Solvent Effects and Metal Ion Recognition in Several Azulenyl-Vinyl-Oxazolones
Abstract
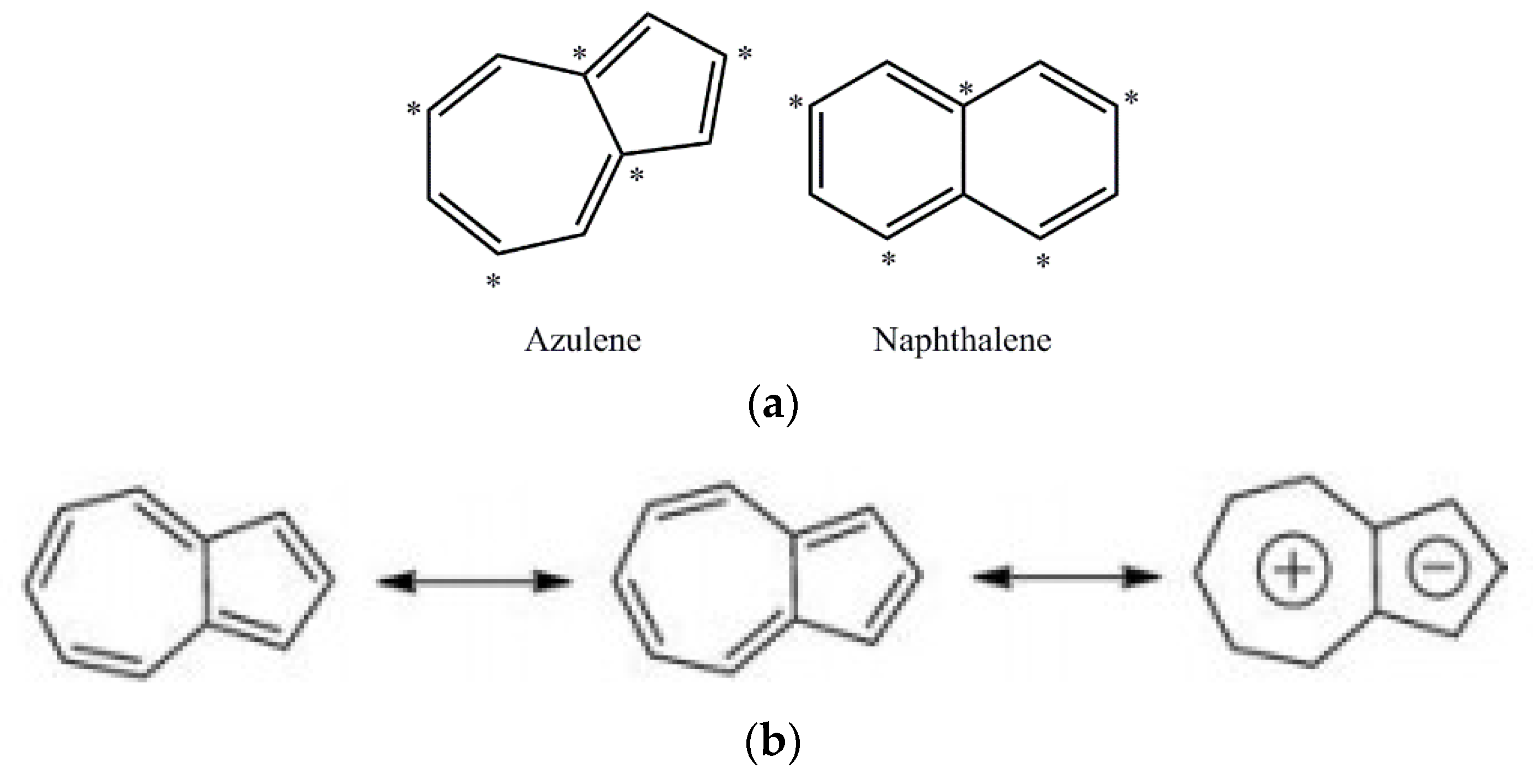
1. Introduction
2. Materials and Methods
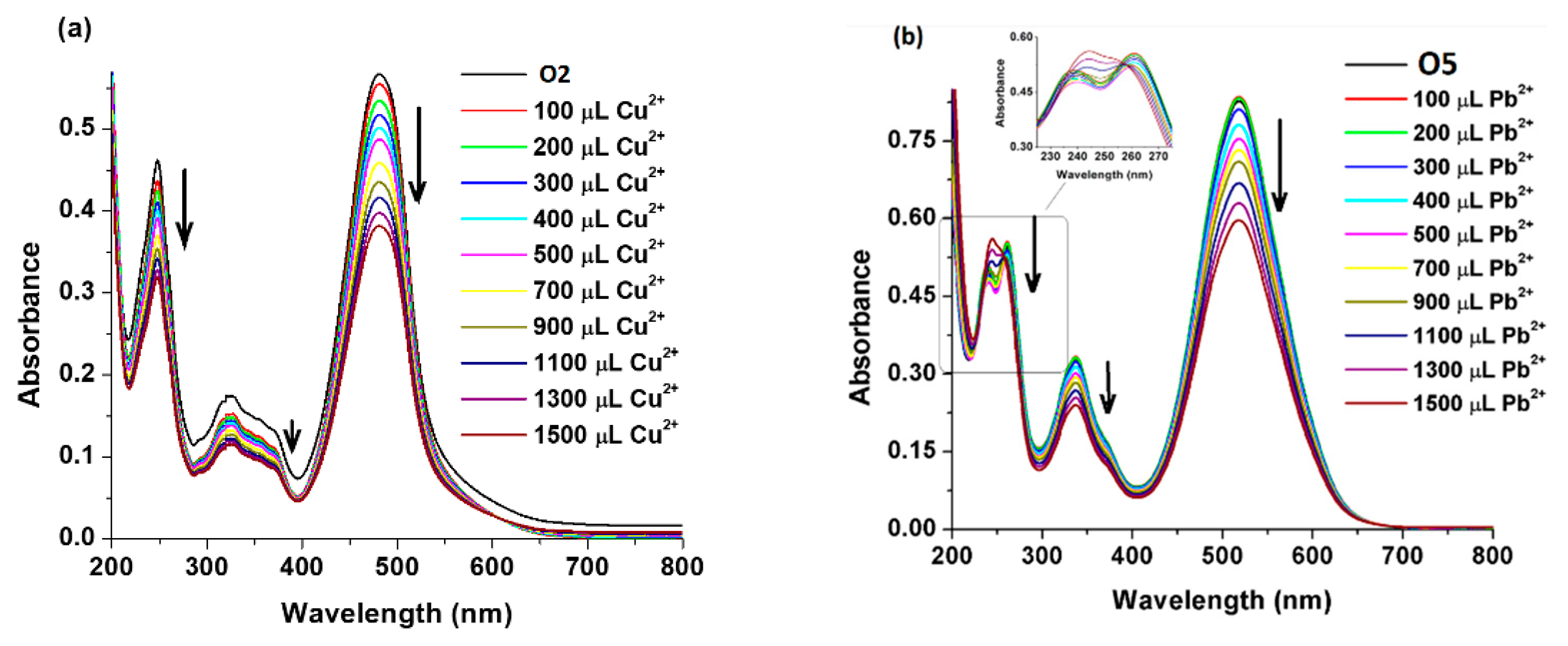
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, H.; Gao, X. Application of azulene in constructing organic optoelectronic materials: New tricks for an old dog. ChemPlusChem 2017, 82, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Amaki, T.; Ishii, A.; Fukuda, K.; Yamasaki, R.; Okamoto, I. Conformational analysis of N-aryl-N-(2-azulenyl)acetamides. Tetrahedron Lett. 2018, 59, 3994–3998. [Google Scholar] [CrossRef]
- Dong, J.X.; Zhang, H.L. Azulene-based organic functional molecules for optoelectronics. Chin. Chem. Lett. 2016, 27, 1097–1104. [Google Scholar] [CrossRef]
- Tao, T.; Fan, Y.; Zhao, J.; Yu, J.; Chen, M.; Huang, W. Reversible alteration of spectral properties for azulene decorated multiphenyl-ethylenes by simple acid base and redox processes. Dye. Pigment. 2019, 164, 346–354. [Google Scholar] [CrossRef]
- Yang, C.C.; Ma, J.Y.; Su, X.; Zheng, X.L.; Chen, H.; He, Y.Y.; Tian, W.Q.; Li, W.Q.; Yang, L. High performance nonlinear materials with simple aromatic hydrocarbons. Flatchem 2022, 33, 100362. [Google Scholar] [CrossRef]
- Damrongrungruang, T.; Rattanayatikul, S.; Sontikan, N.; Wutirak, B.; Teerakapong, A.; Kaewrawang, A. Effect of different irradiation modes of azulene-mediated photodynamic therapy on singlet oxygen and PGE2 formation. Photochem. Photobiol. 2021, 97, 427–434. [Google Scholar] [CrossRef]
- Leino, T.O.; Sieger, P.; Yli-Kauhaluoma, J.; Walen, E.A.A.; Kley, T. The azulene scaffold from a medicinal chemist’s perspective: Physicochemical and in vitro parameters relevant for drug discovery. Eur. J. Med. Chem. 2022, 237, 114379. [Google Scholar] [CrossRef]
- Liu, R.S.H.; Muthyala, R.S.; Wang, X.S.; Asato, A.E.; Wang, P.; Ye, C. Correlation of substituent effects and energy levels of the two lowest excited states of the azulenic chromophore. Org. Lett. 2000, 2, 269–271. [Google Scholar] [CrossRef]
- Murfin, L.C.; Lewis, S.E. Azulene—A bright core for sensing and imaging. Molecules 2021, 26, 353. [Google Scholar] [CrossRef]
- Shevyakov, S.V.; Li, H.; Muthyala, R.; Asato, A.E.; Croney, J.C.; Jameson, D.M.; Liu, R.S.H. Orbital control of the color and excited state properties of formylated and fluorinated derivatives of azulene. J. Phys. Chem. A 2003, 107, 3295–3299. [Google Scholar] [CrossRef]
- Razus, A.C. Azulene moiety as electron reservoir positively charged systems; A short survey. Symmetry 2021, 13, 526. [Google Scholar] [CrossRef]
- Lash, T.D. Out of the blue! Azuliporphyrins and related carbaporphyrinoid systems. Acc. Chem. Res. 2016, 49, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R. Electronic and structural properties of biazulene, terazulene and polyazulenes isomers. J. Phys. Org. Chem. 2007, 20, 395–409. [Google Scholar] [CrossRef]
- Liu, R.S.H. Colorful azulene and its equally colorful derivatives. J. Chem. Educ. 2002, 79, 183–185. [Google Scholar] [CrossRef]
- Ruth, A.A.; Kim, E.K.; Hese, A. The So→S1 cavity ring-down absorption spectrum of jet-cooled azulene dependence of internal conversion on the excess energy. Phys. Chem. Chem. Phys. 1999, 1, 5121–5128. [Google Scholar] [CrossRef]
- Koch, M.; Blacque, O.; Venkatesan, K. Impact of 2,6-connectivity in azulene: Optical properties and stimuli responsive behavior. J. Mater. Chem. C 2013, 1, 7400–7408. [Google Scholar] [CrossRef]
- Foggi, P.; Neuwahl, F.V.R.; Moroni, L.; Salvi, P.R. S1→Sn and S2→Sn absorption of azulene: Femtosecond transient spectra and excited state calculation. J. Phys. Chem. A 2003, 107, 1689–1696. [Google Scholar] [CrossRef]
- Okamoto, M.; Hirayama, S.; Steer, R.P. A reinterpretation of the unsusual barochromism of azulene. Can. J. Chem. 2007, 85, 432–437. [Google Scholar] [CrossRef]
- Itoh, T. Fluorescence and phosphorescence from higher excited states of organic molecules. Chem. Rev. 2012, 112, 4541–4568. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Baryshnikov, G.; Li, X.P.; Zhu, M.J.; Ågren, H.; Zhu, L.L. Anti-Kasha’s rule emissive switching induced by intermolecular H-bonding. Chem. Mater. 2018, 30, 8008–8016. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, Y.; Yue, B.; Wu, B.; Sun, R.; Qu, S.; Zhu, L. Multiwavelength anti-Kasha’s rule emission on self-assembly of azulene-functionalized persulfurated arene. J. Phys. Chem. C 2019, 123, 22511–22518. [Google Scholar] [CrossRef]
- Homocianu, M.; Airinei, A.; Dorohoi, D.O. Solvent effects on the electronic absorption and fluorescence spectra. J. Adv. Res. Phys. 2011, 2, 011105. [Google Scholar]
- Airinei, A.; Isac, D.I.; Homocianu, M.; Cojocaru, C.; Hulubei, C. Solvatochromic analysis and DFT computational study of an azomaleimide derivative. J. Mol. Liq. 2017, 240, 476–485. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Bakhshiev, N.G. Spectroscopy of Intermolecular Interactions; Nauka: Saint Petersburg, Russia, 1972. (In Russian) [Google Scholar]
- Bakhshiev, N.G.; Gubaryan, S.K.; Dobretsov, G.E.; Kirillova, A.Y.; Svetlichnyi, V.Y. Solvatochromism and solvatofluorochromism of the intramolecular charge transfer band of 4-dimethylaminochalcone in the electronic spectra of its solutions. Opt. Spectrosc. 2006, 100, 700–708. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The π* scale of solvent polarities. J. Am. Chem. Soc. 1977, 90, 6027–6038. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Abraham, M.H.; Taft, R. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters π*, α and β, and some methods for simplifying the generalized solvatochromic method. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Catalan, J. Toward a generalized treatment of the solvent effect on the empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA) and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef]
- Catalan, J.; Hopf, H. Empirical components of solute-solvent interactions: The polarizability solvent scale. Eur. J. Org. Chem. 2004, 2004, 4694–4702. [Google Scholar] [CrossRef]
- Laurence, C.; Legros, J.; Chantzis, A.; Phanchat, A.; Jacquemin, D. A database of dispersion-induction DI, electrostatic ES, and hydrogen bonding α1 and β1 solvent-parameters and some applications to the multiparameter correlation analysis of solvent effects. J. Phys. Chem. B 2015, 119, 3174–3184. [Google Scholar] [CrossRef]
- Cristea, M.; Birzan, L.; Dumitrascu, F.; Enache, C.; Tecuceanu, V.; Hanganu, A.; Draghici, C.; Deleanu, C.; Nicolescu, A.; Maganu, M.; et al. 1-Vinylazulenes with oxazolonic ring-potential ligands for metal ion detectors; Synthesis and product properties. Symmetry 2021, 13, 1209. [Google Scholar] [CrossRef]
- Lippert, E. Dipolmoment und Electronenstruktur von angeregten Molekulen. Z. Für Nat. A Phys. Sci. 1955, 10, 541–545. [Google Scholar]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent effects upon fluorescence spectra and dipolmoments of excited states. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Taft, R.W. An examination of linear solvation energy relationships. Progr. Phys. Org. Chem. 1981, 13, 485–630. [Google Scholar]
- Marcus, Y. The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 1993, 22, 409–416. [Google Scholar] [CrossRef]
- Rauf, M.A.; Hisaindee, S. Studies on solvatochromic behavior of dyes using spectral techniques. J. Mol. Struct. 2013, 1042, 45–56. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Arnold, G.L.; Ungureanu, E.M.; Razus, A.C. 1-Vinylazulenes–potential host molecules in ligands for metal ion detectors. Tetrahedron 2016, 72, 2316–2326. [Google Scholar] [CrossRef]
- Arnold, G.L.; Lazar, I.G.; Ungureanu, E.M.; Buica, G.O.; Birzan, L. New azulene modified electrodes for heavy metal ions recognition. Bulg. Chem. Commun. 2017, 49, 205–210. [Google Scholar]
- Muravev, A.; Yakupov, A.; Gerasimova, T.; Islamov, D.; Lazarenko, V.; Shokurov, A.; Ovsyannikov, A.; Dorovaovskii, P.; Zubavichus, Y.; Naummkin, A.; et al. Thiacalixarenes with sulfur functionalized at lower rim: Heavy metal ion binding in solution and 2D-confined space. Int. J. Mol. Sci. 2022, 23, 2341. [Google Scholar] [CrossRef]






| Solvent | Azulene Derivative | ||||
|---|---|---|---|---|---|
| O1 | O2 | O3 | O5 | O6 | |
| 1,4-Dioxane | 21,500 | 20,960 | 20,080 | 20,080 | 20,080 |
| CCl4 | 21,459 | 20,790 | 20,000 | 20,000 | 20,000 |
| Toluene | 21,321 | 20,703 | 19,920 | 19,920 | 19,920 |
| CLF | 21,141 | 20,449 | 19,455 | 19,455 | 19,455 |
| EtAc | 21,551 | 20,876 | 20,161 | 20,161 | 20,161 |
| DCM | 21,321 | 20,533 | 19,493 | 19,493 | 19,493 |
| DCE | 21,186 | 20,576 | 19,841 | 19,841 | 19,841 |
| Acetone | 21,459 | 21,321 | 19,723 | 19,723 | 19,723 |
| Methanol | 21,321 | 20,491 | 19,569 | 19,569 | 19,569 |
| ACN | 21,459 | 20,876 | 19,723 | 19,723 | 19,723 |
| DMF | 21,186 | 20,366 | 19,455 | 19,455 | 19,455 |
| DMSO | 21,008 | 20,202 | 19,193 | 19,193 | 19,193 |
| Method | KAT | Catalan | Laurence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | α | β | π | SA | SB | SP | SdP | DI | ES | α1 | β1 |
| 1,4-Dioxane | 0.0 | 0.37 | 0.55 | 0.000 | 0.444 | 0.737 | 0.312 | 0.77 | 0.36 | 0.00 | 0.44 |
| CCl4 | 0.0 | 0.0 | 0.28 | 0.000 | 0.044 | 0.768 | 0.000 | 0.82 | 0.10 | 0.00 | 0.00 |
| Toluene | 0.0 | 0.11 | 0.54 | 0.000 | 0.128 | 0.782 | 0.284 | 0.86 | 0.20 | 0.00 | 0.15 |
| CLF | 0.44 | 0.0 | 0.58 | 0.047 | 0.071 | 0.783 | 0.614 | 0.80 | 0.40 | 0.20 | 0.00 |
| EtAc | 0.0 | 0.45 | 0.54 | 0.000 | 0.525 | 0.674 | 0.535 | 0.71 | 0.51 | 0.00 | 0.52 |
| DCM | 0.30 | 0.0 | 0.82 | 0.040 | 0.178 | 0.761 | 0.769 | 0.78 | 0.60 | 0.10 | 0.00 |
| DCE | 0.0 | 0.0 | 0.807 | 0.030 | 0.126 | 0.771 | 0.742 | 0.80 | 0.74 | 0.00 | 0.00 |
| Acetone | 0.08 | 0.48 | 0.71 | 0.000 | 0.475 | 0.651 | 0.907 | 0.69 | 0.78 | 0.04 | 0.49 |
| Methanol | 0.93 | 0.62 | 0.60 | 0.605 | 0.545 | 0.608 | 0.904 | 0.64 | 0.84 | 1.00 | 0.54 |
| ACN | 0.19 | 0.31 | 0.75 | 0.044 | 0.286 | 0.645 | 0.974 | 0.67 | 0.84 | 0.23 | 0.37 |
| DMF | 0.0 | 0.69 | 0.87 | 0.031 | 0.613 | 0.759 | 0.977 | 0.78 | 0.87 | 0.00 | 0.69 |
| DMSO | 0.0 | 0.76 | 1.0 | 0.072 | 0.647 | 0.830 | 1.000 | 0.84 | 1.00 | 0.00 | 0.71 |
| Fitting Method | Ligand | y0 × 103 | Correlation Coefficients | R2 | |||
|---|---|---|---|---|---|---|---|
| KAT | aα | bβ | cπ* | ||||
| O1 | 21.70 ± 1.84 | −86.73 ± 1.75 | 56.72 ± 2.17 | −583.31 ± 3.10 | 0.435 | ||
| O2 | 20.60 ± 2.29 | −379.22 ± 2.18 | 200.96 ± 2.70 | −1368.62 ± 3.86 | 0.743 | ||
| O3 | 21.18 ± 2.52 | −228.32 ± 2.40 | −12.44 ± 2.98 | −790.21 ± 4.25 | 0.498 | ||
| O5 | 18.86 ± 2.50 | 376.51 ± 2.31 | 156.34 ± 2.49 | 212.37 ± 3.83 | 0.401 | ||
| O6 | 18.09 ± 2.77 | 110.16 ± 2.63 | 429.86 ± 3.27 | −233.94 ± 4.67 | 0.252 | ||
| Catalan | aSA | bSB | cSP | dSdP | |||
| O1 | 23.39 ± 2.55 | −502.30 ± 1.32 | 85.15 ± 1.00 | −2535.06 ± 3.33 | −308.2 ± 68.26 | 0.943 | |
| O2 | 22.70 ± 2.7 | −822.99 ± 1.42 | 400.93 ± 1.07 | −3447.95 ± 3.58 | −846.20 ± 0.73 | 0.980 | |
| O3 | 23.48 ± 4.16 | −883.34 ± 2.16 | 44.75 ± 1.63 | −3435.86 ± 5.44 | −431.24 ± 1.11 | 0.928 | |
| O5 | 21.35 ± 5.05 | −428.37 ± 2.62 | 415.66 ± 1.98 | −2774.90 ± 6.60 | −484.54 ± 1.35 | 0.861 | |
| O6 | 22.14 ± 13.70 | −1377.65 ± 7.1 | 1141.29 ± 5.38 | −5461.76 ± 17.89 | −336.09 ± 3.66 | 0.743 | |
| Laurence | aDI | bES | cα1 | dβ1 | |||
| O1 | 23.75 ± 4.14 | −2774.5 ± 5.04 | −507.2 ± 1.25 | −305.15 ± 1.14 | 103.52 ± 1.30 | 0.900 | |
| O3 | 23.03 ± 5.14 | −3535.5 ± 6.25 | −1322.9 ± 1.5 | −423.99 ± 1.42 | 588.61 ± 1.61 | 0.955 | |
| O2 | 23.92 ± 6.83 | −3701.7 ± 830 | −754.3 ± 2.06 | −498.44 ± 1.89 | 137.89 ± 2.14 | 0.871 | |
| O5 | 21.61 ± 7.58 | −2869.8 ± 9.21 | −746.93 ± 2.29 | −222.79 ± 2.09 | 480.85 ± 2.37 | 0.792 | |
| O6 | 20.28 ± 8.71 | −2796.9 ± 10.58 | −279.95 ± 2.63 | −175.33 ± 2.40 | 447.12 ± 2.73 | 0.742 | |
| Fitting Method | Ligand | Relative Contribution of Correlation Parameters | Spec.*a (%) | Non-Spec.*b (%) | |||
|---|---|---|---|---|---|---|---|
| Catalan | SA (%) | SB (%) | SP (%) | SdP (%) | |||
| O1 | 14.64 | 2.48 | 73.89 | 8.98 | 17.12 | 82.87 | |
| O2 | 14.91 | 7.26 | 62.48 | 15.33 | 22.18 | 77.81 | |
| O3 | 18.42 | 0.93 | 71.65 | 8.99 | 19.35 | 80.64 | |
| O5 | 10.43 | 10.12 | 67.62 | 11.80 | 20.56 | 79.43 | |
| O6 | 16.56 | 13.72 | 65.67 | 4.04 | 30.28 | 69.71 | |
| Laurence | DI (%) | ES (%) | α1 (%) | β1 (%) | |||
| O1 | 75.18 | 13.74 | 8.26 | 2.80 | 11.07 | 88.92 | |
| O2 | 60.21 | 22.53 | 7.22 | 10.02 | 17.24 | 82.75 | |
| O3 | 72.69 | 14.81 | 9.78 | 2.70 | 12.49 | 87.50 | |
| O5 | 66.42 | 17.28 | 5.15 | 11.12 | 16.28 | 83.71 | |
| O6 | 75.60 | 7.56 | 4.73 | 12.08 | 16.82 | 83.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homocianu, M.; Airinei, A.; Matica, O.-T.; Cristea, M.; Ungureanu, E.-M. Solvent Effects and Metal Ion Recognition in Several Azulenyl-Vinyl-Oxazolones. Symmetry 2023, 15, 327. https://doi.org/10.3390/sym15020327
Homocianu M, Airinei A, Matica O-T, Cristea M, Ungureanu E-M. Solvent Effects and Metal Ion Recognition in Several Azulenyl-Vinyl-Oxazolones. Symmetry. 2023; 15(2):327. https://doi.org/10.3390/sym15020327
Chicago/Turabian StyleHomocianu, Mihaela, Anton Airinei, Ovidiu-Teodor Matica, Mihaela Cristea, and Eleonora-Mihaela Ungureanu. 2023. "Solvent Effects and Metal Ion Recognition in Several Azulenyl-Vinyl-Oxazolones" Symmetry 15, no. 2: 327. https://doi.org/10.3390/sym15020327
APA StyleHomocianu, M., Airinei, A., Matica, O.-T., Cristea, M., & Ungureanu, E.-M. (2023). Solvent Effects and Metal Ion Recognition in Several Azulenyl-Vinyl-Oxazolones. Symmetry, 15(2), 327. https://doi.org/10.3390/sym15020327






