Abstract
Cerebral sulcal infolding exhibits unique species-related lateralization patterns. The present investigation aimed to characterize the asymmetric patterns of sulcal infolding in cynomolgus monkeys and their sexual dimorphism. Three-dimensional magnetic resonance (MR) images were acquired at 7-Tesla from the fixed brains of adult male (n = 5) and female (n = 5) monkeys. The gyrification index (GI) was estimated on MR images throughout the cerebral cortex (global-GI) or in the representative primary sulci (sulcal-GI). The global-GI did not differ between the sexes when the ipsilateral sides were compared. Although there was no sex difference in the sulcal-GI of the ipsilateral sides of any primary sulci, a significant right bias of the sulcal-GI was noted in the inferior rams of the arcuate sulcus and circular sulcus in males but not in females. Secondary sulcal emergence was examined to assess sulcal infolding asymmetry at the individual and population levels. Nonbiased asymmetric emergence was noted in the posterior supraprincipal dimple in both sexes and the rostral sulcus in females. Notably, the emergence of the superior postcentral dimple was significantly right-lateralized in females. The findings revealed right-biased sulcal infolding in male and female cynomolgus monkeys, although the lateralized cortical regions differed between the sexes.
1. Introduction
The cerebral cortex is convoluted to various degrees in many species of mammals [1]. In particular, the complexity of cortical convolution implicates asymmetry of sulcal infolding in higher-order primates, including humans, apes, and old-world monkeys [2]. Although cerebral sulci begin to emerge at midgestation in primates, sulcal morphology changes during postnatal maturation to form asymmetric patterns [3]. Several reports have documented the involvement of sulcal asymmetry in cerebral function in humans, including rightward asymmetry of the superior temporal sulcus and fMRI-defined right-lateralized voice-selective responses [4,5], leftward asymmetry of the central sulcus and right-handedness [6,7], and leftward asymmetry of the paracingulate sulcus and cognition [8,9].
Several approaches, such as the sulcal length, depth, and surface area, have been used to examine the asymmetry of sulcal infolding [10,11]. Our previous study using cynomolgus monkeys revealed that the arcuate sulcus length was right-lateralized in males but symmetrical in females when measuring the sulcal length by placing a cotton thread directly on the entire length of the cerebral sulci (the “cotton thread” method) [10]. However, this method does not provide any information other than sulcal length for evaluating sulcal infolding, that is, the sulcal surface areas and depths. The degree of infolding of specific cerebral sulci can be assessed using the sulcal-gyrification index (sulcal-GI) [12], which is obtained by modifying the gyrification index that quantitatively assesses the convolution throughout the cerebral cortex designed originally by Zilles et al. (1988) [13]. The sulcal-GI fluctuates with changes in the morphology of specific sulci, that is, the length, depth, width, and surface areas. The sulcal-GI is further altered by cortical growth and is correlated highly with cortical expansion, particularly in primary sulci infolded in multimodal association cortices [12]. Here, we applied the sulcal-GI to comprehensively assess asymmetric patterns of cerebral sulcal infolding and characterize the asymmetry in cerebral surface morphology and its sexual dimorphism in adult cynomolgus monkeys using magnetic resonance imaging (MRI)-based morphometry.
2. Materials and Methods
2.1. Samples
This study used three-dimensional (3D) MR images, which were obtained from the fixed brains of sexually mature male (n = 5) and female (n = 5) cynomolgus monkeys (Macaca fascicularis) at 3.5–6.6 years of age [14]. These brain samples were used in our previous study [10], which was approved by the Institutional Animal Care and Use Committee of Shin Nippon Biomedical Laboratories (approval code: B999-178). The samples were fixed using intracardiac perfusion with 0.9% NaCl followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) under deep anesthesia with an intravenous injection of sodium pentobarbital (26 mg/kg; Tokyo Chemical Industry, Tokyo, Japan) and immersed in the same fixative [10].
Ex vivo MRI scans were performed using a horizontal 7.0 T scanner (BioSpec 70/30 USR; Bruker Biospin, Ettlingen, Germany) with an 86 mm volume coil. A transmit/receive quadrature 86 mm volume coil was used for image acquisition. Samples were placed horizontally on an MRI cradle. First, localizer scans were used for the accurate positioning of the brain samples inside the magnet. The sample was positioned to have the center of the brainstem approximately located at the magnet’s isocenter. This was used to acquire 3D short TR/TE (typical T1-weighted parameter setting) MR images covering the entire fixed brain using rapid acquisition with a relaxation enhancement (RARE) sequence and the following parameters: repetition time (TR) = 400 ms; echo time (TE) = 6 ms (effective TE = 19.2 ms); RARE factor = 4; field of view (FOV) = 72 × 64 × 47.5 mm3; acquisition matrix = 288 × 256 × 192; voxel size = 250 × 250 × 250 µm3; number of acquisitions (NEX) = 4; and total scan time = 5 h, 27 min, and 36 s. Short TR/TE images were directly reconstructed at the scan workstation by using Paravision 7.0 (Bruker Biospin, Ettlingen, Germany) and subsequently imported to SliceOmatic software version 4.3 (TomoVision, Montreal, QC, Canada) for visualization and processing.
2.2. MRI-Based Morphometry
All 3D MR images were used to measure the fronto-occipital (FO) length, volume, and surface area of the cerebral cortex and to calculate the global-gyrification index (global-GI) and sulcal-GI. According to our previous study [12], the cortical gray matter of the left and right cerebral hemispheres was segmented semiautomatically on MR images at the coronal (axial) plane using the “Morpho” tool of the SliceOmatic software version 4.3 (TomoVision, Montreal, QC, Canada) based on image contrast. The FO length (length of the cerebral cortex from the frontal pole through the occipital pole) was measured on 3D-rendered images, which were reconstructed based on the segmented images using the 3D-rendering module of the same software. The volume (mm3) was calculated by multiplying the sum of the segmented areas by the slice thickness (250 μm). The cortical surface area in the sulcal grooves was computed from 3D MR images using SliceOmatic software 4.3 (TomoVision). The surface areas of the 26 primary sulci, shown in Figure 1 and Figure S1, were also calculated. Furthermore, the cerebral sulci not involved in gyral demarcations were defined as secondary sulci (see Figure S1). The surface areas of the 12 secondary sulci were then summed up.
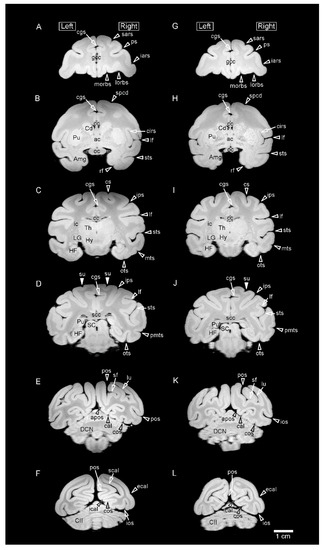
Figure 1.
Representative coronal (axial) MR images (using a RARE sequence with a short TR and the minimum TE settings) obtained from fixed brains of sexually mature cynomolgus monkeys. (A–F) Coronal MR images of the male brain at rostral to caudal levels. (G–L) Coronal MR images of the female brain at rostral to caudal levels. MR images on the same lines are identical levels seen at: (A,G) the rostral end of the genu of corpus callosum (gcc); (B,H) the anterior commissure; (C,I) the lateral geniculate nucleus (LG) of the thalamus; (D,J) the splenium of corpus callosum (scc); (E,K) the deep cerebellar nucleus (DCN); and (F,L) the crus II of ansiform lobule (CII) of cerebellum. Amg, amygdala; apos, anterior parietooccipital sulcus; cal, calcarine sulcus; cgs, cingulate sulcus; cc, corpus callosum; Cd, caudate nucleus; cirs, circular sulcus; cos, collateral sulcus; cs, central sulcus; ecal, external calcarine sulcus; HF, hippocampal formation; Hy, hypothalamus; iars, inferior ram of arcuate sulcus; ic, internal capsule; ical, inferior calcarine sulcus; ios, inferior occipital sulcus; ips, intraparietal sulcus; lf, lateral fissure; lorb, lateral orbital sulcus; lu, lunate sulcus; morb, medial orbital sulcus; mts, middle temporal sulcus; oc, optic chiasma; ots, occipitotemporal sulcus; pmts, posterior middle temporal sulcus; pos, parietooccipital sulcus; ps, principal sulcus; Pu, putamen; Pul, pulvinar of the thalamus; rf, rhinal fissure; sars, superior ram of arcuate sulcus; SC, superior colliculus; scal, superior calcarine sulcus; sf, simian fossa; spcd, superior precentral dimple; sts, superior temporal sulcus; su, superior postcentral dimple; Th, thalamus.
The degree of sulcal infolding was estimated throughout the cerebral cortex (global-GI) or in 26 primary sulci (Figure S1) (sulcal-GI) according to our previous procedure [12]. The global-GI and sulcal-GI were calculated using all MR images in the coronal plane by the proportion of the outer contours of the cerebral cortex to the sum of the sulcal perimeters and the proportion to the perimeters of the specific primary sulci, respectively. Furthermore, the sulcal-GIs of the 12 secondary sulci (Figure S1) were calculated and summed.
2.3. Asymmetric-Quotient Analysis
The asymmetry quotient (AQ) was estimated using the formula ((R − L)/{(R + L) × 0.5}) to assess the leftward or rightward bias of the cortical volume, FO length, cortical surface area, global-GI, and all sulcal-GI examined. The direction of asymmetry was indicated by AQ values: positive value = rightward bias and negative value = leftward bias [15].
2.4. Incidence of Secondary Sulci
The incidence of secondary sulci was separately calculated for the left and right cerebral hemispheres. Furthermore, the percentage of individuals with secondary sulci appearing on either the left or the right side was calculated to assess the asymmetric emergence of secondary sulci at the individual level.
2.5. Statistical Analysis
The left/right-side differences in the cortical volumes, cortical surface area, FO length of the cerebral hemispheres, and global-GI were compared using a paired sample t-test. Sex differences in these four measurements were statistically evaluated using a one-way analysis of variance (ANOVA), followed by a two-tailed Student’s t-test. We further evaluated the left/right side differences in the sulcal surface areas and sulcal-GIs of the 26 primary sulci and the summed values of the surface areas and sulcal-GIs of the secondary sulci using a paired sample t-test. Sex-associated changes in the ipsilateral sides of these sulcal-GIs were assessed using a repeated measures three-way ANOVA with sex as an intergroup factor and the cerebral sulci as an intragroup factor. Sexual differences were assessed on the ipsilateral side of each measurement using Scheffe’s test for post hoc testing, following simple main effects at p < 0.05. The AQ values of the sulcal surface areas and sulcal-GIs were analyzed using a one-sample t-test to determine any significant population-level asymmetry.
The incidence of secondary sulci on the left and right sides of the cerebral cortex was estimated using the chi-square test, and the percentage of individuals with secondary sulci appearing on either the left or the right side was evaluated using a one-sample t-test.
3. Results
3.1. MR Images and 3D-Rendered Images
Representative MR images of the brain from sexually mature male and female cynomolgus monkeys in the coronal plane are shown in Figure 1. An ex vivo high-field MRI using a RARE sequence with a short TR and minimum TE settings (T1W parameter setting) reduced the signal contrast in the white matter of the fixed brain in the paraformaldehyde solution. We further reconstructed the cerebral-cortical surface morphology by 3D-rendered images calculated using MR images (Figure 2). Both the MR and 3D-rendered images could distinguish the primary and secondary sulci (Figure 1 and Figure 2) that had been observed in gross anatomical examinations.
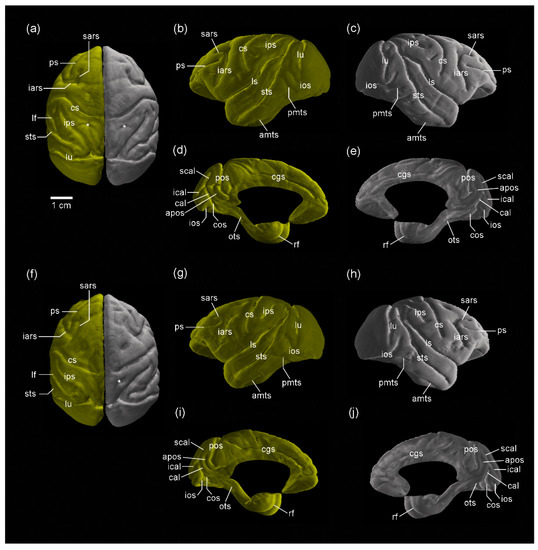
Figure 2.
Three-dimensional volume-rendered images of the cerebral cortex of sexually mature cynomolgus monkeys. (a–e) Dorsal view of the left and right hemispheres, lateral view of the left hemisphere, lateral view of the right hemisphere, medial view of the left hemisphere, and medial view of the right hemisphere of males. (f–j) Dorsal view of the left and right hemispheres, lateral view of the left hemisphere, lateral view of the right hemisphere, medial view of the left hemisphere, and medial view of the right hemisphere of females. The superior postcentral dimple (*) is seen in both the left and right hemispheres in males, amts, anterior middle temporal sulcus; apos, anterior parietooccipital sulcus; cal, calcarine sulcus; cgs, cingulate sulcus; cos, collateral sulcus; cs, central sulcus; iars, inferior ram of arcuate sulcus; ical, inferior calcarine sulcus; ios, inferior occipital sulcus; ips, intraparietal sulcus; lf, lateral fissure; lu, lunate sulcus; ots, occipitotemporal sulcus; pmts, posterior middle temporal sulcus; pos, parietooccipital sulcus; ps, principal sulcus; rf, rhinal fissure; sars, superior ram of arcuate sulcus; scal, superior calcarine sulcus; sts, superior temporal sulcus; *, superior postcentral dimple.
3.2. MRI-Based Morphometry
The left and right sides of the cortical volume, cortical surface area, FO length, and global-GI are shown in Table 1. There were no left/right-sided differences in these four measurements in either male or female cynomolgus monkeys. AQ analysis with a one-sample t-test also indicated no left/right-side bias at the population level (Table 1). When comparing the ipsilateral sides of these measurements between males and females, sexual differences were observed with a significantly greater FO length in males than in females but not in the cortical volume, cortical surface area, and global-GI (Table 1).

Table 1.
Left and right sides of the cortical volume, cortical surface area, fronto-occipital (FO) length of the cerebral hemispheres, global-gyrification index (global-GI), and asymmetry quotient (AQ) values of these four measurements in adult cynomolgus monkeys.
The surface areas were significantly right-lateralized in the circular sulcus and in the medial orbital sulcus in males. There was no left/right-side bias in the surface areas of the other primary sulci examined in males (Table 2). In contrast, the summed surface areas of the secondary sulci were significantly right-lateralized in females, although no primary sulci exhibited significant left/right-side differences in their surface areas (Table 2). A significant population level of asymmetry in the sulcal surface areas was revealed to be leftward in the medial orbital sulcus of males and rightward in the circular sulcus of males and the secondary sulci of females by AQ analysis with a one-sample t-test (Table 3). When comparing the ipsilateral sides of the sulcal surface areas between sexes, there was no sex difference in any primary sulci examined (Table 2). The summed areas of the secondary sulci did not differ between males and females (Table 2).

Table 2.
Sulcal surface areas of the left and right sides of the cerebral cortex in adult cynomolgus monkeys.

Table 3.
Asymmetry quotient (AQ) of the sulcal surface areas of the cerebral cortex in adult cynomolgus monkeys.
The sulcal-GIs were significantly right-lateralized in the circular sulcus and inferior ram of the arcuate sulcus in males. There was no left/right-side bias of the sulcal-GIs of other primary sulci and the summed GI of secondary sulci in males, even in the medial orbital sulcus, where the surface area was significantly left-lateralized (Table 4). In females, there was no left/right-side bias of the sulcal-GI of any primary sulci or the summed GI of secondary sulci (Table 4). AQ analysis with a one-sample t-test also indicated a population level of rightward asymmetry in the circular sulcus and inferior ram of the arcuate sulcus in males but not in females (Table 5). In addition, sex differences were not observed when comparing the ipsilateral sides of the sulcal-GIs and the summed GI of the secondary sulci (Table 4).

Table 4.
Sulcal-gyrification index (sulcal-GI) of the left and right sides of the cerebral cortex in adult cynomolgus monkeys.

Table 5.
Asymmetry quotient (AQ) of the sulcal-gyrification index (sulcal-GI) of the cerebral cortex in adult cynomolgus monkeys.
3.3. Incidence of Secondary Sulci
Small or shallow indentations on the cerebral surface that were not involved in gyral demarcations were defined as secondary sulci. Their emergence varied not only among individuals but also between the left/right hemispheres within individuals (Table 6). Significantly asymmetrical emergences were marked in the posterior supraprincipal dimple in both sexes and the rostral sulcus and superior postcentral dimple only in females (Table 6). Although the incidence of the rostral sulcus and superior postcentral dimple was high (80%) in males, their emergence was symmetrical (Table 6). The emergence of the posterior supraprincipal dimple and rostral sulcus was not biased toward either the left or right side. Notably, the superior postcentral dimple emerged significantly on the right side in females (Table 6).

Table 6.
Incidence of secondary sulci on the left and right sides of the cerebral cortex in adult cynomolgus monkeys.
4. Discussion
The present study revisited sulcal infolding asymmetry in the cerebral cortex of male and female cynomolgus monkeys using MRI-based morphometry. Our previous study revealed rightward lateralization of the length of the arcuate sulcus in males but not in females [10]. In the present study, the inferior ram of the arcuate sulcus was significantly right-lateralized only in males at the population level. The consistent results between our previous and present studies suggest that the sulcal length largely reflects the asymmetry of arcuate sulcus infolding in male cynomolgus monkeys. The rightward asymmetry of the arcuate sulcus length was distinct in male cynomolgus monkeys from adolescence to adulthood, following the completion of primary sulcogenesis [3]. The frontal lobe contained significantly greater levels of androgen receptors on the right side than on the left side in male rhesus monkeys, but this was inconsistent between the left and right sides in females [16]. Arcuate sulcus infolding may lateralize rightward under the influence of androgens in male cynomolgus monkeys.
The inferior ram of the arcuate sulcus in macaques is anatomically identical to the precentral sulcus in humans and the superior precentral sulcus in captive chimpanzees (Pan troglodytes) [1], which forms the posterior boundary of the dorsolateral and ventrolateral prefrontal cortex (dlPFC and vlPFC) [17]. The frontoparietal network, including the dlPFC, has functional connections associated with cognitive control to the contralateral sides of crus II of the ansiform lobule of the cerebellar hemisphere [18]. Contralateral functional connections between the cerebral association cortices and cerebellar hemispheres are known to implicate the complementary lateralized development of these two brain regions [19]. Therefore, right-lateralized infolding of the inferior ram of the arcuate sulcus in male cynomolgus monkeys may be involved in the significant leftward volume laterality of crus II obtained using the same MRI data in our previous study [14].
The present study further revealed a significant right-lateralized infolding of the circular sulcus in male, but not female, cynomolgus monkeys, which had not been demonstrated in our previous study that used the “cotton thread” method [10]. The insular cortex is depicted by the circular sulcus, and the right side of the anterior part is included in the temporoparietal junction with salience-related brain networks, together with the right dlPFC [20]. Therefore, right-lateralized infolding of the circular sulcus in male cynomolgus monkeys may be linked with the ipsilateralized folding of the inferior ram of the arcuate sulcus.
On the other hand, population-level leftward asymmetry of the surface area, rather than the sulcal-GI, was observed in the medial orbital sulcus of male cynomolgus monkeys. However, it is difficult to speculate on the functional and evolutionary implications of the left lateralization of this sulcus. The orbitofrontal cortex is convoluted with high variability in rhesus macaques and humans [21].
Another finding of the present study was the asymmetrical emergence of small or shallow indentations on the cerebral surface, defined as secondary sulci. The majority of the secondary sulci emerged asymmetrically, without left- or right-side biases. Nonbiased asymmetrical development was also found in the secondary sulci emerging on the cerebral cortex of common marmosets [2]. A notable finding of the present study was that the emergence of the superior postcentral dimple, identical to the postcentral sulcus in humans [1], was significantly right-lateralized in females but symmetrical in males. This dimple was observed in 80% of males bilaterally and in females on the right side, suggesting poor development of the left postcentral and supraparietal cortical regions in females. In addition, the central sulcus, superior temporal sulcus, and parietooccipital sulcus emerged as secondary sulci asymmetrically without left- or right-side biases in common marmosets [2]. These sulci are defined as the primary sulci after the split between new-world and old-world monkeys [2]. Therefore, the superior postcentral dimple in old-world monkeys may be infolded by the expansion of surrounding cortical regions in subsequent evolutionary processes and defined as the primary sulcus, named the “postcentral sulcus” after the split into higher-order primates such as apes and humans.
5. Conclusions
The present study quantitatively examined the asymmetrical development of cerebral sulcal infolding in cynomolgus monkeys using MRI-based morphometry and obtained consistent results with our previous study that measured the sulcal length on the cortical surface using the cotton-thread method [10]. On the other hand, no left/right-side bias development of primary sulcal infolding and/or cortical folding has been observed in carnivores [22]. These animals have been found to develop leftward torque asymmetry of the cerebellum [23,24], similar to cynomolgus monkeys [14]. The presence of contralateral cerebro-cerebellar connectivity, which is involved in the lateralized development of the cerebral cortex in primates, is unclear in carnivores [19]. The lateralized infolding of the primary sulci may result from the functional specification of the left/right cerebral hemispheres acquired in the course of primate evolution, with more remarkably developed characteristics compared to other mammalian species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym15061164/s1, Figure S1: Sulcal maps of cynomolgus monkeys.
Author Contributions
Formal analysis, K.S.; Funding acquisition, S.S.; Methodology, K.S. and S.S.; Supervision, K.S.; Validation, K.S. and S.S.; Writing—original draft, K.S.; Writing—review & editing, K.S. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was the result of using research equipment shared by the MEXT Project for promoting public utilization of advanced research infrastructure (program for supporting the construction of core facilities) (grant number JPMXS0450400021, JPMXS0450400022).
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zilles, K.; Palomero-Gallagher, N.; Amunts, K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013, 36, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K. Cerebral sulcal asymmetry in macaque monkeys. Symmetry 2020, 12, 1509. [Google Scholar] [CrossRef]
- Sakamoto, K.; Sawada, K.; Fukunishi, K.; Imai, N.; Sakata-Haga, H.; Fukui, Y. Postnatal change in sulcal length asymmetry in cerebrum of cynomolgus monkeys (Macaca fascicularis). Anat. Rec. (Hoboken) 2014, 297, 200–207. [Google Scholar] [CrossRef]
- Bonte, M.; Frost, M.A.; Rutten, S.; Ley, A.; Formisano, E.; Goebel, R. Development from childhood to adulthood increases morphological and functional inter-individual variability in the right superior temporal cortex. Neuroimage 2013, 83, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Bodin, C.; Takerkart, S.; Belin, P.; Coulon, O. Anatomo-functional correspondence in the superior temporal sulcus. Brain Struct. Funct. 2018, 223, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Foundas, A.L.; Hong, K.; Leonard, C.M.; Heilman, K.M. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry Neuropsychol. Behav. Neurol. 1998, 11, 65–71. [Google Scholar]
- Klöppel, S.; Mangin, J.F.; Vongerichten, A.; Frackowiak, R.S.; Siebner, H.R. Nurture versus nature: Long-term impact of forced right-handedness on structure of pericentral cortex and basal ganglia. J. Neurosci. 2010, 30, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Wood, S.J.; Whittle, S.; Fuller, J.; Adamson, C.; Saling, M.M.; Velakoulis, D.; Pantelis, C.; Yücel, M. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: Associations with cortical thickness, surface area, volume, and sulcal depth. Hum. Brain Mapp. 2008, 29, 222–236. [Google Scholar] [CrossRef]
- Amiez, C.; Wilson, C.R.E.; Procyk, E. Variations of cingulate sulcal organization and link with cognitive performance. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Imai, N.; Sawada, K.; Fukunishi, K.; Sakata-Haga, H.; Fukui, Y. Sexual dimorphism of sulcal length asymmetry in cerebrum of adult cynomolgus monkeys (Macaca fascicularis). Congenit. Anom. (Kyoto) 2011, 51, 161–166. [Google Scholar] [CrossRef]
- Bogart, S.L.; Mangin, J.F.; Schapiro, S.J.; Reamer, L.; Bennett, A.J.; Pierre, P.J.; Hopkins, W.D. Cortical sulci asymmetries in chimpanzees and macaques: A new look at an old idea. Neuroimage 2012, 61, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Fukunishi, K.; Kashima, M.; Imai, N.; Saito, S.; Aoki, I.; Fukui, Y. Regional difference in sulcal infolding progression correlated with cerebral cortical expansion in cynomolgus monkey fetuses. Congenit. Anom. (Kyoto) 2017, 57, 114–117. [Google Scholar] [CrossRef]
- Zilles, K.; Armstrong, E.; Schleicher, A.; Kretschmann, H.J. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. 1988, 179, 173–179. [Google Scholar] [CrossRef]
- Sawada, K.; Saito, S. Sex-related left-lateralized development of the crus II region of the ansiform lobule in cynomolgus monkeys. Symmetry 2022, 14, 1015. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Sholl, S.A.; Kim, K.L. Androgen receptors are dierentially distributed between right and left cerebral hemispheres of the fetal male rhesus monkey. Brain Res. 1990, 516, 122–126. [Google Scholar] [CrossRef]
- Miller, E.K.; Walls, J.D. The frefrontal cortex and executive brain functions. In Fundamental Neuroscience, 2nd ed.; Squire, L., Berg, D., Bloom, F.E., McConnell, S., Roberts, J.L., Spitzer, N.C., Zigmond, M.J., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 1353–1376. [Google Scholar]
- Sang, L.; Qin, W.; Liu, Y.; Han, W.; Zhang, Y.; Jiang, T.; Yu, C. Resting-state functional connectivity of the vermal and hemi-spheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 2012, 61, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Buckner, R.L.; Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J. Neurophysiol. 2013, 109, 46–57. [Google Scholar] [CrossRef]
- Kucyi, A.; Hodaie, M.; Davis, K.D. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J. Neurophysiol. 2012, 108, 3382–3392. [Google Scholar] [CrossRef]
- Chiavaras, M.M.; Petrides, M. Orbitofrontal sulci of the human and macaque monkey brain. J. Comp. Neurol. 2000, 19, 35–54. [Google Scholar] [CrossRef]
- Wosinski, M.; Schleicher, A.; Zilles, K. Quantitative analysis of gyrification of cerebral cortex in dogs. Neurobiology 1996, 4, 441–468. [Google Scholar] [PubMed]
- Koyun, N.; Aydinlio˘ glu, A.; Aslan, K. A morphometric study on dog cerebellum. Neurol. Res. 2011, 33, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 2015, 20, 723–737. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).