Abstract
Single crystals of new non-centrosymmetric iodate Ba(IO3)OH are synthesized hydrothermally and structurally investigated. OD topology-symmetry analysis of layers and their alternation led to conclusions about the similarity with the Bi(IO3)O nonlinear-optical crystal. Both structures are members of a general family with differences in structures and properties. AgBi(IO3)4 and AgBi(SO4)(IO3)2 belong to another family with the two types of layers giving different local symmetry. The properties are estimated semi-quantitatively based on structural data. Such an approach is new and promising. Hypothetical structural variants are predicted.
1. Introduction
Non-centrosymmetric compounds, especially polar, are objects in material science due to their possible properties, such as nonlinear optical, ferroelectric, piezoelectric, pyroelectric and magnetic. Second harmonic generation (SHG) is found for many inorganic crystals as borate, phosphate, sulfate and selenite and they are used as materials. Iodates belong to the inorganic compounds which possess the umbrella-like trigonal [IO3]− anionic group with the apical lone pair of I5+, which provides a strong polarity. “If such building units are aligned in the unit cell, they yield a polar material” [1]. Multiple metal iodate compounds have been obtained and extremely intensively investigated in the last decades in a search of new materials. The results are summarized in reviews on structures and properties of functional metal iodates [2] and in recent reports on second-order nonlinear optical materials based on metal iodates [3]. Special attention is paid to synthesis and investigation of iodates containing lone-pair cations Pb2+, Bi3+ or large polarizable ions such as Ag1+, and one of the best results on high second harmonic generation (SHG) activity is achieved for BiO(IO3) [1]. Umbrella-like iodate groups [IO3]− usually are not condensed because of electrostatic reasons; however, different complicate anions are discovered. The intensive search for new phases includes different metals: alkali, alkali-earth, d0 transition, Ti/Zr, V/Nb/Ta, actinide, Cr/Mo/W, Ln, Pd/Cd/In, Pb/Bi and others [2].
For successful search of new phases, it is necessary not only to enumerate elements, but also to understand the fundamental structural features responsible for the manifestation of properties. Such an important tool is the topology-symmetry analysis of the OD theory of structures [4] that deals with families of structures possessing features of order (O) and disorder (D) character.
The OD theory was suggested from analysis of polytypes such as SiC. Structural variants were explained as a result of proven theorem Z = N/F, where N is the general multiplicity of the symmetry group of a single layer and F is a layer pair. “If the layer has its own local symmetry, differing from the structure as a whole, Z ≠ 1, and structural variants in layer stacking may appear. It has been suggested to characterize local symmetry of a layer as partial operation (PO) of symmetry λ-PO. One says λ-PO does not have continuation in the adjacent layer” [4,5,6]. Let us look at the layer of close-packed spheres of symmetry group P(6/m)mm, but there is no periodicity in the direction of hexad: the second layer uses only one half of the voids because of atomic radius limitation, and the symmetry of the pair is lower, namely P(−3)m1 [5]. The direction of the layer sequences is indicated by parentheses. “For the close packing case Z = N/F = 24/12 = 2 and two principal variants of close-packed layers conjugation exists: hexagonal ABAB… and cubic ABCABC….” [4,5]. The local symmetry operations converting the first layer into the neighboring one have notation σ-PO. “For the description of all structural variants of the OD family, a symmetry groupoid was introduced: the first line is λ-PO symmetry and in the second line σ-PO symmetry” [4,6]. It is essential that a highlighted unit is not necessarily a layer in the structure according to traditional crystal chemistry, and “the structural block can be zero dimensional—bricks, one dimensional—rods, and two dimensional—sheets (layer)” [4].
Being far from material science, Dornberger-Schiff introduced such a topological and symmetrical characteristic as polarity τ and non-polarity ρ of layer (λ-PO) and polarity τ and non-polarity ρ of layer multiplication (σ-PO). It has been proven that “there are three categories of OD-structures of equivalent one type layers [4]: category I (ρ,ρ), category II (τ,τ) and category III (τ,ρ)” [4]. Close packing of equal spheres (metals) with nonpolar layers multiplied in a nonpolar way belongs to the most distributed category I. ZnS and SiC structures of the OD-family possess polar layers also multiplied in a polar way, and the structures are of the rare category II. Borate structures, as it was shown in [6], “demonstrate all three categories with many examples of category III with polar layers multiplied in a nonpolar way”.
The OD theory [4] identifies “polytypes of maximal degree of order (MDO) and periodic polytypes” [4,6]. If one symmetrical variant of layer sequences is realized, there is an MDO polytype. As an example, hexagonal and cubic close packing give each MDO I and MDO II correspondingly; more complicated layer sequences will be called periodic polytypes. “Chaotic layer sequences lead to non-periodic disordered structures” [6].
The problems of in-commensurate unit cells and diffuse scattering were raised first in the pioneer works of Dornberger-Schiff [4,5]. “Now, they belong to the theory and experiments of modern branches of crystallography, namely, modulated structures and diffuse scattering” [6]. It allows complicated disordered structures to be explored. At the same time, the topology-symmetry analysis is still relevant in published form for crystal chemistry. OD-structures of more than one type of layer were analyzed and it is a common case. The modern “modular” approach involves separation of structural building units for any structural family, but symmetry analysis is not used. Another modern term, “polysomatism”, involves description of structures of two types of layers. It is equal to structural homology, but also does not use symmetry analysis.
2. Materials and Methods
The hydrothermal synthesis method is one of the most successful and widespread in the search for new iodate phases (as well as for borate, silicate, phosphate, germanate, etc.). The main features of experiments are reproduced in laboratories with varying temperature intervals, pressures and, of course, composition. Every group has its own “secrets” based on their experience. This method is laborious and time-consuming.
Single crystals of new iodate Ba(IO3)OH were synthesized under hydrothermal conditions from the mixture of oxide components in the mass ratio BaO:I2O5 = 1:1. All the reagents were of analytical grade and were added in standard Teflon-lined stainless steel pressure vessels of 6 mL capacity. The autoclave was filled with a 15% LiNO3 aqueous solution. The weight ratio of solid and liquid phase was 1:5. The synthesis was carried out at a temperature of 280 °C and pressure ~100 atm. The reaction went to completion during heating for 20 days; final cooling after synthesis to the room temperature was done in 24 h. The reaction products—grown crystals—were isolated via filtration of the stock solution, washed with hot water and dried at room temperature.
Several methods were applied for the analysis of phases obtained in the experiments. Because of the amount of crystals in our experiment was small, we used morphological separation, determination of the composition and the X-ray diffraction method with the determination of unit cell parameters on single crystals. Morphological separation was done using an optical microscope with ×32 magnification. Transparent, colorless, flat-prismatic (0.1–0.3 mm), mica-like crystals were found in crystallization products. The yield of the experiment was close to ~90%.
Composition analysis was carried out on crystal surfaces using a Jeol JSM-6480LV scanning electronic microscope combined with WDX analysis. The test showed presence of Ba and I elements.
Nonlinear optical properties were investigated on powder crystalline samples according to the Kurtz and Perry scheme [7] using a Minilite-I YAG:Nd-laser operating in Q-switched mode at the repetition rate of 10 Hz at wavelength λω =1064 nm. The intensity Q = I2ω/I2ω(SiO2) of optical second harmonic generation (SHG) from crystals was approximately 0.7. This result pointed to weak but available non-centrosymmetry of these crystals.
The unit cell was determined on single crystal using an XCalibur S diffractometer equipped with a CCD area detector (ω scanning mode) and graphite-monochromated Mo Kα radiation source (λ = 0.71073 Å). No analogue was found in the ICSD, i.e., it was a new compound. A small, colorless, transparent, flat-prismatic, very thin crystal with a size of 0.12 x 0.072 x 0.009 mm was selected for single-crystal X-ray study. The diffraction experiment was carried out on the same diffractometer. The data were integrated using the CrysAlis Pro Agilent Technologies software [8] and corrected for the Lorentz and polarization factors. The refined monoclinic unit-cell parameters a = 6.0582(4), b = 6.3509(3), c = 10.5825(5) Å, β = 90.338(7)° were close to the orthorhombic one.
The topology-symmetry analysis of OD theory was carried out in accordance with the above-mentioned principles, as well as on the basis of our own experience in the study of structural families.
3. Results and Discussion
3.1. Ba-Iodate and Bi-Iodate Family
3.1.1. Ba(IO3)OH Crystal Structure
A structural model of new iodate was determined using the direct methods with SHELXS [9]. Suggested space group C2 did not give a positive result and did not allow solving of the structure. The analysis of the reflection absence allowed three possible space groups, namely, C2/m, C2 and Cm. The centrosymmetric group C2/m was not confirmed in the structure solving and did not agree also with the SHG signal. The third acentric group, Cm, was true (Table 1) and allowed four heavy atoms to be identified, two Ba-atoms and two I-atoms, in accordance with the composition of crystals. The remaining O sites were found from difference Fourier synthesis and then introduced into the model, which significantly reduced the R-factor. Both I-atoms were coordinated umbrella-like by O1, O2, O4 and O5 with the distances typical for the IO3-group. The O3 atom was identified as oxygen of the hydroxyl group based on Pauling’s balance of valences because it participates only in the coordination of Ba-atoms. The resulting chemical formula is Ba(IO3)OH, Z = 4. Absorption assessment showed the need to take this into account, which was done using numerical absorption correction based on Gaussian integration over a multifaceted crystal model [10].

Table 1.
Crystal data and structure refinement for Ba(IO3)OH 1.
The refinement of the model in SHELXL in isotropic approximation of atomic displacement parameters gave R ~ 9%. According to the Flack parameter x = 0.33, twinning in the crystal was supposed and twin-mirror mz was suggested: it corresponded to the layered nature of the structure—cleavage at (001). Introduction of a twin by matrix 100/010/00-1 improved temperature displacement parameters, interatomic distances and lowered the R-factor to ~6%; the twin component was 0.3.
The final structural model was refined using the least squares procedure in anisotropic approximation of the atomic displacements for heavy cations and isotropic for oxygens with the refinement of the weighting scheme using SHELXL [9]. Atomic coordinates and selected bonds are presented in Table 2 and Table 3. Illustrations were produced using ATOMS [11] and CORELDRAW programs.

Table 2.
Atomic coordinates and equivalent or isotropic displacement parameters for Ba(IO3)(OH). Uequ is defined as one third of the trace of the orthogonalized Uij tensor.

Table 3.
Interatomic distances for Ba(IO3)(OH).
3.1.2. Comparison of Ba(IO3)OH and Bi(IO3)O Structures Using OD-Approach
Among Ba-iodates, two compounds are known: Ba(IO3)2 [12] and Ba(IO3)2H2O [13]. Recently, (Pb,Ba)(IO3)2 was investigated [14]. All the structures demonstrate chess-order distribution of large cations and isolated (IO3) groups, which means they are fundamentally different from the new iodate. The new Ba(IO3)OH structure is composed of two types of layers joined in a triple. The central layer contains Ba-ions and OH-groups. Its optimal description is anion-centered with the anionic OH-group coordinated tetrahedrally by four Ba-cations. The tetrahedra are connected by edges in a layer with the formula {Ba(OH)}+. A similar layer was found in perite PbBiO2Cl [15] and seeligerite [16] minerals. Isolated (IO3)− umbrella-like groups form their own second “layers” {(IO3)}− on both sides of the described first layer. Such an arrangement is typical for mica and other layered minerals; see, for example, Figure 1a,b. It shows the perfect cleavage of crystals described above.
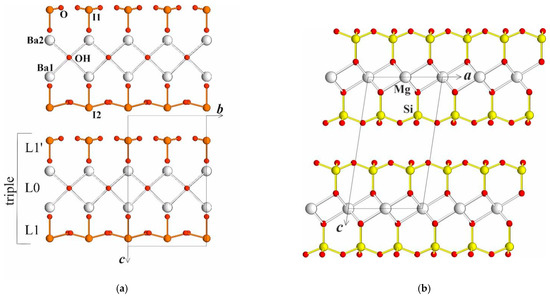
Figure 1.
(a) Ba(IO3)OH structure in bc projection, here and after ball-stick presentation is used for (IO3)-groups, Ba-atoms are shown with large spheres, OH-groups with a smaller one; (b) Talc Mg3(Si4O10)(OH)2 structure in ac projection, ball-stick presentation is used for (SiO4)-tetrahedra, Mg-atoms are shown with large spheres.
In accordance with the topology-symmetry analysis, every layer has its own local symmetry, which may differ from each other and from the whole structure. The central layer denoted as L0 possess tetragonal space group P4/nmm, significantly higher than the structure space group Cm; symmetry elements are given in Figure 2 in ab projection.
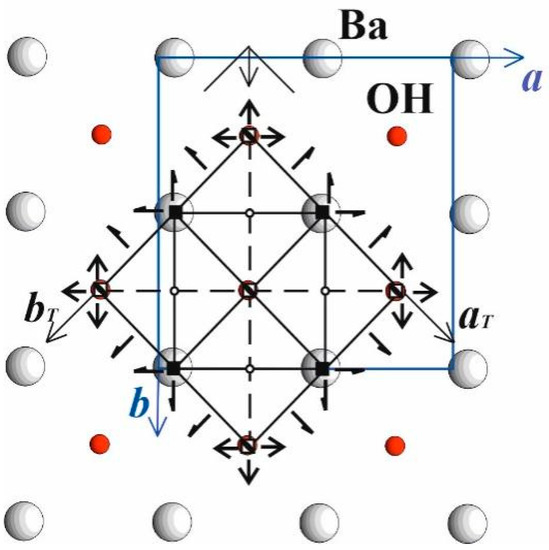
Figure 2.
Central tetrahedral layer L0 in ab projection. The symmetry elements of the group P4/nmm are shown and belong to the smaller unit cell with axes (aT, bT) selected from the main original (a,b) as a half of diagonals.
Iodate “layer” L1 and L1’ have symmetry corresponded to C1m1, equal to the space group of the structure. Using principles of OD theory [4], groupoid of symmetry is:
| L0 | L1 |
| {Ba(OH)}+ | {(IO3)}− |
| λ PO, ρ P(4/n)mm | λ PO, τ C1m(1) |
| σ PO, ρ [0, 21y 0] | |
That means, every type of layer has its own chemical formula and possesses its own local λ PO described by the space group of symmetry, which is valid as a pseudo-symmetry group. A space group is used here because the sliding in symmetry elements is in the layer and as such it actually corresponds to the layer group; parenthesis display the direction of the alternation of the layers. The central layer L0 is non-polar in the direction of alternation along the c-axis, which is denoted by λ PO, ρ. The second layer L1 possesses symmetry C1m1 and is polar along the c-axis and the a-axis too; polarity along the alternation of the layers (c-axis) is denoted by λ PO, τ notation. Multiplication of the L1 layer around L0 to the L1′ into the triple is realized by the 21y axis—the axial subgroup of the P4/nmm group, shown in Figure 3a in side projection with the horizontal axes 4 and −4, lying in projection, thus the 21y axis is running perpendicular to the projection. Such configuration corresponds to category I of structures with >1 type of layers and with the non-polar multiplication of one type of polar L1 and one type of non-polar L0 layer (τ,ρ). Thus, the triple L1-L0-L1′ dipole moments of L1, L1′ layers with iodate umbrella-like groups are located above and below the central none-polar L0 and are directed in the opposite manner, practically compensating for each other. This analysis explains the weak SHG signal.
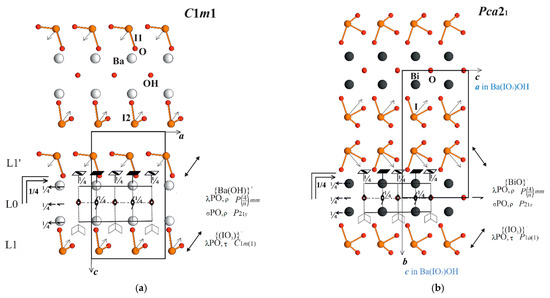
Figure 3.
(a) Ba(IO3)OH structure in ac projection. Layers L0, L1, L1′ are shown with local symmetry elements. Note that tetragonal space group of L0 layer is in side projection, arrows visualize layers L1, L1′ multiplication; (b) BiO(IO3) structure in bc projection, arrows visualize layers L1, L1′ multiplication.
One of the best nonlinear optical crystals, BiO(IO3) [1], has a similar chemical formula to the new iodate, Ba(OH)(IO3). Let us compare both of these crystal structures. Despite different formulas and especially properties, they have many common features. The central layer contains Bi and O instead of Ba and OH. It can be described as composed of anion-centered tetrahedra with four Bi-atoms around the central O atom. IO3-umbrella-like groups are located above and below the central layer L0 forming analogues to Ba-iodate “layers”. BiO(IO3) structure is given in bc-projection (Figure 3b) in accordance with the choice of axes in the X-ray experiment (a,b,c-axes in Ba(IO3)OH correspond to c,a,b-axes in BiO(IO3)). The symmetry of the central layer L0 in Bi-iodate is equal to the symmetry of the layer L0 in Ba-iodate P4/nmm. In Bi-compound the iodate layers L1, L1′ are slightly distorted and do not have a mirror plane (only glide-plane), in contrast to the Ba-compound, but are very similar, having polarity along the layer alternation, τ and inside the layer. However, multiplication of iodate layers L1, L1′ above and below the central L0 layer in both iodates are principally different. All the dipole moments of umbrella-like groups in Bi-iodate are oriented almost identically forming a polar 21 axis of the structure (Pca21). That means, multiplication of the L1 layer to L1′ in the triple is made by another 21 axis of the tetragonal group which is perpendicular to the first, being parallel to the c-axis [1], equal to the a-axis in Ba-iodate. The groupoid of symmetry is principally the same but is added by new σ-PO.
If we compare both structures avoiding different settings, in Ba-iodate L1, L1′ layers multiplication happens via the 21 axis perpendicular to the projection, and in Bi-iodate it is parallel; both directions belong to the equal a,b-axis of the tetragonal group of the L0 layer; thus, both may act equally in the multiplication of layers. A common groupoid can be written as
| L0 | L1 |
| {Ba(OH)}+ or {BiO}+ | {(IO3)}− |
| λ PO, ρ P(4/n)mm λ PO, τ C1m(1) or P1a (1) | |
| σ PO, ρ [21y || 21x] | |
Symmetry operations explain strong SHG in Bi-iodate and very small SHG in Ba-iodate, because dipole moments are oriented opposite each other (Ba-iodate) or almost identically along the polar axis (Bi-iodate) (Figure 3a,b). Two structural variants are so-called MDO polytypes with equal layer alternations or equal σ PO.
It is possible to assume existence of such members of a family, in which a combination of layers alternation will be presented as 21y, 21x, 21y, 21x,21y, 21x… or 21y, 21y, 21x, 21y, 21y, 21x… or in another order. In the absence of a regular alternation of σ PO, disordered structures will appear.
This brings up the question: what determines polar or non-polar iodate “layer’s” L1, L1′ alternation? It is possible to assume that heavy atoms play an important role. In the case of the significantly larger Ba2+ -ion without asymmetry (absence of lone pair), we detect higher symmetry, while in the case of smaller asymmetric Bi3+ -ion with a lone pair, a more asymmetric structure is realized.
3.2. Ag,Bi-Iodate and Ag,Bi-Iodate-Sulfate Family
Numerous Bi-containing compounds have excellent SHG properties. Adding a large polarizable Ag-ion allows good crystals to be obtained such as AgBi(IO3)4 [17]. In a recent work [18] [SO4] groups were chosen to replace a portion of anti-parallel [IO3−] groups with the intention of improving properties. New sulfate-iodate AgBi(SO4)(IO3)2 was successfully discovered and investigated. It possesses a larger SHG effect compared to AgBi(IO3)4. The analysis of structures was carried out including DFT calculations to extract the structure-properties relation. Important consulting of EB (see acknowledgment in [18]) regarding the presence of two types of layers in both structures, polar and non-polar centrosymmetric, allowed authors to discuss their role in the properties relation. That was included in the paper. Here, we add more detailed OD-analysis of this family of iodates. The structures were re-drawn (Figure 4a,b) with the adding of symmetrical relations based on data from [17,18]. Each type of layer has its own formula and local symmetry λ PO. The boundary between the layers shown in the figures as solid lines passes between atoms belonging to two-dimensional fragments with different symmetry. There are no layers in the crystal chemical sense in this family. One type of layer L1 being neutral in charge is equal for both compounds, namely, polar {Ag(IO3)} with a P(1)c1 group in Ag,Bi-iodate or P(1)11 in Ag,Bi-iodate-sulfate. The difference is determined by the presence of a c-glide plane in the iodate structure. The second type of “layers” L2 differs in composition and thickness in compounds. They are centrosymmetric and have a neutral formula {Bi(IO3)3} for iodate and {Bi(IO3)(SO4)} for iodate-sulfate. The λ PO groups are P(1)21/c 1 (presence of c-glade in iodate structure) and P(1)-11, correspondingly, and describe non-polar centrosymmetric layers. Here, we see an unprecedented case when one equal layer is combined with the two types of close but different layers forming two different structures. The SHG response is here determined by the thickness of the non-polar layers: the higher its proportion in the structure, the lower the signal. Accordingly, the ratio of the fraction (thickness) of the non-centrosymmetric layer which is higher in iodate-sulfate to the fraction which is lower in iodate is ~0.55:0.415 = 1.325. This is in satisfactory agreement with the SHG signal (pm/V) ratio ~1.81:1.27 = 1.42. It can be concluded that it is the non-polar component, the L1 layer, that is responsible for the contribution to the nonlinear optical properties.
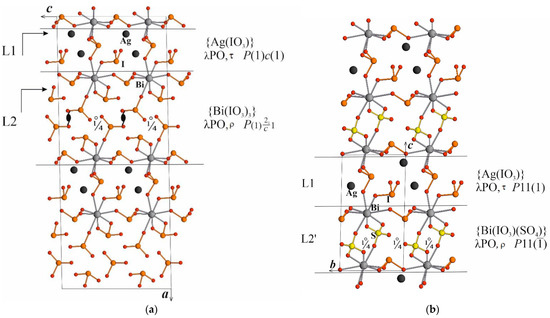
Figure 4.
(a) AgBi(IO3)4 structure in side projection; (b) AgBi(IO3)2(SO4) structure in side projection. Ag and Bi atoms are shown with black and gray spheres. The boundary between L1, L2 layers are shown with the horizontal solid lines. Local symmetry elements are shown.
In the AgBi(IO3)4 compound layer pair L1, L2 is translated by a C-lattice of the structure, while in AgBi(SO4)(IO3)2 it is a simple Tc lattice and both structures are non-centrosymmetric.
Based on OD theory, a different combination of layers is possible in this family. Complicate polytype structures may exist with a large a(c)-axis and the alternation of iodate L2 and iodate-sulfate L2′ after the L1 layer, instead of L1, L2, L1, L2... or L1, L2′, L1, L2′… or L1, L2, L1, L2′… or L1, L2, L1, L2, L1, L2′….
OD theory incudes symbolic letters when the symmetry of letters clearly reflects the symmetry of the polytype, here as example L1 as b, L2 as O: bObObO…-iodate structure, or L2′ as H: bHbHbH…-iodate-sulfate structure, or complex structures bObHbObH… or bObObH…. Such compounds will possess intermediate SGH properties. It is possible to predict the structures with the next polar layer L1 in the triples disposed in a non-polar manner via inversion operation from the centrosymmetric layer: bOqObOq… or bHqHbHq…. Such an iodate- or iodate-sulfate structure will be centrosymmetric. More realistically, asymmetric Bi-O-bonds as well as Ag-O-bonds impede similar variants and synthesized iodate and iodate-sulfate grown in stabile experiments are acentric and polar. Less stable conditions of crystal growth with higher temperatures may produce such disordered polytypes.
| Symmetry groupoids for AgBi(IO3)4 | |
| L1 | L2 |
| Ag(IO3) | Bi(IO3)3 |
| λ PO, τ P (1)c1 | λ PO, ρ P(1)21/c 1 |
| σ PO, τ,ρ [TC || −1] | |
| for AgBi(SO4)(IO3)2 | |
| L1 | L2′ |
| Ag(IO3) | Bi(IO3)(SO4) |
| λ PO, τ P (1)11 | λ PO, ρ P(1)−1 1 |
| σ PO, τ,ρ [Tc || −1] | |
4. Conclusions
Topology-symmetry analysis allows the similarity of crystals to be revealed and assigns them to the common structural family, as was done for Ba(IO3−)OH and Bi(IO3)O. The higher local symmetry of the central layer produces a different combination of layers conjugation in the triple, leading to higher or lower optical non-linearity for two members of the family. Such structures may be ordered (MDO-polytypes) or have significant disorder. The topology-symmetry analysis of another pair of iodates, AgBi(IO3)4 and iodate-sulfate AgBi(SO4)(IO3), led to an exhaustive crystal chemical comparison being obtained including a semi-quantitatively evaluation of nonlinear optical properties based on structural data (thickness of the polar layer L1 along the c-axis). Different predicted structures could be described using groupoid symmetry elements and the most obvious letter symbols. Such an approach is original and promising.
Author Contributions
Conceptualization, E.B. and O.D., methodology E.B. and O.D., software O.R., E.B., investigation O.R., A.V., resources O.D., A.V., E.B., writing-original draft preparation O.R., E.B., O.D., A.V., writing review and editing, E.B., visualization O.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC (CSD) 2095849 contains crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
The authors are grateful to Natalie Zubkova for her aid in collection of experimental diffraction, to the Laboratory of local methods of materials investigation, geological faculty, MSU for determination of compositions, and Sergey Stefanovich for his help in estimation of the SHG signal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nguyen, S.D.; Yeon, J.; Kim, S.H.; Halasyamani, P.S. BiO(IO3): A New Polar Iodate that Exhibits an Aurivillius-Type (Bi2O2)2+ Layer and a Large SHG Response. J. Am. Chem. Soc. 2011, 133, 12422–12425. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-G.; Sun, C.-F.; Yang, B.-P. Structures and properties of functional metal iodates. Sci. China Chem. 2011, 54, 911–922. [Google Scholar] [CrossRef]
- Hu, C.-L.; Mao, J.-G. Recent advances on second-order NLO/materials based on metal iodates. Coord. Chem. Rev. 2015, 288, 1–17. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K. Grundzuege einer Theory der OD-strukturen aus Schichten. Abh. Deutsch. Akad. Wiss. Berlin 1964, 3, 89. [Google Scholar]
- Dornberger-Schiff, K. On Order-Disorder Structures (OD-structures). Acta Cryst. 1956, 9, 593–601. [Google Scholar] [CrossRef]
- Belokoneva, E.L. Borate crystal chemistry in terms of the extended OD-theory: Topology and symmetry analysis. Cryst. Rev. 2005, 11, 151–198. [Google Scholar] [CrossRef]
- Kurtz, S.K.; Perry, T.T. A Powder Technique for the Evaluation of Nonlinear Optical Materials. J. Appl. Phys. 1968, 39, 3798–3813. [Google Scholar] [CrossRef]
- Agilent Technologies, CrysAlisPro Software System; Version 1.171.3735; Agilent Technologies UK Ltd.: Oxsford, UK, 2014.
- Sheldrick, G.M. SHELXL-97, A Program for Crystal Structure Refinement; SHELXS-97, A program for automatic solution of crystal structures; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- CrysAlisPro 1.171.3946; Rigaku Oxford Diffraction, 2018.
- Dowty, E. Atoms 3.2—A Computer Program for Displaying Atomic Structures; Kingpost, TN, USA, 1995. [Google Scholar]
- Petricek, V.; Malya, K.; Kratochvil, B.; Podlahova, J.; Loup, J. Barium diodate. Acta Cryst. 1980, 3, 2130–2132. [Google Scholar] [CrossRef]
- Lutz, H.D.; Alici, E.; Buchmeier, W. Kristallstrukturen des Sr(BrO3)2·H2O, Ba(BrO3)2·H2O, Ba(IO3)2·H2O, Pb(ClO3)2·H2O, Pb(BrO3)2·H2O. Zeit. Anorg. Alg. Chem. 1986, 535, 31–38. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Reutova, O.V.; Dimitrova, O.V.; Volkov, A.S. Synthesis and crystal structure of a new iodate (Pb0.6Ba0.4) (Pb0.4Ba0.6)[IO3]4 Analogous to Sr[IO3]2. Cryst. Rep. 2019, 64, 590–593. [Google Scholar] [CrossRef]
- Gilberg, M. Perite, a new oxihalide mineral from Langban, Sweden. Arkiv foer Mineral. och Geologi 1960, 2, 565–570. [Google Scholar]
- Bindi, L.; Welch, M.D.; Bonazzi, P.; Pratesi, G.; Menchetti, S. The crystal structure of seeligirite, Pb3IO4Cl3, a rare Pb-I-oxychloride from the San Rafael mine, Sierra Gorda, Chile. Mineral. Mag. 2008, 72, 771–783. [Google Scholar] [CrossRef]
- Phanon, D.; Suffren, Y.; Taouti, M.B.; Benbertal, D.; Brenier, A. Gautier-Luneau, I. Optical properties of Nd3+ and Yb3+-dopped AgM(IO3)4 metal iodates: Transparent host matrices for mid-IR lasers and nonlinear materials. J. Mater. Chem. C 2014, 2, 2715–2723. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Q.; Liu, L.; Lin, Z.; Halasyamani, P.S.; Chen, X.; Qin, J. AgBi(SO4)(IO3)2: Aliovalent substitution induces structure dimensionalupgrade and second harmonic generation enhancement. Chem. Comm. 2021, 57, 3712–3715. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).