Design and Catalytic Behaviour of Hosted in Activated Carbon Foam CoxZn1−xFe2O4 Ferrites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Characterization
2.3. Catalytic Test
3. Results and Discussion
3.1. Catalyst Supports Characterization
3.2. Catalysts Characterization
3.2.1. Texture and Surface Parameters
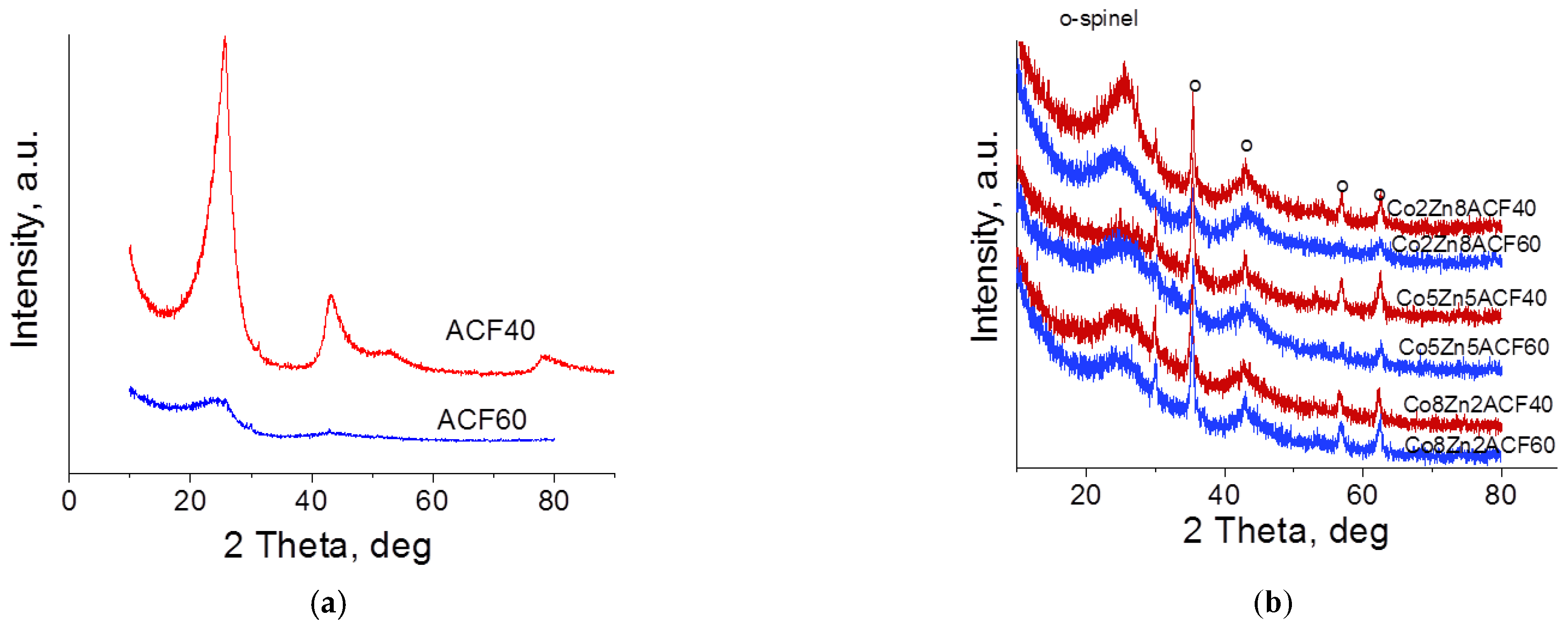
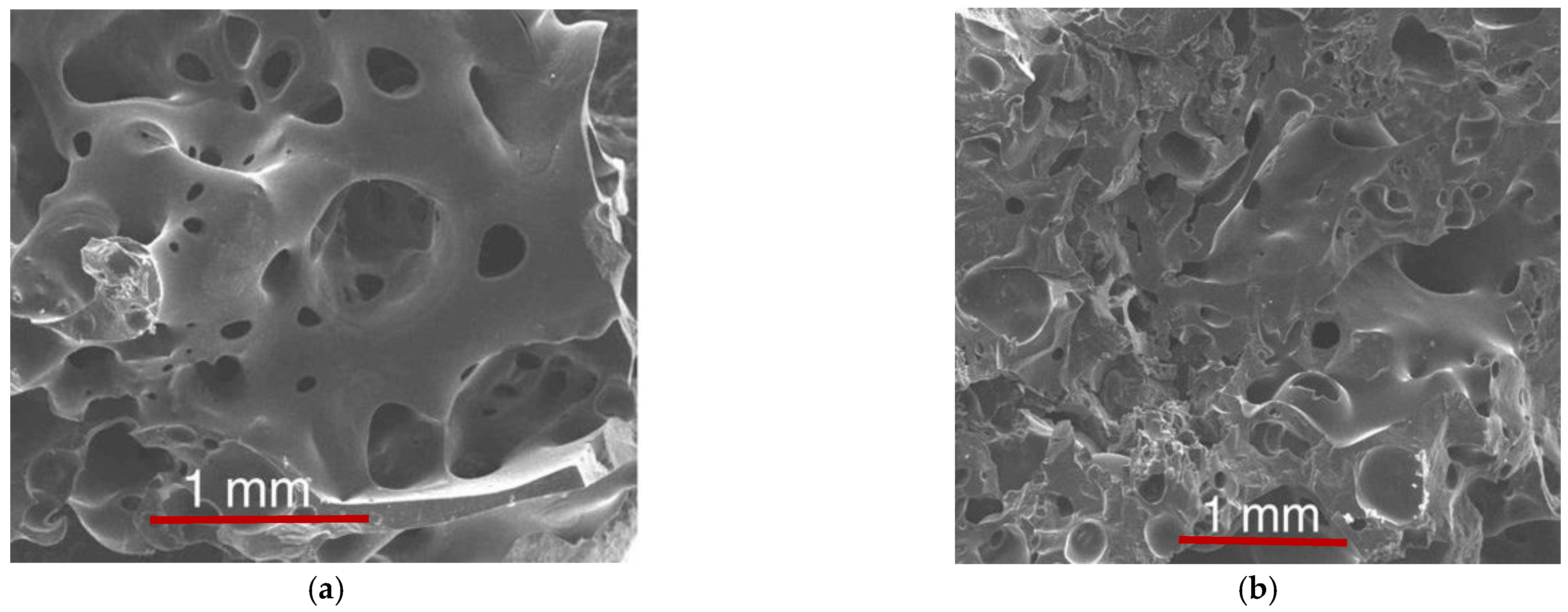
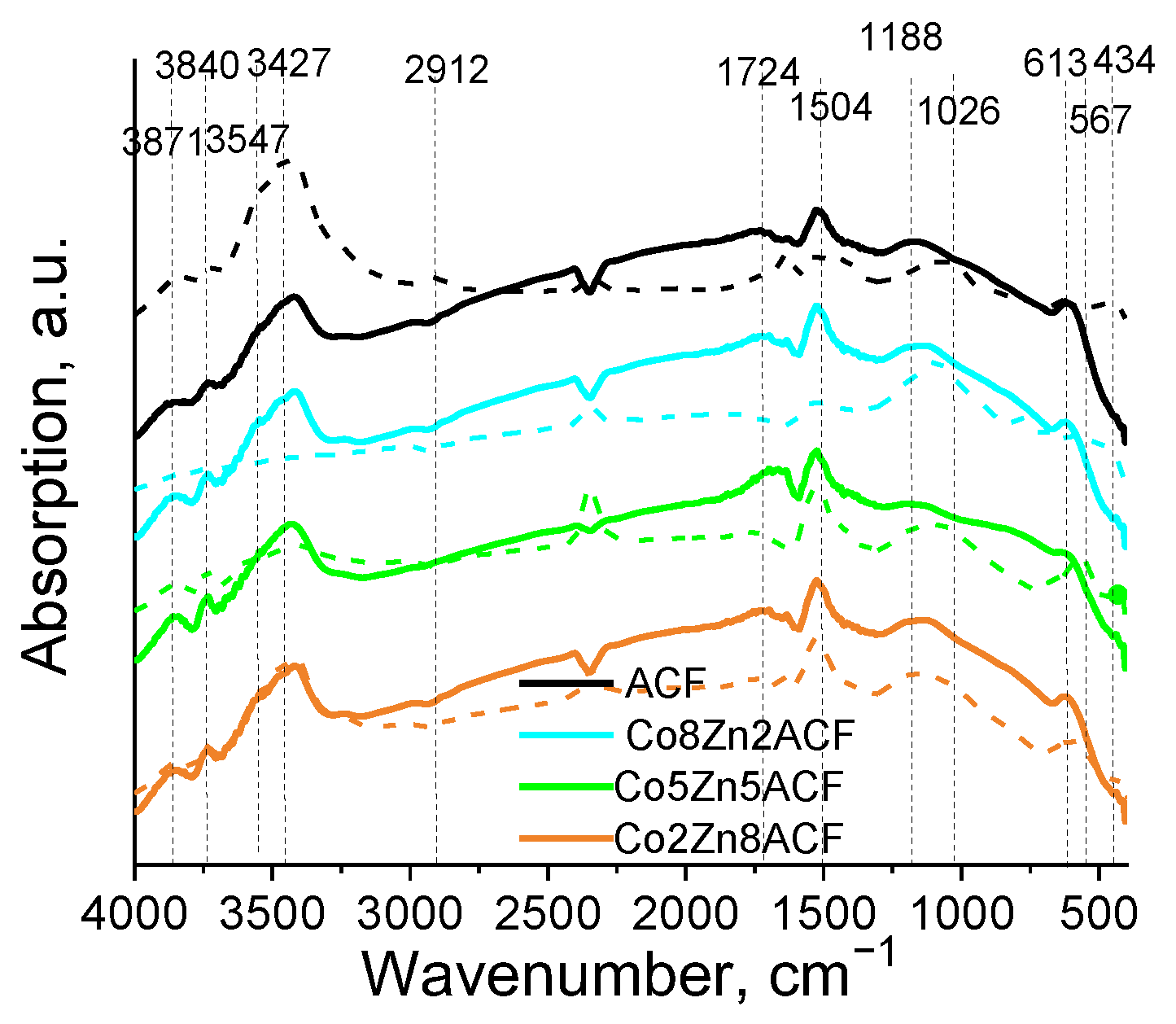
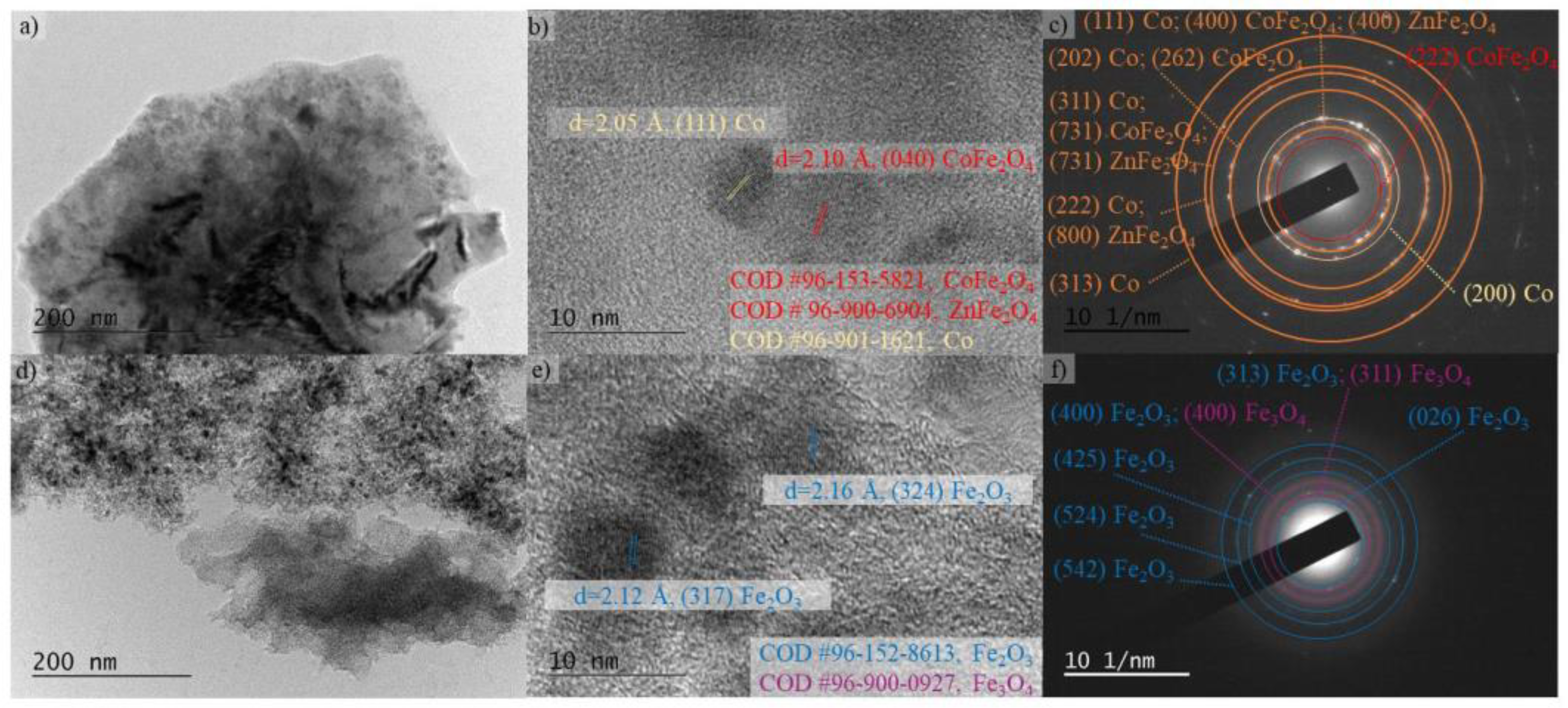
3.2.2. Structure, Morphology and Phase Composition
3.2.3. Temperature-Programmed Reduction-Thermo-Gravimetric Analyses (TPR-TG)
3.2.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roco, M.C. The emergence and policy implications of converging new technologies integrated from the nanoscale. J. Nanopart. Res. 2005, 7, 129–143. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Lehner, R.; Wang, X.; Marsch, S.; Hunziker, P. Intelligent nanomaterials for medicine: Carrier platforms and targeting strategies in the context of clinical application. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 742–757. [Google Scholar] [CrossRef]
- Keçili, R.; Büyüktiryaki, S.; Mustansar, C. Advancement in bioanalytical science through nanotechnology: Past, present and future. Trends Anal. Chem. 2019, 110, 259–276. [Google Scholar] [CrossRef]
- Kaur, A.; Bhargava, G.K. Review paper on nickel-zinc nano ferrite. Mater. Today Proc. 2021, 37, 3082–3086. [Google Scholar] [CrossRef]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V. Mini-review: Ferrite nanoparticles in the catalysis. Arab. J. Chem. 2019, 12, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. Process. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Levada, K.; Pshenichnikov, S.; Abdolrahim, M.; Baricic, M.; Kapitunova, A.; Galieva, A.; Sukhikh, S.; Astakhova, L.; Antipov, S.; et al. Green Synthesis of Co-Zn Spinel Ferrite, Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties. Materials 2020, 13, 5014. [Google Scholar] [CrossRef]
- Ceicilia, A.S.J.; Dinesh, A.; Nirmala, G.F.; Preetha, A.C.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Xavier, B.; Vinosha, P.A.; Manikandan, A. Review on recent advances of zinc substituted cobalt ferrite nanoparticles: Synthesis characterization and diverse applications. Ceram. Int. 2021, 47, 10512–10535. [Google Scholar]
- Tatarchuk, T.R.; Paliychuk, N.D.; Bououdina, M.; Al-Najar, B.; Pacia, M.; Macyk, W.; Shyichuk, A. Effect of cobalt substitution on structural, elastic, magnetic and optical properties of zinc ferrite nanoparticles. J. Alloys Compd. 2018, 731, 1256–1266. [Google Scholar] [CrossRef]
- Narang, S.B.; Pubby, K. Nickel Spinel Ferrites: A review. J. Magn. Magn. Mater. 2021, 519, 167163. [Google Scholar] [CrossRef]
- Parveen, S.; Premkumar, T.; Poornima, S.; Govindarajan, S. Catalytic activity of nanocrystalline ZnM2O4 (M = Fe, Co) prepared via simple and facile synthesis of thermal decomposition of mixed metal complexes of Schiff bases generated from a-ketobutyric acid and diaminoguanidine. J. Saudi Chem. Soc. 2019, 23, 691–701. [Google Scholar] [CrossRef]
- Chahar, D.; Taneja, S.; Bisht, S.; Kesarwani, S.; Thakur, P.; Thakur, A.; Sharma, P.B. Photocatalytic activity of cobalt substituted zinc ferrite for the degradation of methylene blue dye under visible light irradiation. J. Alloys Compd. 2021, 851, 156878. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, S.F.; Tsai, A.P.; Kameoka, S. Catalysts prepared from copper-nickel ferrites for the steam reforming of methanol. J. Power Sources 2015, 281, 138–145. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Nlebedim, I.C.; Snyder, J.E.; Moses, A.J.; Jiles, D.C. Dependence of the magnetic and magnetoelastic properties of cobalt ferrite on processing parameters. J. Magn. Magn. Mater. 2010, 322, 3938–3942. [Google Scholar] [CrossRef]
- Muhammad, A.; Sato-Turtelli, R.; Kriegisch, M.; Grössinger, R.; Kubel, F.; Konegger, T. Large enhancement of magnetostriction due to compaction hydrostatic pressure and magnetic annealing in CoFe2O4. J. Appl. Phys. 2012, 111, 013918. [Google Scholar] [CrossRef]
- Atif, M.; Asghar, M.W.; Nadeem, M.; Khalid, W.; Ali, Z.; Badshah, S. Synthesis and investigation of structural, magnetic and dielectric properties of zinc substituted cobalt ferrites. J. Phys. Chem. Solids 2018, 123, 36–42. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Shyichuk, A.; Sojka, Z.; Gryboś, J.; Naushad, M.; Kotsyubynsky, V.; Kowalska, M.; Kwiatkowska-Marks, S.; Danyliuk, N. Green synthesis, structure, cations distribution and bonding characteristics of superparamagnetic cobalt-zinc ferrites nanoparticles for Pb(II) adsorption and magnetic hyperthermia applications. J. Mol. Liquids 2021, 328, 115375. [Google Scholar] [CrossRef]
- Naik, M.M.; Naik, H.S.B.; Nagaraju, G.; Vinuth, M.; Vinu, K.; Viswanath, R. Green synthesis of zinc doped cobalt ferrite nanoparticles: Structural, optical, photocatalytic and antibacterial studies. Nano Struct. Nano Objects 2019, 19, 100322. [Google Scholar] [CrossRef]
- Andhare, D.D.; Patade, S.R.; Kounsalye, J.S.; Jadhav, K.M. Effect of Zn doping on structural, magnetic and optical properties of cobalt ferrite nanoparticles synthesized via. Co-precipitation method. Phys. B 2020, 583, 412051. [Google Scholar] [CrossRef]
- Rao, P.A.; Rao, K.S.; Raju, T.R.K.; Kapusetti, P.G.; Choppadandi, M.; Varma, M.C.; Rao, K.H. A systematic study of cobalt-zinc ferrite nanoparticles for selfregulated magnetic hyperthermia. J. Alloys Compd. 2019, 794, 60–67. [Google Scholar]
- Hill, J.M. Sustainable and/or waste sources for catalysts: Porous carbon development and gasification. Catal. Today 2017, 285, 204–210. [Google Scholar] [CrossRef]
- García, P.G. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Bader, N.; Ouederni, A. Functionalized and metal-doped biomass-derived activated carbons for energy storage application. J. Energy Storage 2017, 13, 268–276. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chem. Eng. Res. Des. 2018, 129, 271–296. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Mileva, A.; Tsyntsarski, B.; Paneva, D.; Spassova, I.; Kovacheva, D.; Velinov, N.; Karashanova, D.; Georgieva, B.; Petrov, N. Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenergy 2018, 109, 135–146. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Mileva, A.; Paneva, D.; Kovacheva, D.; Spassova, I.; Nihtianova, D.; Markov, P.; Petrov, N.; Mitov, I. Zinc ferrites hosted in activated carbon from waste precursors as catalysts in methanol decomposition. Microporous and Mesoporous Mater. 2016, 229, 59–67. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Mileva, A.; Marinov, S.P.; Paneva, D.; Velinov, N.; Spassova, I.; Kosateva, A.; Kovacheva, D.; Petrov, N. Activated carbons from used motor oil as catalyst support for sustainable environmental protection. Microporous and Mesoporous Mater. 2018, 259, 9–16. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Tsyntsarski, B.; Ivanova, R.; Spassova, I.; Kovacheva, D.; Issa, G.; Paneva, D.; Karashanova, D.; Dimitrov, M.; Georgieva, B.; et al. NixZn1-xFe2O4 modified activated carbons from industrial waste as catalysts for hydrogen production. Microporous and Mesoporous Mater. 2019, 285, 96–104. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, Y.; Ma, X.D.; Shahabadi, S.I.S.; Che, B.; Wang, P.; Lu, X. Biomass-based honeycomb-like architectures for preparation of robust carbon foams with high electromagnetic interference shielding performance. Carbon 2018, 140, 227–236. [Google Scholar] [CrossRef]
- Rao, G.S.; Nabipour, H.; Zhang, P.; Wang, X.; Xing, W.; Song, L.; Hu, Y. Lightweight, hydrophobic and recyclable carbon foam derived from lignin–resorcinol–glyoxal resin for oil and solvent spill capture. J. Mater. Technol. 2020, 9, 4655–4664. [Google Scholar] [CrossRef]
- Min, Z.H.; Cao, M.; Zhang, S.; Wang, X.D.; Yong, Y.G. Effect of precursor on the pore structure of carbon foams. New Carbon Mater. 2007, 22, 75–79. [Google Scholar] [CrossRef]
- Li, Y.; Shen, B.; Pei, X.; Zhang, Y.; Yi, D.; Zhai, W.; Zhang, L.; Wei, X.; Zheng, W. Ultrathin carbon foams for effective electromagnetic interference shielding. Carbon 2016, 100, 375–385. [Google Scholar] [CrossRef]
- Banerjee, C.; Chandaliya, V.K.; Dash, P.S.; Meikap, B.C. Effect of different parameters on porosity and compressive strength of coal tar pitch derived carbon foam. Diam. Relat. Mater. 2019, 95, 83–90. [Google Scholar] [CrossRef]
- Xing, B.; Zeng, H.; Huang, G.; Jia, J.; Yuan, R.; Zhang, C.; Sun, Q.; Cao, Y.; Chen, Z.; Liu, B. Magnesium citrate induced growth of noodle-like porous graphitic carbons from coal tar pitch for high-performance lithium-ion batteries. Electrochim. Acta 2021, 376, 138043. [Google Scholar] [CrossRef]
- Klett, J.W. The role of precursor modification on the production of graphite foam. Carbon 2019, 144, 43–54. [Google Scholar] [CrossRef]
- Inagaki, M.; Qiu, J.; Guo, Q. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Krzesinska, M.; Pusz, S.; Majewska, J.; Tzvetkov, P. Carbon foam derived from pitches modified with mineral acids by a low pressure foaming process. Carbon 2010, 48, 3523–3530. [Google Scholar] [CrossRef]
- Gao, F.; Zang, Y.H.; Wang, Y.; Guan, C.Q.; Qu, J.Y.; Wu, M.B. A review of the synthesis of carbon materials for energy storage from biomass and coal/heavy oil waste. New Carbon Mater. 2021, 36, 34–48. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Wang, B.; Liu, J.; Yu, M. Mesoporous Ni-Co based nanowire arrays supported on three-dimensional N-doped carbon foams as non-noble catalysts for efficient oxygen reduction reaction. Microporous and Mesoporous Mater. 2016, 231, 128–137. [Google Scholar] [CrossRef]
- Stoycheva, I.; Tsyntsarski, B.; Vasileva, M.; Petrova, B.; Georgiev, G.; Budinova, T.; Szeluga, U.; Pusz, S.; Kosateva, A.; Petrov, N. New method for synthesis of carbon foam on the base of mixture of coal tar pitch and furfural without using pressure and stabilization treatment. Diam. Relat. Mater. 2020, 109, 108066. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Zhuang, Q.; Dang, A.; Li, T.; Zhao, T. Preparation and the electromagnetic interference shielding in theX-band of carbon foams with Ni-Zn ferrite additive. J. Eur. Ceram. Soc. 2016, 36, 3939–3946. [Google Scholar] [CrossRef]
- Qin, G.X.; Hao, Y.; Wang, S.; Dong, Y.B. Rapid formation of nitrogen-doped carbon foams by self-foaming as metal free catalysts for selective oxidation of aromatic alkanes. Appl. Catal. A Gen. 2020, 591, 117400. [Google Scholar] [CrossRef]
- Velasco, L.F.; Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Parra, J.B.; Ania, C.O. Carbon foams as catalyst supports for phenol photodegradation. J. Hazard. Mater. 2010, 184, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varila, T.; Makela, E.; Kupila, R.; Romar, H.; Hu, T.; Karinen, R.; Puurunen, R.L.; Lassi, U. Conversion of furfural to 2-methylfuran over CuNi catalysts supported on biobased carbon foams. Catal. Today 2021, 367, 16–27. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Papavasiliou, J.; Ioannides, T. Hydrogen production from methanol over combustion-synthesized noble metal/ceria catalysts. Chem. Eng. J. 2009, 154, 274–280. [Google Scholar] [CrossRef]
- Li, G.; Gu, C.; Zhu, W.; Wang, X.; Yuan, X.; Cui, Z.; Wang, H.; Gao, Z. Hydrogen production from methanol decomposition using Cu-Al spinel catalysts. J. Clean. Prod. 2018, 183, 415–423. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, T.; Liu, Q.; Lei, J.; Gong, L.; Jin, H. Performance investigation of a new cooling, heating and power system with methanol decomposition based chemical recuperation process. Appl. Energy 2018, 229, 1152–1163. [Google Scholar] [CrossRef]
- Pham, T.L.M.; Vo, D.V.N.; Nguyen, H.N.T.; Pham-Tran, N.N. C-H versus O-H bond scission in methanol decomposition on Pt (111): Role of the dispersion interaction. Appl. Surf. Sci. 2019, 481, 1327–1334. [Google Scholar] [CrossRef]
- Jing, J.; Li, L.; Chu, W.; Wei, Y.; Jiang, C. Microwave-assisted synthesis of high performance copper-based catalysts for hydrogen production from methanol decomposition. Int. J. Hydrog. Energy 2018, 43, 12059–12068. [Google Scholar] [CrossRef]
- Fan, M.; Xu, Y.; Sakurai, J.; Demura, M.; Hirano, T.; Teraoka, Y.; Yoshigoe, A. Spontaneous activation behavior of Ni3Sn, an intermetallic catalyst, for hydrogen production via methanol decomposition. Int. J. Hydrog. Energy 2015, 40, 12663–12673. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Petrova, B.; Budinova, T.; Petrov, N.; Velasco, L.F.; Parra, J.B.; Ania, C.O. Porosity development during steam activation of carbon foams from chemically modified pitch. Microporous and Mesoporous Mater. 2012, 154, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS. TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Boehm, H.P. Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: New York, NY, USA, 1966; Volume 16, pp. 179–274. [Google Scholar]
- Papirer, E.; Li, S.; Donnet, J.B. Contribution to the study of basic surface groups on carbons. Carbon 1987, 25, 243–247. [Google Scholar] [CrossRef]
- Yu, M.; Li, C.; Ao, X.; Chen, Q. Fabrication of coal tar pitch–derived reticulated carbon foam as oxidation resistant thermal insulation. J. Anal. Appl. Pyrolysis 2019, 141, 104643. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Materials Science and Engineering of Carbon: Fundamentals; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Liu, H.; Song, H.; Hou, W.; Chang, Y.; Zhang, Y.; Li, Y.; Zhao, Y.; Han, G. Coal tar pitch-based hierarchical porous carbons prepared in molten salt for supercapacitors. Mater. Chem. Phys. 2021, 265, 124491. [Google Scholar] [CrossRef]
- Zhuang, Q.Q.; Cao, J.P.; Zhao, X.Y.; Wu, Y.; Zhou, Z.; Zhao, M.; Zhao, Y.P.; Wei, X.Y. Preparation of layered-porous carbon from coal tar pitch narrow fractions by single-solvent extraction for superior cycling stability electric double layer capacitor application. J. Colloid Interface Sci. 2020, 567, 347–356. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Guan, T.; Zhang, G.; Wang, N.; Li, K. Tailored C-N bond toward defect-rich hierarchically porous carbon from coal tar pitch for high-efficiency adsorptive desulfurization. Fuel 2021, 292, 120251. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Sirajudheen, P.; Nikitha, M.R.; Meenakshi, S. Removal of phosphate and nitrate via a zinc ferrite-activated carbon hybrid composite under batch experiments: Study of isotherm and kinetic equilibriums. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100378. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Bai, Z.Q.; Zhao, R.; Liu, Y.L.; Li, Z.J.; Chu, S.Q.; Zheng, L.R.; Zhang, J.; Zhao, Y.L.; Chai, Z.F.; et al. Introduction of bifunctional groups into mesoporous silica for enhancing uptake of Thorium(IV) from aqueous solution. ACS Appl. Mater. Interfaces 2014, 6–7, 4786–4796. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.R.D.; Pinto, F.G.H.S.; Lima, E.L.F.; Souza, L.D.; Caldeira, V.P.S.; Santos, A.G.D. Influence of synthesis parameters in obtaining KIT-6 mesoporous material. Appl. Sci. 2018, 8, 725. [Google Scholar] [CrossRef] [Green Version]
- Wang, X. Stepwise removal of the copolymer template from mesopores and micropores in SBA-15. J. Porous Mater. 2011, 18, 623–630. [Google Scholar] [CrossRef]
- Sundararajan, M.; Kennedy, L.J.; Aruldoss, U.; Pash, S.K.; Vijay, J.J.; Dunn, S. Microwave combustion synthesis of zinc substituted nanocrystalline spinel cobalt ferrite: Structural and magnetic studies. Mater. Sci. Semicond. Process. 2015, 40, 1–10. [Google Scholar] [CrossRef]
- Greenwood, N.; Gibb, T. Mössbauer Spectroscopy; Capman and Hall Ltd.: London, UK, 1971. [Google Scholar]
- Shipilin, M.A.; Zakharova, I.N.; Shipilin, A.M.; Bachurin, V.I. Mössbauer studies of magnetite nanoparticles. J. Synch. Investig. 2014, 8, 557–561. [Google Scholar] [CrossRef]
- Kopcewicz, M.; Grabias, A.; Kuryliszyn-Kudelska, I.; Dobrowolski, W. Mössbauer effect study of superparamagnetic behavior of ZnFe2O4 nanoparticles formed in ZnO doped with Fe2O3. Phys. Status Solidi B 2019, 256, 1800223. [Google Scholar] [CrossRef]
- Koleva, K.; Velinov, N.; Tsoncheva, T.; Mitov, I.; Kunev, B. Preparation, structure and catalytic properties of ZnFe2O4. Bulg. Chem. Commun. 2013, 45, 434–439. [Google Scholar]
- Koleva, K.; Velinov, N.; Tsoncheva, T.; Mitov, I. Mössbauer study of Cu1-xZnxFe2O4 catalytic materials. Hyperfine Interact. 2014, 226, 89–97. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Pérez-Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J.L.G. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 2006, 243, 199–211. [Google Scholar] [CrossRef]
- Scrimshire, A.; Lobera, A.L.; Kultyshev, R.; Ellis, P.; Forder, S.D.; Bingham, P.A. Variable Temperature 57Fe-Mössbauer Spectroscopy Study of Nanoparticle Iron Carbides. Croat. Chem. Acta 2015, 88, 531–537. [Google Scholar] [CrossRef]
- Roy, P.C.; Doh, W.H.; Jo, S.K.; Kim, C.M. Interaction of methanol and hydrogen on a ZnO (0001) single crystal surface. J. Phys. Chem. C 2013, 117, 15116–15121. [Google Scholar] [CrossRef]
- Pankov, V.V.; Bashkirov, L.A. Mechanism of Ni-Zn ferrite formation. J. Solid State Chem. 1981, 39, 298–308. [Google Scholar] [CrossRef]


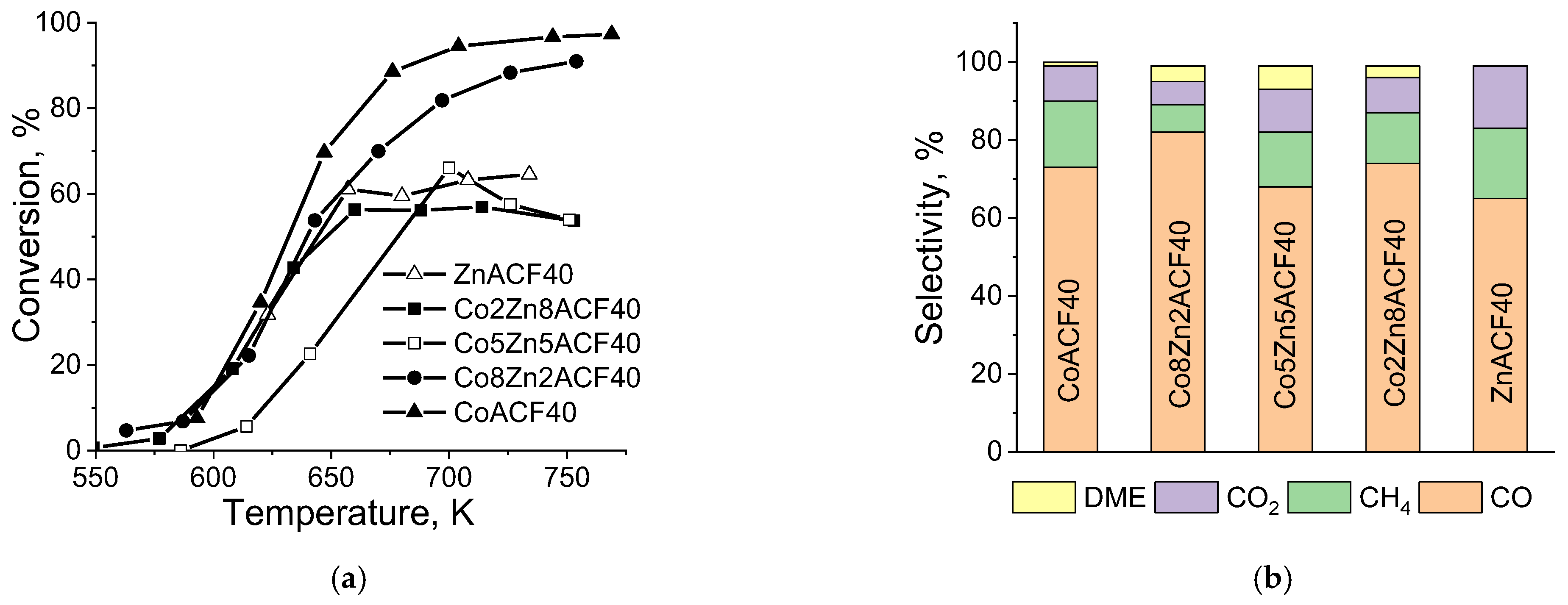
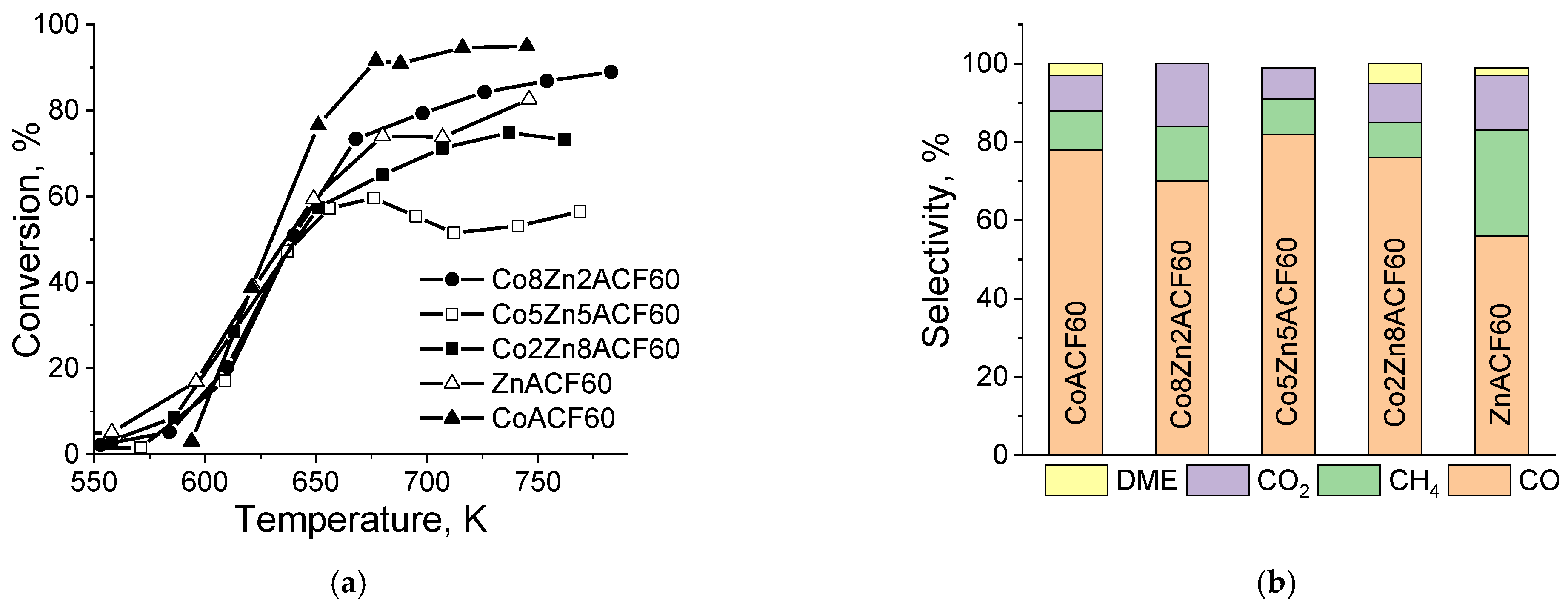

| Sample | SBET m2·g−1 | Vt mL·g−1 | Vmi mL·g−1 | Dav nm | Vmic/ Vmes | Phase Composition | Unit Cell, ×10 nm | Crystallite Size, nm |
|---|---|---|---|---|---|---|---|---|
| ACF40 | 1013 | 0.53 | 0.35 | 2.1 | 1.94 | |||
| ZnACF40 | 466 | 0.27 | 0.22 | 2.3 | 4.40 | spinel | 8.435 (1) | 26 |
| Co2Zn8ACF40 | 548 | 0.31 | 0.24 | 2.2 | 3.43 | spinel | 8.418 (3) | 21 |
| Co5Zn5ACF40 | 661 | 0.33 | 0.24 | 2.0 | 2.67 | spinel | 8.409 (2) | 22 |
| Co8Zn2ACF40 | 583 | 0.36 | 0.25 | 2.4 | 2.27 | spinel | 8.407 (3) | 22 |
| CoACF40 | 712 | 0.45 | 0.30 | 2.6 | 2.00 | spinel | 8.395 (6) | 24 |
| ACF60 | 728 | 0.42 | 0.34 | 2.3 | 4.25 | |||
| ZnACF60 | 672 | 0.38 | 0.30 | 2.3 | 3.75 | spinel | 8.434 (9) | 19 |
| Co2Zn8ACF60 | 558 | 0.34 | 0.24 | 2.5 | 2.40 | spinel | 8.412 (3) | 18 |
| Co5Zn5ACF60 | 677 | 0.45 | 0.28 | 2.6 | 1.64 | spinel | 8.400 (5) | 24 |
| Co8Zn2ACF60 | 692 | 0.45 | 0.28 | 2.6 | 1.64 | spinel | 8.400 (1) | 8 |
| CoACF60 | 992 | 0.49 | 0.36 | 2.0 | 2.77 | spinel | 8.392 (2) | 43 |
| KIT-6 | 912 | 1.29 | 0.11 | 5.6 | 0.09 | |||
| ZnKIT-6 | 601 | 0.94 | 0.04 | 6.2 | 0.04 | hematite | A = 5.063 (4) C = 13.80 (2) | 10 |
| Co2Zn8KIT-6 | 679 | 1.06 | 0.05 | 6.2 | 0.05 | hematite | A = 5.044 (5) C = 13.79 (2) | 10 |
| Co5Zn5KIT-6 | 692 | 1.02 | 0.05 | 5.9 | 0.05 | amorphous | ||
| Co8Zn2KIT-6 | 666 | 0.97 | 0.06 | 5.8 | 0.06 | amorphous | ||
| CoKIT-6 | 600 | 0.92 | 0.06 | 6.1 | 0.07 | amorphous |
| Sample | Surface Functional Groups, mmol·g−1 | |||||
|---|---|---|---|---|---|---|
| Carboxylic | Lactone | Phenolic Hydroxyl | Carbonyl | Total Acidic Groups | Total Basic Groups | |
| ACF40 | 0.01 | 0.01 | 0.03 | 0.60 | 0.64 | 0.59 |
| ACF60 | 0.04 | 0.05 | 0.20 | 1.10 | 1.39 | 0.55 |
| Sample | Components | δ, mm·s−1 (±0.01) | Δ (2ε), mm·s−1 (±0.02) | Beff, T (±0.2) | Γexp, mm·s−1 (±0.04) | G, % |
|---|---|---|---|---|---|---|
| ZnACF60 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.28 | 0.00 | 49.3 | 0.41 | 10 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.62 | −0.06 | 45.6 | 0.54 | 16 | |
| Db-Fe3+ | 0.34 | 0.79 | - | 0.59 | 74 | |
| ZnACF40 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.29 | 0.00 | 49.5 | 0.55 | 14 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.57 | 0.09 | 43.9 | 1.59 | 35 | |
| Db-Fe3+ | 0.35 | 0.47 | - | 0.60 | 51 | |
| ZnKIT-6 | Sx1- Fe3+octa-α-MxFe2−xO3 | 0.38 | −0.21 | 48.9 | 0.43 | 28 |
| Sx2- Fe3+octa-α-MxFe2−xO3 | 0.37 | −0.22 | 46.3 | 0.45 | 53 | |
| Db-Fe3+ | 0.37 | 0.60 | - | 0.64 | 19 | |
| Co2Zn8ACF60 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.31 | 0.00 | 49.0 | 0.35 | 7 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.49 | −0.04 | 43.6 | 1.44 | 41 | |
| Db-Fe3+ | 0.34 | 0.66 | - | 0.62 | 52 | |
| Co2Zn8ACF40 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.30 | 0.00 | 48.8 | 0.45 | 13 |
| Sx2- Fe2.5+octa-Mx Fe3−xO4 | 0.59 | 0.03 | 44.0 | 1.49 | 39 | |
| Db-Fe3+ | 0.34 | 0.60 | - | 0.63 | 48 | |
| Co2Zn8KIT-6 | Sx1- Fe3+octa-α-MxFe2−xO3 | 0.37 | −0.21 | 49.4 | 0.29 | 55 |
| Sx2- Fe3+octa-α-MxFe2−xO3 | 0.38 | −0.22 | 46.6 | 0.62 | 39 | |
| Db-Fe3+ | 0.32 | 0.53 | - | 0.59 | 6 | |
| Co5Zn5ACF60 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.30 | 0.00 | 49.0 | 1.5 | 12 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.50 | 0.00 | 44.0 | 1.5 | 15 | |
| Db-Fe3+ | 0.34 | 0.71 | - | 0.52 | 73 | |
| Co5Zn5ACF40 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.29 | 0.00 | 48.8 | 0.38 | 16 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.54 | 0.00 | 44.7 | 1.37 | 58 | |
| Db-Fe3+ | 0.32 | 0.81 | - | 0.77 | 26 | |
| Co5Zn5KIT-6 | Db-Fe3+ | 0.30 | 0.79 | - | 0.65 | 100 |
| Co8Zn2ACF60 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.33 | 0.00 | 48.9 | 0.50 | 6 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.51 | 0.07 | 44.5 | 1.33 | 24 | |
| Db-Fe3+ | 0.34 | 0.78 | - | 0.54 | 70 | |
| Co8Zn2ACF60, LNT | Sx1- Fe3+tetra-MxFe3−xO4 | 0.41 | 0.00 | 50.2 | 0.45 | 25 |
| Sx2- Fe2.5+octa-Mx Fe3−xO4 | 0.46 | 0.00 | 52.3 | 0.46 | 22 | |
| Sx3- Fe2.5+octa-Mx Fe3−xO4 | 0.54 | 0.00 | 45.4 | 1.25 | 17 | |
| Db-Fe3+ | 0.41 | 0.83 | - | 0.57 | 36 | |
| Co8Zn2ACF40 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.30 | −0.01 | 49.1 | 0.59 | 31 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.44 | 0.03 | 45.0 | 1.30 | 43 | |
| Db-Fe3+ | 0.34 | 0.76 | - | 0.65 | 26 | |
| Co8Zn2KIT-6 | Db-Fe3+ | 0.34 | 0.71 | - | 0.54 | 100 |
| CoACF60 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.30 | 0.00 | 49.8 | 0.48 | 32 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.64 | −0.05 | 46.2 | 0.66 | 18 | |
| Sx3- Fe3+octa-α-Fe2O3 | 0.37 | −0.20 | 52.0 | 0.30 | 8 | |
| Db-Fe3+ | 0.35 | 0.72 | - | 0.50 | 42 | |
| CoACF40 | Sx1- Fe3+tetra-MxFe3−xO4 | 0.25 | 0.03 | 49.0 | 0.47 | 18 |
| Sx2- Fe2.5+octa-MxFe3−xO4 | 0.44 | 0.03 | 45.6 | 0.79 | 29 | |
| Db-Fe3+ | 0.30 | 0.70 | - | 0.53 | 53 | |
| CoKIT-6 | Sx- Fe3+octa-α-MxFe2xO3 | 0.37 | −0.20 | 43.5 | 2.95 | 42 |
| Db-Fe3+ | 0.33 | 0.79 | - | 0.65 | 58 |
| Sample | Components | δ, mm·s−1 (±0.01) | Δ (2ε), mm·s−1 (±0.02) | Beff, T (±0.2) | Γexp, mm·s−1 (±0.04) | G, % |
|---|---|---|---|---|---|---|
| Co5Zn5ACF40(cat) | Sx1- Fe3C | 0.20 | 0.01 | 20.6 | 0.65 | 34 |
| Db-Fe3+octa | 0.34 | 0.84 | - | 0.91 | 66 | |
| Co2Zn8ACF60(cat) | Sx1- Fe3C | 0.18 | 0.05 | 20.4 | 0.45 | 39 |
| Db-Fe3+octa | 0.33 | 0.88 | - | 0.89 | 61 | |
| Co5Zn5ACF60(cat) | Sx1- Fe3C | 0.18 | 0.03 | 20.7 | 0.49 | 31 |
| Db-Fe3+octa | 0.31 | 0.85 | - | 0.87 | 69 | |
| Co8Zn2ACF60(cat) | Sx1- Fe3C | 0.18 | 0.04 | 20.4 | 0.53 | 27 |
| Sx2-α-(Fe,Co) alloy | 0.00 | 0.00 | 34.7 | 0.71 | 8 | |
| Db-Fe3+octa | 0.34 | 0.77 | - | 0.62 | 65 | |
| Co8Zn2ACF60(cat) LNT | Sx1- Fe3C | 0.27 | 0.03 | 23.7 | 0.56 | 34 |
| Sx2- α-(Fe,Co) alloy | 0.09 | −0.03 | 35.7 | 0.79 | 14 | |
| Sx3-Fe3+tetra-MxFe3−xO4 | 0.36 | 0.00 | 48.5 | 0.55 | 5 | |
| Sx4-Fe2.5+octa-MxFe3−xO4 | 0.46 | 0.00 | 45.2 | 0.73 | 5 | |
| Db-Fe3+octa | 0.37 | 0.86 | - | 0.69 | 42 | |
| Co5Zn5KIT-6(cat) | Db1-Fe3+octa | 0.34 | 0.83 | - | 0.69 | 89 |
| Db2-Fe2+octa | 0.64 | 1.86 | - | 0.66 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoncheva, T.; Ivanova, R.; Velinov, N.; Kovacheva, D.; Spassova, I.; Karashanova, D.; Petrov, N. Design and Catalytic Behaviour of Hosted in Activated Carbon Foam CoxZn1−xFe2O4 Ferrites. Symmetry 2021, 13, 1532. https://doi.org/10.3390/sym13081532
Tsoncheva T, Ivanova R, Velinov N, Kovacheva D, Spassova I, Karashanova D, Petrov N. Design and Catalytic Behaviour of Hosted in Activated Carbon Foam CoxZn1−xFe2O4 Ferrites. Symmetry. 2021; 13(8):1532. https://doi.org/10.3390/sym13081532
Chicago/Turabian StyleTsoncheva, Tanya, Radostina Ivanova, Nikolay Velinov, Daniela Kovacheva, Ivanka Spassova, Daniela Karashanova, and Nartzislav Petrov. 2021. "Design and Catalytic Behaviour of Hosted in Activated Carbon Foam CoxZn1−xFe2O4 Ferrites" Symmetry 13, no. 8: 1532. https://doi.org/10.3390/sym13081532
APA StyleTsoncheva, T., Ivanova, R., Velinov, N., Kovacheva, D., Spassova, I., Karashanova, D., & Petrov, N. (2021). Design and Catalytic Behaviour of Hosted in Activated Carbon Foam CoxZn1−xFe2O4 Ferrites. Symmetry, 13(8), 1532. https://doi.org/10.3390/sym13081532








