Abstract
A topological descriptor is a numerical parameter that describes a chemical structure using the related molecular graph. Topological descriptors have significance in mathematical chemistry, particularly for studying QSPR and QSAR. In addition, if a topological descriptor has a reciprocal link with a molecular attribute, it is referred to as a topological index. The use of topological indices can help to examine the physicochemical features of chemical compounds because they encode certain attributes of a molecule. The Randić index is a molecular structure descriptor that has several applications in chemistry and medicine. In this paper, we introduce a new version of the Randić index to the inclusion of the intermolecular forces between bonds with atoms, referred to as an entire Harmonic index (EHI), and we present the entire Harmonic polynomial (EHP) of a graph. Specific formulas have been obtained for certain graph classes, and graph operations have been obtained. Bounds and some important results have been found. Furthermore, we demonstrate that the correlation coefficients for the new index lie between and 1. In the context of enthalpy of formation and -electronic energy, the acquired values are significantly higher than those observed for the Harmonic index and the Randić index.
1. Introduction
We restrict our attention to finite, undirected, and simple graphs, and we refer to such a graph as , where V is the vertex set, and E is the edge set. The symbols , , , and denote a path, cycle, star, and complete graph of order n, respectively. For any element on the graph, either vertex or edge, the degree of the element x is the number of edges joining to x; in particular, the degree of the edge is the sum of the degrees of the vertices u and v minus two. Readers seeking in-depth explanations of the terms or notations not elaborated upon here are directed to [1].
A topological descriptor is a numerical representation of a chemical structure using the molecular graph. When this descriptor also correlates with a molecular property, it is referred to as a topological index. These indices are used to gain insights into the physicochemical properties of chemical compounds. The significance of these indices lies in their ability to capture multiple properties of a molecule in a single numerical value. As a result, numerous topological indices have been developed and analyzed. There is a rich history of topological indices. The relationship between graph properties and chemical characteristics has been explored for decades through the development of diverse topological indices. The pioneering work by Wiener (1947) [2] established the Wiener index, which quantifies the total distance between all pairs of vertices in a graph, and showed its correlation with the boiling point of paraffins. This marked the foundation for distance-based indices. Building on this, Gutman and Trinajstić (1972) [3] introduced the first and second Zagreb indices, based on vertex degrees, to relate to the -electron energy of molecules. These pioneering works inspired a surge in the development of topological indices for diverse chemical applications. Within distance-based indices, degree-based indices have gained notable relevance (cf. [4,5]). For a comprehensive overview of degree and distance-based indices, readers can refer to [6,7,8]. More recently, Ali and Trinajstić (2018) [9] revisited the first, second, and modified first Zagreb connection indices. Subsequently, the connection distance and degree connection indices were investigated in [10]. The value of connection number-based indices was further solidified by Javaid et al. (2014) [11], who demonstrated their strong correlation with various thermodynamic properties. In order to examine the many chemical characteristics of molecular networks (structures), these findings encouraged other mathematicians and chemists to create new topological indices. Extensive research has delved into numerous aspects of the Zagreb indices, including their bounds, extremal graphs, and connections to other graph invariants. For in-depth studies, refer to [12,13,14,15,16,17,18,19,20,21,22,23,24]. Alwardi et al. introduced and studied some versions of entire Zagreb indices of graphs, see [25,26,27]. Some bounds for the first and second entire Zagreb indices were introduced in [28]. The generalization of entire topological indices was introduced very recently in [29]. The Randić index, a versatile topological descriptor [30], developed by Randić in 1975, captures the branching structure of carbon skeletons and exhibits correlations with numerous chemical properties. Initially termed the “branching index” and later the “molecular connectivity index”, it remains a prominent tool in diverse chemical assessments and was defined as:
The Harmonic index, an alternative to the Randić index [31], is defined for a graph G as:
The sum-connectivity index of a graph G [32], denoted by , is defined as
In the same way, in [33], the Harmonic polynomial is defined as
Motivated by the works [25,26,27,29,30,31,32] and the large applications of these topological indices, here, we introduce the entire Harmonic index of the graph along with the entire Harmonic polynomial.
Definition 1.
Consider a simple graph G with vertices V and edges E. We define a set B(G) containing pairs of elements such that x and y are adjacent or incident. Then, the entire Harmonic index is defined as,
Additionally, the entire Harmonic polynomial can be defined as
Precise formulae of this index along with the associated polynomial for significant graph families are derived, and some important properties and relations are established. The motivation for developing this new index is that the existing Harmonic index is limited in its ability to capture the interactions (forces) between edges and vertices, in addition to the interactions between vertices alone.
2. The Informative Power for Chemical Modeling
The field of mathematical chemistry utilizes math and chemistry to analyze chemical reactions and properties. In their work ([34]), Randić and Trinajstić highlight the potential of relating theoretical indices to experimental properties of benchmark datasets to assess the chemical significance of graph invariants. Following this approach, this section explores the applicability of the entire Harmonic index to elucidating structural features of molecules through the quantitative structure–property relationship (QSPR) methodology. Specifically, we focus on evaluating the effectiveness of the entire Harmonic index as a descriptor for QSPR modeling of various physicochemical properties across diverse molecules. The choice of benzenoid hydrocarbons, with their well-defined structures, allows us to represent both cyclic and acyclic chemical systems for this investigation. So, we examine the significance of the entire Harmonic index for predicting for key properties like the total -electronic energy , enthalpy of formation , and boiling points (Bp) of 16 lower benzenoid hydrocarbons, see Figure 1.
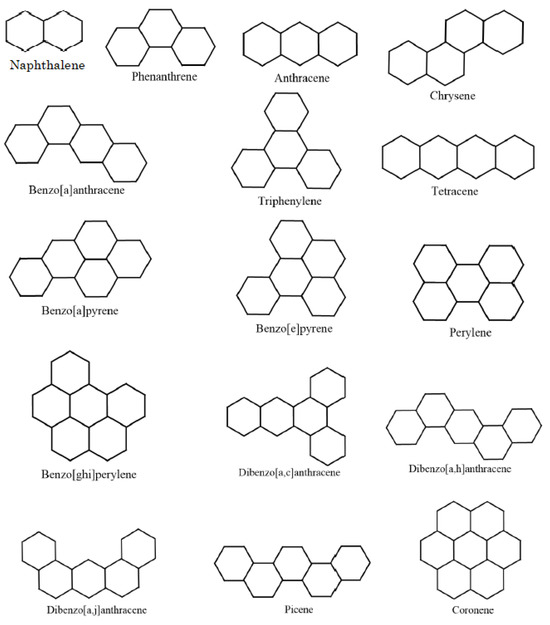
Figure 1.
The 16 lower benzenoid hydrocarbons.
The experimental data of the total -electronic energy , enthalpy of formation , and boiling points (Bp) are taken from NIST databases [35] and retrieved from [36].
Analysis of the data in Table 1 highlights the emergence of the entire Harmonic index as a promising descriptor for diverse physicochemical properties. Constructed linear models in SPSS demonstrate statistically significant correlations with the boiling point , enthalpy of formation , and, notably, a perfect correlation with total -electronic energy , suggesting exceptional predictive potential for this property. Furthermore, Table 2 reveals the consistent advantage of the entire Harmonic index over the traditional Harmonic index, underlining its significance as a novel topological index for modeling chemical and physical properties.

Table 1.
Experimental total -electronic energy (), enthalpy of formation (EF), boiling points (BP) and theoretical indices for benzenoid hydrocarbons.

Table 2.
Correlations of the entire Harmonic index and key descriptors with diverse properties of benzenoid hydrocarbons.
Figure 2 visually portrays the linear relationships between the entire Harmonic index and three critical properties of benzenoid hydrocarbons: the boiling point (BP), enthalpy of formation (EF), and -electronic energy.
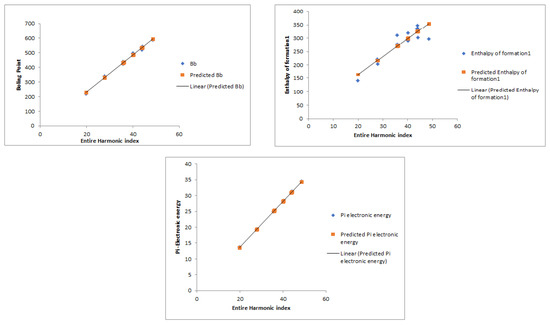
Figure 2.
Linear fitting of the BP, EF, and electronic energy predicted by the entire Harmonic index.
The linear QSPR model yielded the following predictive regression equations, linking the entire Harmonic index (EH) to critical physicochemical properties:
Boiling point (Bp):
Enthalpy of formation (EF):
-electronic energy (E):
3. Mathematical Results on Some Significant Families of Graphs
In this section, we obtain precise formulae for the entire Harmonic index along with the associated polynomial for significant graph families of standard graphs.
Observation 1.
Let G be a graph with q edges and a first Zagreb index . Then,
- 1.
- 2.
- .
- 3.
The line graph captures the relationships between the edges of the original graph. Each vertex in represents an edge in the original graph, and two edges in L(G) are connected if the corresponding edges in the original graph are “neighbors” (meaning they share a common vertex) [1].
Proposition 1.
For a regular graph G of n vertices with degree k greater than 2,
- i.
- ii.
Proof.
G. a regular graph, contains n vertices and each vertex has a degree equal to or more than 2. Hence, it contains edges, and the number of edges on is
Hence,
In the same procedure, we obtain
□
Corollary 1.
For the complete graph and the cycle , where ,
- 1.
- .
- 2.
- .
- 3.
- .
- 4.
- .
Proposition 2.
For any path , with vertices,
- 1.
- .
- 2.
- .
Proof.
In a path with vertices and edges, the degree of each vertex and edge is 2, except for the endpoints. Both the first and last vertices, and , have degree 1, as they only connect to one other vertex. Similarly, the edges connecting them to other vertices, and , also have degree 1. Therefore,
By using the same data about the degrees of the vertices and edges, we have achieved part (ii).
□
Proposition 3.
For any complete bipartite graph , .
Proof.
Let the vertices of be labeled as . Then,
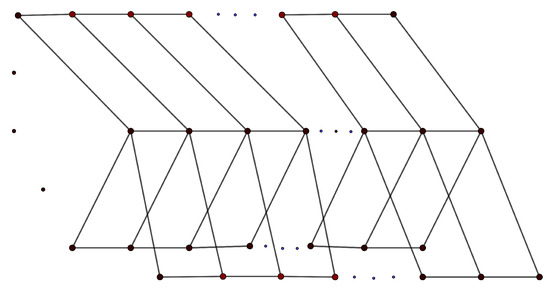
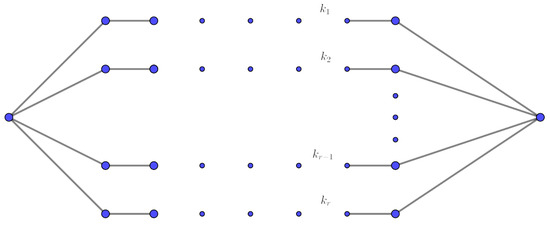
A graph that has r distinct paths connecting two specific vertices is called an -bridge graph. We denote it as , where represent the lengths of each of the r paths. The -bridge is a generalization of theta graphs, see Figure 3. □

Figure 3.
-bridge graph.
Lemma 1.
For any multi-bridge graph ,
Proof.
For a multi-bridge graph , we have
□
Lemma 2.
For the multi-bridge graph , we have
Proof.
By the definition of the edge version of the Harmonic index, we obtain
□
Theorem 1.
Let be the multi-bridge graph. Then,
Proof.
Since , applying Observation 1, we obtain
□
It is easy to see that
So using Lemmas 1 and 2, we obtain
To prove some results, we need some specific sets defined for a graph , as follows:
- , if there is an edge between u and v.
- , if edges e and f share both endpoints and have degrees a and b, respectively.
- , if vertex v and edge f are incident and have degrees a and b, respectively.
In the realm of hydrocarbons, pentacene stands out for its unique structure. Comprised of five fused benzene rings, this vibrant purple powder exhibits semiconducting properties. However, its beauty is fleeting, as exposure to light and air causes pentacene to gradually degrade. The linear form, known as -pentacene, is pictured in Figure 4.

Figure 4.
Linear [n]-Pentacene.
Lemma 3.
For any linear -Pentacene graph G,
Proof.
Let G be the -Pentacene graph, from the definition of the -Pentacene graph G; clearly, it has vertices and edges, and the degrees of the vertices are either two or four. We can see that , , and ; so,
□
Lemma 4.
For any linear -Pentacene graph G,
Proof.
Let G be the -Pentacene graph, from the definition of the -Pentacene graph G; clearly, it has edges, and the degrees of the edges are either two, three, or four. By simple counting, we obtain , , , , and ; so,
Therefore,
□
Theorem 2.
For any linear -Pentacene graph G,
Proof.
Let G be any -Pentacene graph, by using Observation 1, we obtain
To obtain , we have , , , and . Then,
Then by Lemmas 3 and 4, and Equation (1), we obtain
□
Proposition 4.
Let be a wheel graph that contains vertices. Then,
Proof.
For the graph , we can see that , , , , , and . Then, by Observation 1,
□
4. Entire Harmonic Index under Some Common Graph Operations
In this section, we focus on determining the Harmonic index for a variety of graph operations applied to common graph types.
We present the Cartesian product of two graphs , where , , , and are the vertex sets and edge set of and , respectively. The resulting graph has the vertex set , and two vertices and are adjacent, if and only if either ( and ) or ( and ) [1].
Lemma 5.
For any positive integers s and t with , if G is the Cartesian product between the paths and , then
Proof.
Let be any positive integers such that and . Let ; so, we have , and . Then,
Hence, . □
Lemma 6.
If , and , then
Proof.
Let , and . Then, we can see that , , , , , , , and , and then,
Hence,
□
Theorem 3.
For any positive integers s and t with , if G is the Cartesian product between the paths and , then
Proof.
By applying Observation 1, we obtain
To obtain , we have , , , , , and . Then,
Building upon Lemmas 5 and 6, and applying Equation (2), we obtain
□
Lemma 7.
Let S and t be positive integers such that and ; if G is the Cartesian product between the path and the cycle , then
Proof.
Suppose that S and t are positive integers such that and , and G is the Cartesian product between the path and the cycle ; then, it is easy to see that , , and , and then, . Therefore,
□
Lemma 8.
Let S and t be positive integers such that and ; if G is the Cartesian product between the path and the cycle , then
Proof.
Let and ; if , it is easy to see that , , , and . Therefore,
Hence,
□
Theorem 4.
Let S and t be positive integers such that and ; if G is the Cartesian product between the path and the cycle , then
Proof.
Let be any positive integers such that and , if ; then, using Observation 1, we have
To obtain , let be the set of all subsets where v is a vertex in G, e is an edge in G, and v is incident with e, such that and ; then, we have, , , , and , and then,
By virtue of Lemmas 7 and 8 and invoking Equation (3), we obtain
□
Lemma 9.
Let G be the Stacked Book Graph , where and . Then,
Proof.
Let ; then, it is easy to see that , , , , , and , and then,
□
Lemma 10.
Let G be the Stacked Book Graph , where and . Then,
Proof.
Let ; then, we can obtain , , , , , , , , , , , and , and then
□
Theorem 5.
Let G be the Stacked Book Graph , where and . Then,
5. Relationships between the Entire Harmonic Index and Other Indices
Proposition 5.
For any nontrivial connected graph G, . The equality holds, if and only if G is a cycle .
Proof.
Let G be any nontrivial connected graph, and let be a simple graph. Then, for any element in , obviously, . Then,
If the graph is a cycle , then, clearly, . Suppose that . Then, , which implies that , and the only nontrivial connected graph with edges and vertices all of the same degree is the cycle graph . □
Theorem 6.
For the graph G, which has m edges, we obtain
where , and is the first Zagreb index of G.
Proof.
For the graph G with vertices and edges, we obtain
Leveraging the Cauchy–Schwarz inequality, we can deduce
□
6. Conclusions
In this article, we introduced a new version of the Randić index referred to as the entire Harmonic index, along with its associated polynomial. This index was conceptualized, and its discriminating power was investigated with regard to the predictability of the boiling point, enthalpy of formation, and electronic energy of the chemical substances; the correlation coefficients between 0.909 and 1 were acquired, higher than the ones received in the case of the Harmonic index and the Randić index in terms of the enthalpy of formation and -electronic energy. Furthermore, it was higher than the one achieved in the case of the Harmonic index for the boiling point. Specific formulae for some families of graphs and graph operations were achieved; bounds and some important results were found.
Finally, as this represents the initial introduction of the entire Harmonic Index (EHI), several intriguing open problems and potential research avenues require further exploration. These include:
- More mathematical study for this new index to discover its relations with the other graph parameters;
- Investigation of the broader applicability of this new index across diverse network types, including social networks, biological networks, and technological networks;
- Exploration of the potential of this index in various domains, such as drug discovery in medicine and material design in engineering.
Author Contributions
Conceptualization, A.S. and S.H.A.; methodology, A.S.; validation, A.S. and S.H.A.; formal analysis, A.S.; investigation, A.S.; resources, S.H.A.; data curation, S.H.A.; writing—original draft A.S.; preparation, A.S.; writing—review and editing, A.S. and S.H.A.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. and S.H.A. The authorship team wholeheartedly endorses the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under grant No. (UJ-22-DR-11).
Data Availability Statement
The article contains the data that supported the study’s findings.
Acknowledgments
This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under grant No. (UJ-22-DR-11). The authors, therefore, acknowledge with thanks the University of Jeddah for its technical and financial support.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Harary, F. Graph Theory; Addison-Wesley, Reading Mass: Boston, MA, USA, 1969. [Google Scholar]
- Wiener, H. Structural determination of paraffin boiling points. J. Am. Chem. Soc. 1947, 69, 17–20. [Google Scholar] [CrossRef]
- Gutman, I.; Trinajstic, N. Graph theory and molecular orbitals, Total π-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 1972, 17, 535–538. [Google Scholar] [CrossRef]
- Dobrynin, A.A.; Kochetova, A.A. Degree distance of a graph: A degree analog of the Wiener index. J. Chem. Inf. Comput. Sci. 1994, 34, 1082–1086. [Google Scholar] [CrossRef]
- Gutman, I. Selected properties of the Schultz molecular topological index. J. Chem. Inf. Comput. Sci. 1994, 34, 1087–1089. [Google Scholar] [CrossRef]
- Das, K.C.; Gutman, I. On Wiener and multiplicative Wiener indices of graphs. Discret. Appl. Math. 2016, 206, 9–14. [Google Scholar] [CrossRef]
- Das, K.C.; Gutman, I.; Nadjafi-Arani, M.J. Relations between distance-based and degree-based topological indices. Appl. Math. Comput. 2015, 270, 142–147. [Google Scholar] [CrossRef]
- Gutman, I. Degree-based topological indices. Croat. Chem. Acta 2013, 86, 351–361. [Google Scholar] [CrossRef]
- Ali, A.; Trinajstić, N. A novel/old modification of the first Zagreb index. Mol. Inform. 2018, 37, 1800008. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Ali, U.; Siddiqui, K. Novel connection based Zagreb indices of several wheel-related graphs. Comput. J. Comb. Math. 2021, 1, 1–28. [Google Scholar]
- Javaid, M.; Siddique, M.K.; Bonyah, E. Computing gutman connection index of thorn graphs. J. Math. 2021, 2021, 2289514. [Google Scholar] [CrossRef]
- Deng, H.; Sarala, D.; Ayyaswamy, S.K.; Balachandran, S. The Zagreb indices of four operations on graphs. Appl. Math. Comput. 2016, 275, 422–431. [Google Scholar] [CrossRef]
- Ahmed, H.; Saleh, A.; Ismail, R.; Alameri, A. Computational analysis for eccentric neighborhood Zagreb indices and their significance. Heliyon 2023, 9, e17998. [Google Scholar] [CrossRef] [PubMed]
- Wazzan, S.; Saleh, A. New Versions of Locating Indices and Their Significance in Predicting the Physicochemical Properties of Benzenoid Hydrocarbons. Symmetry 2022, 14, 1022. [Google Scholar] [CrossRef]
- da Fonseca, C.M.; Stevanovic, D. Further properties of the second Zagreb index. MATCH Commun. Math. Comput. Chem. 2014, 72, 655–668. [Google Scholar]
- Gutman, I.; Das, K.C. The first Zagreb index 30 years after. MATCH Commun. Math. Comput. Chem. 2004, 50, 83–92. [Google Scholar]
- Khalifeh, M.H.; Yousefi-Azari, H.; Ashrafi, A.R. The first and second Zagreb indices of some graph operations. Discret. Appl. Math. 2009, 157, 804–811. [Google Scholar] [CrossRef]
- Sarala, D.; Deng, H.; Ayyaswamy, S.K.; Balachandran, S. The Zagreb indices of graphs based on four new operations related to the lexicographic product. Appl. Math. Comput. 2017, 309, 156–169. [Google Scholar] [CrossRef]
- Sarkar, P.; De, N.; Pal, A. The Zagreb indices of graphs based on new operations related to the join of graphs. J. Int. Math. Virtual Inst. 2017, 7, 181–209. [Google Scholar]
- Ullah, A.; Zaman, S.; Hamraz, A.; Saeedi, G. Network-based modeling of the molecular topology of fuchsine acid dye with respect to some irregular molecular descriptors. J. Chem. 2022, 2022, 8131276. [Google Scholar] [CrossRef]
- Hakeem, A.; Ullah, A.; Zaman, S. Computation of some important degree-based topological indices for γ-graphyne and Zigzag graphyne nanoribbon. Mol. Phys. 2023, 121, e2211403. [Google Scholar] [CrossRef]
- Ullah, A.; Zaman, S.; Hamraz, A. Zagreb connection topological descriptors and structural property of the triangular chain structures. Phys. Scr. 2023, 98, 025009. [Google Scholar] [CrossRef]
- Zhou, B.; Gutman, I. Further properties of Zagreb indices. MATCH Commun. Math. Comput. Chem. 2005, 54, 233–239. [Google Scholar]
- Alqesmah, A.; Alwardi, A.; Rangarajan, R. On the Distance Eccentricity Zagreb Indices of Graphs. Int. J. Math. Combin. 2017, 4, 110–120. [Google Scholar]
- Alwardi, A.; Alqesmah, A.; Rangarajan, R.; Cangul, I.N. Entire Zagreb indices of graphs. Discret. Math. Algorithms Appl. 2018, 10, 1850037. [Google Scholar] [CrossRef]
- Saleh, A.; Aqeel, A.; Cangul, I.N. On the entire ABC index of graphs. Proc. Jangjeon Math. Soc. 2020, 23, 39–51. [Google Scholar]
- Saleh, A.; Cangul, I.N. On the entire Randic index of graphs. Adv. Appl. Math. Sci. 2021, 20, 1559–1569. [Google Scholar]
- Ghalav, A.; Ashrafi, A.R. Bounds on the entire Zagreb indices of graphs. MATCH Commun. Math. Comput. Chem. 2019, 81, 371–381. [Google Scholar]
- Gutman, I. On vertex and edge degree-based topological indices. Vojnoteh. Glas. Mil. Tech. Cour. 2023, 71, 855–863. [Google Scholar] [CrossRef]
- Randic, M. Characterization of molecular branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar] [CrossRef]
- Zhong, L. The harmonic index for graphs. Appl. Math. Lett. 2012, 25, 561–566. [Google Scholar] [CrossRef]
- Zhou, B.; Nenad, T. On a novel connectivity index. J. Math. Chem. 2009, 46, 1252–1270. [Google Scholar] [CrossRef]
- Iranmanesh, M.A.; Mahboubeh, S. On the Harmonic Index and Harmonic Polynomial of Caterpillars with Diameter Four. Iran. J. Math. Chem. 2015, 5, 35–43. [Google Scholar]
- Randić, M.; Nenad, T. In search for graph invariants of chemical interes. J. Mol. Struct. 1993, 300, 551–571. [Google Scholar] [CrossRef]
- NIST Standard Reference Database. Available online: http://webbook.nist.gov/chemistry/ (accessed on 18 November 2023).
- Hayat, S.; Arif, A.; Zada, L.; Khan, A.; Zhong, Y. Mathematical Properties of a Novel Graph-Theoretic Irregularity Index with Potential Applicability in QSPR Modeling. Mathematics 2022, 10, 4377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).