Abstract
During the past two decades, the interest in new methodologies for the synthesis of chiral N-functionalized indoles has grown rapidly. The review illustrates efficient applications of organocatalytic and organometallic strategies for the construction of chiral α-N-branched indoles. Both the direct functionalization of the indole core and indirect methods based on asymmetric N-alkylation of indolines, isatins and 4,7-dihydroindoles are discussed.
1. Introduction
Heterocyclic compounds are of great interest in medicinal chemistry. According to the U.S. Food and Drug Administration database, approximately 60% of unique small-molecule drugs contain a nitrogen heterocyclic motif [1]. The indole core is the most common nitrogen-based heterocyclic fragment applied for the synthesis of pharmaceutical compounds and agrochemicals [2]. The indole moiety can be found in a wide range of natural products [3]. Therefore, some biologically active indoles contain a substituted α-chiral carbon center on the N1-position (Figure 1) [4,5,6,7].
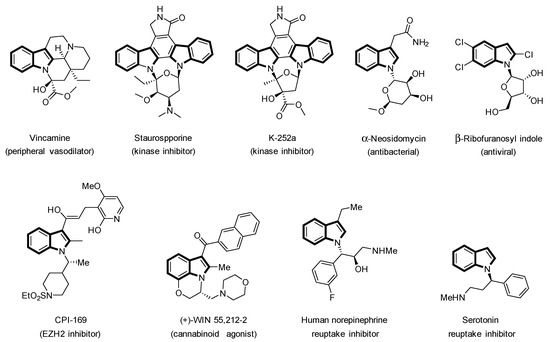
Figure 1.
Biologically active chiral α-N-branched indoles.
The synthesis of enantioenriched indole derivatives is of great importance in organic chemistry. During the past two decades, different strategies have been proposed for the construction of chiral indole derivatives [8,9,10,11]. The most typical synthetic modifications of indoles take place at the C3 position (Scheme 1). The enantioselective electrophilic substitution at C3 is common due to the high nucleophilicity of this position, which is 1013 times more reactive than benzene [12,13]. In contrast to enantioselective C3 transformations, the stereoselective N-alkylation of indole is still a challenge due to the weak nucleophilicity of the nitrogen atom (Scheme 1). Despite this, a number of publications have been published recently that demonstrate new synthetic routes affording chiral N-substituted indole derivatives. In this review, we will introduce and discuss methodologies that provide catalytic stereoselective derivatization at the N-atom of indole.
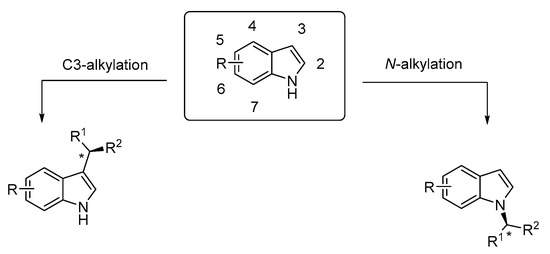
Scheme 1.
Regioselectivity of the asymmetric functionalization of indoles.
On a structural basis, the strategies for the construction of α-N-branched indoles can be classified into two groups: “direct methods” and “indirect methods” (Scheme 2). In the former case, indole derivatives are transformed by transition-metal catalysis or by organocatalysis into chiral compounds (Scheme 2, I). If the structure of the starting compound does not contain an indole moiety, the methods are defined as indirect methods (Scheme 2, II). The indirect methods are subdivided according to the structure of the starting compound and the modifications occurring during the synthesis of α-N-branched indoles (Scheme 2, II). There are several ways to prepare N-functionalized indoles indirectly. This review covers the asymmetric N-alkylation of indolines, isatins and 4,7-dihydroindoles, followed by a redox reaction, which provides the corresponding N-alkylated indoles (Scheme 2, III, IV and V respectively).
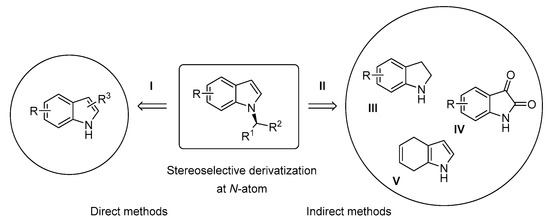
Scheme 2.
Strategies for the enantioselective N-functionalization of indoles.
2. Direct Organocatalytic Methods
During the past two decades, organocatalysis has become a powerful methodology in enantioselective synthesis [14,15,16,17]. In the first part of this review, the direct methods of the stereoselective N-alkylation of indoles based on organocatalysis are discussed. Various electrophiles and different types of organocatalysts have been used to achieve targets with high enantiomeric purities (Scheme 3).
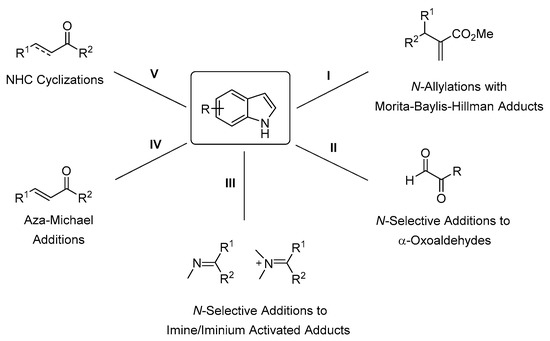
Scheme 3.
Direct organocatalytic derivatization of indole.
2.1. N-Allylations with Morita-Baylis-Hillman Adducts
Chen and co-workers proposed applying Morita-Baylis-Hillman (MBH) tert-butoxy carbonates 1 as electrophiles in a reaction with indole derivatives 2 (Scheme 4, I) [18]. The activation of MBH adducts with a chiral tertiary amine generates in situ tert-butoxy anion which is responsible for the deprotonation of the indole at the N-position. The screening of the reaction revealed that the reaction could be smoothly catalyzed by cinchona alkaloid derived ether 4 (Figure 2) in mesitylene. The substrate scope was performed with either electron-rich or electron-deficient indoles, providing products with moderate to excellent enantiomeric excesses (62–93%). Moreover, the methyl pyrrole-2-carboxylate was examined as a nucleophile, providing the N-substituted product with a good yield (80%) and moderate ee (73%). The C2- and C7-brominated N-allylated indoles can be further converted into fused, cyclic indole systems (Scheme 4, II).
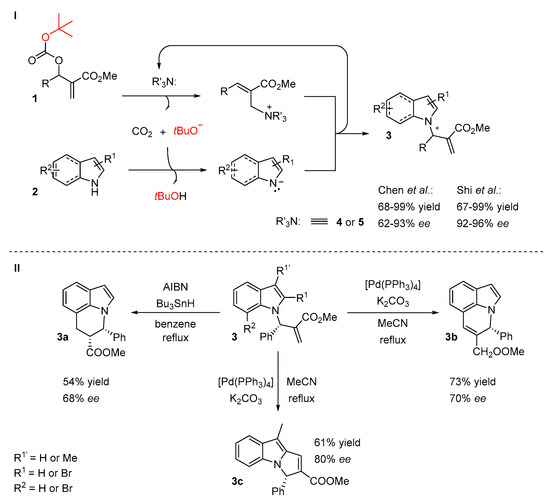
Scheme 4.
The reaction of indole derivatives and Morita–Baylis–Hillman (MBH) carbonates.
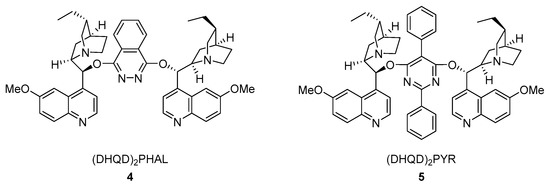
Figure 2.
Chiral Lewis base catalysts.
Shi et al. reported an effective method in which C2-cyano-substituted pyrroles and indoles 2 were subjected to a reaction with O-Boc-protected MBH adducts 1 in the presence of a catalyst 5 [19]. They obtained corresponding N-allylated products 3 under optimal conditions with good to high yields (up to 99%) and moderate to high ee values (up to 96%). Compared with Chen’s work, the introduction of a cyano group at the C2-position of pyrrole instead of a methyl carboxylate group had a positive impact on the reaction stereoselectivty (73% ee vs. 92% ee) and yield (80% vs. 92%). In the case of indoles, only 2-cyanoindole was examined. The ees and yields of the reactions were slightly improved. It should be noted that Chen’s group applied indoles with both electron-withdrawing and electron-donating groups, but Shi’s method was limited to 2-cyanoindoles.
A new method for the activation of pyrroles, indoles and carbazoles was proposed by Vilotijević et al. in 2019 [20]. The silyl-protected indole derivatives 6 can act as latent nucleophiles in the presence of a chiral Lewis base catalyst 4. Latent nucleophiles are compounds that are not nucleophilic, but can be converted into strong nucleophiles when activated. The modification of MBH carbonates by replacing the O-Boc group with a fluoro group affords a new type of fluorinated MBH adducts 1a, which are the source of fluoride ions needed for the desilylation of the indole derivative. The authors performed a mechanistic study and their proposed mechanism is outlined in Scheme 5. The elimination of fluoride ions occurs during the activation of the MBH adduct with catalyst 4. At the same time, fluoride ions deprotect N-silylindole derivatives. As a result, simultaneous activated pairs of electrophiles/nucleophiles occur and the enantioselective N-alkylation of an indole proceeds with excellent regioselectivity and moderate to high enantioselectivity (up to 98%) with a yield up to 98%.
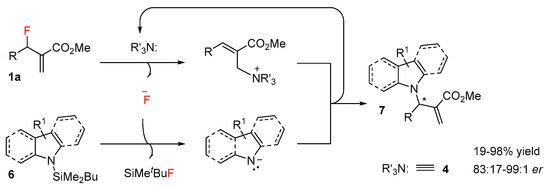
Scheme 5.
The use of a latent nucleophile.
2.2. N-Selective Additions to α-Oxoaldehydes
The chiral N,O-aminal indole structural motif can be found in natural products and pharmaceutical compounds [4,5]. Recently, two organocatalytic approaches to the synthesis of N,O-aminals with indole skeletons were reported (Scheme 6) [4,21]. In both methods, ethyl glyoxylate derivatives 9 were used as electrophiles, but different types of organocatalysts were used. Activation with both a chiral Lewis base and a Lewis acid was exploited efficiently for the same reaction (Scheme 6). Based on the achiral method for the preparation of N,O-aminals of indole derivatives in the presence of a Brønsted base (DABCO), Qin elaborated an asymmetric version of the reaction [21]. The catalytic system derived from BINOL-derived polyether 11 and potassium fluoride provides N,O-aminals with high ee (up to 91%) and with high yields (up to 90%) (Scheme 6, I). Only two examples of an asymmetric reaction were demonstrated. The second approach illustrates the application of a SPINOL-based chiral phosphoric acid 12 in the N-selective alkylation of indole derivatives 8 (Scheme 6, II) [4]. Differently substituted indole derivatives (substitution at both rings) were applied as nucleophiles, affording products with good to excellent enantiomeric excess (up to 99%) and with moderate to high yields (up to 96%). The exception was the product of a 7-fluorosubstituted indole obtained with a 55% yield and 76% ee. The authors concluded that the decrease in stereoselectivity and in the yield of the reaction can be explained by the steric hindrance of the substituent at the C7 position. The transition state of the reaction is outlined in Scheme 6, III. The ethyl glyoxalate and the indole are both activated by chiral phosphoric acid 12. The attack on the aldehyde from the Re-face is favored, affording an (R)-isomer of the product. Remarkably, BINOL-derived chiral phosphoric acid was not as efficient as SPINOL-derived and provided the product with a low level of stereocontrol (ee 5–7%).
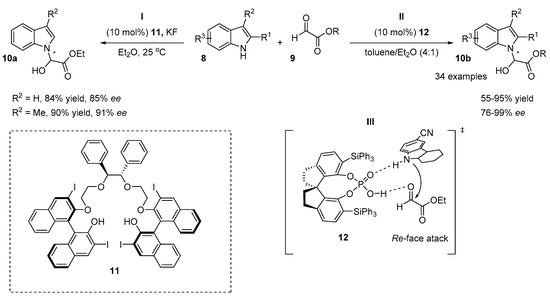
Scheme 6.
Reactions of indoles with glyoxylate derivatives.
2.3. N-Selective Additions of Indoles to Imine/Iminium Activated Adducts
In situ, generating electrophiles such as imines or iminium ions can provide wide access for the construction of chiral indoles. They not only bear substituents at the C3 position, but they are also powerful tools for the synthesis of N-substituted indoles. The activation of electrophiles can be promoted by chiral Brønsted acids such as phosphoric acids. There are a large number of different BINOL- and SPINOL-derived chiral acids and their ability to act as bifunctional catalysts provides unique opportunities for the enantioselective functionalization of the C-N bond.
The first synthesis of chiral α-N-branched indoles 15 via their addition to the acyliminium ion catalyzed by chiral phosphoric acid 17 was proposed by Huang et al. (Scheme 7) [22]. Cyclic N-acyliminium ions are highly reactive electrophiles [23]. They are easily generated from α,β-unsaturated γ-lactams (such as compound 13) by accepting an acidic proton from a chiral phosphoric acid, affording a chiral conjugate base/N-acyliminium ion pair. Huang proposed that the acidic N-H atom of the indole is activated by the conjugate base of chiral phosphoric acid through the hydrogen bond, favoring an attack of the nitrogen atom on the cyclic N-acyliminium ion. The authors conducted a series of labeling and FTIR experiments that explained the formation of the N-acyliminium ion and the indole alkylation step. The reaction was catalyzed by catalyst 17, providing a high level of stereocontrol (ee up to 95%) and high yields of the reaction (up to 98%). The synthetic method afforded chiral indole derivatives 15 with different substituents in both ring systems. The product 15 (R1 = Br, R2 = Me, R3 = H) was further converted into an N-fused polycyclic compound 16 in two additional steps. Interestingly, Boc and phenyl N-protected α,β-unsaturated γ-lactams afforded only C3 alkylated products with low ee values.
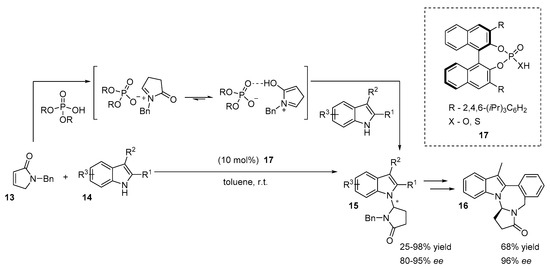
Scheme 7.
N-Acyliminium activated N-alkylation of indoles.
You and co-workers later reported a modified route of Huang’s enantioselective indole N-alkylation with N-acyliminium ions [24]. The new method is based on the cascade reaction, involving a ring-closing metathesis catalyzed by a Ru complex and chiral SPINOL-derived phosphoric acid-catalyzed 21 indole N-alkylation (Scheme 8). The starting N-allyl-N-benzylacrylamide 18 was first converted into α,β-unsaturated γ-lactam 13 by a Ru complex (Zhan-1B) followed by the selective N-alkylation of the indole in the presence of catalyst 21. The authors compared their method with stepwise reactions and found that the sequential catalysis allowed for a more efficient synthesis.
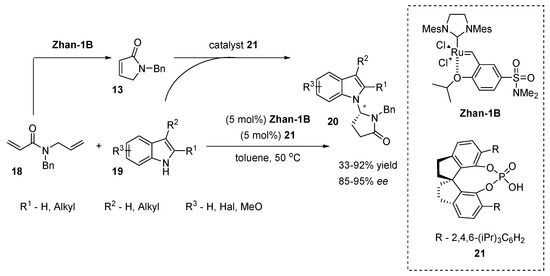
Scheme 8.
Sequential ring-closing/N-alkylation of indoles.
Another example of the application of SPINOL-derived phosphoric acid in the N-alkylation of indoles was demonstrated by Zeng and Zhong [25]. An enantioselective N-addition of indoles to in situ generated cyclic N-acyl imines from hydroxy isoindolinones 23 was efficiently catalyzed by hindered bismesityl-substituted chiral phosphoric acid 25 (Scheme 9). The reaction proceeded smoothly with a broad range of indoles and isoindolinone alcohols affording chiral N-alkylated tetrasubstituted aminals 24 with moderate to good yields (up to 77%) and good to excellent enantioselectivities (up to 98%). The proposed transition state indicates the dual activation mode of the catalyst 25 (Scheme 9).
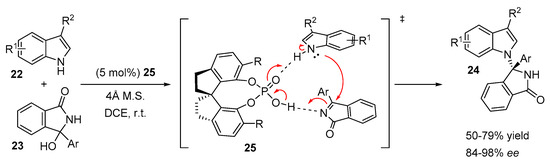
Scheme 9.
Enantioselective addition of indoles to N-acyl imines.
A new class of in situ activated electrophiles for the enantioselective N-alkylation of indoles from the N-protected p-aminobenzylic alcohol 26 was reported by Sun [26]. These alcohols were easily converted to aza-p-quinone methides 26a in the presence of the chiral phosphoric acid 29 and used as alkylating reagents in reactions with 2,3-disubstituted indoles 27 (Scheme 10). The protective group on the nitrogen atom of the electrophile drastically affects the stereoselectivity of the reaction. In the case of bulky aliphatic acyl groups, such as pivaloyl and 1-adamantanecarbonyl groups, excellent enantioselectivities were achieved (ee up to 95%). Other protective groups afforded products with moderate ee values. It is important to mention that C3 unsubstituted indoles gave an exclusive reaction at the C3 position with good ee (74%). The slight modification of the reaction conditions improved the enantioselectivity of the reaction and chiral C3 alkylated indoles were obtained with excellent yields (up to 99%) and high ees (up to 94%). The control experiments proved that the reaction proceeds due to the generation of an aza-p-QM intermediate 26a and, without a nucleophile, the dimerization of the aza-p-QM intermediate occurred. The authors proposed a transition state that demonstrates the bifunctional role of the chiral phosphoric acid in the activation of both an electrophile and a nucleophile.
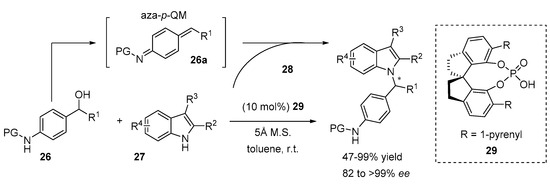
Scheme 10.
Enantioselective N-alkylation of indoles with para-aza-quinone methides.
Recently, an asymmetric N-alkylation of indole derivatives via a Reissert-type reaction catalyzed by a chiral phosphoric acid 34 was reported by You’s group [27]. The authors expected the dearomatization of both reagents, but the reaction proceeded in another manner, providing the N-alkylated adduct 32 (Scheme 11). The method tolerates various protective groups on the amine of tryptamine 31, affording products with good yields (72–98%) and moderate to good enantioselectivities (ee 64–82%). Substituents in the phenyl ring of the tryptamine 31 did not have a negative impact on the ee values (63–73%) or yield (78–89%) of the reaction. Various substituents on the isoquinoline core 30 were tested and their influence on the reaction was studied. Sterically hindered isoquinolines (7- or 8-substituted isoquinolines) afforded lower yields (10–40%) and ee values (29–50% ee). Substituents at other positions were tolerated, leading to N-alkylated products with good to excellent yields (80–98%) and enantioselectivities (80–94%). It is notable that the indole ring bearing a 3-methyl substituent was also tolerated, affording N-alkylated product with excellent yield (98%) and high ee (85%). The chiral N-alkylated product 32 (R1 = R2 = H) was easily modified by the reduction in 1,2-dihydroisoquinoline moiety and the deprotection of the Boc group, leading to free amine 33.
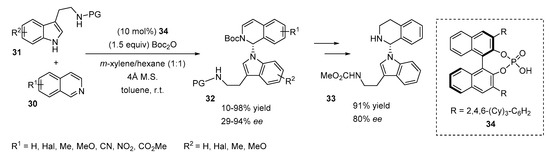
Scheme 11.
Enantioselective N-alkylation of indoles via Reissert-type reaction.
2.4. Aza-Michael Additions
The application of efficient organocatalytic strategies for the synthesis of chiral α-N-branched indoles is limited by the low acidity of the N-H atom and low nucleophilicity of the nitrogen of the indole. Electron-withdrawing groups at C2 or C3 positions increase the acidity of the N-H atom of the indole [28], thus improving its reactivity. Another way to activate the nitrogen atom is the introduction of an electron-donating group at the C3 position, which increases the nucleophilicity of the indole. Sometimes, the functionalization at the C2 and C3 positions of the indole are used to prevent side reactions.
The enantioselective intramolecular ring-closing reaction of 2-substituted indoles 35 with increased acidity under phase transfer catalysis was reported by Bandini and Umani-Ronchi et al. (Scheme 12, I) [29,30]. The authors emphasized the importance of the tight ion pair that occurs between the cinchona-based salt of the quinuclidine ring and the nucleophilic indolate intermediate (Scheme 12, II). The stereocontrol of the reaction was increased by the introduction of electron-withdrawing substituents on the para-position of the benzyl group of the catalyst 37. The substituents at C5 positions of the indole ring did not influence stereoselectivity as indole derivatives 35 with electron-withdrawing or electron-donating groups gave high yields (85–93%) and high ee values (82–89%), which could be increased further by recrystallization.
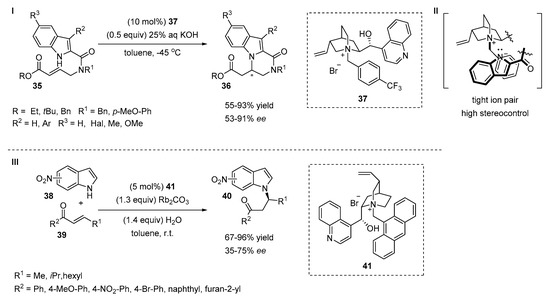
Scheme 12.
Phase transfer-catalyzed N-alkylation of indoles.
The phase transfer-catalyzed asymmetric aza-Michael addition of nitroindoles 38 to α,β-unsaturated carbonyl compounds 39 was investigated by Kanger and co-workers (Scheme 12, III) [31]. The authors determined that the position of the nitro group on the indole core was crucial to control the enantioselectivity of the reaction. The reaction did not proceed with 2- and 7-substituted nitroindoles. The indoles bearing a nitro group at the C5, C6 or C3 positions were non-selective substrates for the reaction as the enantioselectivity was too low (35–42% ee). The reaction between various trans-crotonophenone derivatives and 4-nitroindole afforded products with good to high yields (67–96%) and moderate to good enantioselectivities (59–75%) in the presence of a cinchona alkaloid-based phase transfer catalyst 41. It is important to mention that there was essentially no correlation between the acidity of the indole and its reactivity in the aza-Michael reaction.
The introduction of an electron-withdrawing substituent at the C2-position of the indole ring not only increases the acidity of the N-H atom, but this substitution pattern also opens wide access to cascade reactions that provide chiral N-alkylated polycyclic indoles in a single step. Wang and Ender’s groups separately reported a method where tricyclic chiral indole derivatives were obtained from indole-2-carbaldehyde 42 and various α,β-unsaturated aldehydes 43 in the presence of a Hayashi-Jørgensen catalyst 46 (Scheme 13, I) [32,33]. The cascade reaction is possible due to an iminium/enamine activation mode and consists of an aza-Michael reaction followed by aldol condensation. Despite differences in reaction conditions, both synthetic methods demonstrated moderate to good yields (40–71% and 57–81%) and good to excellent ee values of products (85 to >99% and 71–96%). In the case of 2-furyl enal, the isomeric achiral product 45 was formed.
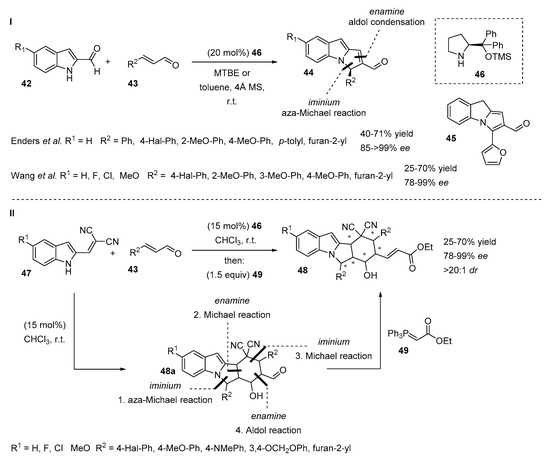
Scheme 13.
Secondary amine catalyzed cascade reactions.
Enders et al. continued to investigate the reactions of 2-substituted indoles with unsaturated aldehydes and reported an asymmetric quadruple cascade reaction (Scheme 13, II) [34]. Indole-2-methylene malononitriles 47 derived from indole-2-carbaldehydes 42 were subjected to reactions with various α,β-unsaturated aldehydes 43 in the presence of the chiral secondary amine 46, providing tetracyclic aldehydes 48a. The domino reaction consists of a tandem aza-Michael-Michael-Michael-aldol reaction, which exploits the iminium-enamine-iminium-enamine activation approach. Because of the enolization during the purification of aldehydes 48a, they were trapped with stabilized Wittig reagent 49. The cascade reaction and olefination were easily completed in a one-pot manner with no impact on the reaction outcome. The reaction scope was performed with both electron rich and electron poor aromatic α,β-unsaturated aldehydes 43 and chiral products 48 were obtained as single diastereoisomers (>20:1 dr) with moderate to good yields (25–70%) and good to excellent enantioselectivities (91–99%). A decrease in enantioselectivity and yield was detected in the case of the heteroaromatic furyl group (33% yield, 78% ee). Indoles with electron-withdrawing and electron-donating substituents at the C5 position tolerated the reaction without affecting yields or stereoselectivities.
An intramolecular reaction of appropriately C2 substituted indole 50 provided selectively N-alkylated tricyclic indole derivatives 51 via an aza-Michael reaction in the presence of a phosphoric acid catalyst 52 (Scheme 14, I) [35]. Under optimal conditions, the reaction scope was broadened with various substituted aromatic enones with electron-donating or electron-withdrawing groups. The authors demonstrated the tolerance of various functionalities such as carbonyl, hydroxyl groups and aromatic rings at the C3 side chain without any impact on the reaction yields (82–96%) or enantioselectivities (88–93%). However, the introduction of an electron-withdrawing group at the C2-position of the indole totally inhibited the reaction. Further investigations of the reaction were concentrated on the combination of mechanistically distinct organocatalysis and transition-metal catalysis (Scheme 14, II). The indolyl olefins 53 and enones 54 reacted smoothly in the presence of the chiral phosphoric acid 52 and ruthenium catalyst Zhan-1B affording the desired products with moderate to excellent yields (45–96%) with 87–93% ees (Scheme 14, II). Notably, if indole 56 was applied as the substrate, both the N-alkylated product 57 and the C3-alkylation product 58 were obtained (Scheme 14, II).
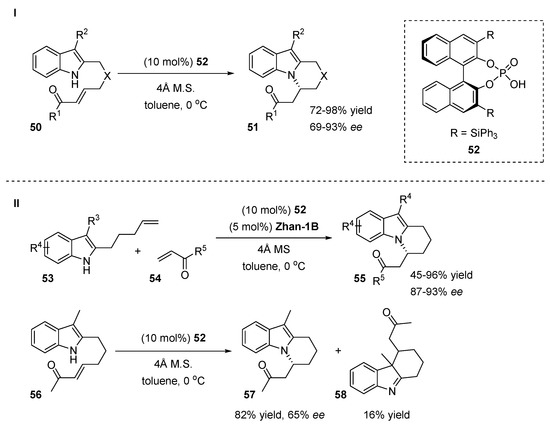
Scheme 14.
Cyclization of electron rich 2-substituted indoles.
2.5. N-Heterocyclic Carbene-Mediated Cyclizations
In recent years, there has been growing interest in the field of N-heterocyclic carbene (NHC) catalysis. The functionalization of the indole core via various NHC-intermediates has been reported by several research groups [36,37,38,39,40]. There are only two articles dedicated to the N-functionalization of indoles [41,42]. Both synthetic methodologies were applied to 7-substituted indole derivatives as a starting material and obtained N-fused tricyclic structures were characteristic.
Biju and co-workers demonstrated an asymmetric NHC-catalyzed domino reaction for the synthesis of pyrroloquinolines (Scheme 15) [41]. The indole substrates 59 used in this reaction had a Michael acceptor moiety at the C7-position and a strong electron-withdrawing group at the C3-position to increase the acidity of the N-H atom of indoles. The cascade reaction was catalyzed by carbene generated from the chiral aminoindanol-derived triazolium salt 62 and proceeded smoothly with various substituted indoles 59 and cinnamaldehyde derivatives 60. The substrate scope revealed that α,β-unsaturated aldehydes bearing electron-withdrawing and electron-donating substituents at the 4-, 3- and 2-positions of the β-aryl ring of enals had no impact on the reaction outcome, affording pyrroloquinoline derivatives with good to high yields (63–95%), good to excellent enantiomeric ratios (87:13 to 99:1) and excellent diastereoselectivities (>20:1). Additionally, heterocyclic enals and disubstitued β-aryl ring enals reacted smoothly with indole derivatives and products were obtained with good yields (67–82%) and high er values (90:10 to 97:3). Cyclic and acyclic alkyl groups at the Michael acceptor moiety of compound 59 could be used without affecting the reactivity/selectivity. In addition, the influence of solvents with different dielectric constants (DEC) on the reaction selectivity and yield was studied. The authors demonstrated that the aprotic solvents with higher dielectric constants afforded better ee and yield. For instance, a poor yield and ee value were obtained in toluene (DEC: 2.38), moderate in THF (DEC: 7.58) and high in DMF (36.7). Therefore, the solvent with higher polarity not only provided good solubility of reactants but it could stabilize zwitterionic intermediates.
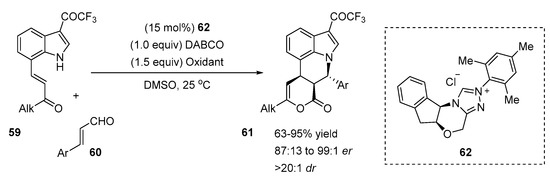
Scheme 15.
N-heterocyclic carbene (NHC)-catalyzed cascade reaction of 7-substituted indoles.
Chi et al. reported the enantioselective functionalization of an indole carbaldehyde N-H group through NHC catalysis (Scheme 16) [42]. The indole derivative 63 was activated by NHC via a carbaldehyde moiety at the C7-position of the indole. The reaction of indole-7-carbaldehyde adducts with derivatives of various carbonyl compounds 64 demonstrated high er values and yields in the presence of a carbene catalyst generated from a triazolium salt 67. The differences in structure and electronic properties of the starting compounds did not affect the reaction selectivity or yield. Both trifluoroacetophenone derivatives and aliphatic trifluoromethyl ketones tolerated the reaction well affording the desired products with high yields and er values (89–98% yield, 94:6 to 96:4). Substitutions on the indole 5- and 6-positions gave the desired products with excellent yields (90–99%) and optical purities (92:8 to 97.5:2.5), regardless of the electronic properties of the substituents. The reaction scope was broadened with the application of isatins 68 as electrophiles (Scheme 16, II). In the case of the isatin derivatives, another precatalyst 70 was used to maintain high yields (up to 95%) and er values (up to 98:2).
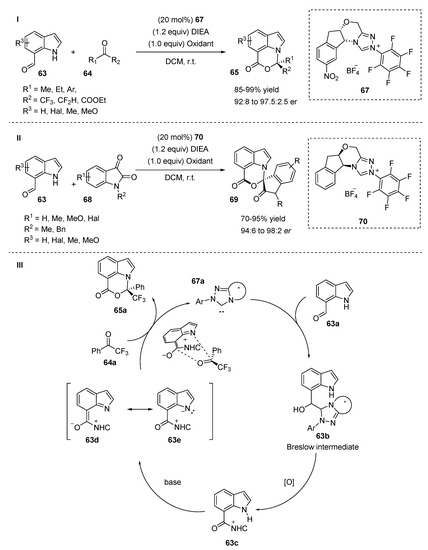
Scheme 16.
NHC mediated N-alkylation of indoles.
The authors proposed the mechanism of the NHC-catalyzed reaction, which is outlined in Scheme 16, III. The nucleophilic attack of NHC 67a on aldehyde 63a forms a Breslow intermediate 63b, which undergoes an oxidation reaction to generate an acylazolium intermediate 63c, followed by its deprotonation, providing the intermediate 63d. Finally, a formal [4 + 2] annulation reaction between intermediate 63d and trifluoroacetophenone 64a affords the desired product 65a and regenerates the NHC catalyst.
3. Organocatalytic Indirect Methods
There are several examples of organocatalytic methods in which the synthesis of chiral N-alkylated indoles was performed indirectly. Three routes for the preparation of N-functionalized indoles are proposed. The first two methods are based on the enantioselective N-alkylation of indoline or isatin and further redox transformation of N-alkylated intermediates into chiral N-functionalized indoles. In the last method, 4,7-dihydroindole was used as the starting material for the C2 Friedel-Crafts alkylation followed the oxidative cyclization, affording N-alkylated indole.
The enantioselective aza-Michael reaction between indoline derivatives 71 and α,β-unsaturated ketones 72 was reported by Ghosh et al. (Scheme 17, I) [43]. A set of N-alkylated indoline adducts 73 were further oxidized to corresponding N-functionalized indole derivatives 75 (Scheme 17, II). The N-alkylation of indolines was investigated and various thiourea and squaramide based bifunctional organocatalysts were tested. The best results were obtained with quinine-derived catalyst 74 in xylene at −20 °C. The influence of substituents in the aromatic rings of α,β-unsaturated ketones 72 was also studied. The authors demonstrated that in the case of electron-withdrawing substituents in the phenyl ring (R3), both the enantioselectivity and yield increased (83–86% yield, 90-96% ee). At the same time, the introduction of an electron-donating group (R3 = 4-Me-Ph) did not affect the selectivity or yield (55% yield, 86% ee). The incorporation of a cyano group on the phenyl ring (R3) afforded a product with a low yield (40%) and high ee (90%). Notably, the heterocyclic furan-2-yl and methyl substituents tolerated the reaction well, providing good yields and stereoselectivities (55–57% yield, 80–84% ee). Indolines with electron-donating substituents at the C5 position afforded products with high yields with high levels of stereocontrol (93–94% yield, 95–99% ee). Electron-withdrawing groups at the C5 position of the indoline decreased the reaction yield and ee value (53–54% yield, 80% ee). The oxidation of chiral N-functionalized indolines 73 to the corresponding N-substituted indoles 75 with DDQ (1.05 equivalent) in THF or MnO2 (10 equivalent) in dichloromethane led to products without any loss in enantioselectivity and with high yields (Scheme 17, II).
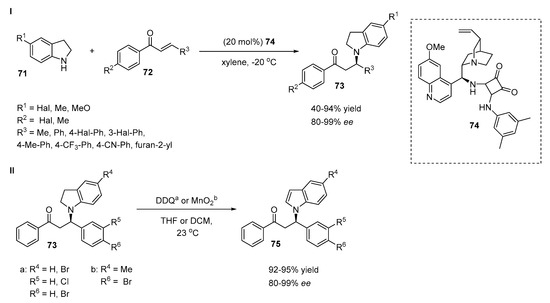
Scheme 17.
Synthesis of N-functionalized indoles via alkylation/oxidation of indolines.
An interesting route for the preparation of chiral N-functionalized indoles 81 from N-alkylated isatin derivatives 80 was proposed by Lu and co-workers (Scheme 18, I) [44]. The method is based on the enantioselective conjugated addition of protected isatin derivatives 76 to α,β-unsaturated enals 77 via the iminium activation of aldehydes by a prolinol-derived catalyst 79. A wide range of various aliphatic, aromatic linear and branched enals 77 tolerated the reaction, providing products with good yields (70–82%) and high enantioselectivities (89–95%). Corresponding N-functionalized indoles 81 were obtained after deprotection and reduction with borane.
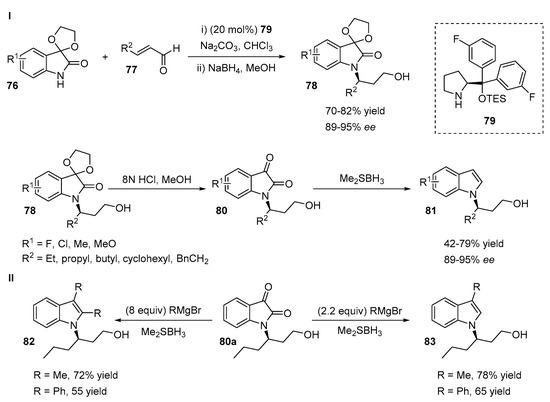
Scheme 18.
Isatin based synthetic route for the preparation of α-N-branched indoles.
The unprotected N-alkylated isatins 80a were further transformed into C2/C3-substituted N-functionalized indoles 82 or 83 (Scheme 18, II).
The application of electron-deficient 4,7-dihydroindole 84 for the construction of N-functionalized indoles 86 was investigated by You et al. (Scheme 19) [45]. Chiral N-functionalized indoles were obtained in a one-pot synthesis. First, the Friedel-Crafts C2-alkylation between the 4,7-dihydroindole 84 and β,γ-unsaturated α-keto ester 85 was catalyzed by chiral N-triflyl phosphoramide 87 at −78 °C in toluene. The authors determined the importance of 4Å molecular sieves in the reaction mixture. Moreover, the stereoselectivty of the reaction was improved when the ester 85 was added by syringe pump to the reaction mixture over 15 min.
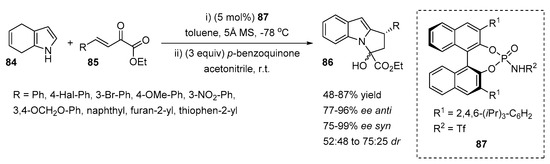
Scheme 19.
C2-alkylation of 4,7-dihydroindoles, followed by oxidative intramolecular cyclization.
The simple work-up with p-benzoquinone after the completion of the first step afforded the oxidative intramolecular cyclization of 2-substituted chiral intermediates. Various N-functionalized products 86 were obtained with moderate to good yields (48–87%), good to excellent enantioselectivities (75–99%) and poor to moderate diastereoselectivities (52:48 to 75:25).
4. Direct Organometallic Methods
Transition metal catalysis is often applied in asymmetric synthesis as a highly efficient method for the construction of chiral compounds [46]. Small loadings of transition metal complexes and the excellent stereocontrol of the reaction make organometallic methods attractive for the stereoselective N-functionalization of indoles. In this part of the review, direct methods of the stereoselective N-alkylation of indoles based on transition metal catalysis are discussed. Various alkylating agents and different types of transition metal complexes were applied to gain a high level of stereocontrol (Scheme 20).
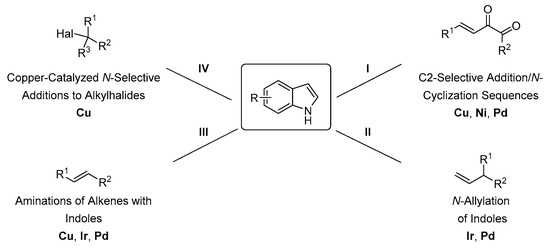
Scheme 20.
Direct transition-metal based stereoselective derivatization of indole.
4.1. C2-Selective Addition/N-Cyclization Sequences
Chen and Xiao applied 3-substituted indoles 88 as N1/C2 dinucleophiles in enantioselective reactions with various β,γ-unsaturated α-ketoesters 89 (Scheme 21, I) [47]. A highly enantioselective cascade reaction consisting of sequential C2 Friedel-Crafts alkylation followed by N-hemiacetalization, providing tricyclic chiral N-functionalized indoles 90, was described. The domino reaction was smoothly catalyzed by copper(II)triflate in the presence of chiral bis(oxazoline) ligand 91 in toluene at 0 °C. The investigation of the substrate scope revealed that the cascade reaction tolerated various esters well, electron-withdrawing and electron-donating groups in the γ-aryl ring of ester, heteroaromatic and vinyl-substituents at the γ-position. γ-Alkyl-substituted β,γ-unsaturated α-ketoesters also reacted smoothly, but in the case of the straight-chain aliphatic substrate (R3 = propyl, R4 = Et) a decrease in the stereoselectivity of the cascade reaction was detected (67% yield, 27% ee, 67:33 dr). Various substituents with different electron and steric properties at the indole core did not affect either the reaction yield or the stereoselectivity of the reaction. The only exception was the reaction with 3-phenylindole, where slight decreases in ee and dr were observed (80% ee, 86:14 dr).
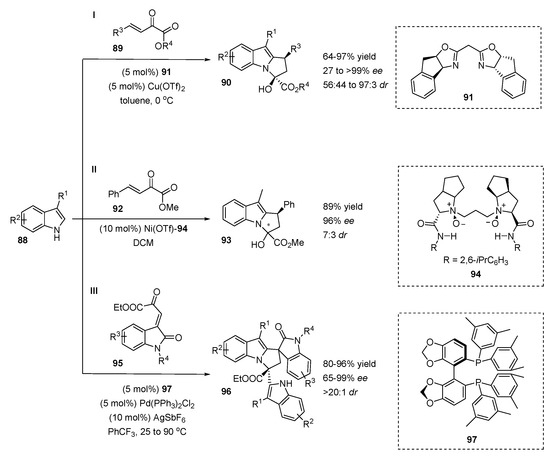
Scheme 21.
C2-alkylation of indoles followed by intramolecular N-cyclization.
Feng et al. investigated the enantioselective intermolecular Friedel-Crafts alkylation reaction at the C2-position of N-methylated indoles with β,γ-unsaturated α-ketoesters [48]. The cascade reaction of the C2-alkylation/N-hemiacetalization of skatole (3-methyl indole) was catalyzed by a chiral N,N′-dioxide 94 Ni(II) complex affording the corresponding product 93 with high yield (89%), excellent ee (96%) and moderate dr (7:3) values (Scheme 21, II). The results were slightly worse than with the chiral Box–copper(II)-catalyzed method discussed above (95 yield, >99% ee, 95:5 dr).
An efficient stereoselective triple cascade reaction of 3-alkylindoles with oxindolyl β,γ-unsaturated α-ketoesters 95 in the presence of a chiral diphosphine 97 palladium(II) catalyst was reported by the Wang group (Scheme 21, III) [49]. The domino reaction consists of asymmetric Friedel-Crafts/N-cyclization/Friedel-Crafts sequential alkylation and provides a wide range of spiro-polycyclic N1/C2 functionalized enantioenriched indoles 96. The various substituents at the phenyl ring and N-atom of oxindolyl β,γ-unsaturated α-ketoesters 95 were well tolerated, affording the spiro-polycyclic products 96 with good to excellent yields (80–96%), high to excellent ee values (86–99%) and excellent diastereoselectivities (>20:1). The indole scope demonstrated some limitations of the reaction: electron-withdrawing or electron-donating substituents at the C5 or C6 positions provided products with high yields (87–94), enantioselectivities and diastereoselectivities (91–98% ee, >20:1 dr) but in the case of the 4-bromo substituted indole a decline in enantioselectivity (65% ee) was detected. Sterically hindered indoles at the C3 position were not the best starting compounds for this cascade reaction. For instance, an n-hexyl substituent negatively affected the ee value of the reaction (81% ee) and the reaction with a bulkier i-Pr substituted indole afforded a product with a low yield (<30%).
Compared with Xiao’s highly enantioselective method based on chiral Box-copper(II) catalysis, Wang’s route, based on a chiral diphosphine 97 palladium(II) catalyst, has its advantages in the use of isatin-derived electrophiles 95, but is not particularly effective for simple γ-aryl substrates 89.
4.2. N-Allylation of Indoles
The enantioselective version of a Tsuji-Trost reaction was applied for the synthesis of chiral indolocarbazole derivatives (Scheme 22, I) [50]. The reaction of protected bis(indole) 98 with cyclopentyl carbonate 99 in the presence of a chiral ligand 102 and palladium catalyst proceeded smoothly, providing products 100 with excellent ee values (99%) and good yields (83% and 75%, depending on the protective group used). The authors conducted a series of NMR experiments and conformational analyses and found that the preferred site of the alkylation was the nitrogen atom of the indole moiety, which was linearly conjugated to the carbonyl function of lactam. Catalytic allylations of (bis)indoles with sugar-derived electrophiles were performed and cyclic products 108 and 109 were successfully obtained (Scheme 22, II).
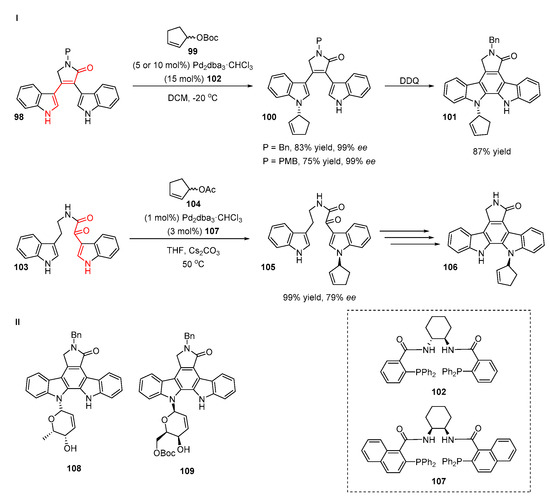
Scheme 22.
Pd-catalyzed N-allylation of (bis)indoles.
The preferred site of the allylation of bis(indole) depends on the acidity of the N-H atom of the indole derivative. When the bis(indole)-bearing conjugated dione moiety 103 at the C3 position was subjected to a reaction, a cyclopentenyl acetate 104 product 105 was formed. The authors also proposed a strategy for the construction of the chiral indolocarbazole 106 from the (bis)indole adduct 105.
Later, Trost et al. demonstrated a general method for the enantioselective N-allylation of electron deficient pyrroles 110 and indoles 111 with vinyl aziridines 112 as electrophiles (Scheme 23, I) [51]. The Pd-catalyzed asymmetric allylic alkylation provided a wide range of the heterocycle-containing chiral 1,2-diamines 113. The desired branched N-alkylated products were obtained with a similar catalytic system that was previously applied for allylation of bis(indole) adducts 98 (Scheme 22, I). The alkylation of indoles and pyrroles was catalyzed by a palladium complex in the presence of a chiral ligand 114 in dichloroethane at room temperature. The authors determined the positive impact of a naphthyl moiety of the chiral ligand on the enantioselectivity of the reaction. The scope of electron-deficient indoles demonstrated exclusively N-alkylation with moderate to high enantioselectivity (73–93%) and moderate to high yields (57–99%). Weak electron-withdrawing groups (such as bromo or chloro) negatively affected the reaction yield and ee. 2-Phenylindole afforded only trace quantities of the desired product. It should be noted that the amide anion of the vinyl aziridine in the π-allyl Pd intermediate was sufficiently basic to deprotonate the indole N-H and facilitate the reaction in most of the cases (Scheme 23, II).
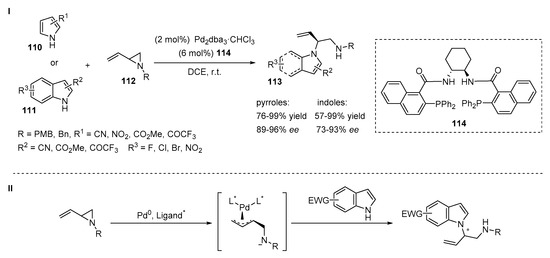
Scheme 23.
Pd-catalyzed N-allylation of electron-deficient pyrroles and indoles.
A highly enantioselective iridium-catalyzed N-allylation of electron-deficient or C3-substituted indoles was reported by Hartwig et al. (Scheme 24) [52]. The authors used a chiral phosphoramidite ligand 119, which was previously studied in N-allylation reactions with more acidic and nucleophilic benzimidazoles, imidazoles and purines. The initial results showed that the reaction proceeded in the presence of metallacycle 120 and cesium carbonate, affording an exclusively N-substituted product 117 with excellent branched-to-linear selectivity (117/118 = 97:3). The reaction scope of the ethyl indole-2-carboxylate 115 with various allylic carbonates 116 revealed that allylation proceeded smoothly at the N-position, providing corresponding products 117 with moderate to excellent regioselectivities (77:23 to 99:1), excellent enantioselectivities (96–99%) and moderate to high yields (54–95%). Indoles bearing various substituents at the C2, C3 and C5 positions were also tested with tert-butyl cinnamyl carbonate, providing excellent branched-to-linear selectivities (94:6 to 99:1), enantioselectivities (96–99%) and yields (21–95%). It should be noted that the parent indoles, 2-methylindole and 2-phenylindole, were allylated selectively at the C3 position. At the same time, 7-azaindole successfully underwent N-allylation in a high N to C3 ratio (9:1) and branched-to-linear selectivity (91:9). The chiral N-substituted 7-azaindole product was isolated with a 79% yield and 99% ee.
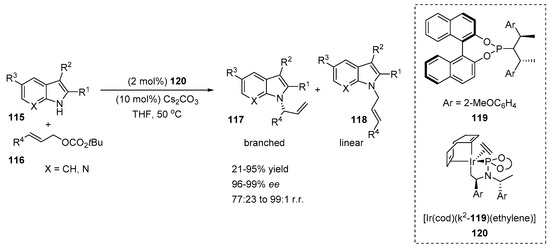
Scheme 24.
Ir-catalyzed N-allylation of indoles.
An efficient route for the synthesis of chiral indolopiperazinones was proposed by You and coworkers (Scheme 25) [53]. The intramolecular allylic amination of indole derivatives 121 was catalyzed by an iridium(I) NHC complex generated from the salt 123 and [Ir(cod)Cl]2 in the presence of DBU in dichloromethane at room temperature. The model reaction provided N-selective products with high yields (82%) and excellent ee values (99%). The amount of Ir complex could be reduced to 1.25 mol% without affecting the reaction outcome (80% yield, 96% ee). Indole derivatives containing electron-donating and electron-withdrawing groups at the C5 or C6 positions were examined in allylation reactions, affording the desired products 122 with good yields (77–91%) and excellent enantioselectivities (97–99% ee). Substrates with various substituents R2 (Bn, Me, allyl and PMB) on the amide nitrogen atom were well tolerated (77–89% yields and 96–99% ee, respectively). The authors also separately synthesized an Ir complex 124 and demonstrated its catalytic efficiency in asymmetric intramolecular cyclization (93% yield, 99% ee). These results were comparable to the results obtained with an in situ formed catalyst (82% yield, 99% ee).
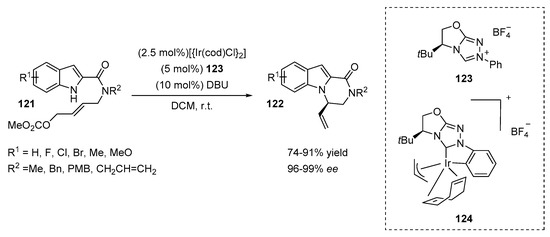
Scheme 25.
Ir-catalyzed synthesis of chiral indolo- and pyrrolopiperazinones.
Xiao et al. reported a highly stereoselective Pd-catalyzed N-functionalization of indoles in the presence of chiral sulfoxide-phosphines (Scheme 26) [54]. The reaction of methyl indole-2-carboxylate with racemic (E)-1,3-diphenylallyl acetate was efficient and stereoselective in the presence of a catalytic system derived from [Pd(C3H5)Cl]2, sulfoxide-phosphine ligand 128 and cesium carbonate in dichloromethane at 40 °C (99% yield, 97% ee). The scope of the reaction was performed with various substituted indoles 125 and allyl acetates 126. Both C2- and C3-substituted indoles tolerated the reaction, demonstrating good to excellent enantioselectivities (75–97%) and moderate to high yields (58–95%) of the corresponding N-alkylated indoles 127. Interestingly, 2-vinyl indole afforded an N/C-dialkylated product as a single diastereomer with good yields (74%) and high enantioselectivity (93%). At the same time, 2-vinyl 7-chloroindole gave an exclusively C3-alkylated product. The scope of allyl acetates revealed that acetates containing phenyl rings afforded products with high yields (89–95%) and excellent ee values (94–96%). The reaction with a sterically less hindered 1-methyl-3-phenylallyl acetate was regioselective and had a high yield (98%), but a decrease in enantioselectivity was detected (65%). The cyclic acetate afforded the desired product with a low ee value (23%).
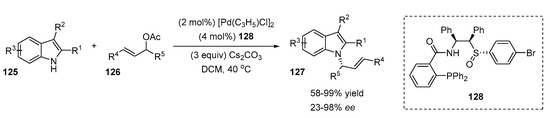
Scheme 26.
Pd-catalyzed N-functionalization of indoles.
Recently, Krische and co-workers demonstrated an efficient asymmetric intermolecular Tsuji–Trost-type indole N-allylation, where complete N-regioselectivity and regioselectivity towards branched products were achieved (Scheme 27, I) [6]. The reaction was smoothly catalyzed by cyclometallated p-allyliridium C,O-benzoates modified with (S)-tol-BINAP 132 under basic conditions. A wide range of various substituted indoles 129 reacted with high levels of enantiomeric enrichment (88–93%), affording products 131 with moderate to excellent yields (60–96%). The parent indole was successfully alkylated with diverse α-substituted allyl acetates 130 containing alkyl groups, phenyl, benzyl ether and methyl sulfide moieties (65–79% yield, 91–93% ee). Furthermore, α-cycloalkyl substituted allyl acetates 130 tolerated the reaction well, affording N-functionalized indoles with good yields (60–76%) and high ee values (90–92%). The authors also demonstrated the intramolecular cyclization of the racemic indole adduct 133 under optimized conditions (Scheme 27, II). The desired chiral tricyclic N-allylated indole 134 was isolated with high yields and high enantioselectivity (80% yield, 89% ee).
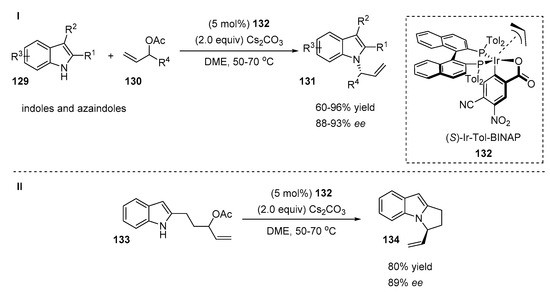
Scheme 27.
Highly selective Ir-catalyzed allylation of indoles.
4.3. Aminations of Alkenes with Indoles
An iridium-catalyzed highly selective intermolecular N-H addition of indoles 135 to inactivated terminal olefins 136 was investigated by Hartwig’s group [55]. The reaction proceeded according to Markovnikov’s selectivity in the presence of a catalytic amount of [Ir(cod)Cl]2 and a chiral bulky DTMB-SEGPHOS ligand 139 (Scheme 28, I). The authors found that the addition of ethyl acetate to the reaction mixture increased the rate and yield of the reaction. Various substituted indoles and α-olefins substrates were examined in an enantioselective hydroamination reaction. The study of the scope of the indoles with 1-octene revealed that C3-, C5- and C6-substituted indoles tolerated the reaction successfully affording products with moderate to good enantioselectivities (45–75% ee) and yields (58–88%). There were no reactions in the case of 2- or 7-substituted indoles. The electron density of the indole core did not noticeably affect the reaction outcome. The scope of α-olefins demonstrated the influence of the β-substituent on the reaction rate, yield and enantioselectivity (39–70%, 4–67% ee, respectively). For instance, yields of products derived from bulky substituted olefins were lower than with 1-octene based products. Moreover, significant amounts of vinylindoles 138 as side products were determined (20–30%). In some cases, the reaction conditions were modified in order to get better results. A drastic decline in ee value (4–5%) was detected when tert-butylpropene was used as a starting compound.
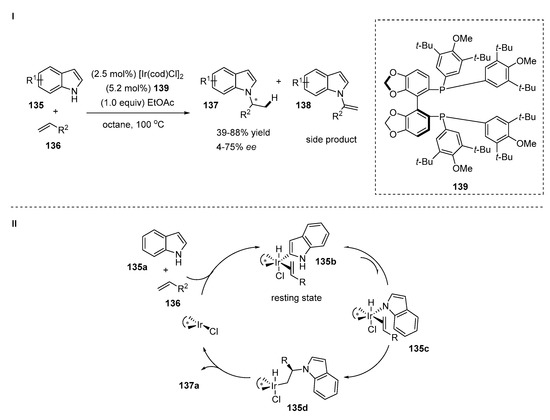
Scheme 28.
Ir-catalyzed hydroamination of alkenes with indoles.
According to mechanistic and computational studies, the authors proposed the mechanism of the reaction that is outlined in Scheme 28, II. The olefin insertion into the Ir−N bond of an N-indolyl complex 135c is faster than the insertion of olefin into the Ir−C bond of the isomeric C-2-indolyl complex 135b (resting state). This feature determines the N-selectivity of the addition of olefin. The formation of vinylindole as a side product and the racemization of the product were also explained and discussed based on mechanistic studies.
Chiral N,O-aminals 143 were obtained by the stepwise metal-catalyzed synthesis from alkoxyallenes 141 and indoles 140 (Scheme 29, I) [5]. The addition of indole 140 to allene proceeded exclusively at the N-position affording unsaturated adducts 142. The obtained N,O-aminals 142 were subjected to a ring-closing metathesis reaction catalyzed by Grubbs 1st generation catalyst. The cyclic products 143 were isolated with a nearly quantitative yield. The authors determined the critical impact of the chiral ligand 102 on the Pd-catalyzed reaction. In the case of ligand 114 the conversion of the reaction nearly stopped. The scope of the reaction demonstrated that indoles with electron-donating or electron-withdrawing groups tolerated the reaction well, providing products with high yields (87–98%) and ee values (85–93%). It is important to mention that the ester group at the C7 position of the indole decreased the rate of the reaction but the product was still isolated with good yields and excellent enantiomeric excess (70%, >99%, respectively). Indoles bearing substituents at C2-position afforded chiral products with good yields (74–93%) and excellent ees (95–98%). The obtained products were successfully converted into various pyranosylated and furanosylated glycosides 144 through stereoselective dihydroxylation by osmium tetraoxide. According to their DFT calculations, the authors proposed the mechanism of the Pd-catalyzed reaction that is outlined in Scheme 29, II.
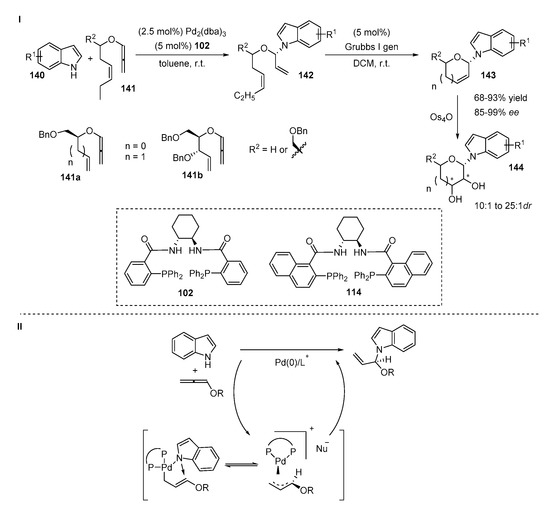
Scheme 29.
Pd-catalyzed synthesis of chiral N,O-aminals.
The enantioselective N-alkylation of indoles via an intermolecular aza-Wacker-type reaction was reported by Sigman et al. (Scheme 30, I) [56]. The formation of the desired product 147 was possible only if selective β-Hb elimination of the unsaturated compound was guaranteed (Scheme 30, II). Otherwise, the classic enamine product was formed. The Pd-catalyzed reaction proceeded between 3-substituted indoles 145 and 1,2-disubstituted alkene 146 in the aprotic solvent (dichloromethane) in the presence of a chiral ligand 148, base (DTBMP) and oxidant (p-benzoquinone). It is important that C2 products were not detected; the alkylation proceeded exclusively at the N-position. The reaction scope was performed with a wide range of substituted alkenols, demonstrating low to good yields (27–78%) and good to high er values (91:1 to 98:2). An excellent functional group tolerance was achieved and highly reactive tosyl- or halide-containing alkenols were compatible with the reaction. The indole scope revealed that the electronic nature of 3-phenylindole did not significantly affect the reaction outcome (69–82% yields, 95:5 to 96:4 er). The authors conducted a series of deuterium-labeled experiments that proved a syn-aminopalladation pathway for this reaction.
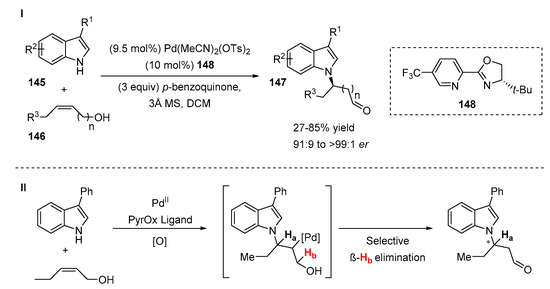
Scheme 30.
N-alkylation of indoles via an aza-Wacker-type reaction.
4.4. Copper-Catalyzed N-Selective Additions of Indoles to Alkylhalides
In recent years, stereoselective visible-light photocatalysis has received considerable attention from the synthetic community due to the unique activation mode of the substrates [57,58]. The photoinduced Cu-catalyzed enantioconvergent N-selective cross-coupling of 3-substituted indoles and carbazoles with racemic tertiary alkyl halides was described by Fu et al. (Scheme 31, I) [59]. The strategy of the reaction is based on the activation of an electrophile via the formation of the stable tertiary radical that is involved in the enantioselective process. A copper salt serves as the photocatalyst and, together with the chiral ligand 152, is responsible for the enantioselective bond-forming process. The reaction was studied with a wide range of carbazole derivatives, 3-substituded indoles 149 and various α-halocarbonyl compounds 150. The scope of the N-coupling partner demonstrated high levels of stereocontrol despite its electronic and steric nature (88–94% ee); the products were obtained with good to excellent yields (79–89%). The electronic effects of the indoline amide group and variations of the amide groups tolerated the reaction well, providing products 151 with excellent enantioselectivities (90–96% ee) and moderate to high yields (73–92%).
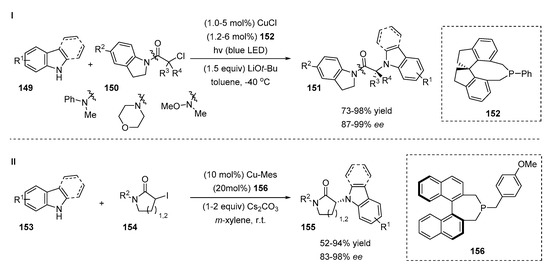
Scheme 31.
Cu-catalyzed N-functionalization of indole derivatives.
Fu and co-workers continued to study the enantioselective Cu-catalyzed N-alkylation of indole derivatives with α-halocarbonyl compounds (Scheme 31, II) [60]. Secondary alkyl iodides 154 were used as coupling partners in a photocatalytic reaction for the preparation of chiral 3-indolyl lactams 155 but the results were unsatisfactory (<1% ee, 24% yield). The screening of the reaction conditions revealed that the reaction occurred under nonphotocatalytic conditions in the absence of light and in the presence of Cu-Mes, a chiral monodentate phosphine ligand 156 and cesium carbonate at room temperature in m-xylene. The catalytic method was not air- or moisture-sensitive. The scope of the electrophiles showed that various N-substituted aromatic and alkyl lactams 154 tolerated the reaction well despite the electronic properties of the substituents. When alkyl bromide was used instead of iodide as an electrophile, a slight decrease in yield (60% vs. 73%) and a comparable value of ee were determined (ee 88%). The yield was improved up to 85% by an increase in the amount of electrophile from 1.5 equivalent to 2.0 equivalent. The authors conducted a series of experiments where the ee of the unreacted electrophile and the yield of the product were monitored during the reaction. These experiments revealed that the asymmetric N-alkylation of an indole with racemic alkyl bromide proceeded via a simple kinetic resolution but, in the case of alkyl iodide, a dynamic kinetic resolution occurred.
5. Other Direct Methods
Chiral N,N′-acyl aminals were prepared from indoles and N-Boc or N-Cbz imines in the presence of a dinuclear zinc–prophenol complex (Scheme 32) [61]. The method was characterized by high N/C3 regioselectivity, which was maintained due to the application of carbamate-protected imines 160. The choice of the solvent also drastically affected the regioselectivity of the reaction. For example, the N-substituted product was formed with a 61% yield if THF was used as a solvent and with a 14% yield with toluene. Although the complete N-selectivity of the alkylation was not achieved, N-alkylated products 161 were easily separable from C3-alkylated products. The study of the substrate scope was performed with a wide range of substituted indoles. Higher yields were obtained when 3-substituted indoles were applied. Moreover, the sterical hindrance of the protection group of the imine affected the reaction yield. Substrates with the Cbz group were more reactive than the Boc-substrates. The reactions of Cbz-imines with indoles proceeded at a lower temperature (4 °C) without any loss of yield and with improved enantioselectivity. To demonstrate the generality of the proposed method, the authors extended the reaction scope to other nitrogen-containing heterocycles, such as carbazole 159 and methyl pyrrole-2-carboxylate 157. The reactions proceeded smoothly affording N-alkylated products 161 with moderate to high yields (52–86%) and with high to excellent er values (96:4 to 99.5:0.5). The chiral N-alkylated products could be efficiently functionalized further, providing new classes of valuable heterocycles.
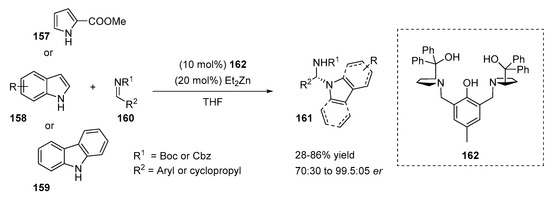
Scheme 32.
Synthesis of chiral N,N′-acyl aminals.
Propargylic compounds are important building blocks in synthetic chemistry because they can be transformed into a wide range of organic derivatives, including heterocycles [62,63]. Shao and coworkers investigated the enantioselective N-propargylation of indoles and carbazoles (Scheme 33) [64]. The method was based on the in situ generation of alkynyl N-Cbz or N-Boc imines 163a from N,O-acetals 163 followed by the nucleophilic N-addition of indole derivatives 164. The reaction was smoothly catalyzed by chiral lithium SPINOL phosphate 166, which was responsible for the elimination of ethanol from N,O-acetal and participated in the transition state of the reaction, providing an excellent level of stereocontrol (ee up to 99 %). The reaction proceeded with a very low catalyst loading without a significant loss of enantioselectivity or yield. Even 0.1 mol% of the catalyst still provided high enantioselectivity and yield (92% and 72%, respectively).
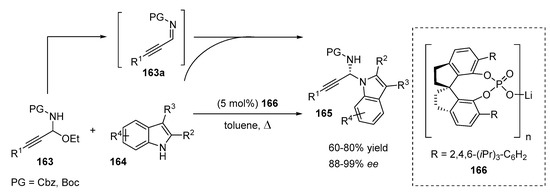
Scheme 33.
Asymmetric N-propargylation of indoles.
A CuH-catalyzed regiodivergent method for the synthesis of chiral indoles was recently reported by Buchwald et al. (Scheme 34) [7]. This synthetic route has two distinctive features: the application of indole 167 as an electrophile and facile access to either N- or C3-alkylated indole products (169 or 172). The regioselectivity of the reaction was efficiently controlled by a chiral ligand 170 or 173 affording either N-alkylated products 169 with high selectivity (>20:1) or C3-alkylated products 172 with a moderate to high ratio (3:1 to >20:1). N-alkylation was achieved via N-oxidative addition of the alkylcopper(I) complex followed reductive elimination.
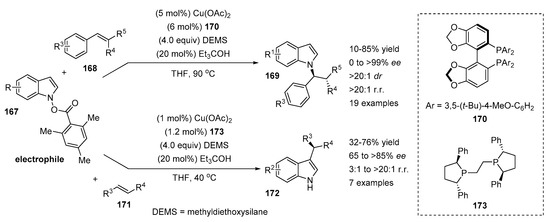
Scheme 34.
Ligand-controlled regiodivergent Cu-catalyzed alkylation of indoles.
The N-alkylation of indoles was performed with various styrene derivatives 168 affording products with moderate to good yields (41–85%) with good to excellent ee values (81–99%). The exceptions were 4-methoxy- and 4-trifluoromethylstyrenes, which gave the desired products with low yields (10% and 17%, respectively). The scope of indole electrophiles revealed that a wide range of substituted indoles tolerated the reaction well. Notably, the 2-carbomethoxyindole was not reactive enough and provided a racemic product with a low yield. Moreover, C3-substituted indole reacted with a low yield and ee (16% yield, 17% ee).
6. Transition-Metal Catalyzed Indirect Methods
Chiral α-N-branched indoles were obtained from the corresponding indolines by transition-metal catalyzed N-alkylation/oxidation sequences (Scheme 35). The application of this route was first reported by You’s group in 2012 (Scheme 35, I) [65]. The chiral N-allylindoles 177 were synthesized via a one-pot iridium-catalyzed allylic amination of indolines 174 followed by dehydrogenation of the resulting N-substituted indolines 176 with DDQ (2,3-dichloro-5,6-dicyano-1,4- benzoquinone). The method was characterized by a broad substrate scope. The electron deficient and electron rich aryl allyl carbonates afforded the desired products with excellent yields (87–92%) with superb ees (96–98%). The 2-thienyl- and alkyl-substituted allylcarbonates tolerated the reaction well and the corresponding products were obtained with good to high yields (up to 86%), with high branched-to-linear selectivity (up to 97:3) and excellent ee-values (up to 99%). Various indolines with electron-donating and electron-withdrawing groups at different positions were tested, demonstrating high levels of stereocontrol (92–99% ee; 96:4 to >99:1 r.r.).
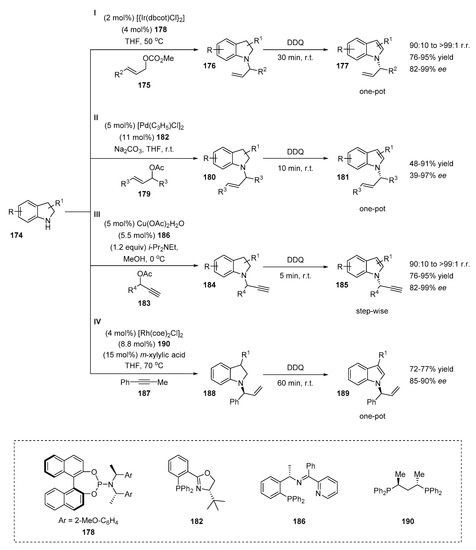
Scheme 35.
Indirect metal-catalyzed strategies for the construction of α-N-branched indoles.
The same group also described the asymmetric one-pot Pd-catalyzed version of the reaction (Scheme 35, II) [66]. The allylation proceeded smoothly in THF in the presence of 5 mol% of the palladium catalyst, 11 mol% of the Phox ligand 182 and 2 equivalents of Na2CO3 as a base. The obtained chiral indolines 180 were oxidized with DDQ in situ affording corresponding α-N-branched indoles 181. The substrate scope was performed with a wide range of indolines. The electronic properties of the substituents of the indoline core did not affect the reaction outcome, providing the desired chiral indoles with moderate to good yields (72–82%) with excellent ees (93–97%). However, the reaction of 2-phenylindoline with 1,3-diaryl allyl acetate gave a moderate yield but still high enantioselectivity (53% yield, 96% ee, respectively). A drastic decrease in ee was detected when (E)-1,3-dimethylallyl acetate was used as a coupling partner (48% yield, 39% ee).
The efficient copper-catalyzed propargylation of indolines with propargylic esters 183 followed by the DDQ oxidation of N-substituted indolines 184 was described by Hu et al. (Scheme 35, III) [67]. Copper salts were used as a metal source which made the method cheaper and easy to handle compared with the two methods discussed above. A disadvantage of this synthetic route is stepwise synthesis. The copper catalyst must be removed, and the methanol evaporated after the propargylation reaction; then the dehydrogenation of the chiral intermediate 184 proceeds in DCM at room temperature in 5 min. The propargylation was catalyzed with the bulky and structurally rigid chiral tridentate ketimine P,N,N-ligand 186 in the presence of Cu salt and base. The authors admitted that the type of copper salt did not noticeably affect the reaction outcome, but the role of the base was critical. When the reaction was performed without a basic additive, the product was formed with a low yield and enantioselectivity (45% yield, 25% ee). The addition of a base increased the yield and stereoselectivity of the reaction. Among the bases, the best results were obtained with Hünig’s base (90% yield, 92% ee). Interestingly, the reaction was also catalyzed by an inorganic base, such as potassium carbonate. A one-pot version of the reaction was possible, but the reaction was low-yielding (35% yield, 92% ee). The scope of propargylic esters revealed that the substitution pattern and electronic properties of the phenyl ring had an impact on the yield and stereocontrol of the reaction. For instance, a 2-choloro substituted substrate gave a corresponding product in decreased yield and ee (79% yield, 85% ee) compared with a 4-choloro substituted substrate (91% yield, 91% ee). The reaction with an electron-rich 4-metoxy substituted indoline was slightly less enantioselective (85% yield, 83% ee). The heterocyclic 2-thienyl and 2-naphtyl tolerated the reaction well, affording the products with high yields (88–89%) with 87–91% ee. Methyl- and fluoro-substituted indolines were also applied as coupling partners, providing high yields and ee values (86–91% yield, 88–94% ee).
The preparation of chiral N-allylindoles was reported by Dong et al. [68]. The synthetic route was based on the hydroamination of alkynes 187 with indolines via rhodium catalysis, followed by the dehydroaromatization of N-substituted indolines 188 (Scheme 35, IV). Both the parent indoline and 3-methylated indoline tolerated the one-pot reaction well, affording the desired N-substituted indoles with good yields and high ee values (72–77% yield, 85–90% ee, respectively).
7. Conclusions
Significant progress has been made over the past two decades in the synthesis of chiral N-alkylated indoles. Direct methods provide an opportunity for the synthesis of chiral N-functionalized indoles in one step from substrates containing an indole core. In some cases, the desired N-regioselectivity could not be gained due to the side reactions that occur at C3- or C2-positions. To avoid this problem C3- or C2-substituted indoles are usually used as starting compounds for the enantioselective N-functionalization. The review illustrates the progress of N-selective functionalization, as only recently was high regioselectivity achieved. Sometimes, the introduction of specific substituents into an indole core is necessary for the activation of the N-position and for the stereocontrol of the reaction. Indirect methods are not as thoroughly studied as direct methods. These methods can afford selective N-alkylation and exclude the problem of regioselectivity. At the same time, multistep synthesis is required for the construction of N-functionalized chiral indoles that make indirect routes less efficient and attractive. The stereoselective functionalization of the N-atom of the indole was successfully achieved by different types of organocatalytic and transition metal catalysis-based methods. These approaches demonstrated efficient routes for the preparation of chiral N-functionalized indoles that could be further modified and could provide facile access to biologically active compounds. Moreover, some methods may be used not only for the construction of chiral N-indoles, but may also find applications in the enantioselective functionalization of other N-heterocyclic compounds, such as indoline, pyrrole and carbazole derivatives.
Author Contributions
Both authors contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Estonian Ministry of Education and Research (grant No. PRG657) and the Centre of Excellence in Molecular Cell Engineering (2014–2020.4.01.15-0013).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vitaku, E.; Smith, D.T.; Njardsrson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Singh, O.M. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini-Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Ding, T.-M.; Yan, Z.-Q.; Hou, S.-H.; Zhu, G.-D.; Zhang, S.-Y. Asymmetric N-Hydroxyalkylation of Indoles with Ethyl Glyoxalates Catalyzed by a Chiral Phosphoric Acid: Highly Enantioselective Synthesis of Chiral N,O-Aminal Indole Derivatives. Org. Lett. 2019, 21, 2795–2799. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, H.W.; Jeong, W.; Moon, D.; Rhe, Y.H. Palladium-Catalyzed Asymmetric Nitrogen-Selective Addition of Indoles to alkoxyallenes. Org. Lett. 2018, 20, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Schempp, T.T.; Znieg, J.R.; Stivala, C.E.; Krische, M.J. Regio-and Enantioselective Iridium-Catalyzed N-Allylation of Indoles and Related Azoles with Racemic Branched Alkyl-Substituted Allylic Acetates. Angew. Chem. Int. Ed. 2019, 58, 7762–7766. [Google Scholar] [CrossRef]
- Ye, Y.; Kim, S.-T.; Jeong, J.; Baik, M.-H.; Buchwald, S.L. CuH-Catalyzed Enantioselective Alkylation of Indole Derivatives with Ligand-Controlled Regiodivergence. J. Am. Chem. Soc. 2019, 141, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Eichholzer, A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef]
- Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010, 39, 4449–4465. [Google Scholar] [CrossRef]
- Dalpozzo, R. Strategies for the asymmetric functionalization of indoles: An update. Chem. Soc. Rev. 2015, 44, 742–778. [Google Scholar] [CrossRef]
- Karchava, A.V.; Melkonyan, F.S.; Yurovskaja, M.A. New Strategies for the synthesis of N-alkylated indoles. Chem. Heterocycl. Compd. 2012, 48, 391–407. [Google Scholar] [CrossRef]
- Lakhdar, S.; Westermaier, M.; Terrier, F.; Goumont, R.; Boubaker, T.; Ofial, A.R.; Mayr, H. Nucleophilic Reactivities of Indoles. J. Org. Chem. 2006, 71, 9088–9095. [Google Scholar] [CrossRef] [PubMed]
- Otero, N.; Mandado, M.; Mosquera, R.A. Nucleophilicity of Indole Derivatives: Activating and Deactivating Effects Based on Proton Affinities and Electron Density Properties. J. Phys. Chem. A 2007, 111, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, D. The advent and development of organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Gröger, H.; MacMillan, D. Asymmetric Organocatalysis; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Torres, R.R. Stereoselective Organocatalysis, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Dalko, P.I. (Ed.) Comprehensive Enantioselective Organocatalysis; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Cui, H.-L.; Feng, X.; Peng, J.; Lei, J.; Jiang, K.; Chen, Y.-C. Chemoselective Asymmetric N-Allylic Alkylation of Indoles with Morita–Baylis–Hillman Carbonates. Angew. Chem. Int. Ed. 2009, 48, 5737–5740. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wei, Y.; Shi, M. Asymmetric substitutions of O-Boc-protected Morita–Baylis–Hillman adducts with pyrrole and indole derivatives. Org. Biomol. Chem. 2012, 10, 1396–1405. [Google Scholar] [CrossRef]
- Zi, Y.; Lange, M.; Schultz, C.; Vilotijević, I. Latent Nucleophiles in Lewis Base Catalyzed Enantioselective N-Allylations of N-Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 10727–10731. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Chen, Z.; Qin, W. Direct Preparation of Indole Hemiaminals through Organocatalytic Nucleophilic Addition of Indole to Aldehydes. Synthesis 2018, 50, 4063–4070. [Google Scholar]
- Xie, Y.; Zhao, Y.; Qian, B.; Yang, L.; Xia, C.; Huang, H. Enantioselective N–H Functionalization of Indoles with α,β-Unsaturated γ-Lactams Catalyzed by Chiral Brønsted Acids. Angew. Chem. Int. Ed. 2011, 50, 5682–5686. [Google Scholar] [CrossRef]
- Wu, P.; Nielsen, T.E. Scaffold Diversity from N-Acyliminium Ions. Chem. Rev. 2017, 117, 7811–7856. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Wang, S.-G.; Yin, Q.; You, S.-L. N-alkylation of indole via ring-closing metathesis/isomerization/Mannich cascade under ruthenium/chiral phosphoric acid sequential catalysis. Org. Chem. Front. 2014, 1, 39–43. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Chen, Z.; Hu, J.; Zeng, X.; Zhong, G. Chiral phosphoric acid catalyzed enantioselective N-alkylation of indoles with in situ generated cyclic N-acyl ketimines. Chem. Commun. 2018, 54, 9230–9233. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, J. Catalytic Asymmetric N-Alkylation of Indoles and Carbazoles through 1,6-Conjugate Addition of Aza-para-quinone Methides. Angew. Chem. Int. Ed. 2017, 56, 4583–4587. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Gu, Q.; You, S.-L. Chemoselective N–H functionalization of indole derivatives via the Reissert-type reaction catalyzed by a chiral phosphoric acid. Org. Biomol. Chem. 2018, 16, 6146–6154. [Google Scholar] [CrossRef]
- Yagil, G. The Proton Dissocation Constant of Pyrrole, Indole and Related Compounds. Tetrahedron 1967, 23, 2855–2861. [Google Scholar] [CrossRef]
- Bandini, M.; Eichholzer, A.; Tragni, M.; Umani-Ronchi, A. Enantioselective Phase-Transfer-Catalyzed Intramolecular Aza-Michael Reaction: Effective Route to Pyrazino-Indole Compounds. Angew. Chem. Int. Ed. 2008, 47, 3238–3241. [Google Scholar] [CrossRef]
- Bandini, M.; Bottoni, A.; Eichholzer, A.; Miscione, G.P.; Stenta, M. Asymmetric Phase-Transfer-Catalyzed Intramolecular N-Alkylation of Indoles and Pyrroles: A Combined Experimental and Theoretical Investigation. Chem. Eur. J. 2010, 16, 12462–12473. [Google Scholar] [CrossRef]
- Trubitsõn, D.; Martõnova, J.; Erkman, K.; Metsala, A.; Saame, J.; Kõster, K.; Järving, I.; Leito, I.; Kanger, T. Enantioselective N-Alkylation of Nitroindoles under Phase-Transfer Catalysis. Synthesis 2020, 52, 1047–1059. [Google Scholar] [CrossRef]
- Wang, C.; Raabe, G.; Enders, D. Enantioselective Synthesis of 3H-Pyrrolo[1,2-a]indole-2-carbaldehydes via an Organocatalytic Domino Aza-Michael/Aldol Condensation Reaction. Synthesis 2009, 24, 4119–4124. [Google Scholar]
- Hong, L.; Sun, W.; Liu, C.; Wang, L.; Wang, R. Asymmetric Organocatalytic N-Alkylation of Indole-2-carbaldehydes with α,β-Unsaturated Aldehydes: One-Pot Synthesis of Chiral Pyrrolo[1,2-α]indole-2-carbaldehydes. Chem. Eur. J. 2010, 16, 440–444. [Google Scholar] [CrossRef]
- Greb, A.; Deckers, K.; Selig, P.; Merkens, C.; Enders, D. Quadruple Domino Organocatalysis: An Asymmetric Aza-Michael/Michael/ Michael/Aldol Reaction Sequence Leading to Tetracyclic Indole Structures with Six Stereocenters. Chem. Eur. J. 2012, 18, 10226–10229. [Google Scholar]
- Cai, Q.; Zheng, C.; You, S.-L. Enantioselective Intramolecular Aza-Michael Additions of Indoles Catalyzed by Chiral Phosphoric Acids. Angew. Chem. Int. Ed. 2010, 49, 8666–8669. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.J.; Zhang, H.; Grossmann, A.; Loh, C.C.J.; Merkens, C.; Enders, D. Asymmetric Synthesis of Pyrroloindolones by N-heterocyclic Carbene Catalyzed [2+3] Annulation of α-Chloroaldehydes with Nitrovinylindoles. Angew. Chem. Int. Ed. 2013, 52, 13562–13566. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Daniliuc, C.G.; Studer, A. Oxidative N-heterocyclic Carbene Catalyzed Dearomatization of Indoles to Spirocyclic Indolenines with a Quaternary Carbon Stereocenter. Angew. Chem., Int. Ed. 2017, 56, 7402–7406. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Yang, S.; Xu, W.; Liu, J.; Perveen, S.; Kong, X.; Zehra, S.T.; Fang, X. Carbene-catalyzed asymmetric Friedel–Crafts alkylation-annulation sequence and rapid synthesis of indole-fused polycyclic alkaloids. Commun. Chem. 2019, 2, 85. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Zhang, Y.; Chen, X.-F.; Huang, J.; Shi, S.-H.; Hui, X.-P. Highly enantioselective synthesis of functionalized azepino[1,2α]indoles via NHC-catalyzed [3+4] annulation. Chem. Commun. 2019, 55, 4363–4366. [Google Scholar] [CrossRef]
- Sun, S.; Lang, M.; Wang, J. N-Heterocyclic Carbene-Catalyzed β-Indolylation of α-Bromoenals with Indoles. Adv. Synth. Catal. 2019, 361, 5704–5708. [Google Scholar] [CrossRef]
- Mukherjee, S.; Shee, S.; Poisson, T.; Besset, T.; Biju, A.T. Enantioselective N-Heterocyclic Carbene-Catalyzed Cascade Reaction for the Synthesis of Pyrroloquinolines via N-H functionalization of indoles. Org. Lett. 2018, 20, 6998–7002. [Google Scholar] [CrossRef]
- Yang, X.; Luo, G.; Zhou, L.; Liu, B.; Zhang, X.; Gao, H.; Jin, Z.; Chi, Y.R. Enantioselective Indole N−H Functionalization Enabled by Addition of Carbene Catalyst to Indole Aldehyde at Remote Site. ACS Catal. 2019, 9, 10971–10976. [Google Scholar] [CrossRef]
- Zhou, B.; Ghosh, A.K. Bifunctional cinchona alkaloid-squaramide-catalyzed highly enantioselective aza-Michael addition of indolines to α,β-unsaturated ketones. Tetrahedron Lett. 2013, 54, 3500–3502. [Google Scholar]
- Dou, X.; Yao, W.; Jiang, C.; Lu, Y. Enantioselective N-alkylation of isatins and synthesis of chiral N-alkylated indoles. Chem. Commun. 2014, 50, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhang, W.; You, S.-L. One-Pot Synthesis of Pyrrolo[1,2-a]indoles by Chiral N-Triflyl Phosphoramide Catalyzed Friedel-Crafts Alkylation of 4,7-Dihydroindole with β,γ-Unsaturated α-Keto Esters. Chin. J. Chem. 2012, 30, 2615–2623. [Google Scholar]
- Pellissier, H. Recent Developments in Enantioselective Metal-Catalyzed Domino Reactions. Adv. Synth. Catal. 2019, 361, 1733–1755. [Google Scholar] [CrossRef]
- Cheng, H.-G.; Lu, L.-Q.; Wang, T.; Yang, Q.-Q.; Liu, X.-P.; Li, Y.; Deng, Q.-H.; Chen, J.-R.; Xiao, W.-J. Highly Enantioselective Friedel–Crafts Alkylation/N-Hemiacetalization Cascade Reaction with Indoles. Angew. Chem. Int. Ed. 2013, 52, 3250–3254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zhao, X.; Zhang, J.; Zhou, L.; Lin, L.; Feng, X. Enantioselective Friedel–Crafts alkylation for synthesis of 2-substituted indole derivatives. Chem. Commun. 2013, 49, 11311–11313. [Google Scholar] [CrossRef]
- Li, N.-K.; Zhang, J.-Q.; Sun, B.-B.; Li, H.-Y.; Wang, X.-W. Chiral Diphosphine−Palladium-Catalyzed Sequential Asymmetric Double-Friedel−Crafts Alkylation and N-Hemiketalization for Spiropolycyclic Indole Derivatives. Org. Lett. 2017, 19, 1954–1957. [Google Scholar] [CrossRef]
- Krische, M.; Berl, V.; Grenzer, E.M.; Trost, B.M. Chemo-, Regio-, and Enantioselective Pd-Catalyzed Allylic Alkylation of Indolocarbazole Pro-aglycons. Org. Lett. 2002, 4, 2005–2008. [Google Scholar]
- Osipov, M.; Dong, G.; Trost, B.M. Palladium-Catalyzed Dynamic Kinetic Asymmetric Transformations of Vinyl Aziridines with Nitrogen Heterocycles: Rapid Access to Biologically Active Pyrroles and Indoles. J. Am. Chem. Soc. 2010, 132, 15800–15807. [Google Scholar]
- Levi, M.; Hartwing, J.F. Iridium-Catalyzed Regio- and Enantioselective N-Allylation of Indoles. Angew. Chem. Int. Ed. 2009, 48, 7841–7844. [Google Scholar]
- Ye, K.-Y.; Cheng, Q.; Zhou, C.-X.; Dai, L.-X.; You, S.-L. An Iridium(I) N-Heterocyclic Carbene Complex Catalyzes Asymmetric Intramolecular Allylic Amination Reactions. Angew. Chem. Int. Ed. 2016, 55, 8113–8116. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Yu, X.-Y.; Chen, J.-R.; Feng, B.; Zhang, H.; Qi, Y.-H.; Xiao, W.-J. Enantioselective Direct Functionalization of Indoles by Pd/SulfoxidePhosphine-Catalyzed N-Allylic Alkylation. Org. Lett. 2015, 17, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Sevov, C.S.; Zhou, J.; Hartwig, J.F. Iridium-Catalyzed, Intermolecular Hydroamination of Unactivated Alkenes with Indoles. J. Am. Chem. Soc. 2014, 136, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R.; Bahamonde, A.; Farukawa, Y.; Sigman, M.S. Enantioselective N-Alkylation of Indoles via an Intermolecular AzaWacker-Type Reaction. J. Am. Chem. Soc. 2019, 141, 8670–8674. [Google Scholar] [CrossRef]
- Abreu, D.; Belmont, M.; Brachet, E. Synergistic Photoredox/Transition-Metal Catalysis for Carbon–Carbon Bond Formation Reactions. Eur. J. Org. Chem. 2020, 2020, 1327–1378. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, W.; Zheng, W.-H.; Lu, H. Advances in asymmetric visible-light photocatalysis, 2015–2019. Org. Biomol. Chem. 2019, 17, 8673–8689. [Google Scholar] [CrossRef] [PubMed]
- Kainz, Q.M.; Matier, C.D.; Bartoszewicz, A.; Zultanski, S.L.; Peters, J.C.; Fu, G.C. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 2016, 351, 681–684. [Google Scholar] [CrossRef]
- Bartoszewicz, A.; Matier, C.D.; Fu, G.C. Enantioconvergent Alkylations of Amines by Alkyl Electrophiles: Copper-Catalyzed Nucleophilic Substitutions of Racemic α-Halolactams by Indoles. J. Am. Chem. Soc. 2019, 141, 14864–14869. [Google Scholar] [CrossRef]
- Gnanamani, E.; Hung, C.-I.; Trost, B.M. Controlling Regioselectivity in the Enantioselective N-Alkylation of Indole Analogues Catalyzed by Dinuclear Zinc-ProPhenol. Angew. Chem. Int. Ed. 2017, 56, 10451–10456. [Google Scholar]
- Roy, R.; Saha, S. Scope and advances in the catalytic propargylic substitution reaction. RSC Adv. 2018, 8, 31129–31193. [Google Scholar] [CrossRef]
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and Reactivity of Propargylamines in Organic Chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Shan, W.; Shao, Z. Direct asymmetric N-propargylation of indoles and carbazoles catalyzed by lithium SPINOL phosphate. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-B.; Zhang, X.; Dai, L.-X.; You, S.-L. Asymmetric N-Allylation of Indoles Through the Iridium-Catalyzed Allylic Alkylation/Oxidation of Indolines. Angew. Chem. Int. Ed. 2012, 51, 5183–5187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhuo, C.-X.; You, S.-L. Enantioselective synthesis of N-allylindoles via palladium-catalyzed allylic amination/oxidation of indolines. RSC Adv. 2014, 4, 10875–10878. [Google Scholar] [CrossRef]
- Zhu, F.; Hu, X. Enantioselective N-propargylation of indoles via Cu-catalyzed propargylic alkylation/dehydrogenation of indolines. Chin. J. Catal. 2015, 36, 86–92. [Google Scholar] [CrossRef]
- Chen, Q.-A.; Chen, Z.; Dong, V.M. Rhodium-Catalyzed Enantioselective Hydroamination of Alkynes with Indolines. J. Am. Chem. Soc. 2015, 137, 8392–8395. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).