Abstract
Hand preference in non-human primates has been studied extensively with the aim of understanding the evolution of hemispheric asymmetry and hand preferences in humans. However, the focus has been on hand preferences expressed in adulthood, with a surprising lack of studies on hand preferences in infants and changes that occur during the development of other, potentially associated, asymmetries in the brain and behaviour. This paper reports on the development of hand preference for grasping food and taking it to the mouth in common marmosets. It considers the development of other types of behaviour, such as head cocking and anogenital licking, that parallel and might influence the development of hand preferences during the first months of life. It then discusses behavioural differences between left- and right-handed adult marmosets, including response to novel stimuli, social behaviour and cognitive bias. The need to study the development of hand preferences together with the development of these other expressions of cognitive function is highlighted. The question to be addressed by empirical studies is whether hand preference is a downstream manifestation of the development of hemispheric differences in sensory processing and cognition, or whether it is instrumental in the development of functional differences between the hemispheres. Comparison is made to paw preference and associated behaviour in non-primate species.
1. Introduction
Hand preferences are widespread amongst primate species, although not as group biases within populations, as is the case of handedness in humans [1,2,3,4]. Nevertheless, revealing the factors that influence the development of hand preferences in primates, as well as the ways in which hand preference might influence the development of lateral asymmetries of cognitive processing, relies on the study of the development of hand preferences in selected primate species. The common marmoset (Callithrix jacchus) is one such species. This paper is primarily concerned with development of hand preferences in marmosets and development of other lateralized behaviour. The potential role of hand preferences in the development of social and cognitive traits is discussed, as well as the influence of specific types of experience on the development of hand preference. For comparison, the development of paw preference in non-primate mammals and the association between paw preference and other behaviour in non-primate species is considered.
Throughout adult life, common marmosets have stable hand preferences for simple reaching [5,6], scored by repeatedly assessing the hand used to pick up pieces of food and take them to the mouth to be eaten. This is usually referred to as simple reaching, and that term will be used in this paper even though it involves not only reaching for the food but also holding it and taking it to the mouth to eat. Note that hand preference for simple reaching may differ, in strength at least [7], from hand preference expressed in manipulation using coordination of both hands [4,8,9,10,11], but bimanual coordination has not yet been investigated in marmosets. One way to do so would be to modify the TUBE test [12] so that a tube containing food could be held steady with one hand, allowing the marmoset to use the other hand to reach inside the tube to obtain a food reward. So far, this test has been used for primates with better control of the hand and fingers in picking up food and objects than the simpler grasping performed by marmosets. However, it is worth noting that it has been possible to test another New World primate, the spider monkey (Ateles geoffroyi), on somewhat modified TUBE tests [13].
Currently, for common marmosets, we have data only on unimanual hand use in simple tasks but, as this paper will discuss, simple unimanual hand use has advantages in revealing associated behaviour, including proactive versus reactive behaviour and cognitive bias. Unlike bimanual coordination, simple hand use is unconstrained and reflects which side of the brain is in control, or active, during the performance of a task or in expressing a particular behaviour.
Hand preference of marmosets for simple reaching (and holding) in adulthood is considered first. In the marmoset colony at the University of New England, UNE (for housing conditions see Pines et al. [14]), a longitudinal study was conducted on hand preferences for reaching in 48 marmosets, scored at least once per year until death, or at a maximum age of 12 or 13 years [6,15]. At each age, hand preference was scored over 10 days, at the normal time of feeding to obtain a total of 100 scores per individual (10 scores per day). A score was recorded each time a marmoset grasped a piece of food and took it to its mouth. Repeated taking of the food to the mouth while remaining stationary and without dropping it was scored as one act. On rare occasions, the marmoset took the food to the mouth and then switched to holding it with the other hand, taking the food to the mouth in that hand: this act received a score of one left and one right. Picking up a piece of food with both hands was scored separately and found to be of very low occurrence in adult marmosets (2% of total food holding, see later).
All marmosets in the colony had a significant hand preference, 21 with a left-hand preference and 17 with a right-hand preference [6]. For each individual marmoset, hand preference for simple reaching remained unchanged throughout adulthood [5,6]. The same hand preferences were also largely maintained across other tasks involving reaching and grasping with one hand [16]. This was also found to be the case in another study of common marmosets in a different colony [17]. Although some marmosets changed their hand preference when tested on a task with increased visuospatial demands [16], this occurred only in a small minority of the group.
Across the adult life span, the strength hand preference remains unchanged in those marmosets with a right-hand preference, but the strength of hand preference declines gradually in those marmosets with a left-hand preference [15]. However, despite this gradual weakening of hand preference in aged, left-handed marmosets, they still retained their left-hand preference throughout adulthood [6,15]. Possibly, this declining strength of hand preference for holding food and eating it has something to do with declining manual dexterity, as found in humans with minor age-related cognitive decline [18].
In the UNE colony, there were no adults without a significant left- or right-hand preference. Similarly, in another colony of common marmosets, tested on a slightly different unimanual task, Cordeiro de Sousa et al. [19] found that all but one of forty-six marmosets had either a left- or right-hand preference. However, more adult marmosets without significant hand preference for simple reaching have been reported to occur in other colonies (for example, see [17,20,21]). The reasons for these differences between marmoset colonies in captivity are unknown but worth investigating. The first questions addressed in this paper are, “How do these hand preferences develop and what factors might influence their development?”.
2. Hand Preferences in Infant Marmosets
Hand use for food-holding in marmosets begins at about four weeks postnatally [22,23,24]. This was confirmed in a study of 15 infant marmosets in the UNE colony, involving observational recording during four sessions per day, each with 30 min duration, for the first month postnatal and for two such sessions per day in the second month postnatal [5]. In the second month postnatal and using the same method of scoring as for the adults (see above), the infants were scored for an average of 30 unimanual acts of simple food holding [5]. Holding food with both hands was a separate measure and it made up 50% of acts of food holding in the second month postnatal, followed by a decline to 5% in months five to eight and 2% thereafter [5]. Possibly relevant to this decline in holding the food with both hands is the evidence that the jaw of the marmoset grows during the first two or so months postnatal and then ceases to grow [25]. Conceivably, it is easier to hold food in one hand at the mouth once the jaw is stronger and so can operate in coordination with one hand to hold the food. The decline with age in holding food with both hands together might also be due to increasing strength of the hand and digits, and hence improved grasping ability [26]. In fact, grip strength could be measured across age from birth to, say, two months of age to determine whether it has a significant role in the development of unimanual hand preference.
Using only scores of unimanual simple-reaching and food-holding, no significant hand preferences were found in any of the fifteen infant marmosets aged one to two months postnatal [27]. By five to eight months postnatal, all but two of these same marmosets had developed significant hand preferences and all maintained these with increased strength at 10 to 12 months, at an age when they are reaching sexual maturity [28], and at 22 months [27], as well as thereafter [5]. The two marmosets without significant hand preferences at five to eight months developed significant preferences at 10–12 months and maintained these thereafter.
In infant marmosets of five to eight months of age, body posture was found to differ between left- and right-hand preferring marmosets. Left-hand use was positively associated with adopting a suspended posture (e.g., hanging on the cage wire by the right hand and arm) and right-hand use was positively associated with tripedal standing [5]. This result may reflect greater strength of the right hand and arm, as postulated for primates by MacNeilage et al. [29], thereby influencing hand preference when the body is suspended using the stronger right hand. However, this was a transient effect since marmosets at 10 to 12 months and older preferred the tripedal posture while feeding, and they retained either their left- or right-hand preference [5].
These results show that the first few months after birth are critical in the development of hand preferences in marmosets. It is plausible that developmental changes in brain and behaviour over these first months of life influence the development of hand preferences, or vice versa. In humans, hand preferences develop over the first two years of life and, as suggested by Michel et al. [30], this shapes lateralization of cognition. Noting that scoring simple hand-reaching in marmosets is not the same as the method usually used to assess hand preference in humans, it is still possible to apply a similar hypothesis to the development of hand preferences and cognition in marmosets, and this could be tested empirically. Moreover, although it is recognised that the approximately equal distribution of left- and right-hand preference in adult marmosets differs from the predominance of right-handedness in adult humans [31], research on the development of hand preference in marmosets and the interactive influence of sensory stimulation will assist in understanding the cascade of events leading to the development of hand preferences in humans [32] and other primates, in both New and Old World monkeys and prosimians. In fact, as in marmosets, when reaching for an object, humans in their first few years of life have weak and inconsistent hand preferences, transitioning to the use of a preferred hand by seven to ten years of age [33].
3. Development of Other Behaviour in Early Postnatal Life
Since a broad range of neural and behavioural development takes place in the postnatal period and during infancy, the next question addressed is whether any of these other developmental changes interact with the development of hand preference. Firstly, it could be suggested that the development of hand preference in infants might be influenced by the side of the mother on which the infant suckles. Since suckling involves clinging to the mother’s body in a way that is likely to require differential use of the hands and forelimbs, a side preference for suckling could influence the development of hand preference for simple reaching. In fact, marmosets have strong preferences for suckling at one of their mother’s teats and the same preference is seen both when a marmoset suckles at the same time as its twin and when it suckles alone [27]. However, in a study by Rogers and Kaplan [27], no significant association was found between the preferred side of suckling and hand preference measured at any age.
Another important form of stimulation received during the early postnatal period is anogenital licking. Marmoset parents lick the anogenital region of their infants, particularly between days 16 and 45 post-birth and this licking is mainly performed by the mother [34]. Those infants receiving more anogenital licking exhibit more exploration in later life [34]. Additionally, those that receive more licking of their anogenital region were later less fearful of a stimulus resembling snakes, presented on a platform and inside a glass container [34]. In a study of 15 marmosets, the number of times a marmoset was on the platform and the total time spent on the platform correlated positively, and strongly, with the amount of anogenital licking received by the marmoset in infancy [34]. In the same study, however, no significant relationship was found between the amount of anogenital licking received by offspring and hand preference measured in adulthood [34]. Nevertheless, as discussed below, right-handed adult marmosets do interact with novel stimuli more than do left-handed marmosets [35], which suggests that it would be worth further investigating anogenital licking, as a measure of parental attention, and its potential influence on development of hand preference. Indeed, it seems that anogenital stimulation may be a widespread feature of development in vertebrates and it is, potentially, a form of stimulation that affects development of behaviour in infants. Earlier studies on rodents showed that anogenital licking by the mother influences development of sexual behaviour [36] and the importance of tactile stimulation in early life has been shown to affect behaviour of humans and other non-human species in later life [37].
A possible association between developing hand preference and visual exploration could be investigated by measuring head cocking behaviour. Head cocking behaviour is a type of visual exploratory behaviour, involving tilting the head to different angles when examining a visual stimulus [38]: Figure 1. In marmosets, this behaviour begins between days 12 and 14 postnatal, and it increases up until day 24 postnatal and continues to develop for the next two months in parallel with development of the visual system [39,40]. The amount of head cocking performed by infants from birth to two months was found to correlate strongly with the amount of anogenital licking received in the first 60 days of life, particularly so for anogenital licking by the mother [39]. Although no association was found between the total number of head cocks recorded and hand preference, there was a difference between left- and right-handed marmosets in the angle to which the head was tilted anticlockwise: right-handed marmosets had a preference to tilt the head to an angle of 45°, whereas left-handed marmosets titled their head to a preferred angle of 90° [39]. Whether this difference in angle represents a difference in type or degree of visual exploration is unknown, but it is potentially so, given that visual sampling of objects at different angles is used in recognition and memory encoding [41]. Of course, the angle of head cocking might be physically constrained by hand preference, which must affect body posture and balance, and future research on hand preference and head cocking could take that into account. These initial findings suggest that further investigation of head cocking in relation to the development of hand preference is warranted.
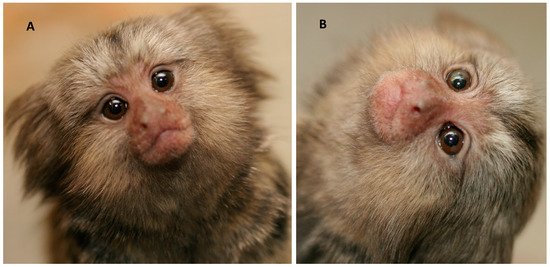
Figure 1.
Head cocking in infant, common marmosets. (A). 45 degrees to the right. (B). 90 degrees to the left. Photographs taken by David Elkins, University of New England.
Research on the development of exploratory behaviour and its opposite, avoidance, (i.e., approach-withdrawal behaviour) over the life stages when marmosets develop hand preferences might lead to interesting results that could be relevant to understanding the development of hand preference in humans and other primates. Indeed, there is convincing evidence that hand preference is associated with approach-avoidance in adult marmosets (discussed next).
4. Behaviour Associated with Hand Preference in Adulthood
In adult marmosets, behavioural type is associated with hand preference (summarised in [2]). Right-handed, adult marmosets approach novel objects sooner and touch them more often than do left-handed marmosets [35,42]. A similar relationship between hand preference and response to unfamiliar food has been found in Geoffroy’s marmosets, Callithrix geoffroyi: right-handed marmosets sampled unfamiliar food more readily than did left-handed marmosets [43]. Moreover, the researchers found that the right-handed marmosets exhibited less fear when they heard the calls of an avian predator than did left-handed marmosets: left-handed marmosets remained immobile (referred to as, freezing) for longer after hearing the calls than did right-handed marmosets [43]. In general, therefore, it can be said that left-handed marmosets are reactive and right-handed marmosets are proactive.
One could explain these results by the likelihood that a preference to use one hand reflects activity in and processing by the contralateral hemisphere of the brain, as shown in humans [44]. Increased fear with the predominant use of the left hand and, hence, control by the right hemisphere would then be consistent with the role of the right hemisphere in fear responses, as shown in some vertebrate species [45] and discussed further by Gainotti [46]. In support of a link between the left hand (right hemisphere) and fear behaviour, Tomaz et al. [47] and Pereira et al. [48,49] have shown that increased fear responses in marmosets are coupled with changes in tympanic membrane temperature of the right ear only. This measure depends on blood flow to the ipsilateral hemisphere and, therefore, demonstrates that the fear response involves activity in the right hemisphere, thus explaining its association with left-hand preference.
Predominant use of the left hand and right hemisphere is also associated with negative cognitive bias. Left-handed marmosets trained to expect a reward from a bowl with a white lid and not from one with a black lid (or trained vice versa) treat a bowl with a grey lid (an ambiguous stimulus) as negative, whereas right-handed marmosets treat it as positive [50]. This result is consistent with greater fear/negativity when there is a bias to control behaviour using the right hemisphere.
Preferred use of the left hand and right hemisphere is also associated with other differences in behaviour compared to marmosets with a right-hand and left-hemisphere preference. Compared to right-handed marmosets, left-handed marmosets show less social facilitation when they interact with each other while catching insects [51] and they utter fewer vocalizations in communication with conspecifics both when catching insects and when mobbing an unfamiliar stimulus [51]. Consistent with this finding of higher levels of social interaction in right- than left-handed marmosets, Vaughan et al. [21] have reported higher social behaviour in right-handed and ambidextrous marmosets, compared to left-handed marmosets. Hence, it seems that right-hand preference goes together with sociability or at least with certain aspects of social behaviour.
At this stage, it would be premature to argue that hand preference has anything specific to do with ‘personality’ in marmosets. In fact, Masilkova et al. [52] found no association between hand preference and personality scores. Moreover, Tomassetti et al. [20] have reported that it is strength but not direction of hand preference that is associated with behaviour that these researchers labelled as ‘inquisitiveness’. In their study, both right- and left-handed marmosets were more inquisitive than ambidextrous marmosets. This result appears to be contradictory to those reported by Cameron and Rogers [35], Braccini and Caine [43] and Pereira et al. [48,49] (discussed above) but very different methods were used to assess exploration in these studies compared to assessment of inquisitiveness in the study by Tomassetti et al. [20]. Exploration was measured empirically as proximity of approach, touching and other active interactions with novel stimuli, whereas inquisitiveness was deduced from questionnaires filled in by humans familiar with the marmosets.
At this stage of knowledge, a more precise assessment of behaviour and behaviour-type is needed to understand the possible association between hand preference and expressed behaviour. However, one could borrow terminology from evolutionary psychology to say that left- and right-handed marmosets have different “behavioural syndromes”, which refers to suites of correlated traits [53]. Evidence suggests that these correlated traits are due to the predominant use of the left or right hemisphere.
5. Is Development of Cognitive Performance Associated with Hand Preference?
In marmosets, the trajectory of the development of hand preference appears to be paralleled by the development of head cocking, and, hence, visual exploration, but it is not known whether one of these influences the development of the other, or whether they could both be part of the same behaviour system, controlled by similar brain regions. Convincing evidence from testing adult marmosets associates exploration, measured as an approach to and touching of novel objects, with right-hand preference and, hence, a bias to process information and control behaviour by the left hemisphere (above). Now, it would be worthwhile to test whether visual exploration, as in head cocking, has the same right-hand (left hemisphere) association.
As in other species, the early life of the marmoset is characterised by a cascade of developing patterns of behaviour (summarised in [54]). Whether any of these patterns of behaviour are related directly to each other or whether any of them have deep homology has not yet been determined. In marmosets, no research has yet investigated specifically the development of responding to novel objects, and, hence, exploration versus avoidance and even fear, or whether the pattern of development of these responses is associated with hand preference.
One might even question whether hand preference, or rather, lack thereof, in infant marmosets is homologous to hand preference measured in adulthood, as discussed by Michel [30] for the development of handedness in humans. Michel [30] concluded that handedness in humans is, indeed, a homologous trait expressed “serially across development”, firstly as head orientation preference, then hand preference to reach for an object, followed by role-differentiated bimanual manipulation. In rhesus macaques, the sequence of development does not predict later hand preference on either unimanual or bimanual tasks, suggesting a difference from humans [55]. In marmosets, hand preference for simple reaching expressed at different adult ages is likely to be a homologous trait, but this may not be so for hand preference in infancy since at this stage of development there are no significant hand preferences. As already discussed, because the trait of hand preference reflects the use of one or the other hemisphere, it is associated with other behavioural traits specialised to the hemisphere controlling the hand in use. These traits include exploration versus avoidance, proactive versus reactive behaviour, social versus non-social or even asocial behaviour. These behaviours may, or may not, be homologous with hand preference. However, one recognises that behaviours can be expressed with differing features that are organised hierarchically and that homology may occur at different levels of neural organisation [56].
Human children that have consistent hand preference in early life show more advanced patterns of cognitive development compared to children who develop hand preference later [57]. In other words, hand preferences in early life could influence the development of functional lateralization of the hemispheres and, hence, shape cognitive function [57]. Alternatively, developing functional lateralization of the brain could influence the expression of hand preference. Concerning the latter hypothesis, Marcori et al. [58], looking at the development of various types of lateralization in humans (hand, foot, trunk, auditory and visual lateralization), have suggested that a single and general lateralization process occurs in early life (seven to twelve years of age), probably stemming from differentiation of the hemispheres. After this age, according to Marcori et al. [58], each type of laterality follows its own separate trajectory, each involving a different lateralization process. It is conceivable that, in marmosets also, a general pattern of lateralization develops as a consequence of differentiation of the cerebral hemispheres and then it is manifested in several types of behaviour, including hand preference.
6. Behaviour Associated with Hand/Paw Preference in Non-Primates
Although this paper has featured the development of hand preferences in primates, common marmosets specifically, and it has discussed whether hand preferences are associated with other expressions of behaviour, it is worth considering whether any parallel examples are known to occur in non-primate species. In fact, lateralization of brain function characterises a broad range of vertebrate species other than primates (summarised in [45,59]) and, in some of those species, sensory experience in early life has been found to have a crucial role in the development of lateralization; for example, the role of stimulation of the embryo by light in the development of visual lateralization in chicks [60,61,62] and pigeons [63] and the effect of tactile stimulation on the development of lateralization in rats [64,65].
The equivalent of hand preference in primates is seen in those species of non-primates with paws that are used to hold or manipulate objects. The first example was found by testing rodents in a task requiring them to reach into a tube to grasp pellets of food [66]. As found in common marmosets, rodents show either left- or right-paw preference in this task, and this has been confirmed recently by a comprehensive meta-analysis of studies assessing paw preference in rats and mice [67]. Similarly, tree shrews tested on some reaching tasks are either left- or right-paw preferent [68] and a study of small-clawed otters found that they too are either left- or right-pawed in a simple task requiring them to reach for objects [69]. Hence, in rodents, tree shrews, otters and in marmosets (see above) tested for simple reaching, paw and hand use is lateralized but there is no population bias.
On a task requiring small-clawed otters to reach into a hole, and therefore a more difficult task than simply reaching for food or objects, they were significantly right-pawed [69]. As suggested previously, whether hand preference is biased at the population level may depend on the nature of the task and what cognitive demands it requires [6]. If the specific processing abilities of one hemisphere are required for the successful performance of the task (e.g., those of the right hemisphere for spatial reaching or the left hemisphere for fine manipulation or discrimination), a population bias of hand or paw preference is manifested. However, for simple tasks not demanding use of specialised processing by a particular hemisphere, animals may use either the left or right hand/paw. Some will be left-paw preferent, some right-paw preferent and some, although fewer, may use both limbs equally and so be recorded as ambilateral.
Left-, right- or ambilateral preference is characteristic of dogs and cats assessed by scoring the paw used to reach into a stable container to obtain food or, for dogs, to hold steady an unstable container (a Kong) so that they can lick out the food inside [70,71,72]. In a meta-analysis of data on paw preferences in dogs and in cats, Ocklenburg et al. [73] found that most dogs and cats have either a left- or right-paw preference, with no population bias, whereas ambilateral preference is much less common, although the proportion of ambilateral animals varies between samples and the tasks used to score paw preference [74].
As discussed earlier in this paper, in the simple task of picking up food items and taking them to the mouth, marmosets are either left- or right-hand preferring and hand preferences in this task are associated with specialised patterns of behaviour. To my knowledge, amongst non-primate species, it is only in domestic dogs that researchers have, so far, investigated an association between paw preference and other behaviour. As found in marmosets, left-pawed dogs display a negative cognitive bias compared to right-pawed and ambilateral dogs [75]. One study found that right-pawed dogs are more successful in completing Guide Dog Training than left-pawed or ambilateral dogs [76]. Another study found that left-pawed dogs were less likely to direct aggression at a stranger [77]. From these examples, we can conclude that a pattern of behaviour in left- versus right-pawed dogs is consistent with that found in marmosets, but this does not apply to all types of behaviour.
By contrast, other studies of the relationship between paw preference and behaviour have reported that ambilateral dogs differ from both left- and right-pawed dogs in being more reactive to threatening sounds (e.g., the rumbling of a thunderstorm) [71]. Ambilateral dogs are also less aggressive to strangers [78] and show higher levels of fear, playfulness and sociability [79]. Not clearly consistent with these findings, in another study, ambilateral dogs were found to approach and obtain food in a novel task more readily than left- or right-pawed dogs [80]. In cats, ambilateral paw preference is associated with more aggression, less obedience and being less affectionate [81] and being less able to solve a problem task than either left- or right-pawed cats [82]. Tests of wild squirrels, however, found that learning a task was performed better by individuals with weaker strength of paw preference [83]. Thus far, the evidence for behavioural differences between ambilateral preferent animals and those with significant paw preferences is mixed and sometimes contradictory.
Hence, there is a need to study paw preferences and associated patterns of behaviour in a broader range of species. Possibly, at least part of the reason for the differences between studies of paw preference and behaviour in dogs results from the range of different breeds tested in each task and also differences in the domestic environments in which the dogs have been raised or in which they are living at the time of testing. Breed and previous experience are both important factors influencing the behaviour of dogs. In fact, stress causes dogs to shift to ambilateral paw-preference [84] and this may explain the pattern of behaviour in ambilateral dogs discussed above (i.e., elevated fear and reactivity to unexpected, fear-inducing stimuli).
Another area of research thus far not investigated is the development of paw preferences in non-primate species. Only in dogs and cats has the development of paw preferences been investigated to some extent. Wells and Millsopp [85] assessed paw preference in cats at three, six and twelve months of age on a task that required them to reach into a glass jar containing food. This longitudinal study found that at three and six months the cats were more likely to be ambilateral than they were to have either a left- or right-paw preference. Significant paw preferences in cats did not develop until they were 12 months old when males showed a tendency to prefer their left paw and females to prefer their right paw. This ambilateral preference in early life that develops into significant preference for left or right is similar to the development of hand preferences found for marmosets (see above).
In dogs, scored for lifting a paw to reach for a food reward offered by a human, Charlton and Frasnelli [86] found a shift in paw preference from left in male puppies to right in adulthood, whereas females had consistent right-paw preferences as puppies and adults. This was a cross-sectional study (different dogs made up the puppy and adult groups) and the hand-preference of the owner/tester was associated with the dog’s paw preference. Obviously, a longitudinal investigation of paw preferences of dogs at different ages is needed.
In addition, it is timely to investigate the potential developmental events that may precede or accompany the development of paw preferences (for the possible role of hormones see discussion in [85]). From the currently available literature, it is evident that more longitudinal studies on the development of paw preferences in non-primate species are needed, particularly with the measurement of associated patterns of behaviour from birth to adulthood. Furthermore, studies on the development of hand/paw preference in non-domesticated or laboratory-bred species are needed.
7. Conclusions
This paper has focused on simple, unimanual reaching for and holding of food in marmosets and it has discussed evidence that such unimanual preference expressed in a relatively easy task is associated with other patterns of behaviour relying on activation of the hemisphere being used to control the preferred hand. Some evidence suggests that, in non-primate mammals also, paw preference is also associated with behaviour controlled by the hemisphere contralateral to the preferred hand.
It is known that hand preference in the common marmoset develops during the postnatal period and that it does so in parallel with the development of several other patterns of behaviour. The question remaining unanswered is whether these separate, though parallel, developing behaviours are homologous with hand preference for simple reaching and whether any one behaviour has discernible influences on another pattern of behaviour. From the perspective of hand preferences, we need to discover whether developing hemispheric differences lead to hand preference, along with other expressed behaviour, including fearfulness, exploration, ‘inquisitiveness’ and cognitive bias. Whereas there is convincing evidence that hand preference in adults is associated with a constellation of these behaviours (proactive versus reactive, positive versus negative cognitive bias, social versus non-social or asocial behaviour), more research is needed to discover interrelationships of these patterns of behaviour during development, including whether sensory experience during infancy influences the development of hand (or paw) preference [87].
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Westergaard, G.C.; Lussier, I.D.; Higley, J.D. Between-species variation in the development of hand preference among macaques. Neuropsychologia 2001, 39, 1373–1378. [Google Scholar] [PubMed]
- Rogers, L.J. Manual bias, behavior, and cognition in common marmosets and other primates. Prog. Brain Res. 2018, 238, 91–113. [Google Scholar]
- Lilak, A.L.; Phillips, K.A. Consistency of hand preference across low-level and high-level tasks in capuchin monkeys (Cebus apella). Am. J. Primatol. 2008, 70, 254–260. [Google Scholar] [CrossRef]
- Chapelain, A.S.; Hogervorst, E.; Mbonzo, P.; Hopkins, W.D. Hand preferences for bimanual coordination in 77 bonobos (Pan paniscus): Replication and extension. Int. J. Primatol. 2011, 32, 491–510. [Google Scholar]
- Hook, M.A.; Rogers, L.J. Development of hand preferences in marmosets (Callithrix jacchus) and effects of ageing. J. Comp. Psych. 2000, 114, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. R. Soc. Lond. B 2009, 364, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Maille, A.; Belbeoc’h, C.; Rossard, A.; Bec, P.; Blois-Heulin, C. Which are the features of the TUBE task that make it so efficient in detecting manual asymmetries? An investigation in two Cercopithecine species (Cercopithecus neglectus and Cercocebus torquatus). J. Comp. Psychol. 2013, 127, 436–444. [Google Scholar] [CrossRef]
- Soto, C.; Gáqyuez, J.M.M.; Llorente, M. Hand preferences in co-ordinated bimanual tasks in non-human primates: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 141, 104822. [Google Scholar] [PubMed]
- Caspar, K.R.; Pallasdies, F.; Mader, L.; Sartorelli, H.; Begall, S. The evolution and biological correlates of hand preferences in anthropoid primates. eLife 2022, 11, e77875. [Google Scholar]
- Rogers, L.J.; Kaplan, G. Hand preferences and other lateral biases in rehabilitated orang-utans (Pongo pygmaeus pygmaeus). Anim. Behav. 1996, 51, 13–25. [Google Scholar] [CrossRef]
- Meguerditchian, A.; Vauclair, J.; Hopkins, W.D. On the origins of human handedness and language: A comparative review of hand preferences for bimanual coordinated actions and gestural communication in nonhuman primates. Dev. Psychobiol. 2013, 55, 637–650. [Google Scholar]
- Hopkins, W.D.; Phillips, K.A.; Bania, A.; Calcutt, S.E.; Gardner, M.; Russell, J.; Schaeffer, J.; Lonsdorf, E.V.; Ross, S.R.; Schapiro, S.J. Hand preferences for coordinated bimanual actions in 777 great apes: Implications for the evolution of handedness in Hominins. J. Human Evol. 2011, 60, 605–611. [Google Scholar]
- Motes Rodrigo, A.; Ramirez Torres, C.E.; Hernandez Salazar, L.T.; Laska, M. Hand preferences in two unimanual and two bimanual coordinated tasks in the black-handed spider monkey (Ateles geoffroyi). J. Comp. Psychol. 2018, 132, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pines, M.K.; Kaplan, G.; Rogers, L.J. A note on indoor and outdoor housing preferences of common marmosets (Callithrix jacchus). Appl. Anim. Behav. Sci. 2007, 108, 348–353. [Google Scholar]
- Rogers, L.J. Differential ageing of the brain hemispheres: Evidence from a longitudinal study of hand preferences in common marmosets. Symmetry 2021, 13, 2349. [Google Scholar] [CrossRef]
- Hook, M.A.; Rogers, L.J. Visuospatial reaching preferences of common marmosets: An assessment of individual biases across a variety of tasks. J. Comp. Psychol. 2008, 122, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yamazaki, Y.; Iriki, A. Hand preference depends on posture in common marmosets. Behav. Brain Res. 2013, 248, 144–150. [Google Scholar] [CrossRef]
- Vasyleno, O.; Gorecka, M.M.; Waterloo, K.; Rodríguez-Aranda, C. Reduction in manual asymmetry and decline in fine manual dexterity in right-handed older adults with mild cognitive impairment. Laterality 2022, 27, 581–604. [Google Scholar] [CrossRef]
- Cordeiro de Sousa, M.B.C.; Xavier, N.S.; da Silva, H.P.A.; de Oliviera, M.S. Hand preference study in marmosets (Callithrix jacchus) using food reaching tests. Primates 2001, 42, 57–66. [Google Scholar] [CrossRef]
- Tomassetti, D.; Caracciolo, S.; Manciocco, A.; Chiarotti, F.; Vitale, A.; De Filippis, B. Personality and lateralization in common marmosets (Callithrix jacchus). Behav. Proc. 2019, 167, 103899. [Google Scholar]
- Vaughan, E.; Le, A.; Casey, M.; Workman, K.P.; Lacreuse. Baseline cortisol levels and social behavior differ as a function of handedness in marmosets (Callithrix jacchus). Am. J. Primatol. 2019, 81, e23057. [Google Scholar] [CrossRef]
- Box, H.O. Quantitative studies of behaviour within captive groups of marmoset monkeys (Callithrix jacchus). Primates 1975, 16, 155–174. [Google Scholar] [CrossRef]
- Missler, M.; Wolff, J.R.; Rothe, H.; Heger, W.; Merker, H.-J.; Treiber, A.; Scheid, R.; Crook, G.A. Developmental biology of the common marmoset: A proposal for “postnatal staging”. J. Med. Primatol. 1992, 21, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.E. From dependence to sexual maturity: The behavioural ontogeny of callitrichidae. In Marmosets and Tamarins: Systematics, Behaviour and Ecology; Rylands, A.B., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 235–250. [Google Scholar]
- Wilson, N.H.F.; Speight, P.M.; Gardner, D.L. Growth of the mandible in the common marmoset (Callithrix jacchus). J. Med. Primatol. 1982, 11, 242–251. [Google Scholar] [CrossRef]
- Fox, D.M.; Mundinano, I.-C.; Bourne, J.A. Prehensile kinematics of the marmoset monkey: Implications for the evolution of visually-guided behaviors. J. Comp. Neurol. 2019, 527, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Kaplan, G. Teat preference for suckling in common marmosets: Relationship to side of being carried and hand preference. Laterality 1998, 3, 269–281. [Google Scholar]
- Box, H.O. Social behavior in the common marmoset monkey (Callithrix jacchus). Biol. Human Affairs 1978, 43, 51–64. [Google Scholar]
- MacNeilage, P.F.; Studdert-Kennedy, M.J.; Lindblom, B. Primate handedness reconsidered. Behav. Brain Sci. 1987, 10, 247–303. [Google Scholar]
- Michel, G.F. The concept of homology in the development of handedness. Dev. Psychobiol. 2013, 55, 84–91. [Google Scholar] [CrossRef]
- Papadatou-Pastou, M.; Ntolka, E.; Schmitz, J.; Martin, M.; Munafo, M.R.; Ocklenburg, S.; Paracchini, S. Human handedness: A meta-analysis. Psychol. Bull. 2020, 146, 481–524. [Google Scholar] [CrossRef]
- Nelson, E.L. Developmental cascades as a framework for primate handedness. Front. Behav. Neurosci. 2022, 16, 1063348. [Google Scholar] [CrossRef] [PubMed]
- Scharoun, S.; Bryden, P.J. Hand preference, performance abilities, and hand selection in children. Front. Psychol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Kaplan, G.; Rogers, L.J. Parental care in marmosets (Callithrix jacchus): Development and effect of anogenital licking on exploration. J. Comp. Psychol. 1999, 113, 269–276. [Google Scholar] [CrossRef]
- Cameron, R.; Rogers, L.J. Hand preference of the common marmoset, problem solving and responses in a novel setting. J. Comp. Psychol. 1999, 113, 149–157. [Google Scholar] [CrossRef]
- Moore, C.L. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev. Psychobiol. 1984, 17, 347–356. [Google Scholar] [CrossRef]
- Bales, K.L.; Witczak, L.R.; Simmons, T.C.; Savidge, L.E.; Rothwell, E.S.; Rogers, F.D.; Manning, R.A.; Heise, M.J.; Englund, M.; del Razo, A. Social touch during development: Long-term effects on brain and behavior. Neurosci. Biobehav. Rev. 2018, 95, 202–219. [Google Scholar] [PubMed]
- Menzel, C.R.; Menzel, E.W., Jr. Head cocking and visual exploration in marmosets (Sanguinus fuscicollis). Behaviour 1980, 75, 219–233. [Google Scholar] [CrossRef]
- Kaplan, G.; Rogers, L.J. Head-cocking as a form of exploration in the common marmoset and its development. Dev. Psychobiol. 2006, 48, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Izumi, A.; Tsuchida, J.; Yamaguchi, C. Effects of rearing conditions on early visual development in common marmosets. Dev. Psychobiol. 2012, 54, 700–705. [Google Scholar] [CrossRef]
- Harris, I.M.; Harris, J.A.; Corballis, M.C. Binding identity and orientation in object recognition. Atten. Percept. Psychophys. 2020, 82, 153–167. [Google Scholar] [CrossRef]
- Fernández-Lázaro, G.; Latorre, R.; Alonso-García, E.; Núñez, I.B. Nonhuman primate welfare: Can there be a relationship between personality, lateralization and physiological indicators? Behav. Proc. 2019, 166, 103897. [Google Scholar]
- Braccini, S.N.; Caine, N.G. Hand preference predicts reactions to novel foods and predators in marmosets (Callithrix geoffroyi). J. Comp. Psychol. 2009, 123, 18–25. [Google Scholar] [CrossRef]
- Sainburg, R.L. Convergent models of handedness and brain lateralization. Front. Psychol. 2014, 5, 1092. [Google Scholar] [PubMed]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Gainotti, G. Hemispheric asymmetries for emotions in non-human primates: A systematic review. Neurosci. Biobehav. Rev. 2022, 141, 104830. [Google Scholar]
- Tomaz, C.; Verburg, M.S.; Boere, V.; Pianta, T.F.; Belo, M. Evidence of hemispheric specialization in marmosets (Callithrix penicillate) using tympanic membrane thermometry. Braz. J. Med. Biol. Res. 2003, 36, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Duarte, R.B.; Maior, R.S.; Barros, M. Natural predator and a human stimulus differently affect the behavior, cortisol and cerebral hemisphere activity of marmoset monkeys. Physiol. Behav. 2018, 195, 112–117. [Google Scholar]
- Pereira, L.C.; Maior, R.S.; Barros, M. Time-dependent changes in cortisol and tympanic temperature lateralization during food deprivation stress in marmoset monkeys. Front. Behav. Neurosci. 2020, 14, 123. [Google Scholar] [PubMed]
- Gordon, D.J.; Rogers, L.J. Cognitive bias, hand preference and welfare in common marmosets. Behav. Brain Res. 2015, 287, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Rogers, L.J. Differences in social and vocal behavior between left- and right-handed common marmosets. J. Comp. Psychol. 2010, 124, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Masilkova, M.; Šlipogor, V.; Silva, G.H.L.M.; Haová, M.; Lhota, S.; Bugnyar, T.; Konecná, M. Age, but not hand preference, is related to personality traits in common marmosets (Callithrix jacchus). R. Soc. Open Sci. 2022, 9, 220797. [Google Scholar]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar]
- Schultz-Darken, N.; Braun, K.M.; Emborg, M.E. Neurobehavioral development of common marmoset monkeys. Dev. Psychobiol. 2016, 58, 141–158. [Google Scholar]
- Nelson, E.L.; Emery, M.S.; Babcock, S.M.; Novak, M.F.X.; Suomi, S.J.; Novak, M.A. Head orientation and handedness trajectory in rhesus monkey infants (Macaca mulatta). Dev. Psychobiol. 2011, 53, 246–255. [Google Scholar] [CrossRef]
- Hall, B.K. Homology, homoplasy, novelty, and behavior. Dev. Psychobiol. 2013, 55, 4–12. [Google Scholar]
- Michel, G.F.; Campbell, J.M.; Marcinowski, E.M.; Nelson, E.L.; Babik, I. Infant hand preference and the development of cognitive abilities. Front. Psychol. 2016, 7, 410. [Google Scholar] [CrossRef]
- Marcori, A.J.; Monteiro, P.H.M.; Brussolo, A.D.; Okazaki, V.H.A. The development of hand, foot, trunk, hearing, and visual lateral preference throughout the lifespan. Neuropsychologia 2023, 178, 108444. [Google Scholar]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain lateralization: A comparative perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [PubMed]
- Rogers, L.J. Light experience and asymmetry of brain function in chickens. Nature 1982, 297, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008, 76, 235–244. [Google Scholar] [PubMed]
- Chiandetti, C.; Galliussi, J.; Andrew, R.J.; Vallortigara, G. Early light stimulation suggests a second route, via gene activation, to cerebral lateralization in vertebrates. Sci. Rep. 2013, 3, 2701. [Google Scholar] [CrossRef]
- Manns, M.; Römling, J. The impact of asymmetrical light input on cerebral hemispheric specialization and interhemispheric cooperation. Nat. Commun. 2012, 3, 696. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V.H.; Garbanati, J.A.; Sherman, G.; Yutzey, D.A.; Kaplan, R. Infantile stimulation induces brain lateralization in rats. Science 1978, 201, 1150–1152. [Google Scholar] [CrossRef]
- Denenberg, V.H. Hemispheric laterality in animals and the effects of early experience. Behav. Brain. Sci. 1981, 4, 1–49. [Google Scholar] [CrossRef]
- Collins, R.L. On the inheritance of direction and degree of asymmetry. In Cerebral Lateralization in Nonhuman Species; Glick, S.D., Ed.; Academic Press: New York, NY, USA, 1985; pp. 41–71. [Google Scholar]
- Manns, M.; Basbasse, Y.L.; Freund, N.; Ocklenburg, S. Paw preferences in mice and rats: Meta-analysis. Neurosci. Biobehav. Rev. 2021, 127, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Joly, M.; Scheumann, M.; Zimmermann, E. Posture does not matter! Paw usage and grasping paw preference in a small-bodied quadrupedal mammal. PLoS ONE 2012, 7, e38228. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.; Ströckens, F.; Stavenhagen, P.; Ocklenburg, S. Paw preferences in the Asian small-clawed otter—Using an inexpensive, video-based protocol to study laterality of rare species in the zoo. Laterality 2018, 23, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L. Paw preference as a tool for assessing emotional functioning and welfare in dogs and cats: A review. Appl. Anim. Behav. Sci. 2021, 236, 105148. [Google Scholar] [CrossRef]
- Branson, N.J.; Rogers, L.J. Relationship between paw preference strength and noise phobia in Canis familiaris. J. Comp. Psychol. 2006, 120, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; d’Ingeo, S.; Quaranta, A. Lateralized functions in the dog brain. Symmetry 2017, 9, 71. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Isparta, S.; Peterburs, J.; Papadatou-Pastou, M. Paw preference in cats and dogs: Meta-analysis. Laterality 2019, 24, 647–677. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.S.; Barnard, S. Stability of motor bias in the domestic dog, Canis familiaris. Behav. Proc. 2018, 149, 1–7. [Google Scholar]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.S.; Barnard, S. Cognitive bias and paw preference in the domestic dog (Canis familiaris). J. Comp. Psychol. 2017, 131, 317–325. [Google Scholar] [CrossRef]
- Tomkins, L.M.; Thomson, P.C.; McGreevy, P.D. Associations between motor, sensory and structural lateralisation and guide dog success. Vet. J. 2012, 192, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.S.; Barnard, S. Lack of association between paw preference and behaviour problems in the domestic dog, Canis familiaris. Appl. Anim. Behav. Sci. 2019, 210, 81–87. [Google Scholar] [CrossRef]
- Schneider, L.A.; Delfabbro, P.H.; Burns, N.R. Temperament and lateralization in the domestic dog (Canis familiaris). J. Vet. Behav. 2013, 8, 124–134. [Google Scholar] [CrossRef]
- Barnard, S.; Wells, D.L.; Hepper, P.G.; Milligan, A.D.S. Association between lateral bias and personality traits in the domestic dog (Canis familiaris). J. Comp. Psychol. 2017, 131, 246–256. [Google Scholar]
- Marshall-Pescini, S.; Barnard, S.; Branson, N.J.; Valsecchi, P. The effect of preferential paw usage on dogs’ (Canis familiaris) performance in a manipulative problem-solving task. Behav. Proc. 2013, 100, 40–43. [Google Scholar] [CrossRef]
- McDowell, L.J.; Wells, D.L.; Hepper, P.G.; Dempster, M. Lateral bias and temperament in the domestic cat (Felis silvestris). J. Comp. Psychol. 2016, 130, 313–320. [Google Scholar] [CrossRef]
- Isparta, S.; Demirbas, Y.S.; Bars, Z.; Kul, B.C.; Güntürkün, O.; Ocklenburg, S.; Pereira, G.D.G. The relationship between problem-solving ability and laterality in cats. Behav. Brain Res. 2020, 391, 112691. [Google Scholar] [CrossRef] [PubMed]
- Leaver, L.A.; Ford, S.; Miller, C.W.; Yeo, M.K.; Fawcett, T.W. Learning is negatively associated with strength of left/right paw preference in wild grey squirrels (Sciurus carolinensis). Learn. Behav. 2020, 48, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, Y.S.; Isparta, S.; Saral, B.; Yilmaz, N.K.; Adiay, D.; Matsui, H.; Töre-Yargin, G.; Musa, A.A.; Atilgan, D.; Öztürk, H.; et al. Acute and chronic stress alter behavioral laterality in dogs. Sci. Rep. 2023, 13, 4092. [Google Scholar]
- Wells, D.L.; Millsopp, S. The ontogenesis of lateralized behavior in the domestic cat, Felis silvestris catus. J. Comp. Psychol. 2012, 126, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Frasnelli, E. Does owner handedness influence paw preference in dogs? Anim. Cogn. 2023, 26, 425–433. [Google Scholar] [PubMed]
- Rogers, L.J. Unfolding a sequence of sensory influences and interactions in the development of functional brain laterality. Front. Behav. Neurosci. 2023, 16, 1103192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).