase analyses. The partitioning behavior of Mg in calcium carbonates from aqueous solutions can be assessed with the apparent partitioning coefficient (D) defined by the following Equation [17]:
where {Mg}sol and {Ca}sol denote the concentration of Mg and Ca in the solutions. {Mg}solid and {Ca}solid denote the concentration of Mg and Ca in solids. Therefore, {Mg}solid/{Ca} solid corresponds to Mg/Ca ratio in Table 1. The log D obtained at 25 °C [11,15] widely varies from −3.5 to −2.4. The MHCs with the higher Mg/Ca generally exhibit the higher log D (Table 1). The XANES spectra showed that the AMC contents in the solids with MHC increased with the Mg/Ca ratio of the solids. Therefore, the dependency of the log D on the Mg/Ca ratio at 25 °C may come from the contribution of AMC with MHC in the sample rather than the partitioning in the structure of the MHC, because the increase in AMC simply results in the increase in {Mg}solid relative to {Ca}solid in (1). On the other hand, the log D obtained from the low-temperature synthesis at 5 °C takes almost constant value at −4.2 (Table 1). The XANES spectrum suggests that the AMC formation is almost negligible at 5 °C. The constant partitioning coefficients represent the partitioning of Mg in the structure of MHC at 5 °C.
The simultaneous formation of AMC is essential for the formation of MHC, and it has been suggested that AMC likely plays a role in preventing the dehydration of MHC [11,15,18]. However, we found that MHC formation at low temperature produced little AMC. During MHC formation, when the initial Na2CO3 concentration exceeds the Ca concentration in the presence of a large amount of Mg, after all of the Ca2+ has reacted with CO32−, Mg2+ in the solution reacts with the remaining CO32− to form AMC [11]. In the present study, the initial solution conditions were the same as those used in the experiments of Nishiyama et al. [11] that were conducted at 25 °C. Therefore, AMC most likely forms together with MHC at the start of synthesis. In a preliminary study, we confirmed an initial decrease in Mg concentration in the solution at the start of synthesis (Figure A1 in Appendix A); however, the AMC that formed was gradually dissolved during the aging processes. The higher concentrations of Mg observed in the solutions after synthesis in the present study compared with those reported by Nishiyama et al. [11] (Table 1) suggest that the solubility of AMC is higher at 5 °C than it is at 25 °C. It has been also documented that the solubility of nesquehonite (MgCO3·3H2O), a hydrous Mg carbonate, increases with the decrease in temperature from 25 °C to 5 °C [19]. In addition, the transformation rate of MHC has been shown to be significantly retarded with decreasing temperature [20]. Therefore, the MHC formed during the initial stages of the reaction is able to persist even after the dissolution of AMC.
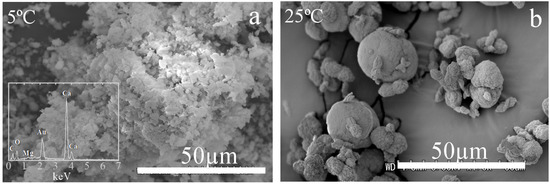
The MHC with low Mg obtained from this study can provide the implication of the role of the Mg on the crystal growth and the morphology of MHC. The peak widths of XRD peak are related to the crystallite size according to Scherrer Equation [21]:
where L denotes the crystallite size, β denotes full width at half maximum of the selected diffraction peak, K represents the Scherrer constants (0.94), and θ denotes the position of the selected diffraction peak. Fukushi et al. [15] also measured the crystallite size of the MHC obtained at 25 °C. They showed that the crystallite size negatively correlates with the Mg/Ca ratio (Table 1). The crystallite size of the MHC obtained at 5 °C takes almost constant at around 37 nm regardless of the initial solution conditions (Table 1). The size is significantly higher than any synthesized MHC at 25 °C, which always exhibits the higher Mg/Ca. This suggests that the presence of AMC with MHC acts as the inhibitor for the MHC crystallite growth, as suggested by Fukushi et al. [15]. Figure 3 shows the SEM image of the MHC with the EDS spectrum obtained from the initial Na2CO3 concentration of 0.11 mol/kg (#9 in Table 1). The MHC obtained at 5 °C was the aggregates of irregularly shaped particles. The morphology is very different from the MHC obtained at 25 °C, which commonly exhibits the two connected spherules with a diameter from several to 30 μm [15]. The crystallite sizes of MHC obtained at 5 °C were not significantly different from those at 25 °C. The difference of MHC between at 5 and 25 °C was the Mg contents in MHC. This suggests that the AMC may play a role in controlling the morphology.
4. Conclusions
Here, we report that MHC with low Mg content (Mg/Ca < 0.01) can be synthesized at low temperature (5 °C) using conditions reported previously [9] (i.e., initial concentration of CO3 exceeding the total concentration of Ca in the presence of a large amount of Mg). The X-ray absorption near near-edge structure spectrum of the solid obtained at 5 °C with an initial Na2CO3 concentration of 0.10 mol/kg showed that the Mg in the solid was structurally incorporated into the MHC and that the chemical speciation of this Mg was similar to that of Mg in aragonite. Thus, low-temperature synthesis can be used to produce MHC without the production of AMC effectively.
Author Contributions
Conceptualization, T.K. and K.F.; Methodology; T.K., M.Y., Y.T. (Yasuo Takeichi), and Y.T. (Yoshio Takahashi); Writing-Original Draft Preparation, T.K. and K.F.; Writing-Review and Editing, M.Y., Y.T. (Yasuo Takeichi), and Y.T. (Yoshio Takahashi); Funding Acquisition, K.F. M.Y. and Y.T. (Yoshio Takahashi). All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by Grants-in-Aid for Scientific Research from the Japan Society for Promotion of Science (no. 17H06458) and by a cooperative research program of the Institute of Nature and Environmental Technology, Kanazawa University (nos. 19035 and 20061). This work was performed under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2018S1-001).
Acknowledgments
We would like to thank S. Tan for the experimental support for the X-ray absorption near-edge structure analysis. Discussions with Y. Sekine are also greatly appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
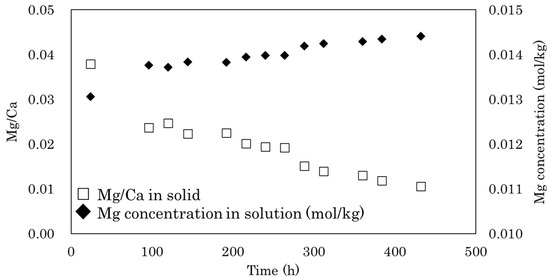
Figure A1.
Changes in Mg concentration and Mg/Ca ratio over time during monohydrocalcite synthesis at 5 °C. The initial solutions were 0.015 mol/kg in MgCl2, 0.015 mol/kg in CaCl2, and 0.08 mol/kg in Na2CO3.
References
- Fukushi, K.; Munemoto, T.; Sakai, M.; Yagi, S. Monohydrocalcite: A Promising Remediation Material for Hazardous Anions. Sci. Technol. Adv. Mater. 2011, 12, 64702. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The Role of Mg in the Crystallization of Monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Taylor, G.F. The Occurrence of Monohydrocalcite in Two Small Lakes in the South-East of South Australia. Am. Mineral. 1975, 60, 690–697. [Google Scholar]
- Last, F.M.; Last, W.M.; Halden, N.M. Carbonate Microbialites and Hardgrounds from Manito Lake, an Alkaline, Hypersaline Lake in the Northern Great Plains of Canada. Sediment. Geol. 2010, 225, 34–49. [Google Scholar] [CrossRef]
- Fukushi, K.; Matsumiya, H. Control of Water Chemistry in Alkaline Lakes: Solubility of Monohydrocalcite and Amorphous Magnesium Carbonate in CaCl2–MgCl2–Na2CO3 Solutions. ACS Earth Space Chem. 2018, 2, 735–744. [Google Scholar] [CrossRef]
- Fukushi, K.; Sakai, M.; Munemoto, T.; Yokoyama, Y.; Takahashi, Y. Arsenate Sorption on Monohydrocalcite by Coprecipitation during Transformation to Aragonite. J. Hazard. Mater. 2016, 304, 110–117. [Google Scholar] [CrossRef]
- Munemoto, T.; Fukushi, K.; Kanzaki, Y.; Murakami, T. Redistribution of Pb during Transformation of Monohydrocalcite to Aragonite. Chem. Geol. 2014, 387, 133–143. [Google Scholar] [CrossRef]
- Yagi, S.; Fukushi, K. Removal of Phosphate from Solution by Adsorption and Precipitation of Calcium Phosphate onto Monohydrocalcite. J. Colloid Interface Sci. 2012, 384, 128–136. [Google Scholar] [CrossRef]
- Sakai, M.; Munemoto, T.; Fukushi, K. Arsenate Uptake by Monohydrocalcite. Dep. Bull. Pap. K-INET Kanazawa Univ. 2010, 66–68, In Japanese. Available online: https://kanazawa-u.repo.nii.ac.jp/?action=pages_view_main&active_action=repository_view_main_item_detail&item_id=29857&item_no=1&page_id=13&block_id=21 (accessed on 13 April 2020).
- Yagi, S.; Fukushi, K. Phosphate Sorption on Monohydrocalcite. J. Mineral. Petrol. Sci. 2011, 106, 109–114. [Google Scholar] [CrossRef]
- Nishiyama, R.; Munemoto, T.; Fukushi, K. Formation Condition of Monohydrocalcite from CaCl2-MgCl2-Na2CO3 Solutions. Geochim. Cosmochim. Acta 2013, 100, 217–231. [Google Scholar] [CrossRef]
- Blue, C.R.; Giuffre, A.; Mergelsberg, S.; Han, N.; De Yoreo, J.J.; Dove, P.M. Chemical and Physical Controls on the Transformation of Amorphous Calcium Carbonate into Crystalline CaCO3 polymorphs. Geochim. Cosmochim. Acta 2017, 196, 179–196. [Google Scholar] [CrossRef]
- Kimura, T.; Koga, N. Monohydrocalcite in Comparison with Hydrated Amorphous Calcium Carbonate: Precipitation Condition and Thermal Behavior. Cryst. Growth Des. 2011, 11, 3877–3884. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yao, Q.-Z.; Zhou, G.-T.; Fu, S.-Q. Transformation of Amorphous Calcium Carbonate into Monohydrocalcite in Aqueous Solution: A Biomimetic Mineralization Study. Eur. J. Mineral. 2015, 27, 717–729. [Google Scholar] [CrossRef]
- Fukushi, K.; Suzuki, Y.; Kawano, J.; Ohno, T.; Ogawa, M.; Yaji, T.; Takahashi, Y. Speciation of Magnesium in Monohydrocalcite: XANES, Ab Initio and Geochemical Modeling. Geochim. Cosmochim. Acta 2017, 213, 457–474. [Google Scholar] [CrossRef]
- Lin, C.Y.; Turchyn, A.V.; Steiner, Z.; Bots, P.; Lampronti, G.I.; Tosca, N.J. The Role of Microbial Sulfate Reduction in Calcium Carbonate Polymorph Selection. Geochim. Cosmochim. Acta 2018, 237, 184–204. [Google Scholar] [CrossRef]
- Morse, J.W.; Bender, M.L. Partition coefficients in calcite: Examination of factors influencing the validity of experimental results and their application to natural systems. Chem. Geol. 1990, 82, 265–277. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Xu, X.; Pan, H.; Tang, R. Transformation of Amorphous Calcium Carbonate into Aragonite. J. Cryst. Growth 2012, 343, 62–67. [Google Scholar] [CrossRef]
- Harrison, A.L.; Mavromatis, V.; Oelker, E.H.; Bénézeth, P. Solubility of the hydrated Mg-carbonates nesquehonite and dypingite from 5 to 35 °C: Implications for CO2 storage and the relative stability of Mg-carbonates. Chem. Geol. 2019, 504, 123–135. [Google Scholar] [CrossRef]
- Munemoto, T.; Fukushi, K. Transformation Kinetics of Monohydrocalcite to Aragonite in Aqueous Solutions. J. Mineral. Petrol. Sci. 2008, 103, 345–349. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Röntgen. Nachr. Ges. Wiss. Göttingen. 1918, 2, 96–100. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).