Deep MLP-CNN Model Using Mixed-Data to Distinguish between COVID-19 and Non-COVID-19 Patients
Abstract
1. Introduction
2. Dataset and Methodology
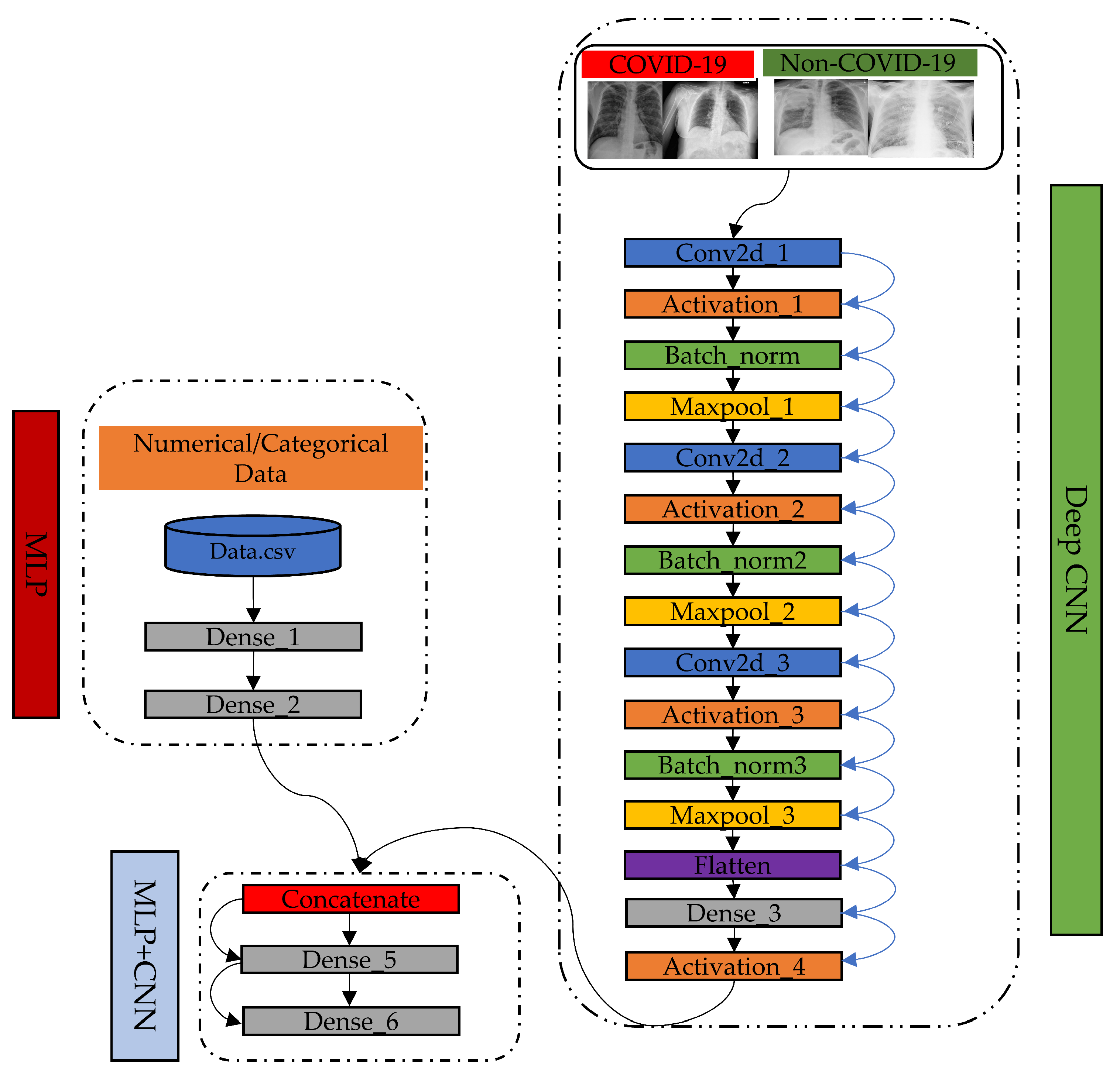
2.1. Proposed Model
2.2. How Our Proposed MLP-CNN Model Works
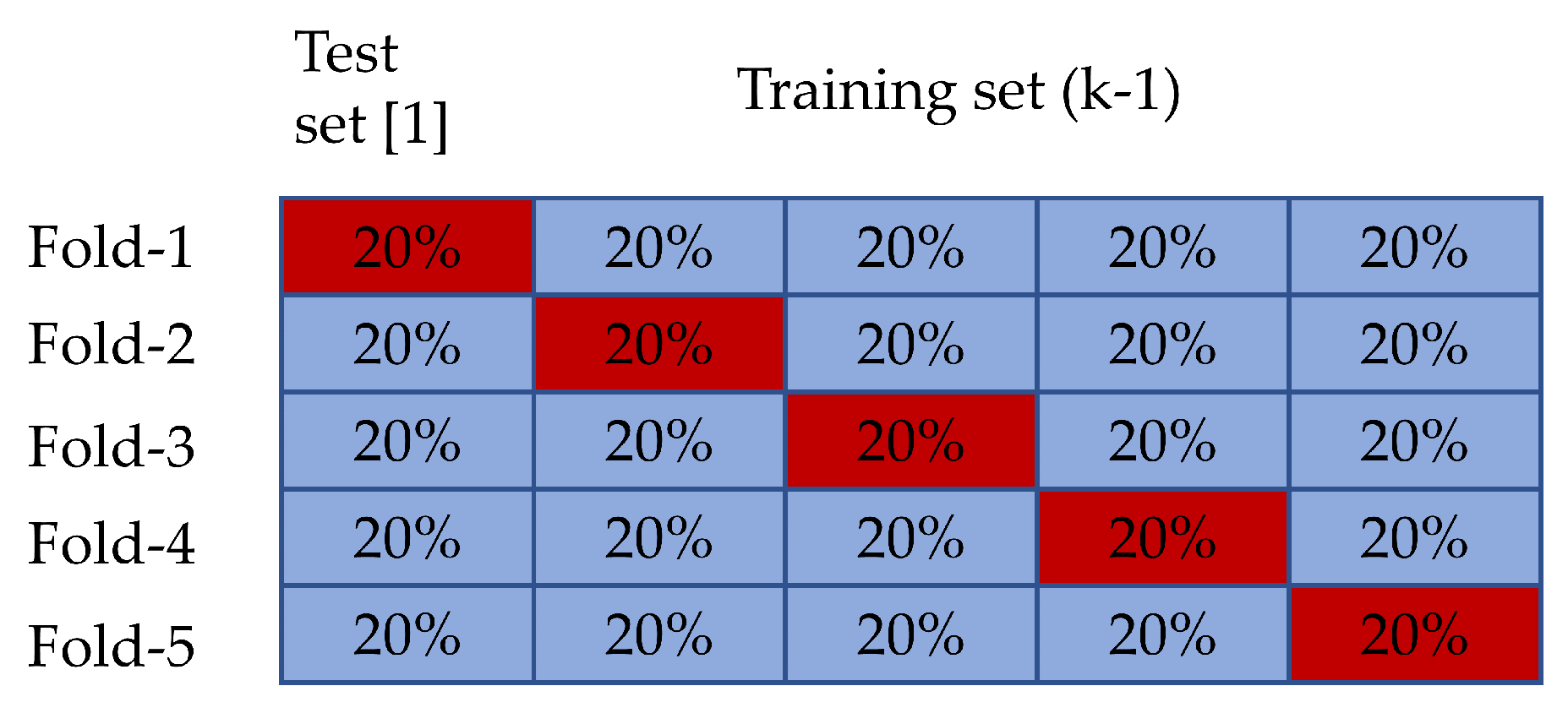
2.3. Experiment Setup
- True Positive ()= COVID-19 patient classified as patient
- False Positive ()= Healthy individuals classified as patient
- True Negative ()= Healthy individuals classified as healthy
- False Negative ()= COVID-19 patients classified as healthy
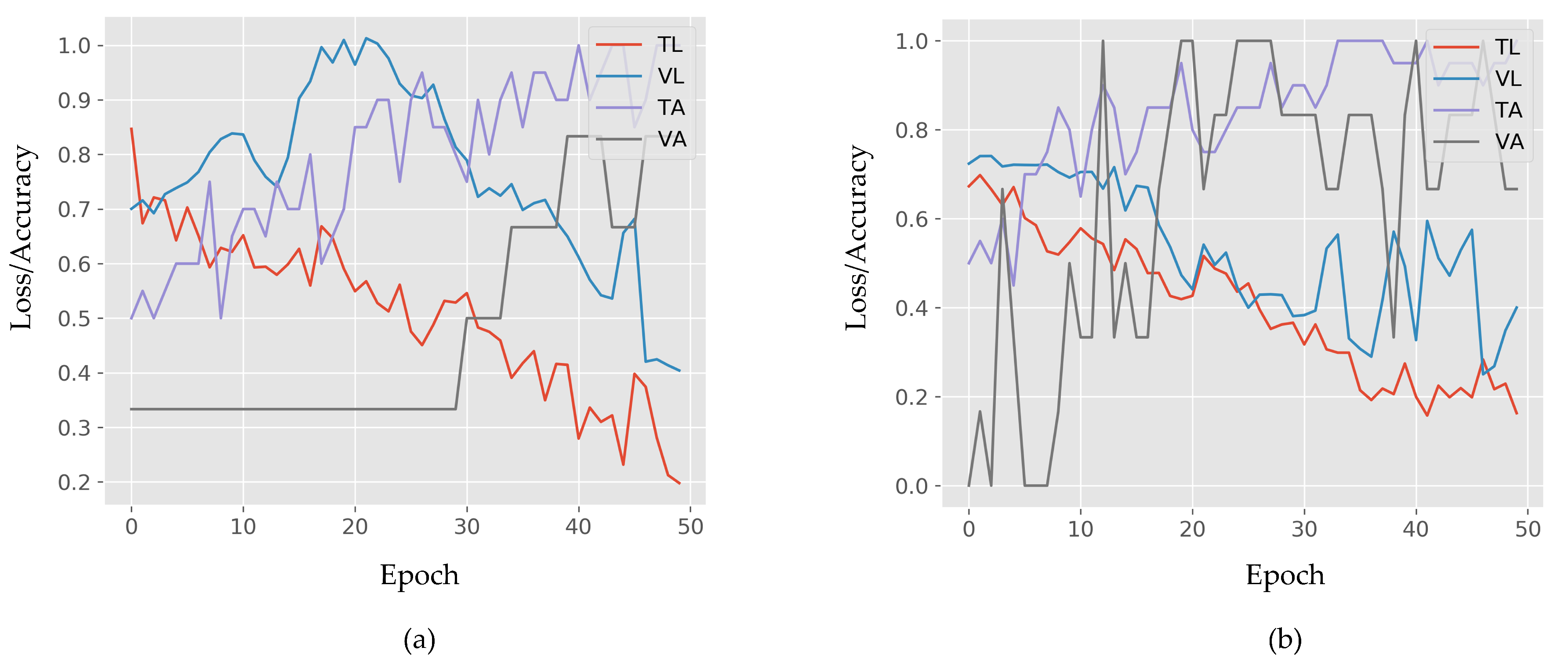
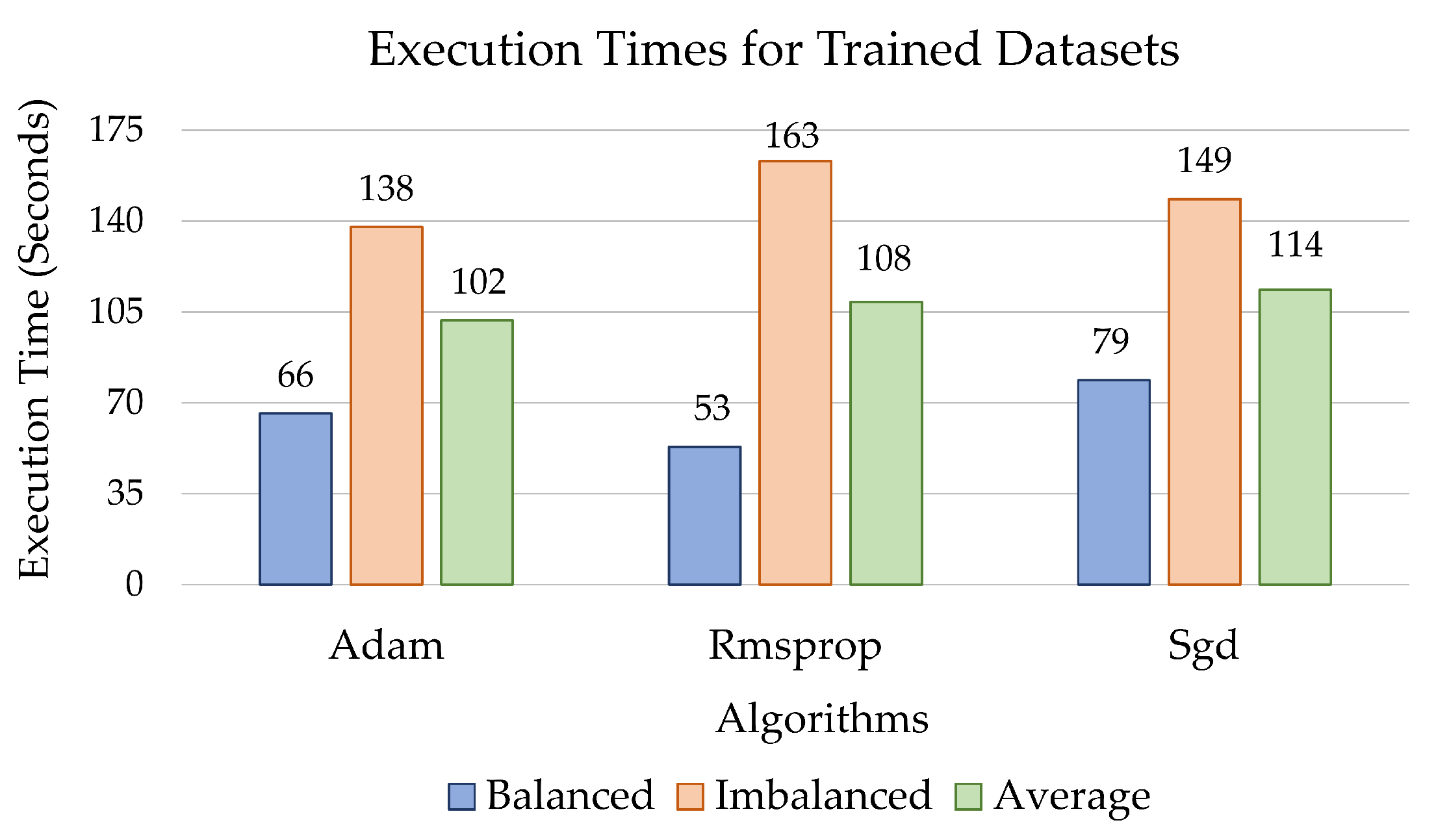
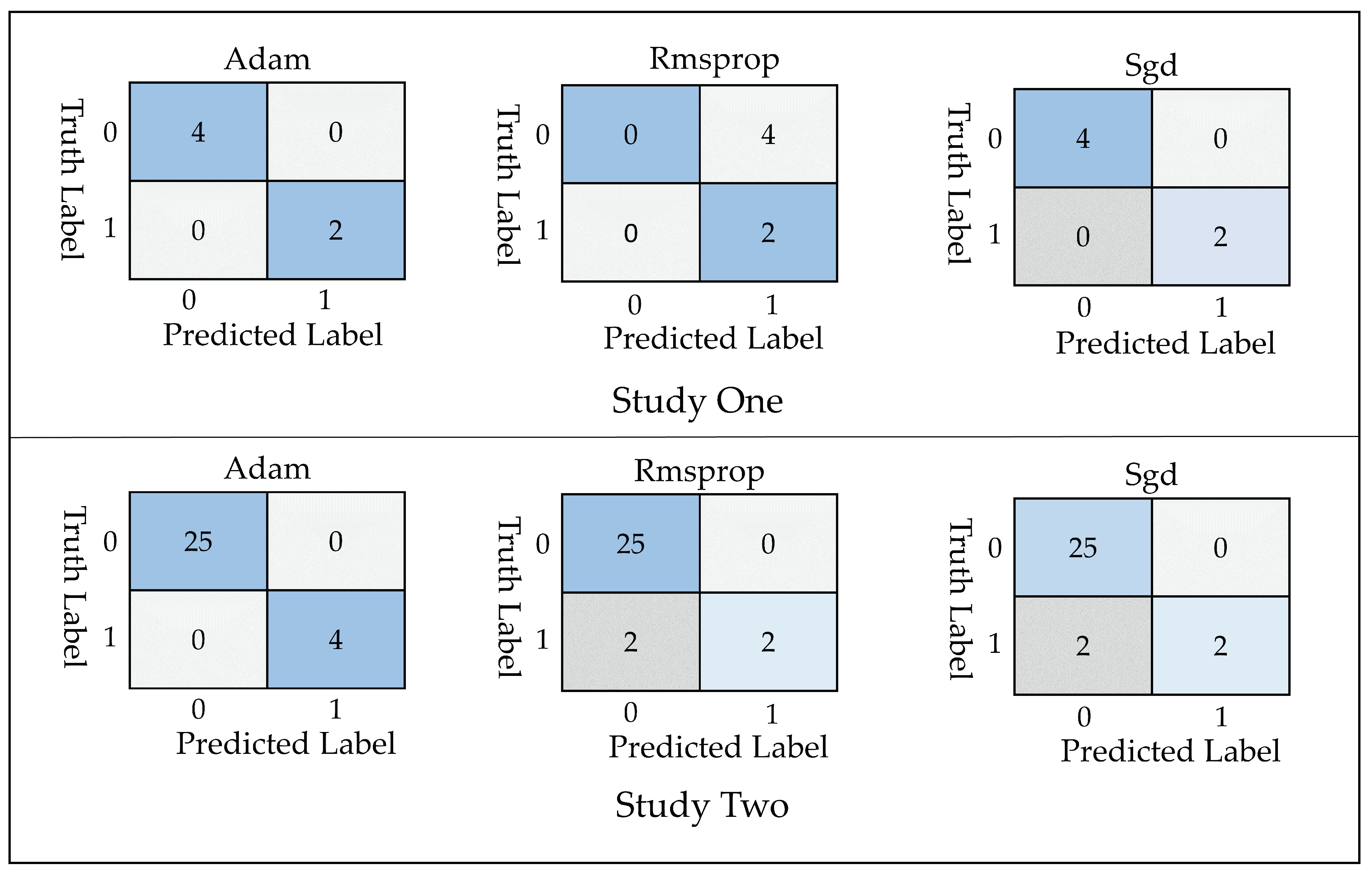
3. Computational Results
4. Discussion
- the size of the dataset adopted is comparatively small, and
- only four numerical and categorical parameters were considered.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roosa, K.; Lee, Y.; Luo, R.; Kirpich, A.; Rothenberg, R.; Hyman, J.; Yan, P.; Chowell, G. Real-Time Forecasts of the COVID-19 Epidemic in China from February 5th to February 24th, 2020. Infect. Dis. Model. 2020, 5, 256–263. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.T.; Xiao, Y.; Wang, M.; Sun, C.; Liang, J.; Li, S.; Zhang, M.; Guo, Y.; Xiao, Y. Prediction of Criticality in Patients with Severe Covid-19 Infection Using Three Clinical Features: A Machine Learning-Based Prognostic Model with Clinical Data in Wuhan. MedRxiv 2020. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Narin, A.; Kaya, C.; Pamuk, Z. Automatic Detection of Coronavirus Disease (covid-19) Using x-ray Images and Deep Convolutional Neural Networks. arXiv 2020, arXiv:2003.10849. [Google Scholar]
- Dashbord. Covid-19 WorldMeter. August 2020. Available online: https://www.worldometers.info/coronavirus/ (accessed on 30 August 2020).
- Yuen, K.S.; Ye, Z.W.; Fung, S.Y.; Chan, C.P.; Jin, D.Y. SARS-CoV-2 and COVID-19: The Most Important Research Questions. Cell Biosci. 2020, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liang, T. Handbook of COVID-19 Prevention and Treatment; Compiled According to Clinical Experience; The First Affiliated Hospital, Zhejiang University School of Medicine: Hangzhou, China, 2020. [Google Scholar]
- Zhao, W.; Zhong, Z.; Xie, X.; Yu, Q.; Liu, J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. Am. J. Roentgenol. 2020, 214, 1072–1077. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Covid-19 Situation in Bangladesh. Preprints 2020. [Google Scholar] [CrossRef]
- Alam, M.S.; Alam, M.Z.; Nazir, K.N.H.; Bhuiyan, M.A.B. The Emergence of Novel Coronavirus Disease (COVID-19) in Bangladesh: Present Status, Challenges, and Future Management. J. Adv. Vet. Anim. Res. 2020, 7, 198–208. [Google Scholar] [CrossRef]
- Weron, R. Electricity Price Forecasting: A Review of the State-of-the-Art with a Look into the Future. Int. J. Forecast. 2014, 30, 1030–1081. [Google Scholar] [CrossRef]
- Ponta, L.; Puliga, G.; Oneto, L.; Manzini, R. Identifying the Determinants of Innovation Capability with Machine Learning and Patents. IEEE Trans. Eng. Manag. 2020. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; Van Der Laak, J.A.; Van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.; Wang, L.; Rao, J.; Lim, T. Deep Learning Applications in Medical Image Analysis. IEEE Access 2017, 6, 9375–9389. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Faust, O.; Hagiwara, Y.; Hong, T.J.; Lih, O.S.; Acharya, U.R. Deep Learning for Healthcare Applications Based on Physiological Signals: A Review. Comput. Methods Programs Biomed. 2018, 161, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Murat, F.; Yildirim, O.; Talo, M.; Baloglu, U.B.; Demir, Y.; Acharya, U.R. Application of Deep Learning Techniques for Heartbeats Detection Using ECG Signals-Analysis and Review. Comput. Biol. Med. 2020, 644, 103726. [Google Scholar] [CrossRef]
- Rizvi, A.S.; Murtaza, G.; Yan, D.; Irfan, M.; Xue, M.; Meng, Z.H.; Qu, F. Development of Molecularly Imprinted 2D Photonic Crystal Hydrogel Sensor for Detection of L-Kynurenine in Human Serum. Talanta 2020, 208, 120403. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaur, I. Artificial Intelligence, Machine Learning and Deep Learning: Definitions and Differences. Clin. Exp. Dermatol. 2020, 45, 131–132. [Google Scholar] [CrossRef]
- Greenspan, H.; Van Ginneken, B.; Summers, R.M. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Trans. Med. Imaging 2016, 35, 1153–1159. [Google Scholar] [CrossRef]
- Yildirim, O.; Talo, M.; Ay, B.; Baloglu, U.B.; Aydin, G.; Acharya, U.R. Automated Detection of Diabetic Subject Using Pre-Trained 2D-CNN Models with Frequency Spectrum Images Extracted from Heart Rate Signals. Comput. Biol. Med. 2019, 113, 103387. [Google Scholar] [CrossRef]
- Saba, T.; Mohamed, A.S.; El-Affendi, M.; Amin, J.; Sharif, M. Brain Tumor Detection Using Fusion of Hand Crafted and Deep Learning Features. Cogn. Syst. Res. 2020, 59, 221–230. [Google Scholar] [CrossRef]
- Dorj, U.O.; Lee, K.K.; Choi, J.Y.; Lee, M. The Skin cancer Classification Using Deep Convolutional Neural Network. Multimed. Tools Appl. 2018, 77, 9909–9924. [Google Scholar]
- Kassani, S.H.; Kassani, P.H. A Comparative Study of Deep Learning Architectures on Melanoma Detection. Tissue Cell 2019, 58, 76–83. [Google Scholar]
- Ribli, D.; Horváth, A.; Unger, Z.; Pollner, P.; Csabai, I. Detecting and Classifying Lesions in Mammograms with Deep Learning. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Talo, M.; Yildirim, O.; Karabatak, M.; Acharya, U.R. Automated Invasive Ductal Carcinoma Detection Based Using Deep Transfer Learning with Whole-Slide Images. Pattern Recognit. Lett. 2020. [Google Scholar] [CrossRef]
- Ozturk, T.; Talo, M.; Yildirim, E.A.; Baloglu, U.B.; Yildirim, O.; Acharya, U.R. Automated Detection of COVID-19 Cases Using Deep Neural Networks with X-ray Images. Comput. Biol. Med. 2020, 121, 103792. [Google Scholar] [CrossRef]
- Kanne, J.P.; Little, B.P.; Chung, J.H.; Elicker, B.M.; Ketai, L.H. Essentials for Radiologists on COVID-19: An Update—Radiology Scientific Expert Panel. Radiology 2020. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, 200432. [Google Scholar] [CrossRef] [PubMed]
- Haghanifar, A.; Majdabadi, M.M.; Ko, S. COVID-CXNet: Detecting COVID-19 in Frontal Chest X-ray Images using Deep Learning. arXiv 2020, arXiv:2006.13807. [Google Scholar]
- National Heart Foundation of Bangladesh. Available online: http://www.nhf.org.bd/hospital_charge.php?id=6 (accessed on 29 August 2020).
- Health System Tracker. Available online: https://www.healthsystemtracker.org/indicator/access-affordability/percent-insured/ (accessed on 28 August 2020).
- How Much Does an X-ray Cost. Available online: https://health.costhelper.com/x-rays.html (accessed on 28 August 2020).
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Zhang, J.; Zhang, L.; Gong, D.; Zhao, Y.; Hu, S.; Wang, Y.; Hu, X.; Zheng, B. Deep Learning-Based Model for Detecting 2019 Novel Coronavirus Pneumonia on High-Resolution Computed Tomography: A Prospective Study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ghoshal, B.; Tucker, A. Estimating Uncertainty and Interpretability in Deep Learning for Coronavirus (COVID-19) Detection. arXiv 2020, arXiv:2003.10769. [Google Scholar]
- Wang, L.; Wong, A. Covid-net: A Tailored Deep Convolutional Neural Network Design for Detection of Covid-19 Cases from Chest X-ray Images. arXiv 2020, arXiv:2003.09871. [Google Scholar]
- Jin, C.; Chen, W.; Cao, Y.; Xu, Z.; Zhang, X.; Deng, L.; Zheng, C.; Zhou, J.; Shi, H.; Feng, J. Development and Evaluation of an AI System for COVID-19 Diagnosis. MedRxiv 2020. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, S.; Li, L.; Zhang, X.; Zhang, X.; Huang, Z.; Chen, J.; Zhao, H.; Jie, Y.; Wang, R. Deep Learning Enables Accurate Diagnosis of Novel Coronavirus (COVID-19) with CT Images. MedRxiv 2020. [Google Scholar] [CrossRef]
- Butt, C.; Gill, J.; Chun, D.; Babu, B.A. Deep Learning System to Screen Coronavirus Disease 2019 Pneumonia. arXiv 2020, arXiv:2002.09334. [Google Scholar]
- Shi, F.; Xia, L.; Shan, F.; Wu, D.; Wei, Y.; Yuan, H.; Jiang, H.; Gao, Y.; Sui, H.; Shen, D. Large-Scale Screening of Covid-19 from Community Acquired Pneumonia Using Infection Size-Aware Classification. arXiv 2020, arXiv:2003.09860. [Google Scholar]
- Gong, J.; Ou, J.; Qiu, X.; Jie, Y.; Chen, Y.; Yuan, L.; Cao, J.; Tan, M.; Xu, W.; Zheng, F. A Tool to Early Predict Severe 2019-Novel Coronavirus Pneumonia (COVID-19): A Multicenter Study Using the Risk Nomogram in Wuhan and Guangdong, China. MedRxiv 2020. [Google Scholar] [CrossRef]
- COVID-19 Diagnostic Imaging Recommendations. Available online: https://www.appliedradiology.com/articles/covid-19-diagnostic-imaging-recommendations (accessed on 27 June 2020).
- ACR Issues Statement for Use of Chest Radiography, CT for Suspected COVID-19 Infection. Available online: https://www.appliedradiology.com/communities/CT-Imaging/acr-issues-statement-for-use-of-chest-radiography-ct-for-suspected-covid-19-infection (accessed on 27 June 2020).
- Force, A.D.T.; Ranieri, V.; Rubenfeld, G.; Thompson, B.; Ferguson, N.; Caldwell, E. Acute Respiratory Distress Syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Lau, A.L.; Chi, I.; Cummins, R.A.; Lee, T.M.; Chou, K.L.; Chung, L.W. The SARS (Severe Acute Respiratory Syndrome) Pandemic in Hong Kong: Effects on the Subjective Wellbeing of Elderly and Younger People. Aging Ment. Health 2008, 12, 746–760. [Google Scholar] [CrossRef]
- The New Coronavirus Appears to Take A Greater Toll on Men Than on Women. Available online: https://www.npr.org/sections/goatsandsoda/2020/04/10/831883664/the-new-coronavirus-appears-to-take-a-greater-toll-on-men-than-on-women (accessed on 25 June 2020).
- Everything You Should Know About the 2019 Coronavirus and COVID-19. Available online: https://www.healthline.com/health/coronavirus-covid-19#symptoms (accessed on 20 June 2020).
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.B.; Wang, D.C.; Mei, J. Performance of Radiologists in Differentiating COVID-19 from Viral Pneumonia on Chest CT. Radiology 2020, 200823. [Google Scholar] [CrossRef]
- Cohen, J.P.; Morrison, P.; Dao, L. COVID-19 Image Data Collection. arXiv 2020, arXiv:2003.11597. [Google Scholar]
- Nakada, R.; Imaizumi, M. Adaptive Approximation and Estimation of Deep Neural Network to Intrinsic Dimensionality. arXiv 2019, arXiv:1907.02177. [Google Scholar]
- Zhou, L.; Pan, S.; Wang, J.; Vasilakos, A.V. Machine Learning on Big Data: Opportunities and Challenges. Neurocomputing 2017, 237, 350–361. [Google Scholar] [CrossRef]
- Chollet, F. Deep Learning with Python; Apress: Berkeley, CA, USA, 2017. [Google Scholar]
- Ahmed, E.; Moustafa, M. House Price Estimation from Visual and Textual Features. arXiv 2016, arXiv:1609.08399. [Google Scholar]
- Law, S.; Paige, B.; Russell, C. Take a Look Around: Using Street View and Satellite Images to Estimate House Prices. ACM Trans. Intell. Syst. Technol. (TIST) 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Wang, F.; Zou, Y.; Zhang, H.; Shi, H. House Price Prediction Approach Based on Deep Learning and ARIMA Model. In Proceedings of the 2019 IEEE 7th International Conference on Computer Science and Network Technology (ICCSNT), Dalian, China, 19–21 October 2019; pp. 303–307. [Google Scholar]
- Koch, D.; Despotovic, M.; Leiber, S.; Sakeena, M.; Döller, M.; Zeppelzauer, M. Real Estate Image Analysis—A Literature Review. J. Real Estate Lit. 2019, 27, 269–300. [Google Scholar]
- Kumar, E.S.; Talasila, V.; Rishe, N.; Kumar, T.S.; Iyengar, S. Location Identification for Real Estate Investment Using Data Analytics. Int. J. Data Sci. Anal. 2019, 8, 299–323. [Google Scholar] [CrossRef]
- Sutskever, I.; Martens, J.; Dahl, G.; Hinton, G. On the Importance of Initialization and Momentum in Deep Learning. In Proceedings of the International Conference on Machine Learning, Atlanta, GA, USA, 16–21 June 2013; pp. 1139–1147. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for Stochastic Optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Zhang, C.; Liao, Q.; Rakhlin, A.; Miranda, B.; Golowich, N.; Poggio, T. Theory of Deep Learning IIb: Optimization Properties of SGD. arXiv 2018, arXiv:1801.02254. [Google Scholar]
- Bengio, Y. Rmsprop and Equilibrated Adaptive Learning Rates for Nonconvex Optimization. arXiv 2015, arXiv:1502.04390v1. [Google Scholar]
- Tang, Y. Deep Learning Using Linear Support Vector Machines. arXiv 2013, arXiv:1306.0239. [Google Scholar]
- Ahsan, M.M. Real Time Face Recognition in Unconstrained Environment; Lamar University-Beaumont: Beaumont, TX, USA, 2018. [Google Scholar]
- Mohanty, S.P.; Hughes, D.P.; Salathé, M. Using Deep Learning for Image-Based Plant Disease Detection. Front. Plant Sci. 2016, 7, 1419. [Google Scholar] [CrossRef] [PubMed]
- Menzies, T.; Greenwald, J.; Frank, A. Data Mining Static Code Attributes to Learn Defect Predictors. IEEE Trans. Softw. Eng. 2006, 33, 2–13. [Google Scholar] [CrossRef]
- Stolfo, S.J.; Fan, W.; Lee, W.; Prodromidis, A.; Chan, P.K. Cost-Based Modeling for Fraud and Intrusion Detection: Results from the JAM Project. In Proceedings of the DARPA Information Survivability Conference and Exposition, DISCEX’00, Hilton Head, SC, USA, 25–27 January 2000; Volume 2, pp. 130–144. [Google Scholar]
- Khan, A.I.; Shah, J.L.; Bhat, M.M. Coronet: A Deep Neural Network for Detection and Diagnosis of COVID-19 from Chest X-ray Images. Comput. Methods Programs Biomed. 2020, 196, 105581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Y.; Li, Y.; Shen, C.; Xia, Y. Covid-19 Screening on Chest X-ray Images Using Deep Learning Based Anomaly Detection. arXiv 2020, arXiv:2003.12338. [Google Scholar]
- Rapid Assistance in Modelling the Pandemic: RAMP. Available online: https://epcced.github.io/ramp/ (accessed on 8 September 2020).
- Bellomo, N.; Bingham, R.; Chaplain, M.A.; Dosi, G.; Forni, G.; Knopoff, D.A.; Lowengrub, J.; Twarock, R.; Virgillito, M.E. A Multi-Scale Model of Virus Pandemic: Heterogeneous Interactive Entities in a Globally Connected World. arXiv 2020, arXiv:2006.03915. [Google Scholar]
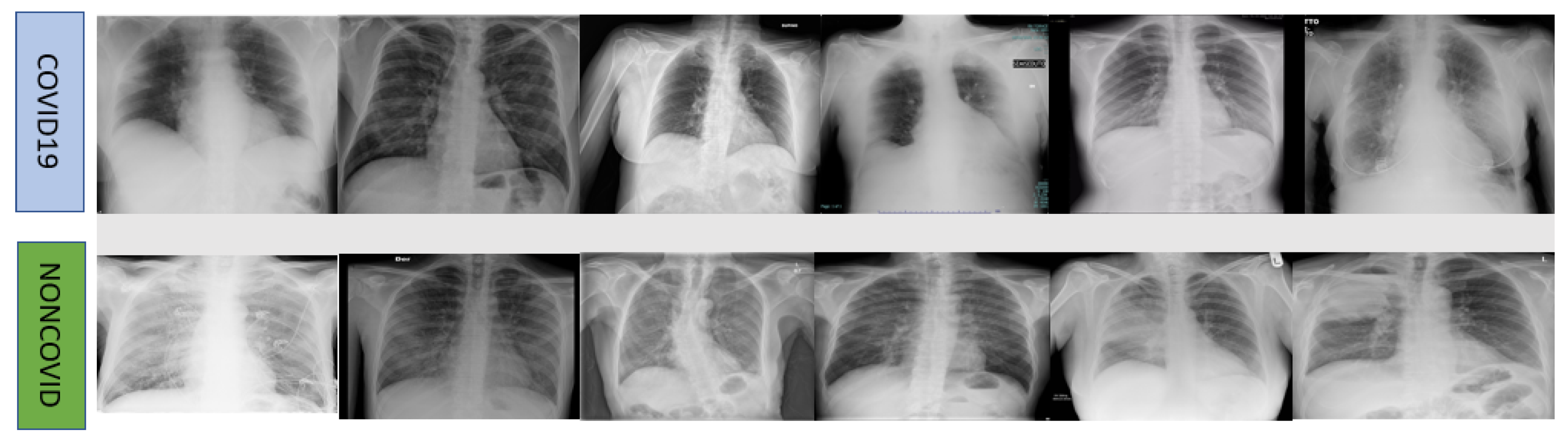
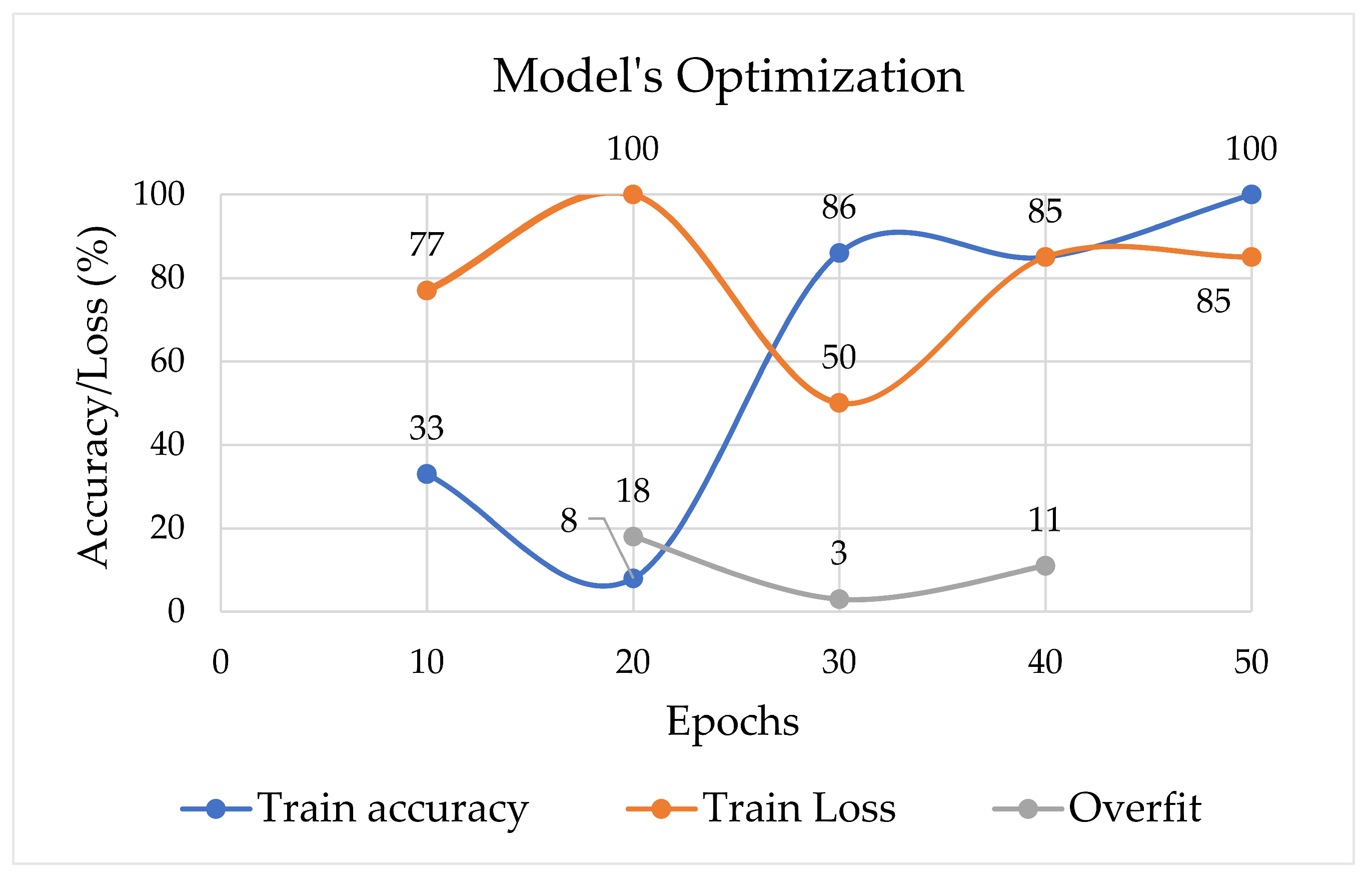
| Dataset | Label | Training Set | Testing Set | Total | Mean± SD | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (Years) | Temperature (Celsius) | ||||||
| Study One | COVID-19 | 9 | 4 | 13 | 51.29 ± 16.72 | 38.26 ± 0.85 | 0.49 |
| (Balanced dataset) | Non-COVID-19 | 11 | 2 | 13 | |||
| Study Two | COVID-19 | 87 | 25 | 112 | 55.73 ± 16.66 | — | 0.06 |
| (Imbalanced dataset) | Non-COVID-19 | 26 | 4 | 30 | |||
| Number of Hidden Layers | Neuron | Accuracy (%) | Loss (%) |
|---|---|---|---|
| 1 | 4 | 42 | 76 |
| 1 | 8 | 56 | 100 |
| 2 | 4 | 100 | 100 |
| 2 | 8 | 35 | 76 |
| 3 | 4 | 33 | 77 |
| 3 | 8 | 64 | 100 |
| Data Ratio (%) | Study One | Study Two | ||
|---|---|---|---|---|
| Training/Testing | Training Accuracy | Testing Accuracy | Training Accuracy | Testing Accuracy |
| 75/25 | 94% | 71% | 90% | 70% |
| 70/30 | 66% | 50% | 95% | 60% |
| 60/40 | 73% | 45% | 70% | 71% |
| 80/20 | 100% | 100% | 100% | 96% |
| Algorithm | Accuracy (%) | Precision (%) | Recall (%) | F1 Score (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | CI | CI | CI | |||||||||
| Adam | 96.3 | 92.9 | 94.6 ± 3.4 | 97.2 | 89.9 | 93.5 ± 3.7 | 96.3 | 92.8 | 94.5 ± 3.5 | 96.4 | 90.7 | 93.5 ± 3.7 |
| Rmsprop | 82.5 | 95.4 | 88.9 ± 4.7 | 79.4 | 92.5 | 85.9 ± 5.3 | 82.9 | 95 | 88.9 ± 4.8 | 79.6 | 93.6 | 86.6 ± 5.2 |
| Sgd | 91.8 | 85.1 | 88.4 ± 4.9 | 95.4 | 82.1 | 88.7 ± 4.8 | 91.8 | 86.1 | 88.5 ± 4.8 | 82.4 | 83.6 | 83 ± 5.7 |
| Model | Accuracy |
|---|---|
| Ghoshal and Tucker [36] | 92.9% |
| Zhang et al. [69] | 96% |
| Wang and Wong [37] | 83.5% |
| Proposed model | 96.3% with Adam |
| Reference | Data Type | Method | Database Size | Accuracy |
|---|---|---|---|---|
| Jin et al. [38] | CT | CNN | 497 COVID-19, 1385 others | 94.1% |
| Song et al. [39] | CT | ResNet50 | 88 COVID-19, 186 others | 82.9% |
| Butt et al. [40] | CT | CNN | 219 COVID-19, 399 others | 86.7% |
| Shi et al. [41] | CT | RF | 1658 COVID-19, 1027 others | 90.7% |
| Wang and Wong [37] | X-ray | CNN | 45 COVID-19, 2794 others | 83.5% |
| Khan et al. [68] | X-ray | Xception | 284 COVID-19, 967 others | 89.6% |
| Proposed model | X-ray | MLP-CNN + Rmsprop | 112 COVID-19, 30 others | 95.38% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, M.M.; E. Alam, T.; Trafalis, T.; Huebner, P. Deep MLP-CNN Model Using Mixed-Data to Distinguish between COVID-19 and Non-COVID-19 Patients. Symmetry 2020, 12, 1526. https://doi.org/10.3390/sym12091526
Ahsan MM, E. Alam T, Trafalis T, Huebner P. Deep MLP-CNN Model Using Mixed-Data to Distinguish between COVID-19 and Non-COVID-19 Patients. Symmetry. 2020; 12(9):1526. https://doi.org/10.3390/sym12091526
Chicago/Turabian StyleAhsan, Md Manjurul, Tasfiq E. Alam, Theodore Trafalis, and Pedro Huebner. 2020. "Deep MLP-CNN Model Using Mixed-Data to Distinguish between COVID-19 and Non-COVID-19 Patients" Symmetry 12, no. 9: 1526. https://doi.org/10.3390/sym12091526
APA StyleAhsan, M. M., E. Alam, T., Trafalis, T., & Huebner, P. (2020). Deep MLP-CNN Model Using Mixed-Data to Distinguish between COVID-19 and Non-COVID-19 Patients. Symmetry, 12(9), 1526. https://doi.org/10.3390/sym12091526





