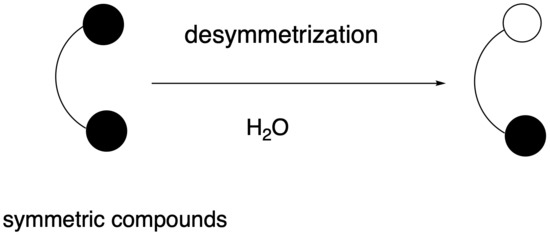
Non-Enzymatic Desymmetrization Reactions in Aqueous Media
Abstract
1. Introduction
2. Water-Mediated Non-Enzymatic Desymmetrization
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SDS | sodium dodecyl sulfate |
| DMAP | N,N-dimethylaminopyridine |
| DMT-MM | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride |
| THP | tetrahydropyran |
| ROMP | ring opening metathesis polymerization |
| TFA | trifluoroacetic acid |
| DBSA | p-dodecylbenzenesulfonic acid |
| 4-NBA | 4-nitrobenzoic acid |
| Ns | nosyl |
| ppy | (2-pyridinyl) phenyl |
| dtbbpy | 4,4′-Di-tert-butyl-2,2′-bipyridine |
| LED | light-emitting diode |
References
- Ohno, M.; Otsuka, M. Chiral synthons by ester hydrolysis catalyzed by pig liver esterase. Org. React. 1989, 37, 1–55. [Google Scholar] [CrossRef]
- Schoffers, E.; Golebiowski, A.; Johnson, C.R. Enantioselective synthesis through enzymatic asymmetrization. Tetrahedron 1996, 56, 3769–3826. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- García-Urdiales, E.; Alfonso, I.; Gotor, V. Enantioselective enzymatic desymmetrizations in organic synthesis. Chem. Rev. 2011, 111, PR110–PR180. [Google Scholar] [CrossRef] [PubMed]
- Niwayama, S. Recent desymmetrization reactions by CALB. SSRG Int. J. Appl. Chem. 2020, 7, 37–39. [Google Scholar] [CrossRef]
- Patti, A.; Sanifilippo, C. Breaking molecular symmetry through biocatalytic reactions to gain access to valuable chiral synthons. Symmetry 2020, 12, 1454. [Google Scholar] [CrossRef]
- Akai, S. Development of novel asymmetric reactions oriented to next-generation enzymatic organic syntheses. Yakugaku Zasshi 2003, 123, 919–931. [Google Scholar] [CrossRef][Green Version]
- Hudlicky, T.; Reed, J.W. Application of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem. Soc. Rev. 2009, 38, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Akai, S.; Kita, Y. Recent progress on the lipase-catalyzed asymmetric syntheses. J. Synth. Org. Chem. Jpn. 2007, 65, 772–782. [Google Scholar] [CrossRef]
- Borissov, A.; Davies, T.Q.; Ellis, S.R.; Fleming, T.A.; Richardson, M.S.W.; Dixon, D.J. Organocatalytic enantioselective desymmetrisation. Chem. Soc. Rev. 2016, 45, 5474–5540. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-García, Á.; Kündig, E.P. Desymmetrisation of meso-diols mediated by non-enzymatic acyl transfer catalysis. Chem. Soc. Rev. 2012, 41, 7803–7831. [Google Scholar] [CrossRef]
- Peterson, K.S. Nonenzymatic enatioselective synthesis of all-carbon quaternary centers through desymmetrization. Tetrahedron Lett. 2015, 56, 6523–6535. [Google Scholar] [CrossRef]
- Quintavalla, A.; Cerisoli, L.; Montroni, E. Enantioselective desymmetrization promoted by bifunctional organocatalysts. Curr. Organocatalysis 2014, 1, 107–171. [Google Scholar] [CrossRef]
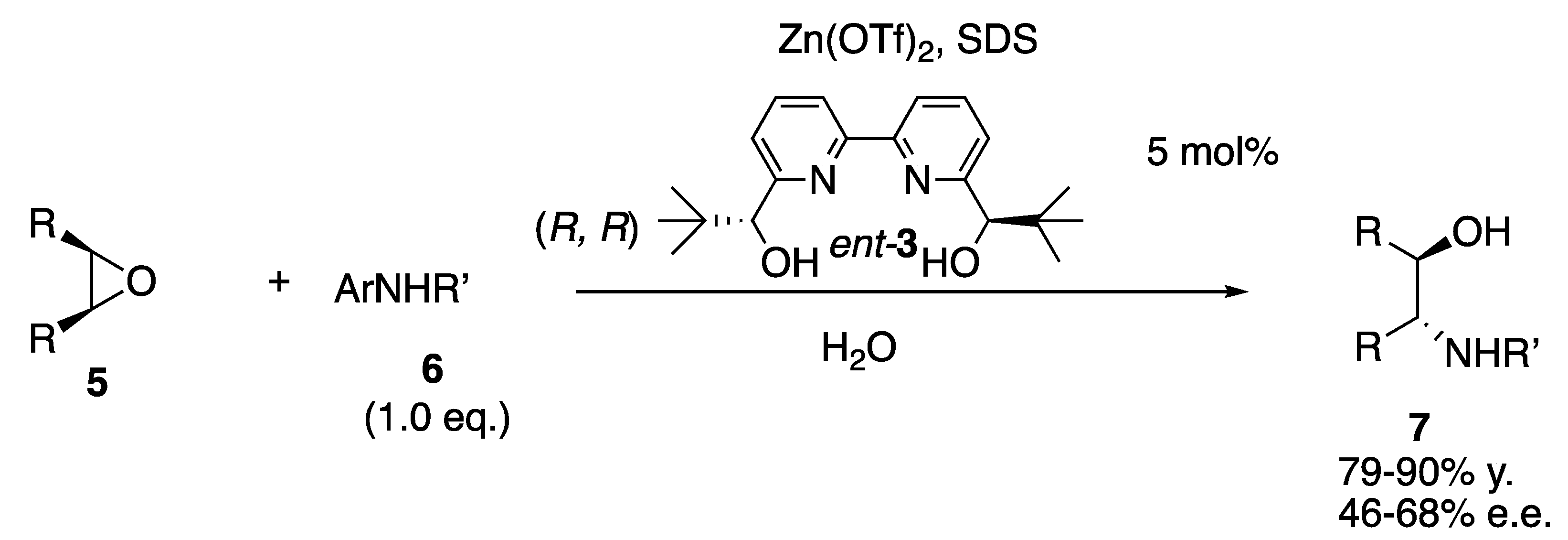
- Kokubo, M.; Naito, T.; Kobayashi, S. Chiral zinc (II) and copper (II)-catalyzed asymmetric ring-opening reactions of meso-epoxides with aniline and indole derivatives. Tetrahedron 2010, 66, 1111–1118. [Google Scholar] [CrossRef]
- Ogawa, C.; Kokubo, M.; Kobayashi, S. Asymmetric ring-opening reactions of meso-epoxides using metal-chiral bipyridine complexes. J. Synth. Org. Chem. Jpn. 2010, 68, 718–728. [Google Scholar] [CrossRef]
- Bonollo, S.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. Zn (II)-catalyzed desymmetrization of meso-epoxides by aromatic amines in water. Synlett 2008, 10, 1574–1578. [Google Scholar] [CrossRef]
- White, D.E.; Tadross, P.M.; Lu, Z.; Jacobsen, E.N. A broadly applicable and practical oligomeric (salen)co catalyst for enantioselective epoxide ring-opening reactions. Tetrahedron 2014, 70, 4165–4180. [Google Scholar] [CrossRef]
- Kumar, M.; Kureshy, R.I.; Ghosh, D.; Khan, N.H.; Abdi, S.H.R.; Bajaj, H.C. Synthesis of chiral ligands with multiple stereogenic centers and their application in titanium (IV)-catalyzed enantioselective desymmetrization of meso-epoxides. ChemCatChem 2013, 5, 2336–2342. [Google Scholar] [CrossRef]
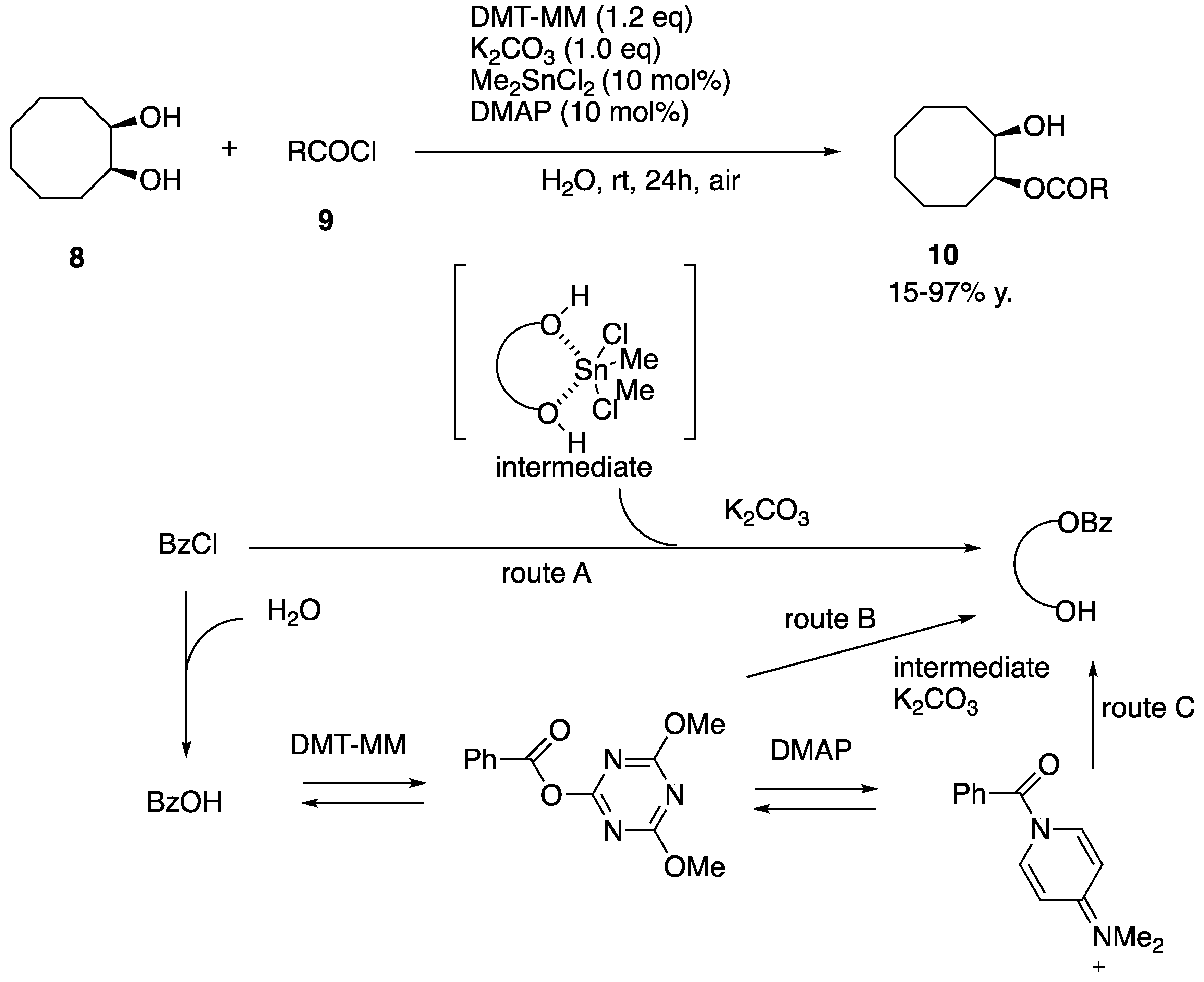
- Muramatsu, W.; William, J.M.; Onomura, O. Selective monobenzoylation of 1,2- and 1,3-diols catalyzed by Me2SnCl2 in water (organic solvent free) under mild conditions. J. Org. Chem. 2012, 77, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Matsumoto, A.; Asano, K.; Matsubara, S. Desymmetrization of gem-diols via water-assisted organocatalytic enantio- and diastereoselective cycloetherification. Chem. Commun. 2020, 56, 12335–12338. [Google Scholar] [CrossRef]
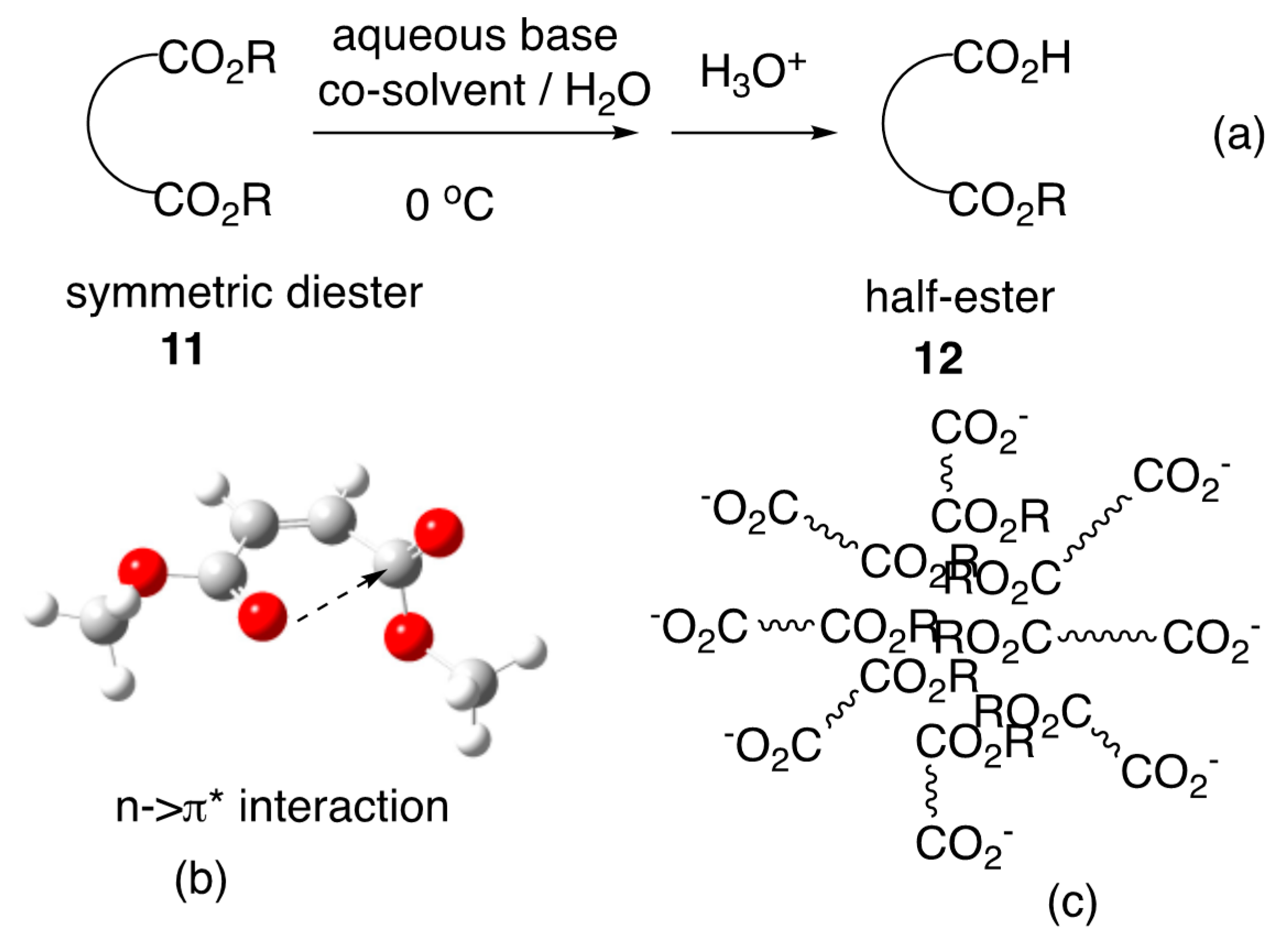
- Niwayama, S. Highly efficient selective monohydrolysis of symmetric diesters. J. Org. Chem. 2000, 65, 5834–5836. [Google Scholar] [CrossRef]
- Cho, H.; Alexander, R.B.; Niwayama, S. Conformational analysis of symmetric diesters. Curr. Org. Chem. 2012, 16, 1151–1158. [Google Scholar] [CrossRef]
- Niwayama, S. Highly efficient and practical selective monohydrolysis of symmetric diesters: Recent progress and scope. J. Synth. Org. Chem. Jpn. 2008, 66, 983–994. [Google Scholar] [CrossRef]
- Niwayama, S.; Wang, H.; Hiraga, Y.; Clayton, J.C. Studies of solvent effects in the highly efficient selective monohydolysis of symmetric diesters. Tetrahedron Lett. 2007, 48, 8508–8510. [Google Scholar] [CrossRef]
- Niwayama, S.; Rimkus, A. Effects of counter cations in selective monohydrolyses of symmetric diesters. Bull. Chem. Soc. Jpn. 2005, 78, 498–500. [Google Scholar] [CrossRef]
- Niwayama, S.; Cho, H.; Lin, C. Highly efficient selective monohydrolysis of dialkyl malonates and their derivatives. Tetrahedron Lett. 2008, 49, 4434–4436. [Google Scholar] [CrossRef]
- Niwayama, S.; Cho, H. Practical large scale synthesis of half-esters of malonic acid. Chem. Pharm. Bull. 2009, 57, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Niwayama, S.; Cho, H.; Zabet-Moghaddam, M.; Whittlesey, B.R. Remote exo/endo selectivity in selective monohydrolysis of dialkyl bicyclo[2.2.1]heptane-2,3-dicarboxylate derivatives. J. Org. Chem. 2010, 75, 3775–3780. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, T.; Niwayama, S. Practical selective monohydrolysis of bulky symmetric diesters: Comparing with sonochemistry. Tetrahedron 2018, 74, 6815–6820. [Google Scholar] [CrossRef]
- Shi, J.; Niwayama, S. Practical selective monohydrolysis of bulky symmetric diesters. Tetrahedron Lett. 2018, 59, 799–802. [Google Scholar] [CrossRef]
- Niwayama, S.; Hiraga, Y. New exo/endo selectivity observed in monohydrolysis of dialkyl bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylates. Tetrahedron Lett. 2003, 44, 8567–8570. [Google Scholar] [CrossRef]
- Shi, J.; Hayashishita, Y.; Takata, T.; Nishihara, Y.; Niwayama, S. Syntheses of polynorbornadienes by ring-opening metathesis polymerizations (ROMP) of symmetric and non-symmetric 2,3-bis(alkoxycarbonyl)norbornadienes and their conversion to half-ester derivatives. Org. Biomol. Chem. 2020, 18, 6585–6746. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.M.; Heuft, M.A.; Rabbat, P. Solvent effects on the monobromination of α, ω-diols: A convenient preparation of ω-bromoalkanols. J. Org. Chem. 2000, 65, 5837–5838. [Google Scholar] [CrossRef] [PubMed]
- Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C.F., III. Organocatalytic direct asymmetric aldol reactions in water. J. Am. Chem. Soc. 2006, 128, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Sumiya, T.; Takahashi, J.; Gotoh, H.; Urushima, T.; Shoji, M. Highly diastereo- and enantioselective direct aldol reactions in water. Angew. Chem. Int. Ed. 2006, 45, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Maya, V.; Raj, M.; Singh, V.K. Highly enantioselective organocatalytic direct aldol reaction in an aqueous medium. Org. Lett. 2007, 9, 2593–2595. [Google Scholar] [CrossRef]
- Luo, S.; Xu, H.; Li, J.; Zhang, L.; Mi, X.; Zheng, X.; Cheng, J.-P. Facile evolution of asymmetric organocatalysis in water assisted by surfactant Brønsted acids. Tetrahedron 2007, 63, 11307–11314. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, X.; Fu, S. Rationally designed 4-phenoxy substituted prolineamide phenols organocatalyst for the direct aldol reaction in water. Tetrahedron Lett. 2009, 50, 1173–1176. [Google Scholar] [CrossRef]
- Bisticha, A.; Triandafillidi, I.; Kototos, C.G. tert-Butyl esters of peptides as organocatalysts for the asymmetric aldol reaction. Tetrahedron Asymmetry 2015, 26, 102–108. [Google Scholar] [CrossRef]
- Aresu, E.; Fioravanti, S.; Pellacani, L.; Sciubba, F.; Trulli, L. Water-controlled chiral inversion of a nitrogen atom during the synthesis of diazirines from α-branched N, N’-dialkyl α-diimines. New J. Chem. 2013, 37, 4125–4129. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Toullec, P.Y.; Díez, J.; Conejero, S.; Michelet, V.; Cadierno, V. Cycloisomerization versus hydration reactions in aqueous media: A Au (III)-NHC catalyst that makes the difference. Org. Lett. 2012, 14, 2520–2523. [Google Scholar] [CrossRef]
- Benniston, A.C.; Sirbu, D.; Turta, C.; Probert, M.R.; Clegg, W. A simple method for desymmetrizing 1,1′-ferrocenedicarboxaldehyde. Tetrahedron Lett. 2014, 55, 3777–3780. [Google Scholar] [CrossRef][Green Version]
- Uetake, U.; Uwamori, M.; Nakada, M. Enantioselective approach to polycyclic polyprenylated acylphloroglucinols via catalytic asymmetric intramolecular cyclopropanation. J. Org. Chem. 2015, 80, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Yu, S. Synthesis of chiral fluorides by sequential organocatalyzed desymmetrization of glutaric anhydrides and photoredox-catalyzed dicarboxylic fluorination. Synlett 2021, 32, 391–394. [Google Scholar] [CrossRef]
- García-García, C.; Ortiz-Rojano, L.; Álavarez, S.; Álavarez, R.; Ribagorda, M.; Carreño, M.G. Friedel–Crafts alkylation of indoles with p-quinols: The role of hydrogen bonding of water for the desymmetrization of the cyclohexadienone system. Org. Lett. 2016, 18, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Z.; Sun, J. Enantioselective oxetane ring opening with chloride: Unusual use of wet molecular sieves for the controlled release of HCl. Angew. Chem. Int. Ed. 2016, 55, 6954–6958. [Google Scholar] [CrossRef] [PubMed]

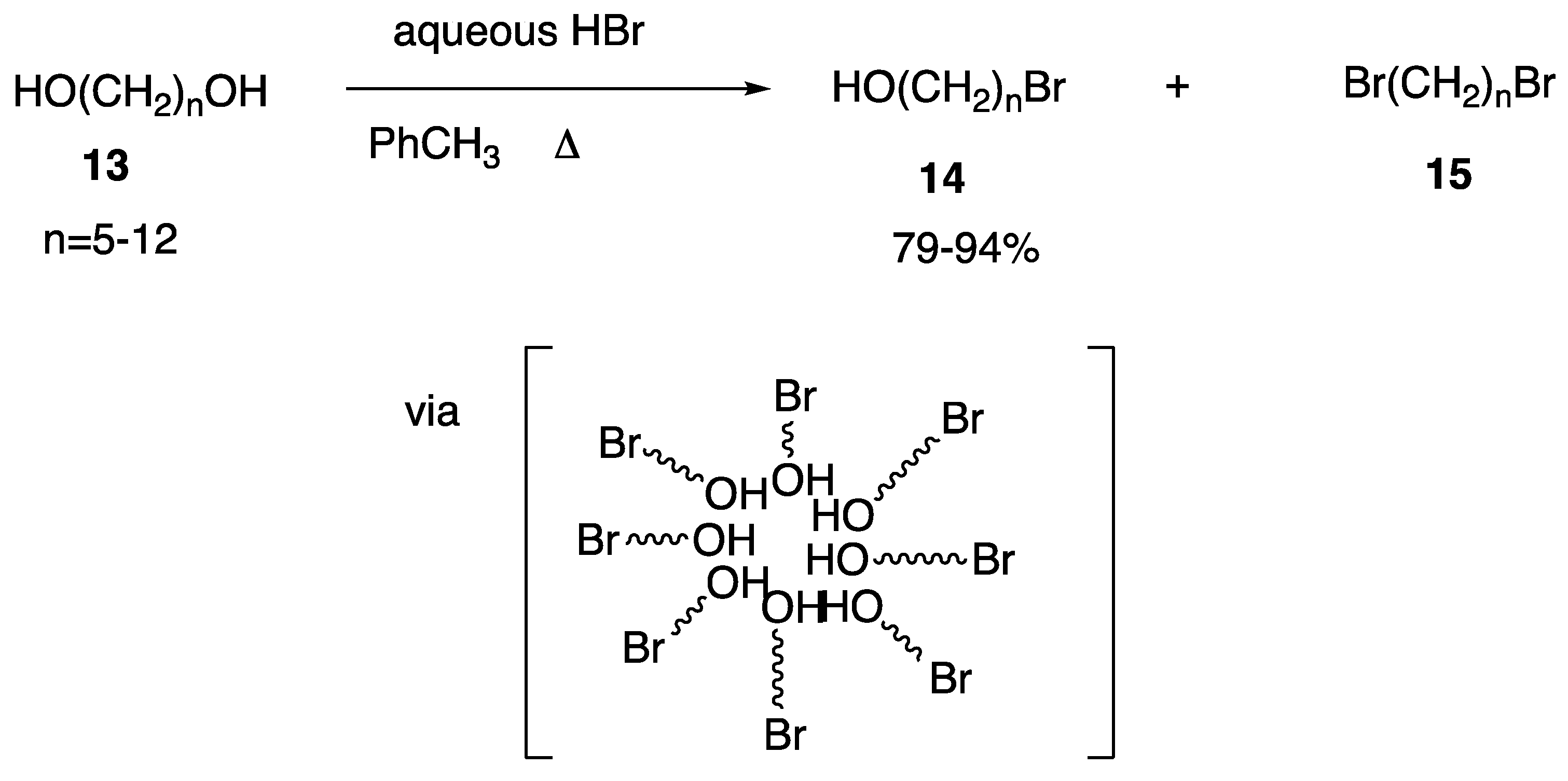

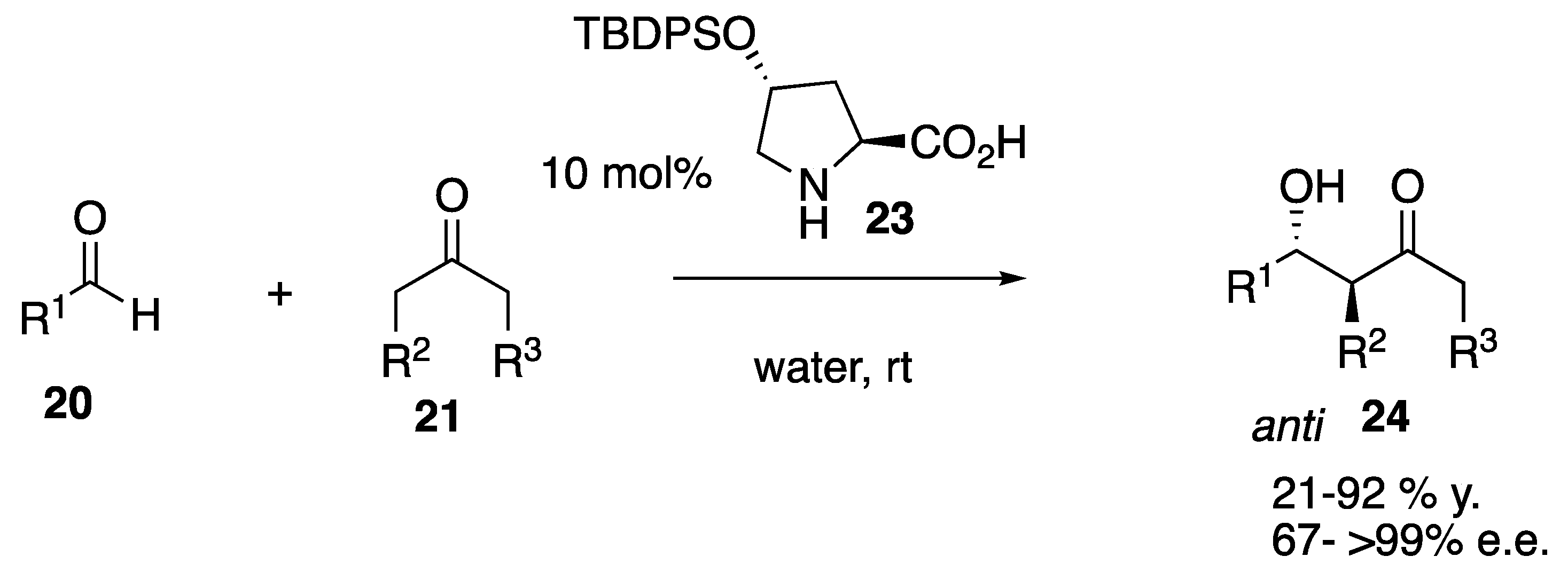
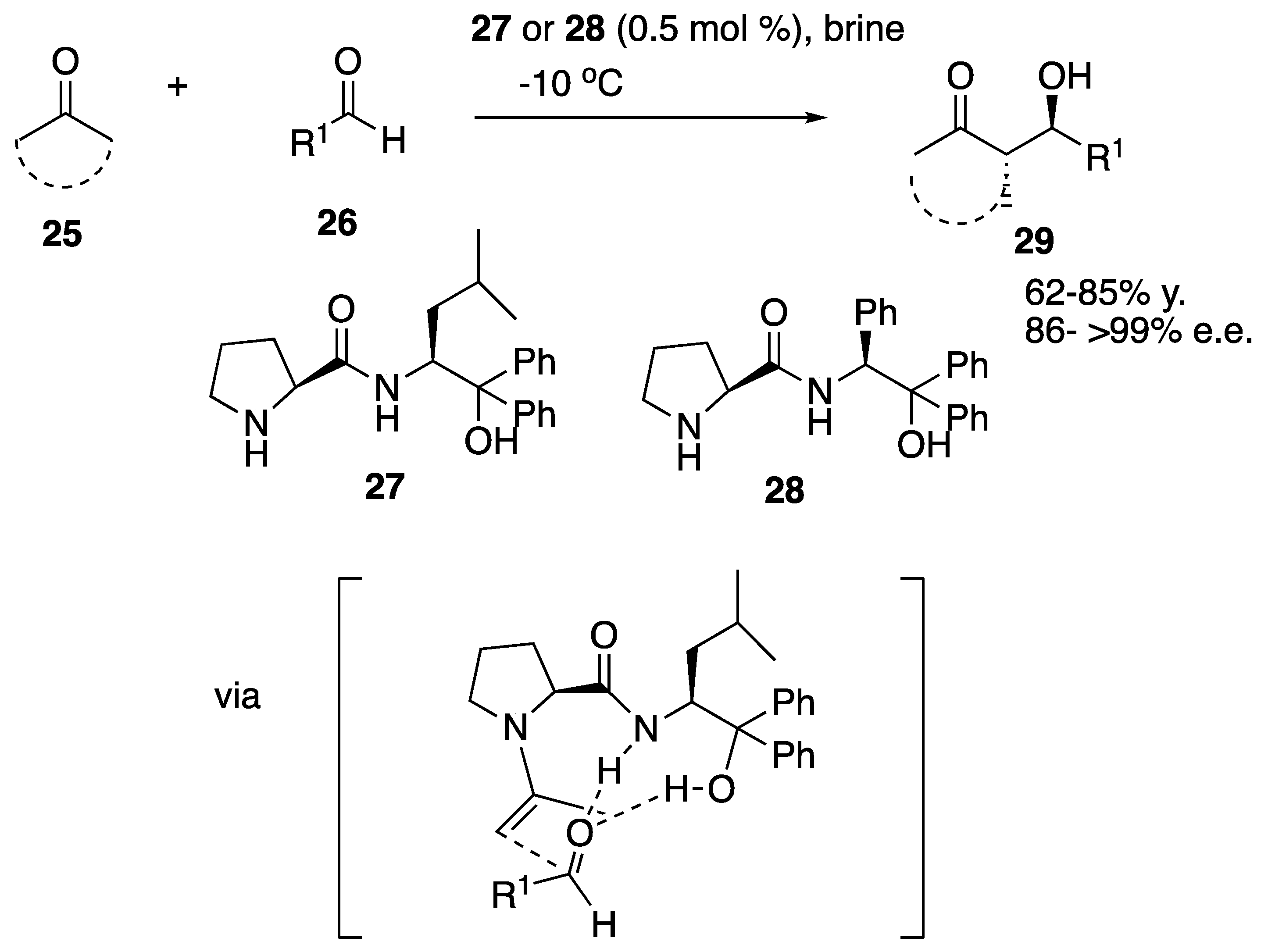

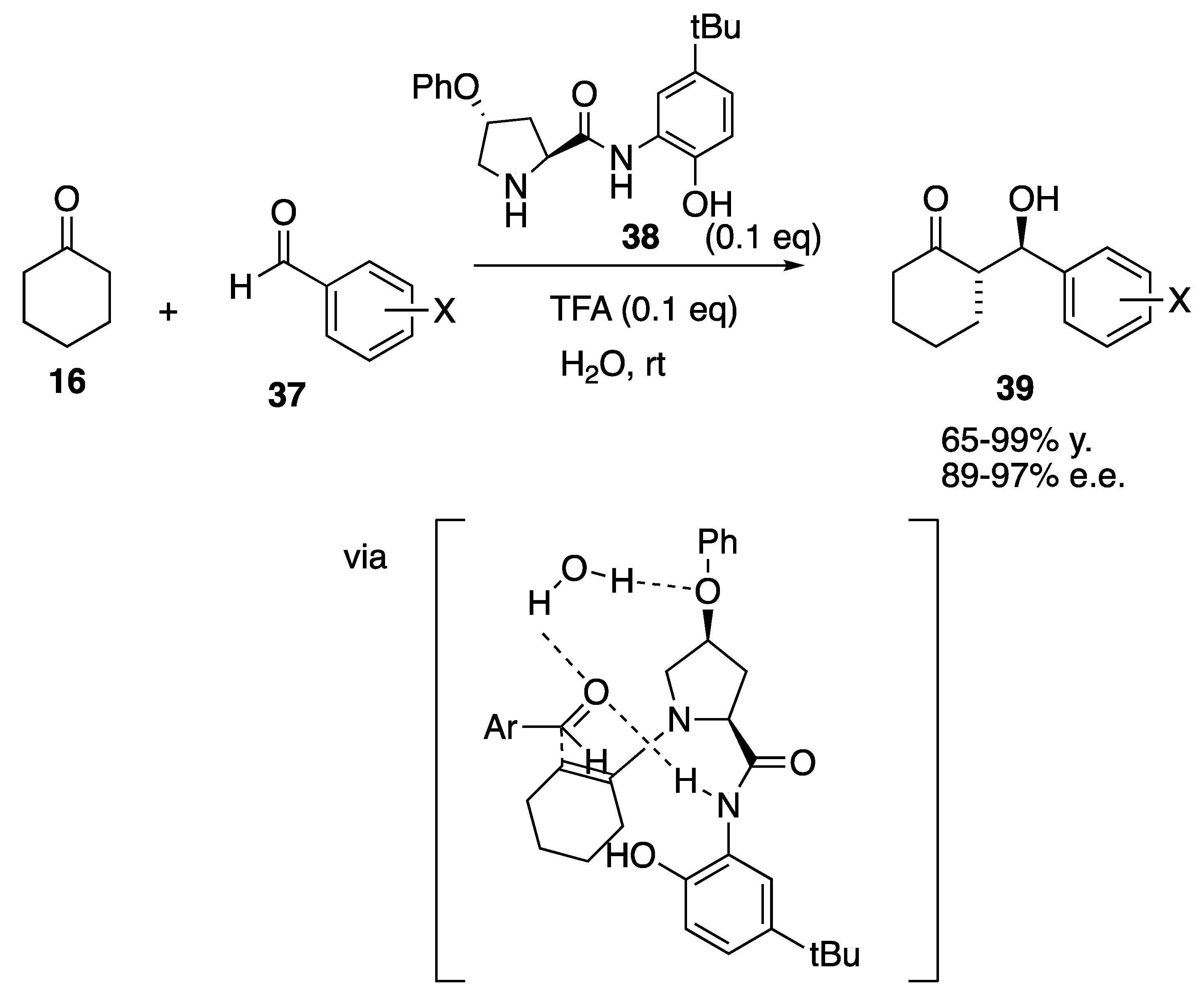
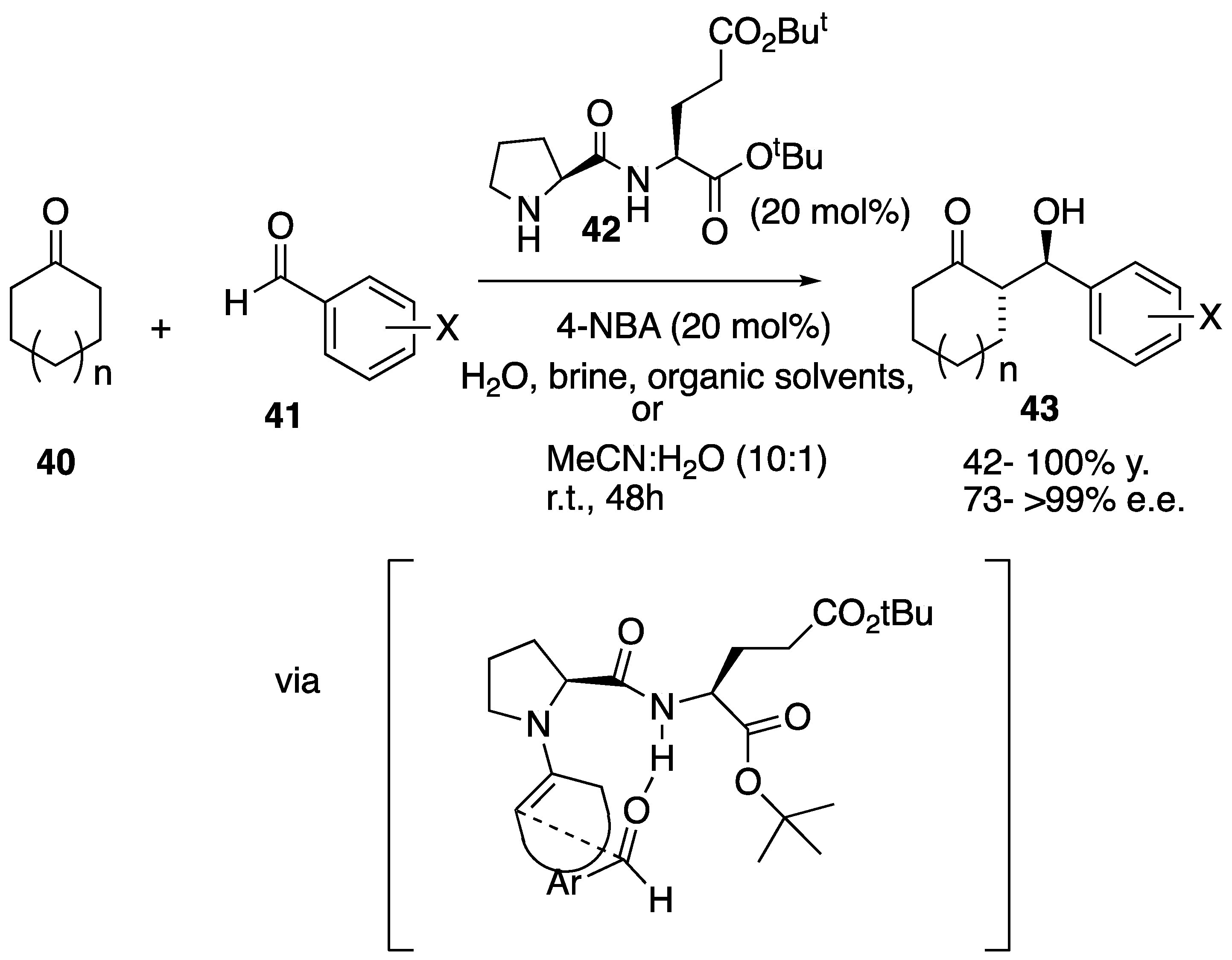
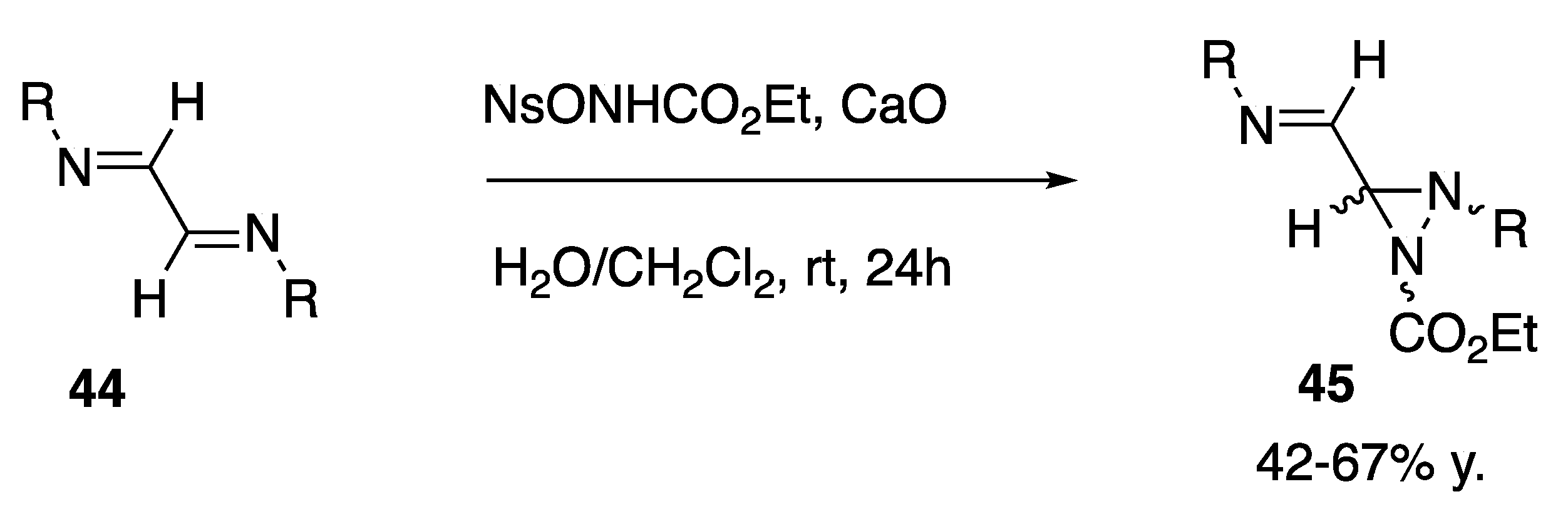
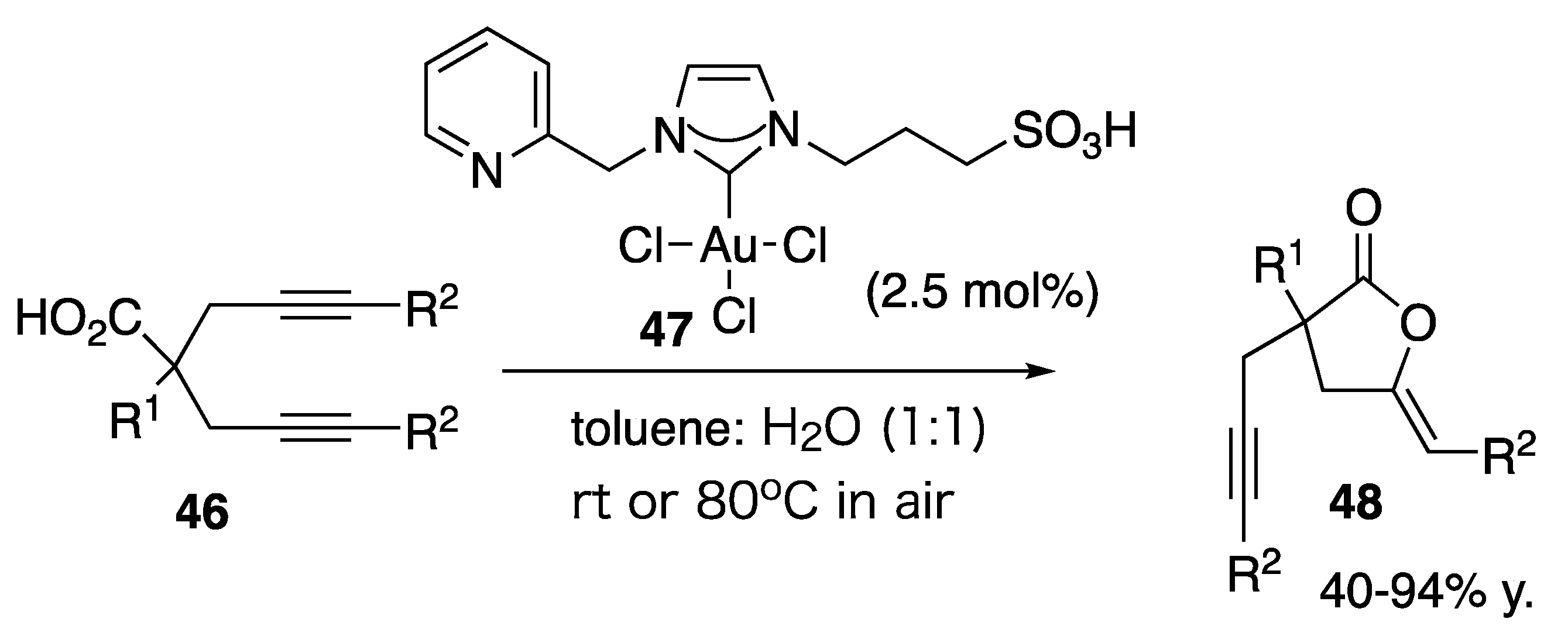
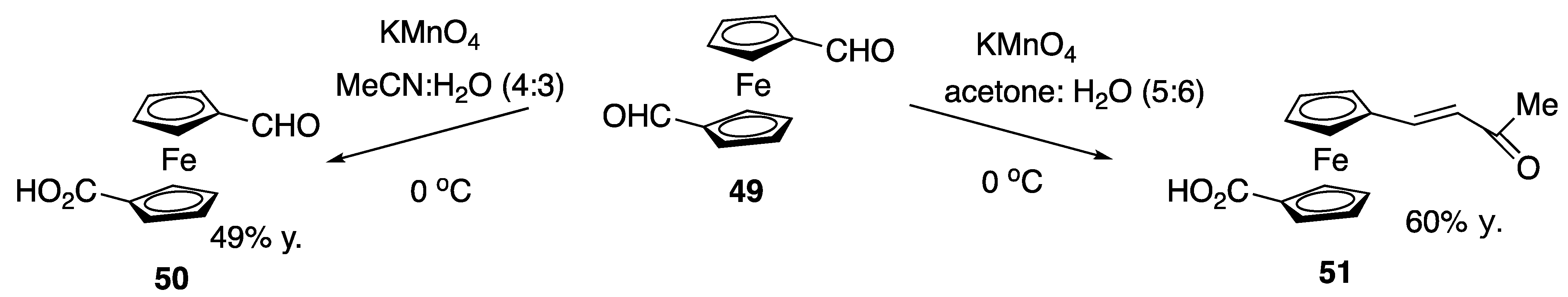
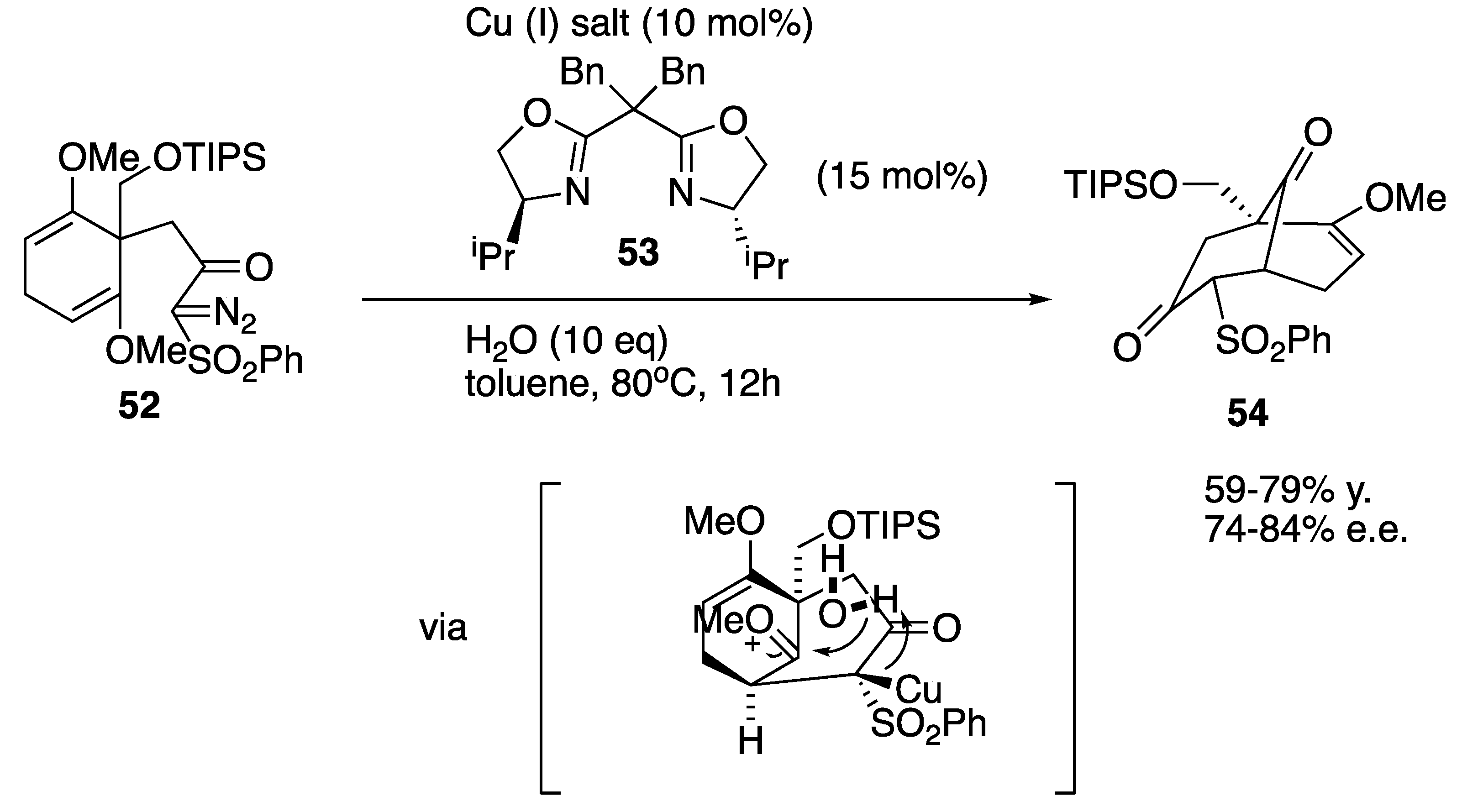
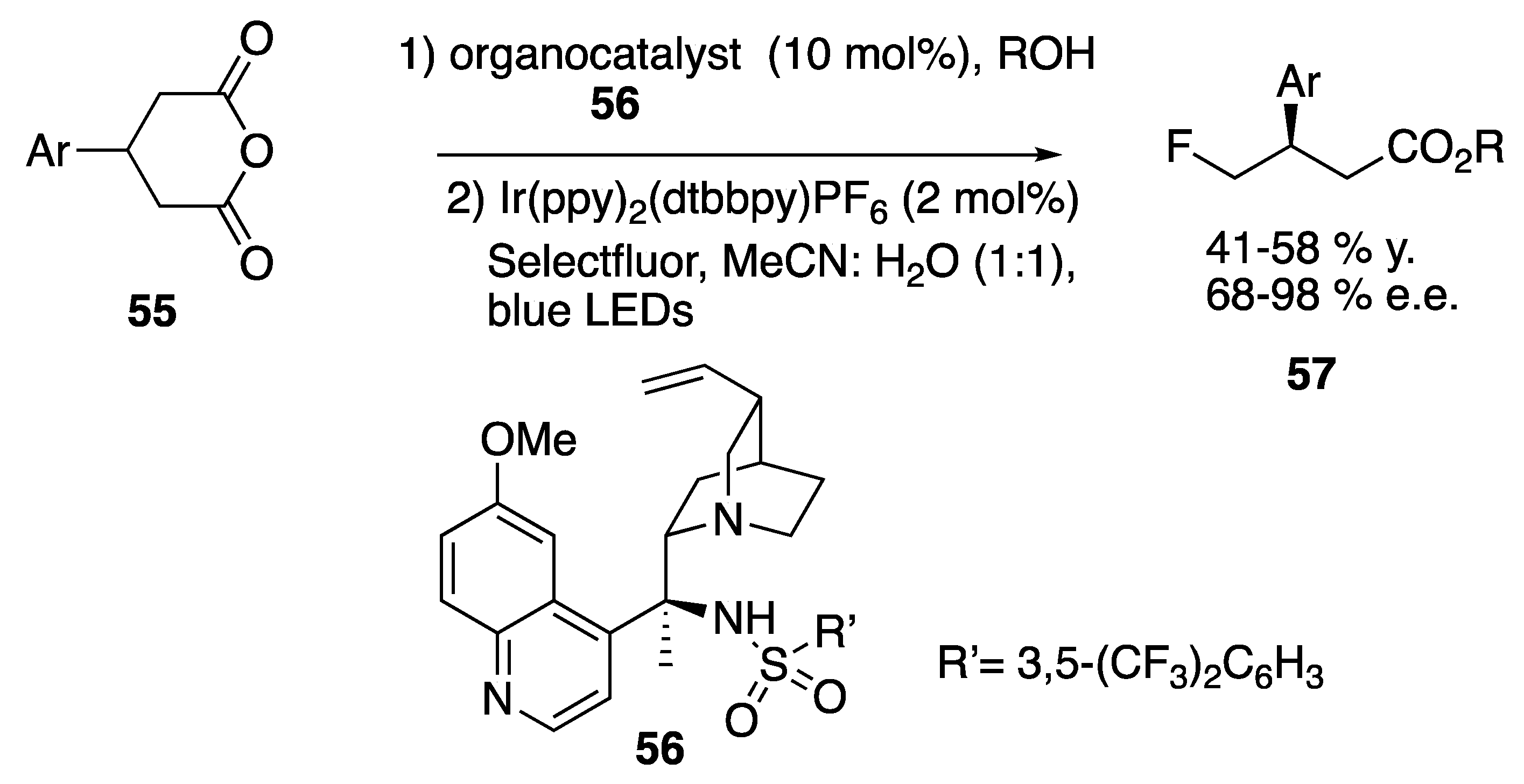
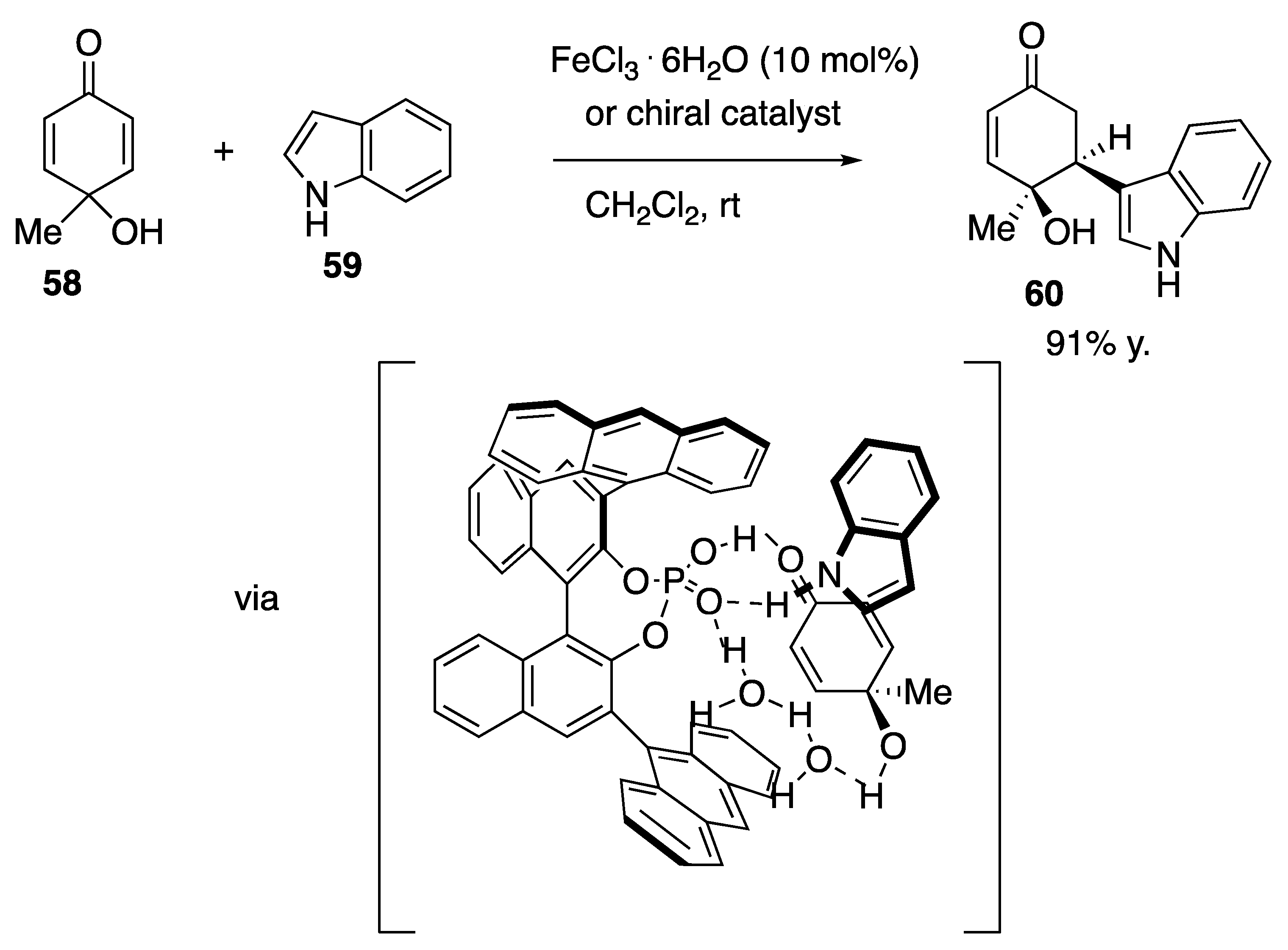
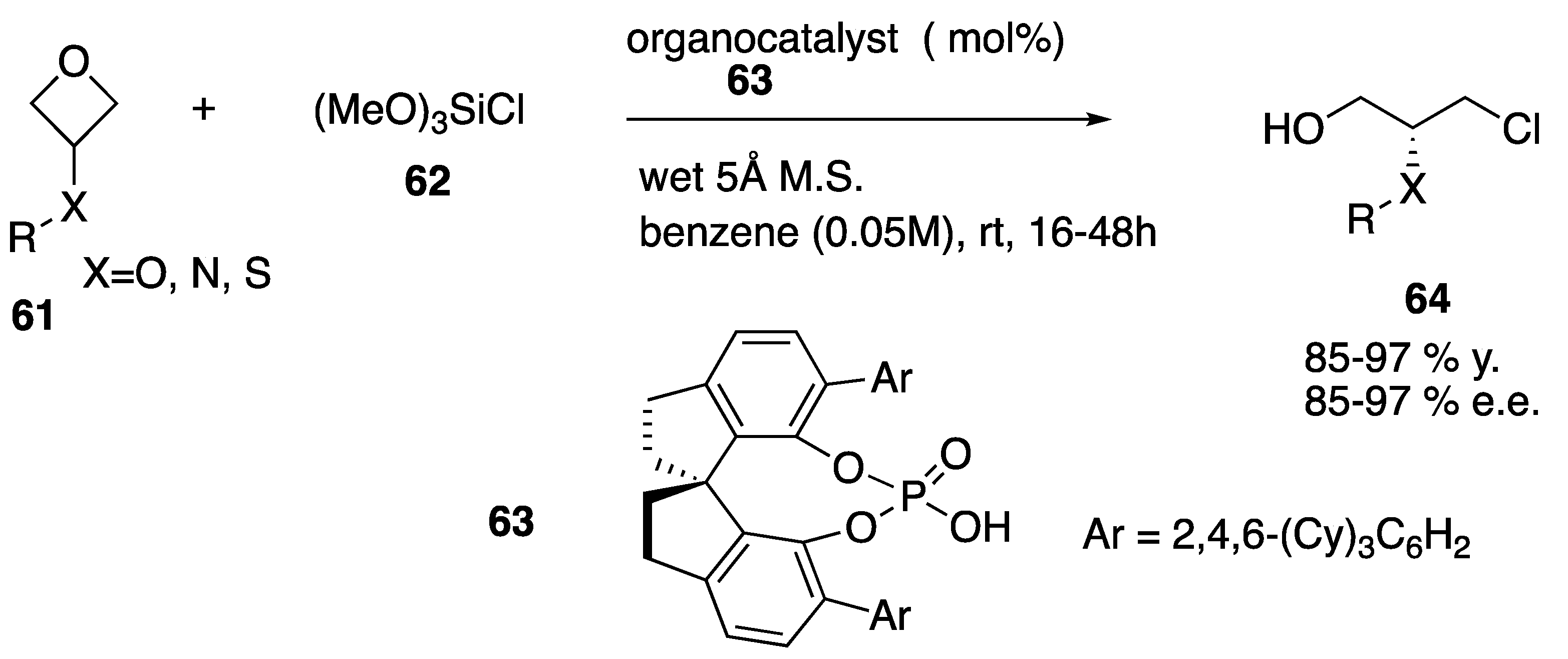
| Starting Symmetric Compound | Reaction | Best Yield (Best Optical Purity) |
|---|---|---|
| epoxide | aminolysis (ring opening) | 100% (95% e.e.) |
| diol | monobenzoylation | 97% |
| monobromination | 94% | |
| diester | monohydrolysis | >99% |
| ketone | aldol | 100% (>99% e.e.) |
| diimine | diaziridine formation | 67% |
| bispopargylic carboxylic acid | cycloisomerization | 94% |
| dicarboxaldehyde | monooxidation | 49% |
| aldol | 60% | |
| cyclohexa-1,4-diene | cyclopropanation | 79% (84% e.e.) |
| anhydride | alkolysis + fluorination | 58% (98% e.e.) |
| p-quinol | Friedel–Crafts alkylation | 91% |
| oxetane | chlorination (ring opening) | 97% (97% e.e.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niwayama, S. Non-Enzymatic Desymmetrization Reactions in Aqueous Media. Symmetry 2021, 13, 720. https://doi.org/10.3390/sym13040720
Niwayama S. Non-Enzymatic Desymmetrization Reactions in Aqueous Media. Symmetry. 2021; 13(4):720. https://doi.org/10.3390/sym13040720
Chicago/Turabian StyleNiwayama, Satomi. 2021. "Non-Enzymatic Desymmetrization Reactions in Aqueous Media" Symmetry 13, no. 4: 720. https://doi.org/10.3390/sym13040720
APA StyleNiwayama, S. (2021). Non-Enzymatic Desymmetrization Reactions in Aqueous Media. Symmetry, 13(4), 720. https://doi.org/10.3390/sym13040720