Abstract
Background: A large body of research has shown brain asymmetries in spatial attention. Specifically, there is an attention-processing advantage for the left visual field in healthy, right-handed subjects, known as “pseudoneglect.” Several studies have revealed that emotions modulate this basic spatial phenomenon, but the direction of the effect is still unclear. Here we systematically review empirical evidence on the behavioral effects of emotion on pseudoneglect. Methods: We searched through Pubmed, Scopus, PsycINFO, and PsychArticles. Original peer-reviewed articles published until February 2021 were included if they (1) were written in English; (2) were conducted on adults; (3) included at least one task to measure pseudoneglect, and (4) included at least one task with emotional stimuli or employed a measure of emotional state/trait, as they relate to pseudoneglect. Results: Fifteen studies were included, and 784 healthy participants took part in all studies reviewed. Discussion: The results show some evidence of emotion modulation of pseudoneglect, but evidence on the direction of the effect is mixed. We discuss the role of methodological factors that could account for the available findings and the implications for emotion asymmetry hypotheses such as the right-hemisphere hypothesis, the valence-specific hypothesis, as well as neural and arousal frameworks of attention–emotion interactions.
1. Introduction
Humans show systematic spatial asymmetries when exploring the visual environment. Indeed, many people have the tendency not to attend equally to the left and right sides of the visual field. Averaging across the population, the bias is usually toward the left, but individuals vary, with some exhibiting a left and others exhibiting a right bias. This spatial processing bias in the left hemifield in neurologically typical individuals has been named “pseudoneglect”, as it shows an opposite direction from the rightward bias of patients with right hemisphere damage and visual neglect [1,2]. Despite a large number of behavioral and neurophysiological studies, the causal factors underlying this phenomenon are still unclear. According to the activation–orientation theories, each individual has a more dominant hemisphere (i.e., the left hemisphere is dominant in right-handed individuals) that determines pseudoneglect in the contralateral hemifield, more often the right hemifield [3,4]. Other theories suggest mechanisms that call upon object-based bias [5] or global and local processing, where global spatial tasks predominantly recruit the right hemisphere [6]. Regardless of the theoretical accounts, a common task to assess asymmetries in visuo-spatial attention is visual line bisection, where subjects are asked to manually bisect a horizontal line drawn on paper or select the middle point of a computerized line. The task requires both visual and motor information to make judgments about line length. Although much smaller than that measured in patients with visual neglect, the magnitude of bisection errors in typical individuals appears to be a real phenomenon, not related to methodological artifacts [7].
There are two other common variations of the line bisection task that are generally employed to assess pseudoneglect: rod tactile and landmark tasks. In the rod tactile task, subjects are blindfolded and asked to bisect a centrally aligned rod, usually using their left and right fingers. This task requires tactile and motor information to perform the bisection. Conversely, in the landmark task, lines are pre-bisected (i.e., a mark indicates the bisection point), and participants’ task is to indicate whether the mark is closer to the left or right or whether the left or right side of the pre-bisected line is shorter or longer [7,8]. The landmark task is a non-motor adaptation of the line bisection task, and it requires purely visual information. In fact, in the landmark task, the contribution of spatial attention asymmetries is not entangled with the asymmetry derived from the unilateral motor action [9].
In general, pseudoneglect has been observed across several experimental tasks and sensory modalities, and it seems to be modulated by many biological and cultural factors. In an influential review, Jewell and McCourt [7] showed how pseudoneglect depends on parameters such as gender, age, performing hand, and the direction in which participants initiate motor scanning, either by hand or eye.
Over the last two decades, much research has focused on the influence of emotion on spatial biases in both patients and neurologically intact individuals, based on the strong influence that emotion has on attention in everyday life, on the tight interconnection between the neural mechanisms that mediate these two phenomena, and on the brain lateralization of emotion processing. In this context, spatial attention tasks such as the line bisection have been used in an attempt to disentangle the issue of emotion and attention lateralization by assessing the modulatory effect of emotion on pseudoneglect. The rationale is that if attention is right-lateralized and emotion processing is also right-lateralized (i.e., “right-hemisphere hypothesis” [10]), then both functions concur in shifting the activation balance in favor of the right hemisphere, enhancing the pseudoneglect in the left hemifield. In contrast, if positive emotion is left-lateralized and negative emotion is right-lateralized (i.e., the “valence-specific hypothesis” [11]), only negative emotion should increase the activation of the right hemisphere and enhance pseudoneglect. By the same token, positive emotion should attenuate pseudoneglect in the contralateral hemifield by increasing the activation of the left hemisphere.
The association between emotion and the right hemisphere goes back to the very early neurology literature when Mills [12] observed that patients with a lesion in the right side of the brain had an impairment in emotional expression. The right-hemisphere hypothesis states that the perception of emotional stimuli is related to the activity of the right hemisphere, regardless of affective valence [13,14]. Conversely, the valence-specific hypothesis was based on evidence that lesions in the left frontal lobe were related to negative emotional states while lesions in the right hemisphere were more associated with positive or maniac emotional states [15,16,17]. According to the valence-specific hypothesis, the left hemisphere processes positive emotions, whereas the right hemisphere processes negative emotions [11,18,19]. An alternative view that can be considered a variant of this hypothesis, the “approach–withdrawal” hypothesis, proposes that brain asymmetries observed for positive and negative emotions are related to the underlying motivational system to which positive and negative emotions are linked [20,21,22]. Accordingly, the left prefrontal cortex is involved in processing emotions, such as happiness and anger, which entail an approach toward the stimuli. Conversely, the right prefrontal cortex processes emotions, such as sadness and fear, related to withdrawal from aversive stimuli.
In this context, tasks used to measure the naturally occurring spatial attention biases, such as the line bisection task, have been used to assess the relative contribution of each hemisphere to emotion. This line of research is based on the more or less implicit neural assumptions that: (a) the right posterior temporo-parietal cortex is dominant for allocating attention to contralateral left hemifield; (b) the neural mechanisms underlying emotion and attention are strongly interconnected; (c) processing emotional stimuli induces asymmetrical alterations in brain activation, which in turn cause an attentional bias toward the contralateral hemifield. Therefore, the activated network of regions involved in emotion processing produces a modulation in the attention network that is reflected in a shift in spatial biases. These assumptions make it possible to make some predictions on the possible experimental outcomes concerning the different hypotheses. For instance, the right-hemisphere hypothesis implies that attention and emotion processing engages the same neural substrates in the right hemisphere. Accordingly, processing emotional stimuli (regardless of whether they are faces, words, pictures, or sounds) and orienting attention would additively activate the same brain areas and circuits in the right hemisphere. This joint activation would shift the balance between the left and right hemispheres in favor of the right, enhancing the attention bias toward the left visual field. Importantly, this prediction requires that the effect occurs regardless of emotional valence (and type of stimuli).
Conversely, for the valence-specific hypothesis, processing positive stimuli (or positive affect in general) would preferentially engage the left hemisphere, shifting the balance in activation between the two hemispheres in favor of the left hemisphere, engendering an attention bias toward the right visual field or at least, reducing the bias toward the left. By the same token, processing negative stimuli (or negative affect in general) by preferentially engaging the right hemisphere would shift the activation balance in favor of the right hemisphere, enhancing the attention bias toward the left visual field. However, note that according to the approach-withdrawal hypothesis, these predictions would apply to approach/withdrawal motivation rather than to valence. Therefore, angry and happy faces as approach-related stimuli would shift the balance in hemispheric activation in favor of the left hemisphere.
Despite a large body of research, evidence on the interaction between emotion and spatial attention is still not well understood, and a review of the literature examining whether and how emotion modulates pseudoneglect is lacking. Understanding how spatial attentional biases are affected by emotional processing can also assist clinicians and researchers to understand how this interaction can modulate the strength of contralesional deficits in patients with visual neglect and extinction and ultimately how to employ attentional resources to overcome the deficit.
Here, we review the available research on the influence of emotional processing on pseudoneglect and discuss these results in the context of the theoretical frameworks such as the right-hemisphere hypothesis and the valence-specific hypothesis. A critical analysis of this evidence will contribute to understanding the interplay between emotion and attention and the possible role of brain asymmetries in these two functions.
2. Materials and Methods
The systematic review was conducted according to the PRISMA guidelines (retrieved from http://www.prisma-statement.org/, accessed on 19 January 2021 [23,24,25,26]). The PRISMA protocol consists of a 27-item checklist and a 4-phase flow diagram that guides the systematic review process (see Figure 1).
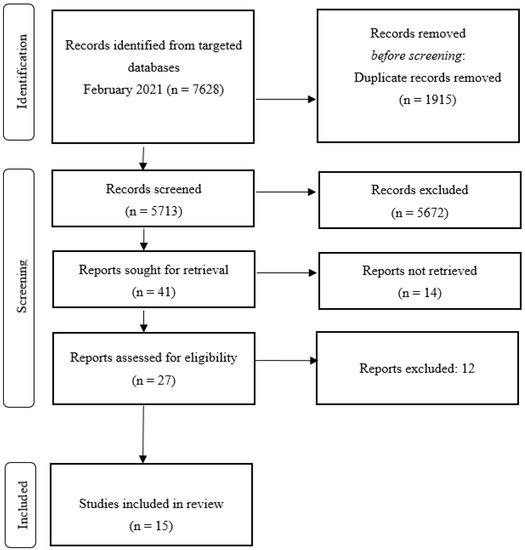
Figure 1.
Flowchart of selection process for included articles.
2.1. Research Strategies
We conducted a systematic search of articles published in peer-reviewed journals in the following electronic databases: PubMed (1949 to February 2021), Scopus (1788 to February 2021), PsycINFO (1806 to February 2021), PsychArticles (1800 to February 2021), and Web of Science (1900 to February 2021). The research was conducted in February 2021. Mendeley reference manager software was used to import the references from the databases and to remove duplicates. The first screening was made by reading the title and abstract. The full text of the selected studies was read. The search strategy used Boolean combinations of the following keywords: “line bisection”, “landmark task”, “pseudoneglect”, “greyscales task”, “grating scales task”, “tactile rod bisection task”, “lateralized visual detection”, “cancellation task”, “emotion*”, “picture”, “word”, “music”, “approach”, “avoidance”, “withdraw”, and “affect”. In addition to systematic searches in the above databases, we also searched for additional articles in the reference lists (i.e., backward research) and the citation searches of the selected articles (i.e., forward research).
2.2. Eligibility Criteria
In this systematic review on the relation between pseudoneglect and emotion, we included studies that fulfilled the following criteria: (1) original, peer-reviewed articles; (2) written in English; (3) conducted on adults; (4) included at least one task to measure pseudoneglect (line bisection task, landmark task, greyscales task, grating scales task, tactile rod bisection task, lateralized visual detection, cancellation task; and (5) included at least one task with emotional stimuli or employed a measure of emotional state/trait as they relate to pseudoneglect (see Table 1). Articles from all publication years were accepted. Exclusion criteria were: studies with samples diagnosed with psychiatric or neuropsychological disorders (e.g., anxiety, depression, bipolar disorder, visual neglect, etc.) and studies conducted with children.

Table 1.
Synthetic description of studies examining the influence of emotion processing on spatial attention biases.
2.3. Data Collection
Descriptive data extraction was performed from each study and included: (a) metadata (i.e., authors and year of publication); (b) information related to the sample (i.e., sample size, age, gender, and handedness); (c) methodological information (emotional and spatial attention measurements); and (d) results. Quality check and accuracy of the first reviewer’s (FS) data extraction was performed by the second reviewer (AP).
3. Results
3.1. Study Selection
The literature search strategy and inclusion criteria yielded 15 studies that measured the relationship between emotional processing and spatial attention pseudoneglect. As shown in Figure 1, the literature search generated 5713 potentially relevant articles (after removing 1915 duplicates).
After title and abstract screening, 5672 were excluded, either because they did not meet the inclusion criteria or they were qualitative studies, reviews, or commentaries. The full texts of the remaining 41 eligible studies were retrieved and reviewed; 26 articles were excluded because they did not employ any emotional measure related to pseudoneglect or were conducted on patients with neuropsychological or neurological disease. This screening resulted in the inclusion of 15 articles for qualitative review.
The sample sizes ranged from 17 to 160, and the sample age from 20 to 66 years old; 56% were women. Almost all articles reported that the participants were right-handed. In total, 784 healthy participants took part in all studies reviewed.
The spatial attention measures of pseudoneglect were as follows: perceptual line bisection task (11 studies), perceptual landmark task (two studies), cancellation task (one study), bisection task, and haptic line bisection (one study). Eleven studies employed valenced stimuli (four with faces, four with words, one with objects and scenes, three abstract and figurative paintings, two with sounds and music). Six studies employed questionnaires to assess participants’ emotional state or trait, one of which related the non-pathological, claustrophobic fear to attentional biases in healthy individuals. Overall, of the 15 selected studies, 11 report a relationship between pseudoneglect and some emotional measure, whereas three studies did not find a significant relationship, and one study found mixed results.
3.2. Visual Stimuli
3.2.1. Faces
Four studies investigated how the presentation of emotional faces with positive and negative valence modulated the performance on the perceptual line bisection and landmark task for a total of nine experiments. These studies showed considerable variability in their results, but also in the methodology used, with one study pointing to emotion enhancing the leftward bias [27], two studies showing that positive emotion induces a rightward bias (i.e., attenuates the leftward bias) [28,29], and one study reporting mixed results [30].
Specifically, Armaghani et al. [27] used a paper-and-pencil line bisection flanked, on one or both sides, by emotional and neutral faces. Faces were black-and-white photographs of the same actress presented near the extremities of the line. When two faces were presented, they could have the same or different expressions. The authors found that happy and sad faces enhanced the leftward bias compared to neutral faces. In contrast, Cattaneo et al. [28] used a computerized line bisection task flanked by identical emotional faces presented at the two extremities and found that happy faces reduced the leftward bias compared to sad and neutral faces. Similarly, Hatin and Tottenham [29] used a line bisection task, in which the line was made of words or faces, and found that happy and angry faces elicited a rightward bias.
Finally, Leggett et al. [30] used emotional primes (happy, angry, and neutral faces) and a perceptual landmark task. Differently from the other studies, which used a self-paced bisection task, the line was presented for a brief interval (500 ms), and emotional faces were presented before (rather than concurrently with) the line. In the first of five experiments, they found that angry face-primes yield a leftward shift compared to happy face-primes, although happy and angry faces did not elicit biases that were different from those elicited by neutral faces. However, these findings were not replicated when combining the data of all experiments, including experiment 5, which was a direct replication of the first experiment.
Importantly, the methodology used differed substantially between these studies, including aspects such as the spatial position of the faces in relation to the line, the temporal presentation of the stimuli (sequential vs. simultaneous), and the spatial attention measure (line bisection vs. landmark task).
3.2.2. Words
Four studies investigated whether the presentation of emotional words with positive and negative valence modulated the performance on the perceptual line bisection and landmark task. Of these, one study reported that emotional words enhance the rightward bias [31], one reported that negative words attenuate the rightward bias [32], one study reported that emotional words enhance the leftward bias [29], and one reported a positive relationship between the detection of negative words and leftward bias [33].
More specifically, Mohr et al. [31] and Hatin and Tottenham [29] employed a variation of the Character-Line Bisection Task (CLBT, [34,35]), originally created to assess visual neglect, combining the line bisection and cancellation tasks. They used lines made of strings of letters that spelled out words with neutral or emotional connotations, and subjects were required to mark the center of the line. Using this task, Mohr et al. [31] conducted four experiments and found that lines made of emotional words enhanced rightward biases compared to lines made of neutral words. In contrast, Hatin and Tottenham [29], using positive words, reported that positive words significantly enhanced the leftward bias. The other two studies presented a word-prime before the line bisection [33] or before the landmark task [32]. Tamagni et al. [33] asked participants to perform a lateralized word detection task. Words were individually presented for 116 ms, and the subjects’ task was to detect them as fast as possible. Subjects also performed a paper-and-pencil line bisection task. Findings showed that better detection of negative words correlated with a leftward bias. Consistent with this result, Milhau et al. [32] presented negative emotional prime-words for 500 ms, and subjects were instructed to memorize as many words as possible for a subsequent recall task at the end of the session and perform the landmark task using either their right or left hand. The authors reported that negative words reduced the rightward bias for right-handers (i.e., shifted the bias leftwards), but positive words reduced rightward bias for left-handers.
Overall, and again with much variability across studies, one study reported that emotions enhance the rightward bias [31], two of the four studies found that negative words shift the bias leftward [32,33], and one showed that positive words enhance the leftward bias [29].
In summary, and regardless of whether studies used emotional faces or words, three reported that emotion induces a rightward bias (or attenuates the leftward bias): one study with emotional words [31], one with angry and happy faces [29], and one with happy faces [28]. Four studies reported that emotion induces a leftward bias (or attenuates the rightward bias): one study with happy and sad faces [27] and three studies with negative words [29,32,33]. One study with faces and words reported mixed results [30]. These findings would suggest that the category of stimuli used (faces vs. words) may also be an important factor to consider (see Discussion).
3.2.3. Artwork
Three studies examined how aesthetic judgments of paintings with emotional connotations affect pseudoneglect [36,37,38]. Drago et al. [36] asked subjects to rate the evocative impact of each painting and to perform a paper-and-pencil line bisection task after painting viewing. They found that the evocative impact of the painting correlated with the line bisection bias (i.e., stronger emotional evocation—regardless of valence—was related to smaller rightward bias). However, Hatin and Tottenham [37] reported different findings when using the same paintings as Drago et al., presented in a mirrored and non-mirrored version. Using visual-analog and numeric scales, either ascending and descending, subjects rated the evocative impact of each painting and its mirror version, after which they performed a paper-and-pencil line bisection task. There was no effect of the painting on the line bisection. They showed that left-hand line bisection bias, not line bisection accuracy, is mainly related to the ratings and that the line bisection bias interacts with the symmetry in the paintings (mirrored/non-mirrored) and with the rating scale direction (ascending/descending). Therefore, the authors argued that these factors, rather than the emotional impact of the paintings, account for the results reported by Drago et al. [36]. Finally, Ciricugno et al. [38] asked subjects to bisect a line, which could be superimposed on a gray screen, painting, or photograph of real-world scenes with emotionally neutral connotations and found that paintings enhanced the bias to the left compared to the photographs and baseline.
In summary, studies using artwork do not provide clear evidence on the effects of emotion on pseudoneglect.
3.3. Auditory Stimuli
Two studies have assessed whether auditory stimuli modulate participants’ current emotional state and how this, in turn, affects pseudoneglect. Cattaneo et al. [28] employed a haptic line bisection paradigm while listening to emotional vocal sounds. They found that laughing attenuated the leftward bias compared to crying and neutral sounds. Similarly, Hausman et al. [39] asked subjects to listen for 10 min to happy or sad classical music or sit in silence and then to perform a line bisection task. They found that listening to classical music with a happy connotation produced a rightward bias compared to sitting in silence and listening to sad music.
In summary, both studies conducted with auditory stimuli revealed that listening to happy or sad auditory stimuli significantly changed emotional states in the predicted direction, inducing positive or negative emotions. Importantly, these changes in the subjects’ positive emotional states affected spatial biases in the rightward direction.
3.4. Emotional Traits, Hormones, Stress, and Spatial Distance
One study [40] investigated the relationship between emotional traits as measured by the Positive and Negative Affect Schedule, PANAS [41], and pseudoneglect. In particular, they assessed how individuals described themselves as having more positive or negative emotions in everyday life. The results showed that individuals with self-reported positive affect showed a rightward bias in the line bisection task (i.e., positive correlation between positive affect and rightward bias).
Another study [42] assessed the influence of sex hormones on emotions, as measured by the State–Trait–Cheerfulness Inventory (STCI-S18; [43]), and pseudoneglect during menses the mid-luteal cycle phase in normally cycling women. The authors found no effects of mood on a paper-and-pencil line bisection task.
Somma et al. [44] conducted a longitudinal study on the effects of stress on pseudoneglect induced by the strict lockdown rules during the COVID-19 pandemic in Italy. The authors asked subjects to perform a computerized cancellation task (i.e., to cancel all the stimuli as fast as possible using a stylus pen touch or a mouse click) one week before the start of the lockdown and during the following two months during the lockdown. They found that better-coping participants—as assessed by Positive Attitude and Problem Solving COPE-NIV subscales [45]—showed a smaller leftward bias in the cancellation task compared to low-coping participants.
Finally, Lourenco et al. [46] examined whether trait feelings of claustrophobic fear, measured with the CLQ claustrophobia questionnaire [47], predicted spatial biases. Subjects performed a line bisection task using a laser pointer from nine different distances. Lines were centered on a legal-sized paper and attached horizontally to a wall. Results showed that subjects who reported greater claustrophobic fear had more gradual rightward shifts at a shorter distance (i.e., greater for near spaces) than those with less claustrophobic fear.
In summary, evidence on the effects of self-reported affect and traits on pseudoneglect show that positive affect [40] and positive attitude [44] are correlated with a rightward bias. Moreover, greater self-reported claustrophobic fear is related to a rightward bias when the line bisection is performed at a short distance [46].
4. Discussion
This systematic qualitative review is the first to evaluate the effect of emotion on pseudoneglect in healthy adults. Of the 15 studies meeting the inclusion criteria, 11 studies used visual stimuli, such as faces, words, and pictures with emotional connotations. The main finding is that the majority of the studies found that pseudoneglect was modulated by emotional stimuli or by participants’ self-reported emotional state or trait. However, evidence on the direction of these effects is less clear-cut, as some studies show that emotion enhances the leftward spatial attention bias, others report effects in the opposite direction, while others find that the direction of the effect is valence dependent. Importantly, one issue makes strict comparisons difficult, and this is related to the existence of substantial methodological differences across studies. This aspect implies that the heterogeneity in the observed findings could be due to the effect of emotion on pseudoneglect being inconsistent or to the different tasks and stimuli used. Therefore, the following discussion aims at clarifying the role of the different factors in accounting for the extant evidence on emotion modulation of pseudoneglect.
We will first discuss the findings with regard to the theoretical frameworks of the right-hemisphere hypothesis and the valence-specific hypothesis; secondly, we will relate them to the neural and arousal frameworks of attention–emotion interactions.
Brain Asymmetries in Emotion Processing
In recent decades, much debate has addressed the extent to which the left and right hemispheres contribute to emotion processing. Despite new insights provided by several behavioral, neuropsychological, and neurophysiological studies, the role of brain asymmetries in emotional processing remains uncertain. While there is some evidence of a relationship between emotion processing and the right hemisphere (e.g., [48]), other evidence suggests that the processing of positive emotions and approach motivation relies on the left hemisphere (e.g., [49,50]). These diverging findings have led to the formulation of two main proposals on brain lateralization of emotional perception: the “right-hemisphere hypothesis” [48] and the “valence-specific hypothesis” [11].
The present review shows that the findings of three studies are compatible with the right-hemisphere hypothesis, five with the valence-specific hypothesis, and six with neither of these hypotheses, as they report biases in directions opposite to those expected by the right and valence hypotheses (Table 2). One could argue whether using spatial attention tasks, such as line bisection, is the best strategy for disentangling the relative contributions of the two hemispheres to emotion and attention and their interactions. However, it is important to note that the emotion lateralization hypotheses are grounded upon neurophysiological studies and cannot be easily dismissed. In fact, a large body of research suggests a right hemisphere dominance for the perception and expression of emotions across primate phylogeny, from Old World monkeys to humans [51]. In particular, in humans, it has been shown that the right temporo-parietal regions contain three maps coding polarity, complexity, and intensity of emotional experiences [52]. Nevertheless, there is also neuroimaging evidence suggesting a more complex interaction between subcortical and cortical regions—in the anterior and posterior parts of the brain as well as in the left and right hemispheres—underlying emotion processing [53]. Most likely, what contributes to the complex picture that emerges from the literature is an additional neural factor to consider, which is related not only to which hemisphere may be preferentially involved in processing emotion but also to which hemisphere is preferentially involved in processing the specific category (e.g., faces, words, sounds, etc.) of the stimuli used and their relative position in the visual field (i.e., central vs. peripheral presentation). For instance, visual stimuli such as faces and words likely activate networks of non-parietal visual category-selective regions that include the right fusiform face area [54] and the left visual word form area [55]. Therefore, findings could be more clear-cut and in favor of the valence-specific hypothesis when the category of stimuli used (i.e., words) and emotion (i.e., positive or approach-related) rely on the activity of the left brain hemisphere, which will be additively activated, reducing pseudoneglect. By the same token, findings could be more in favor of the right-hemisphere hypothesis when the category of stimuli used (i.e., faces) and emotion rely on the activity of the right brain hemisphere, enhancing pseudoneglect. On the other hand, when stimuli activate one brain hemisphere (e.g., words and the left hemisphere) but emotion activates the other, the relative difference in brain activation between the two hemispheres may be insufficiently substantial to affect pseudoneglect. In addition, while visual processing shows contralateral dominance in the early visual cortex, auditory processing shows weak contralateral dominance in the auditory cortex. These factors might explain the different findings across studies performed using auditory vs. visual stimuli. Further studies are needed to assess the differential contribution of these visual and auditory networks in the neural emotion–attention interaction when using different categories of stimuli.

Table 2.
Attention biases. Summary of the evidence on emotion effects on pseudoneglect and direction of the effects. The second column shows the type of emotional measure or stimulus used. “State-trait” refers to the use of questionnaires. The third column shows the average spatial attention bias at baseline. The fourth column reports the direction (leftward or rightward) of the emotion modulation on the attention biases. The last two columns report the findings in relation to the right-hemisphere hypothesis and the valence hypothesis.
Aside from this issue, a further complication stems from other methodological differences between studies that may contribute to the findings. First, there are substantial differences between studies using the experimental design and analysis approaches. For instance, the time between presenting the emotional stimuli and spatial attention tasks varies, with some employing simultaneous and others sequential presentation. This difference does not rule out low-level variables (such as surround suppression) due to simultaneous versus sequential stimulus presentation that might contribute to the attention bias [56,57]. In addition, some studies present the line flanked by two emotional stimuli and some others flanked by just one stimulus on the left or right side of the line. Importantly, there is evidence that contextual stimuli may influence the localization of the subjective midpoint, biasing the bisection away from the location of the flanker [58]. Indeed, using one flanker seems to increase the attentional load for extracting the segment from the background and reduce the salience of the flanked-line segment [59].
At a statistical level, quantitative comparisons between studies are difficult to make because even considering only a few studies with similar tasks or paradigms; many do not report a measure of the effect size or comparable statistics (e.g., [27,28]). Moreover, positive and negative stimuli were not always matched according to specific parameters such as emotional intensity or arousal and recognizability (for facial or vocal expressions). In particular, arousal represents a core dimension for emotional processing that helps detect a threat and, consequently, initiate appropriate approach/avoidance behaviors (fight or flight response) [60]. The interactions between emotional arousal and attention encompass many stages of processing, from early to higher-order (e.g., [61]). These interactions have been explained within the framework of the arousal-biased competition theory [62], largely based on the biased competition theory (e.g., [63,64,65]). According to this theory, arousal can enhance the processing of salient stimuli or impair the processing of non-salient stimuli. Despite the pervasive connection between emotional arousal and attention, most studies reviewed here did not control or manipulate arousal, probably due to the intrinsic difficulty of separating arousal from valence. Indeed, the stimuli employed to induce negative and positive emotions also modulate arousal levels [66]. Moreover, although arousal and valence traditionally have been considered separate psychological dimensions [67], some recent studies have challenged this view, suggesting that the two dimensions cannot be disentangled at the neural level [68].
Finally, there are individual differences in the attention bias at baseline, with some studies reporting a leftward and others a rightward bias. This variability does not seem to predict the direction of changes driven by the emotional modulation of the bisection bias (see Table 2, third and fourth column). A fruitful approach for future studies would be to factor in the experimental design the individual difference at baseline and compare brain activation asymmetries at baseline and during the task.
5. Conclusions
Several limitations should be considered in the interpretation of our results. First, there is a possible language bias since the research strategy was limited to articles published in English, and we did not include unpublished studies. Furthermore, the small number of studies available, together with the methodological difference in design, paradigms, statistics, and stimuli, only allowed a qualitative review, and a meta-analysis was not possible.
In general, the present review shows some evidence of an interactive effect between emotion and attention biases, although the direction of this effect is variable. We discussed the different factors that may contribute to this variability, and we hope that it would prompt researchers to design studies aimed at disentangling the relative contribution of stimulus category, stimuli presentation, task, as well as the role of arousal and of individual differences at baseline. Importantly, the research question as to how emotion and attention interact in biasing the activation of one hemisphere over the other has implications not only for understanding the typical brain functioning across the lifespan, particularly with regard to the changes in brain asymmetries occurring with aging but also for clinical application in case of neuropsychological deficits. Indeed, several studies have shown that emotion improves visual attention (e.g., [69]) and awareness (e.g., [70]), and reduces bisection error associated with visual neglect syndrome (e.g., [71,72,73,74,75]).
Regardless of the specific neural mechanism involved and of cortical asymmetric dominance, the present review points to at least some modulatory effect of emotion on attention. Future research focusing on replicability and generalizability of the effects could also help clarify the relative contribution of factors related to task and stimuli used and determine the optimal paradigm to assess the modulation of systematic shifts in the individual’s spatial biases.
Author Contributions
Conceptualization, A.P. and F.S.; methodology, A.P. and F.S.; software, A.P. and F.S.; validation, A.P. and F.S.; formal analysis, A.P. and F.S.; investigation, A.P. and F.S. resources, A.P.; data curation, A.P. and F.S.; writing—original draft preparation, F.S.; writing—review and editing, A.P., F.S. and G.G.; visualization, F.S.; supervision, A.P.; project administration, A.P.; F.S.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
AP is funded by the Italian Ministry of University and Research: RM120172B77EE5F8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data retrieved using the specified criteria is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowers, D.; Heilman, K.M. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia 1980, 18, 491–498. [Google Scholar] [CrossRef]
- Toba, M.N.; Cavanagh, P.; Bartolomeo, P. Attention biases the perceived midpoint of horizontal lines. Neuropsychologia 2011, 49, 238–246. [Google Scholar] [CrossRef]
- Nicholls, M.E.; Roberts, G.R. Can free-viewing perceptual asymmetries be explained by scanning, pre-motor or attentional biases? Cortex 2002, 38, 113–136. [Google Scholar] [CrossRef]
- Bultitude, J.H.; Davies, A.M.A. Putting attention on the line: Investigating the activation–orientation hypothesis of pseudoneglect. Neuropsychologia 2006, 44, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.A.; Nicholls, M.E. The nature and contribution of space-and object-based attentional biases to free-viewing perceptual asymmetries. Exp. Brain Res. 2005, 162, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Dapretto, M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. NeuroImage 2006, 29, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]
- Milner, A.D.; Brechmann, M.; Pagliarini, L. To halve and to halve not: An analysis of line bisection judgements in normal subjects. Neuropsychologia 1992, 30, 515–526. [Google Scholar] [CrossRef]
- Learmonth, G.; Papadatou-Pastou, M. A meta-analysis of line bisection and landmark task performance in older adults. Neuropsychol. Rev. 2021, 1–20. [Google Scholar] [CrossRef]
- Borod, J.C.; Cicero, B.A.; Obler, L.K.; Welkowitz, J.; Erhan, H.M.; Santschi, C.; Grunwald, I.S.; Agosti, R.M.; Whalen, J.R. Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology 1998, 12, 446. [Google Scholar] [CrossRef]
- Ahern, G.L.; Schwartz, G.E. Differential lateralization for positive versus negative emotion. Neuropsychologia 1979, 17, 693–698. [Google Scholar] [CrossRef]
- Mills, C.K. The cerebral mechanisms of emotional expression. Trans. Coll. Physicians 1912, 34, 381–390. [Google Scholar]
- Gainotti, G. Studies on the functional organization of the minor hemisphere. Int. J. Ment. Health 1972, 1, 78–82. [Google Scholar] [CrossRef]
- Levy, J.; Heller, W.; Banich, M.T.; Burton, L.A. Are variations among right-handed individuals in perceptual asymmetries caused by characteristic arousal differences between hemispheres? J. Exp. Psychol. Hum. Percept. Perform. 1983, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Silberman, E.K.; Weingartner, H. Hemispheric lateralization of functions related to emotion. Brain Cogn. 1986, 5, 322–353. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Greenberg, M.S.; Weiman, A.L.; Gur, R.C.; Hungerbuhler, J.P.; Geschwind, N. Hemispheric asymmetry in the expression of positive and negative emotions: Neurologic evidence. Arch. Neurol. 1982, 39, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, K. The significance of special mental tests for diagnosis and prognosis in schizophrenia. Am. J. Psychiatry 1939, 96, 575–588. [Google Scholar] [CrossRef]
- Adolphs, R.; Jansari, A.; Tranel, D. Hemispheric perception of emotional valence from facial expressions. Neuropsychology 2001, 15, 516. [Google Scholar] [CrossRef] [PubMed]
- Wedding, D.; Stalans, L. Hemispheric differences in the perception of positive and negative faces. Int. J. Neurosci. 1985, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Emotion and affective style: Hemispheric substrates. Psychol. Sci. 1992, 3, 39–43. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992, 20, 125–151. [Google Scholar] [CrossRef]
- Carver, C.S.; Harmon-Jones, E. Anger is an approach-related affect: Evidence and implications. Psychol. Bull. 2009, 135, 183. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Linee guida per il reporting di revisioni sistematiche e meta-analisi: Il PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Hetrick, S.E.; Page, M.J. Updated reporting guidance for systematic reviews: Introducing PRISMA 2020 to readers of the Journal of Affective Disorders. J. Affect. Disord. 2021, 292, 56–57. [Google Scholar] [CrossRef]
- Armaghani, S.J.; Crucian, G.P.; Heilman, K.M. The influence of emotional faces on the spatial allocation of attention. Brain Cogn. 2014, 91, 108–112. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Lega, C.; Boehringer, J.; Gallucci, M.; Girelli, L.; Carbon, C.C. Happiness takes you right: The effect of emotional stimuli on line bisection. Cogn. Emot. 2014, 28, 325–344. [Google Scholar] [CrossRef]
- Hatin, B.; Sykes Tottenham, L. What’s in a line? Verbal, facial, and emotional influences on the line bisection task. Laterality: Asymmetries of Body. Brain Cogn. 2016, 21, 689–708. [Google Scholar]
- Leggett, N.C.; Thomas, N.A.; Nicholls, M.E. End of the line: Line bisection, an unreliable measure of approach and avoidance motivation. Cogn. Emot. 2016, 30, 1164–1179. [Google Scholar] [CrossRef]
- Mohr, C.; Leonards, U. Rightward bisection errors for letter lines: The role of semantic information. Neuropsychologia 2007, 45, 295–304. [Google Scholar] [CrossRef]
- Milhau, A.; Brouillet, T.; Dru, V.; Coello, Y.; Brouillet, D. Valence activates motor fluency simulation and biases perceptual judgment. Psychol. Res. 2017, 81, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Tamagni, C.; Mantei, T.; Brugger, P. Emotion and space: Lateralized emotional word detection depends on line bisection bias. Neuroscience 2009, 162, 1101–1105. [Google Scholar] [CrossRef]
- Na, D.L.; Adair, J.C.; Choi, S.H.; Seo, D.W.; Kang, Y.; Heilman, K.M. Ipsilesional versus contralesional neglect depends on attentional demands. Cortex 2000, 36, 455–467. [Google Scholar] [CrossRef]
- Lee, B.H.; Kang, S.J.; Park, J.M.; Son, Y.; Lee, K.H.; Adair, J.C.; Heilman, K.M.; Na, D.L. The Character-line Bisection Task: A new test for hemispatial neglect. Neuropsychologia 2004, 42, 1715–1724. [Google Scholar] [CrossRef]
- Drago, V.; Finney, G.R.; Foster, P.S.; Amengual, A.; Jeong, Y.; Mizuno, T.; Crucian, G.P.; Heilman, K.M. Spatial-attention and emotional evocation: Line bisection performance and visual art emotional evocation. Brain Cogn. 2008, 66, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hatin, B.; Sykes Tottenham, L. The relationship between line bisection performance and emotion processing: Where do you draw the line? Laterality Asymmetries Body Brain Cogn. 2016, 21, 709–731. [Google Scholar] [CrossRef]
- Ciricugno, A.; Ferrari, C.; Rusconi, M.L.; Cattaneo, Z. Viewing of figurative paintings affects pseudoneglect as measured by line bisection. Atten. Percept. Psychophys. 2020, 82, 3795–3803. [Google Scholar] [CrossRef]
- Hausmann, M.; Hodgetts, S.; Eerola, T. Music-induced changes in functional cerebral asymmetries. Brain Cogn. 2016, 104, 58–71. [Google Scholar] [CrossRef]
- Drake, R.; Myers, L. Visual attention, emotion, and action tendency: Feeling active or passive. Cogn. Emot. 2006, 20, 608–622. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063. [Google Scholar] [CrossRef]
- Hausmann, M. Hemispheric asymmetry in spatial attention across the menstrual cycle. Neuropsychologia 2005, 43, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Ruch, W.; Köhler, G.; Van Thriel, C. Assessing the “humorous temperament”: Construction of the facet and standard trait forms of the State-Trait-Cheerfulness-Inventory—STCI. Humor 1996, 9, 303–340. [Google Scholar] [CrossRef]
- Somma, F.; Bartolomeo, P.; Vallone, F.; Argiuolo, A.; Cerrato, A.; Miglino, O.; Mandolesi, L.; Zurlo, M.C.; Gigliotta, O. Further to the left: Stress-induced increase of spatial pseudoneglect during the COVID-19 lockdown. Front. Psychol. 2021, 12, 573846. [Google Scholar] [CrossRef] [PubMed]
- Sica, C.; Magni, C.; Ghisi, M.; Altoè, G.; Sighinolfi, C.; Chiri, L.R.; Franceschini, S. Coping Orientation to Problems Experienced-Nuova Versione Italiana (COPE-NVI): Uno strumento per la misura degli stili di coping. Psicoter. Cogn. E Comport. 2008, 14, 27. [Google Scholar]
- Lourenco, S.F.; Longo, M.R.; Pathman, T. Near space and its relation to claustrophobic fear. Cognition 2011, 119, 448–453. [Google Scholar] [CrossRef]
- Radomsky, A.S.; Rachman, S.; Thordarson, D.S.; McIsaac, H.K.; Teachman, B.A. The claustrophobia questionnaire. J. Anxiety Disord. 2001, 15, 287–297. [Google Scholar] [CrossRef]
- Demaree, H.A.; Everhart, D.E.; Youngstrom, E.A.; Harrison, D.W. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance”. Behav. Cogn. Neurosci. Rev. 2005, 4, 3–20. [Google Scholar] [CrossRef]
- Jansari, A.; Rodway, P.; Goncalves, S. Identifying facial emotions: Valence specific effects and an exploration of the effects of viewer gender. Brain Cogn. 2011, 76, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.T.; Aznar-Casanova, J.A.; Fukusima, S.S. Patterns of brain asymmetry in the perception of positive and negative facial expressions. Laterality Asymmetries Body Brain Cogn. 2009, 14, 256–272. [Google Scholar] [CrossRef]
- Lindell, A.K. The silent social/emotional signals in left and right cheek poses: A literature review. Laterality Asymmetries Body Brain Cogn. 2013, 18, 612–624. [Google Scholar] [CrossRef]
- Lettieri, G.; Handjaras, G.; Ricciardi, E.; Leo, A.; Papale, P.; Betta, M.; Pietrini, P.; Cecchetti, L. Emotionotopy in the human right temporo-parietal cortex. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.C.; Nimmo-Smith, I.A.N.; Lawrence, A.D. Functional neuroanatomy of emotions: A meta-analysis. Cogn. Affect. Behav. Neurosci. 2003, 3, 207–233. [Google Scholar] [CrossRef]
- Kanwisher, N.; Yovel, G. The fusiform face area: A cortical region specialized for the perception of faces. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 2109–2128. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, M.; Hutzler, F.; Wimmer, H.; Mair, A.; Staffen, W.; Ladurner, G. The visual word form area and the frequency with which words are encountered: Evidence from a parametric fMRI study. Neuroimage 2004, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kastner, S.; De Weerd, P.; Desimone, R.; Ungerleider, L.G. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 1998, 282, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Kastner, S.; De Weerd, P.; Pinsk, M.A.; Elizondo, M.I.; Desimone, R.; Ungerleider, L.G. Modulation of sensory suppression: Implications for receptive field sizes in the human visual cortex. J. Neurophysiol. 2001, 86, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Ricci, M. Influence of contextual stimuli on line bisection. Percept. Mot. Ski. 2002, 95, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Iavarone, A.; Viggiano, A.; Monda, M.; Carlomagno, S. Effect of a visual distractor on line bisection. Exp. Brain Res. 2012, 219, 489–498. [Google Scholar] [CrossRef]
- Colibazzi, T.; Posner, J.; Wang, Z.; Gorman, D.; Gerber, A.; Yu, S.; Zhu, H.; Kangarlu, A.; Duan, Y.; Russell, J.A.; et al. Neural systems subserving valence and arousal during the experience of induced emotions. Emotion 2010, 10, 377. [Google Scholar] [CrossRef]
- Pessoa, L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Sutherland, M.R. Arousal-biased competition in perception and memory. Perspect. Psychol. Sci. 2011, 6, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Vecera, S.P.; Farah, M.J. Does visual attention select objects or locations? J. Exp. Psychol. Gen. 1994, 123, 146. [Google Scholar] [CrossRef] [PubMed]
- Desimone, R.; Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Lee, T.S. A unified model of spatial and object attention based on inter-cortical biased competition. Neurocomputing 2002, 44, 775–781. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Satpute, A.B.; Wager, T.D.; Weber, J.; Barrett, L.F. The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex 2016, 26, 1910–1922. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Haj-Ali, H.; Anderson, A.K.; Kron, A. Comparing three models of arousal in the human brain. Soc. Cogn. Affect. Neurosci. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Petrucci, M.; Pecchinenda, A. Sparing and impairing: Emotion-induced modulation of the attentional blink and the extended sparing in a 3-targets RSVP task. Atten. Percept. Psychophys. 2018, 80, 439–452. [Google Scholar] [CrossRef]
- Pecchinenda, A.; Monachesi, B.; Laeng, B. Fearful expressions of rapidly presented hybrid-faces modulate the lag 1sparing in the attentional blink. Acta Psychol. 2020, 209, 103124. [Google Scholar] [CrossRef]
- Tamietto, M.; Latini Corazzini, L.; Pia, L.; Zettin, M.; Gionco, M.; Geminiani, G. Effects of emotional face cueing on line bisection in neglect: A single case study. Neurocase 2005, 11, 399–404. [Google Scholar] [CrossRef]
- Soto, D.; Funes, M.J.; Guzmán-García, A.; Warbrick, T.; Rotshtein, P.; Humphreys, G.W. Pleasant music overcomes the loss of awareness in patients with visual neglect. Proc. Natl. Acad. Sci. USA 2009, 106, 6011–6016. [Google Scholar] [CrossRef]
- Tsai, P.L.; Chen, M.C.; Huang, Y.T.; Lin, K.C.; Chen, K.L.; Hsu, Y.W. Listening to classical music ameliorates unilateral neglect after stroke. Am. J. Occup. Ther. 2013, 67, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Thaut, M.H. Musical neglect training for chronic persistent unilateral visual neglect post-stroke. Front. Neurol. 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Niedeggen, M. Pleasant music improves explicit but not implicit processing in spatial neglect and extinction. Psychomusicology Music Mind Brain 2020, 30, 189–201. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).