Abstract
Meta-control describes an interhemispheric response conflict that results from the perception of stimuli that elicit a different reaction in each hemisphere. The dominant hemisphere for the perceived stimulus class often wins this competition. There is evidence from pigeons that meta-control results from interhemispheric response conflicts that prolong reaction time when the animal is confronted with conflicting information. However, recent evidence in pigeons also makes it likely that the dominant hemisphere can slow down the subdominant hemisphere, such that meta-control could instead result from the interhemispheric speed differences. Since both explanations make different predictions for the effect of commissurotomy, we tested pigeons in a meta-control task both before and after transection of the commissura anterior. This fiber pathway is the largest pallial commissura of the avian brain. The results revealed a transient phase in which meta-control possibly resulted from interhemispheric response conflicts. In subsequent sessions and after commissurotomy, however, the results suggest interhemispheric speed differences as a basis for meta-control. Furthermore, they reveal that meta-control is modified by interhemispheric transmission via the commissura anterior, although it does not seem to depend on it.
1. Introduction
Meta-control refers to the one hemisphere taking charge of response selection when the two hemispheres are brought into conflict [1,2,3]. This phenomenon was first demonstrated in split-brain patients and healthy people [1,4], but was also later revealed in monkeys [5], chicken [6], and pigeons [2,3,7]. It is often assumed that meta-control results from one hemisphere inhibiting the other via the various commissures that connect the two halves of the brain at the midbrain and telencephalic level [8,9].
Meta-control becomes especially visible in species with pronounced brain asymmetries. Depending on the type of stimulus, one or the other hemisphere regularly gains control. Birds are ideal subjects for these studies [10]. Their left hemisphere is superior in discrimination, categorization, and memorization of visual patterns (chicks: [11]; quail: [12]; pigeons: [13,14]) and visuomagnetic cues (pigeons: [15]; chicks: [16]), while their right hemisphere is superior in visually guided interactions with emotionally charged stimuli (chicks: [17]), attentional shifts (chicks and pigeons: [18]), social interactions (chicks: [19]), as well as in relational and spatial analyses of visual information (chicks: [20]; pigeons: [14,21]).
Meta-control could result from either inter-hemispheric response conflict or differences in hemisphere-specific speed. If inter-hemispheric response conflict was the cause, situations in which each half-brain competes to present a different response should produce longer reaction times than non-conflicting situations [2,8]. This is because decision making with two incompatible options usually requires a longer processing time [10]. If, however, meta-control simply results from hemisphere-specific processing speed, the outcome would be different. The decision time would be determined solely by the faster hemisphere, which would always win. Two competing hemispheres would then be as fast as the faster hemisphere.
A recent study conducted by Ünver & Güntürkün [2] in pigeons collected evidence for the inter-hemispheric response conflict model. In their study, pigeons were trained by a forced-choice color discrimination task monocularly, and each hemisphere learned to discriminate between its own stimulus pair. Then, under binocular conditions, the birds were exposed to two types of test stimuli. These test stimuli were created by combining positive and negative patterns learned by each hemisphere. If the animal had to discriminate between a stimulus pair that consisted of two positive (left- and right-hemispheric) patterns on one pecking key and two negative patterns on the other, the choice was easy. Both hemispheres agreed to peck the pattern combination that was positive for both half-brains. Consequently, the animals responded quickly to this “super stimulus”. The situation was different when each stimulus was composed of the positive pattern of one hemisphere and the negative pattern of the other hemisphere. In the case of such an “ambiguous stimulus”, the overall pattern signaled an interhemispheric reward history conflict. As it turned out, the ambiguous stimulus caused a significant response delay. This makes it likely that meta-control rests mainly on an inter-hemispheric response conflict and not on hemisphere-specific speed.
A recent study, however, proposed a different mechanism. Qian & Güntürkün [22] recorded signals from the sensorimotor arcopallium of pigeons while the birds were conducting a color discrimination task under monocular conditions. All birds in their study learned faster and responded more quickly with their right eye/left hemisphere. The arcopallium not only harbors descending premotor neurons but also commissural neurons that constitute the commissura anterior—the largest avian interhemispheric connection at the pallial level. As shown by Letzner et al. [23], the commissura anterior originates from the telencephalic arcopallium/amygdala-complex and contains a small cluster of non-GABAergic sensorimotor and amygdaloid fibers that project onto a wide range of contralateral structures such as the posterior amygdala, the sensorimotor arcopallium, as well as further sensory and motor components of the nidopallium. We chose this commissure for our study due to these widespread projections onto the contralateral hemisphere. Qian & Güntürkün [22] transiently blocked the arcopallial activity of one hemisphere and recorded from the contralateral arcopallium during color discrimination to determine the effect of left-to-right and right-to-left information transfer. They discovered that the left hemisphere was able to modify the timing of individual activity patterns of the neurons in the right hemisphere via asymmetrical commissural interactions. In contrast to that, right arcopallial neurons were hardly able to alter the activity pattern of left arcopallial cells. Thus, under conditions of interhemispheric competition, left arcopallial neurons could delay the contralateral spike time of those in the right hemisphere. As a result, the neurons of the right hemisphere would come too late to control a response and the left hemisphere would govern decisions. This finding could imply that hemispheric dominance in birds is realized at least in part by time shifts of the neural activity of one or the other hemisphere.
The studies by Ünver & Güntürkün [2] and Qian & Güntürkün [22] make contradictory predictions of the mechanisms of meta-control. Both would assume that the commissura anterior plays a decisive role in inter-hemispheric response conflicts but would predict different choice patterns from birds in a meta-control task after commissurotomy. Ünver & Güntürkün [2] would infer that the loss of the commissura anterior should reduce reaction times when presented with an ambiguous stimulus because an inter-hemispheric response conflict could no longer result in an inter-hemispheric delay in processing time. In contrast, Qian & Güntürkün [22] would not expect a change in reaction times under the ambiguous stimulus because the dominant hemisphere already determines the response. They would, however, expect that the dominance of the left hemisphere would weaken after commissurotomy because the left-to-right control of the neuronal spike times could no longer be executed. To test these predictions, we conducted a meta-control study as published by Ünver & Güntürkün [2], and subsequently transected the commissura anterior to re-test the animals with the same task.
2. Materials and Method
2.1. Subjects
Nine naïve pigeons of unknown sex were used in the study. All pigeons were housed in single cages with other conspecifics and maintained on a 12:12 h light–dark cycle. Their body weight was maintained at 80–90% of their free-feeding weight by feeding diet food on weekdays and a mixture of peas, corn, and sunflower seeds on the weekends. Water was provided ad libitum. For the monocular sessions, velcro rings were fixed around the eyes of the pigeons using glue that was non-irritating to the skin. Cone-shaped eye caps that were attached to the other sides of the velcro rings at their bases and were created using cardboard. These eye caps could be easily attached and removed from the rings surrounding the eyes for monocular testing (Figure 1). All procedures were conducted in compliance with the guidelines for the care and use of laboratory animals and approved by the local committee (LANUV).
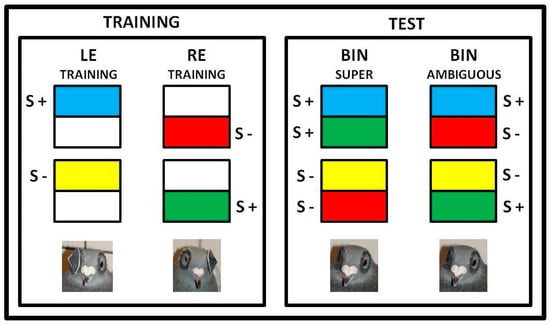
Figure 1.
The stimuli used in the experiment. Super stimuli consisted of a combination of two positive and two negative stimuli presented to the left eye (LE) and right eye (RE) during the training phase. Ambiguous stimuli were created by combining a negative stimulus for one hemisphere and a positive stimulus for the other. Both eyes (BIN = binocular) were open during the test phase. The color combinations shown in the figure are merely examples of the various combinations used. Below are photographs showing the animals with a cap on one eye (left) or both eyes uncovered (right).
2.2. Apparatus
A custom-made operant chamber measuring 40 × 35 × 35 cm (W × D × H) in size was used for the experiment. The chamber was equipped with a feeder and illuminated using a house light. The feeder was immediately illuminated when food was presented. The stimuli (5 × 5 cm in size) were introduced on a TFT LCD touchscreen monitor with 1024 × 768 resolution. The monitor was placed on the same side of the chamber as the feeder to ensure that the pigeons could easily reach the feeder immediately after pecking at the stimuli on the screen. The experimental sessions were controlled by a custom-written MATLAB program (MathWorks, Natick, MA, USA) using the Biopsy Toolbox [24].
2.3. Procedure
Before learning the color discrimination task, all pigeons were trained in autoshaping sessions consisting of 40 trials. In these sessions, the pigeons were made to peck on a white square presented on the screen under monocular conditions. The white square was presented for 4 s, and food was delivered immediately following a single peck on the white square. These sessions were conducted according to a fixed ratio (FR1) schedule. The birds were trained in a counterbalanced manner—on one day, only the left eye (LE) was blocked, whereas on the next day, only the right eye (RE) was blocked. Response to the white square in >85% of the trials in two consecutive sessions per eye condition was set as the criterion for progress to the subsequent schedules. Once the birds met this criterion, their training progressed to a variable ratio (VR) schedule wherein they were progressively trained with variable ratios VR2, VR4, and VR8 under monocular conditions again, with the same criterion. All the sessions in the VR schedule consisted of 40 trials.
Once the birds met the response criterion for the VR, we commenced the color discrimination training. Rectangles of four different colors (red, yellow, green, or blue) were used as stimuli. The color discrimination sessions were conducted under monocular conditions, and the color combinations were balanced among pigeons to prevent color preferences. As shown in Figure 1, they were always placed in a compound at the upper or lower position of a larger white rectangle. Each eye of the pigeons was exposed to a different pair of stimuli (e.g., red and yellow for the LE; blue and green for the RE). One of these colors served as S+ and the other as S− for each eye. The pigeons had to choose between an upper and a lower compound stimulus that each consisted of a colored and a white rectangle. Pecks on the S+ compound were rewarded regardless of whether the peck location was on the colored or on the white part of the compound. The same rule was applied for the S− compound. The monocular sessions were conducted in a counterbalanced manner, similar to the autoshaping sessions.
The stimuli were presented for 4 s. A single peck on the S+ compound immediately activated the feeder for 2 s, whereas a peck on the S− compound resulted in switching off the house lights for 5 s and playing a loud noise for 1 s. Once the birds responded to the S+ compound in >85% of the trials in two consecutive sessions for each eye condition, the number of trials per session was increased to 200 in steps of 20. The criteria that was applied in each step was that the pigeons had to make at least 85% correct choices (responses to the S+ compound) for each eye condition in a single session. As the number of trials in each session was increased, the reward ratio (responses to S+) was decreased in steps of 10% until reaching 40%. This procedure was employed to prevent extinction learning in subsequent catch trials. As a final step, a new stimulus pair, a white (S+) square and a gray (S−) square, were introduced. Because the birds had already been trained to respond to the white square during the autoshaping sessions, we expected them to be able to rapidly discriminate between this new stimulus pair. This white/gray “dummy” discrimination procedure was necessary to maintain the birds’ responses during the critical test sessions that included catch trials. In the catch trials, the colored stimuli were re-arranged to create “super” and “ambiguous” stimuli that were not rewarded. Each of the final sessions consisted of 200 trials, with 80% of the stimuli being presented as white (S+) and gray (S−) dummy stimuli. As outlined above, both S+ (the S+ of the LE and the S+ of the RE) on one pecking key and both S− on the other key were termed super stimuli. Unlike the other sessions, the critical test sessions were performed under binocular conditions. The gray/white stimuli represented a common associative background for both stimuli. This was not applied to the ambiguous stimuli. On each key, the S+ of one hemisphere was always combined with the S− of the other hemisphere. The proportion of catch trials in the final session was 20% (i.e., the number of catch trials was 40, with 20 being ambiguous and 20 being super stimuli). The remaining trials consisted of the white/gray stimuli pair (the number of white/gray stimuli was 160). No feedback for the catch trials was available, whereas the white/gray stimuli discrimination had a 40% reward probability. Following the first critical test session that included catch trials, the pigeons were further trained using the well-known training stimuli under monocular conditions. These sessions using the well-known training stimuli between each critical test session were conducted because it was necessary to maintain the pigeons’ response at a stable level during the subsequent critical test sessions. Therefore, this sequence was repeated until enough catch trial responses were collected.
After six sessions at most of testing for meta-control, pigeons underwent a commissurotomy operation. After a two-week recovery period, the same task and procedure were applied, and data were collected.
2.4. Surgery
Before surgery, nine birds participating in the experiment were given a mixture of ketamine (ketamine hydrochloride, 100 mg/mL; Zoetis, Berlin, Germany) and xylazine (xylazine hydrochloride, 23.32 mg/mL, methyl-4-hydroxybenzoate, 1.5 mg/mL; Bayer Vital, Leverkusen, Germany) by intramuscular injection (7:3 ratios, 0.12 mL/100 g body weight). The anesthetized birds were placed on a warming pad in a stereotaxic device. Their heads were fixed at a 45° angle in the head holder according to the coordinates of the pigeon brain atlas [25]. Prior to the commissurotomy, the scalp was opened and a window was opened in the skull with a drill, centered at the anterior 7.75 and lateral 0.0 coordinates. Then, the dura mater was removed. The main vessel in the gap between the two hemispheres was delicately pulled aside with a hand-made hook. Finally, a 2-mm-wide, 0.3 mm thick blade was slowly lowered into the region with the following coordinates: Anterior 7.75, lateral 0.0 at a depth of 9.0 mm from the surface of the brain [25]. The blade was lowered in increments of 1 mm, with a 2 min pause between each increment. Thus, the risk of damage to the brain due to the pressure caused by the blade was minimized. At the end of the operation, the knife was removed in the same manner, i.e., by lifting 1 mm every 2 min. The skin was stitched after a medical sponge was placed on the operation area. Finally, a painkiller was sprayed over the operation area and an antibacterial powder (Tyrasor; Engelhard Arzneimittel, Niederdorfleben, Germany) was applied. In addition, an intramuscular painkiller (Rimadyl, 0.04 mL/100 g body weight; Pfizer, GmbH, Münster, Germany) was administered. The pigeons were kept in their individual cages for one week to allow them to overcome the effects of the operation. Then, the tests were conducted.
2.5. Histology
The pigeons were deeply anesthetized with equithesin (0.55 mL/100 g body weight) and perfused with 4% paraformaldehyde (VWR Prolabo Chemicals, Leuven, Belgium) after the last post-operation tests. The brain was removed, immersed in gelatin (Merck, Darmstadt, Germany) and sectioned into 40-μm frontal slices using a freezing microtome (Leica Microsystems Nussloch GmbH, Nussloch, Germany). Sections were mounted, nissl and klüver-barrera stained, and the success of the commissurotomy was verified microscopically. In all nine birds, the commissura anterior was verified to be completely sectioned (Figure 2). In some animals the blade had been successfully lowered along the midline (Figure 2b), in others it was slightly off the midline and had damaged the medial most parts of the hemispheres in the medial meso- and nidopallium, as well the area above the commissura anterior (Figure 2a). These are not areas associated with the visual system and we could not see any correlation between our histological verifications and our behavioral results.
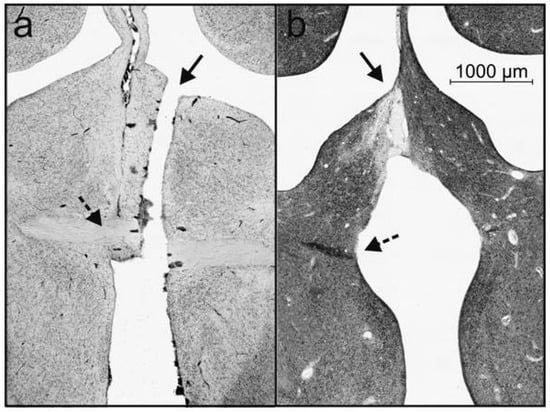
Figure 2.
A nissl (a) and a nissl/klüver-barrera (b) stained frontal section of two pigeons with transections of the commissura anterior. The straight arrows point to the tissue rupture resulting from the passing of the blade, while the broken arrows indicate remaining fibers of the commissura. Note that in (a) the blade has damaged the area above the commissure since it was slightly off the midline. This is not the case in (b). Scale bar in (b) also applies to (a).
3. Results
Two variables were important in studying the effect of the commissurotomy on meta-control. First, how many individuals display significant meta-control before vs. after commissurotomy? Meta-control in our task is defined as a significantly higher number of choices that are dominated by one hemisphere being faced with an ambiguous pattern. Second, how did the reaction times to ambiguous- and super-stimuli change after the commissurotomy?
Meta-control: A meta-control effect was observed in three out of nine birds before commissurotomy (for each individual: chi square test, p <0.05). In two birds the right eye dominated the decisions of the animal, and in one bird the left eye was dominant. Overall, this number was not sufficient to produce a significant meta-control effect at the population level (paired-sample t-test, t = 0.246, p = 0.812, n = 9). These three birds all ceased to demonstrate meta-control after commissurotomy. On the other hand, post-commissurotomy meta-control was observed in two different animals (one left, one right eye) that had not exhibited meta-control before the operation (chi square test, each p < 0.05). During the post-commissurotomy period, no significant meta-control at the population level was observed (paired-sample t-test, t = 0.939, p = 0.375, n = 9).
Reaction times: When first confronted with the ambiguous stimulus, the birds showed significantly higher reaction times to the ambiguous (1.14 s) than to the super stimulus (1.03 s) (paired-sample t-test, t = 2.540, p = 0.035, n = 9). In the second and subsequent sessions, however, this effect disappeared, such that the reaction time responses to super and ambiguous stimuli were no longer significantly different from each other (super stimulus: 1.07 s; ambiguous stimulus: 1.1 s; (paired-sample t-test, t = 0.479, p = 0.646, n = 8)). There were no significant reaction time differences to the super stimulus between session 1 and sessions 2–6 (paired sample t-test; t = 0.755, p = 0.475, n = 8). The same applied to the ambiguous stimulus (paired-sample t-test; t = 0.033, p = 0.975, n = 8). Note that the average values of sessions 2–6 were derived from 8 birds, since one pigeon stopped working on the task after session 1 (and then restarted after surgery). Similarly, in the post-surgery tests, no significant differences in the reaction times between super and ambiguous signals were observed (super stimulus: 1.24 s; ambiguous stimulus: 1.29 s; (paired-sample t-test, t = 0.614, p = 0.556, n = 9)). Moreover, there was no significant difference between the response times to the two stimulus types in the pre-surgery sessions (excluding session 1) and post-surgery sessions (mean of super stimulus sessions 2–6: 1.07 s; post-surgery session: 1.15 s; (paired-sample t-test, t = 0.680, p = 0.518, n = 8); mean of ambiguous stimulus sessions 2–6: 1.1 s; post-surgery session: 1.22 s; (paired-sample t-test, t = 1.097, p = 0.309, n = 8)) (Figure 3).
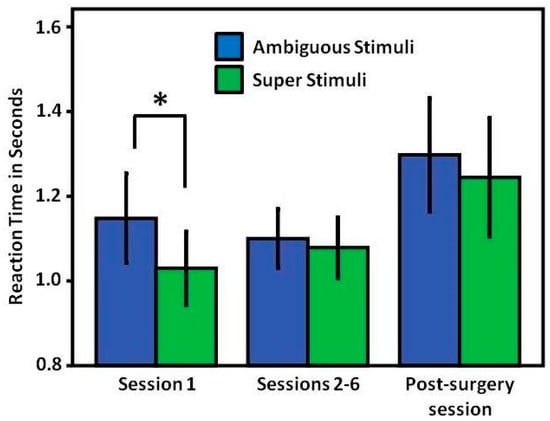
Figure 3.
Average reaction times of subjects to ambiguous and super stimuli during sessions prior to the commissurotomy and in the first session after the commissurotomy. Significant differences are indicated by an asterisk (p <0.05). Error bars are ±1 SEM. Note that the averages of sessions 2–6 were derived from 8 birds, because one pigeon stopped working on the task after session 1, but restarted after surgery.
4. Discussion
Meta-control can occur when the two hemispheres compete with each other to produce a hemisphere-specific response [1,2,4,5,7]. In studies with birds working on color discrimination tasks, the dominant hemisphere is usually the left [10,11,13]. Concomitantly, there is some evidence for a higher incidence of left-hemispheric meta-control in such tasks with pigeons [7]. The present study tested two different possible mechanisms of meta-control. One of these assumes that meta-control results from each hemisphere inhibiting the other [8]. Such a mechanism should cause conflicting (in our case ambiguous) stimuli to produce longer processing times, resulting in longer reaction times. A recent study found evidence supporting this prediction, and therefore suggested that meta-control results from the interhemispheric conflict [2]. An electrophysiological study, however, found evidence for a different mechanism: Qian & Güntürkün [22] discovered that arcopallial neurons of the left hemisphere dominate the response of the animal during color discrimination through a faster activation of motor responses. Furthermore, the left hemisphere controls the right hemispheric spike times, and is thus able to delay reaction times of the other hemisphere. This effect would increase the advantage of the left hemisphere. These findings make different predictions for the effect of the commissurotomy on meta-control. The mechanism based on the interhemispheric conflict would imply that a section of the commissura anterior should reduce reaction times to ambiguous stimuli (no commissural exchange → no interhemispheric conflict), whereas the model based on hemisphere specific speed would not predict post-surgery changes in reaction time to ambiguous stimuli (no commissural exchange → no change in hemisphere-specific speed). At the same time, the results of Qian & Güntürkün [22] suggest that the advantage of the left hemisphere would be smaller after commissurotomy (no commissural exchange → no possibility to further delay response execution of the right hemisphere). Our findings suggest that the birds only experience interhemispheric conflict on the first session with ambiguous stimuli, and the effect disappears in the following sessions. A subsequent commissurotomy does not alter reaction times to ambiguous stimuli but does modify meta-control. Overall, our data would be compatible with a model according to which interhemispheric conflict occurs in a short, initial period, but then gives way to lateralized reaction patterns determined by hemisphere-specific speed.
As visible in Figure 3, reaction times to super and ambiguous stimuli were the most different in the first session in which the animals were first presented these two stimulus types under binocular conditions. However, in subsequent sessions reaction times became increasingly similar. Ünver & Güntürkün [2] had based their conclusion of interhemispheric conflict on the first session after introducing ambiguous stimuli. This conclusion may remain valid but is obviously restricted to this initial session. In subsequent sessions, a different mechanism seems to prevail. It is indeed conceivable that the animals quickly learned about the absence of negative or positive feedback when responding to the ambiguous stimuli. It is known that pigeons are extremely sensitive to reward alterations in operant categorization tasks, and subsequently tend to bias their choices towards initially favored alternatives [26]. Similar findings were also observed in studies with monkeys [27,28]. This makes it likely that our commissurotomy was performed at a point in time in which the pigeons were no longer pondering response conflicts but instead biased their choices according to mechanisms based on hemisphere-specific speed. Consequently, response times to ambiguous stimuli were not altered by commissurotomy.
This scenario is compatible with the explanation that each hemisphere rushes with its own hemisphere-specific speed to motor areas. During color discrimination, the left hemisphere usually produces faster reaction times. This has been observed in various studies with pigeons [29] and other birds [17,30]. This was also observed by Qian & Güntürkün [22] when recording from the pigeon arcopallium during color discrimination. This study also offers a mechanistic explanation of this observation by revealing that the left hemisphere can modify the spike time of the right hemisphere. Thus, under conditions of conflict, the left hemisphere could delay the right hemispheric response speed, thereby accelerating its own advantage. From this point of view, a transection of the commissura anterior should reduce, but not completely terminate the left hemispheric superiority. Indeed, we observed major alterations of meta-control after surgery. Usually, an individually significant extent of meta-control is observed in only a fraction of pigeons [2,3,7]. With the procedure used in this study, it was mostly the left hemisphere that evinced meta-control [2,7]. In the current experiment, three out of nine birds demonstrated meta-control before commissurotomy (two left hemispheric, one right hemispheric). This is a typical result pattern [2,7]. After transecting the commissura anterior, however, all three birds lost their hemisphere-specific advantage. Instead, two other birds displayed significant meta-control (one left, one right). Although this is certainly not a strong proof of the conclusion of Qian & Güntürkün [22], it is conceivable that the changes observed in meta-control in our nine pigeons resulted from the loss of a left hemispheric advantage that resulted in biased interhemispheric interactions. If indeed neuronal speed differences cause the bias towards the right eye in metacontrol studies, the large individual differences may result from the fact that neurons show within the pigeon’s visual system substantial latency differences between individual birds [22,31,32,33].
It is known that the commissura anterior connects with the anterior and intermediate arcopallium. These structures project onto a wide cluster of visual and sensorimotor areas. Our study focused on the contribution of the commissura anterior to visual asymmetries. However, further commissural systems may also play a role in metacontrol since studies of both chicks [34] and pigeons [35,36,37] suggested that subpallial commissures also play key roles in visually-guided lateralized behavior. The supraoptic decussation (DSO) is one such subpallial connection, and is known to be responsible for interocular transfer during visual discrimination [38]. This may be due to the indirect connection of the DSO to telencephalic visual structures such as Wulst. More recently, it has been shown that the nucleus of the lateral ponto-mesencephalic tectum (nLPT), a midbrain structure, contains GABAergic neurons and its projections terminate in the contralateral optic tectum (TeO) via the commissura tectalis [39]. Therefore, this midbrain commissure may also play a crucial role during meta-control. Thus, the present study must be complemented by further experiments to reveal the full scenario of interhemispheric interactions of lop-sided bird brains.
Although our study was centered on the mechanisms of meta-control, it might also offer some more general insights on the behavior of organisms with lateralized brains. A key problem of these species is the production of a single response from two asymmetrically specialized hemispheres. Our results suggest that the default option in such situations could be to let both hemispheres compete based on hemisphere-specific processing speed. Because the dominant hemisphere for a certain stimulus class usually produces faster responses [22], the most competent half-brain would primarily determine the response. The commissural slowing mechanism discovered by Qian & Güntürkün [22] would amplify this interhemispheric speed difference to ensure that the dominant hemisphere controls the overall response.
Author Contributions
Conceptualization: Q.X. and O.G.; designed experiment: Q.X. and O.G.; performed experiment: E.Ü.; statistical analysis: E.Ü. and O.G.; manuscript preparation: E.Ü. and O.G.; funding acquisition and project supervision: O.G. All authors revised and approved the paper.
Acknowledgments
We are grateful for the support of Annika Simon during surgery and the conduct of the histological procedure. We also thank Felix Ströckens and Sarah von Eugen for help during documentation of histological results. Supported by the Deutsche Forschungsgemeinschaft through SFB 874.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levy, J.; Trevarthen, C. Metacontrol of hemispheric function in human split-brain patients. J. Exp. Psychol. Hum. 1976, 2, 299–312. [Google Scholar] [CrossRef]
- Ünver, E.; Güntürkün, O. Evidence for interhemispheric conflict during meta-control in pigeons. Behav. Brain Res. 2014, 270, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Güntürkün, O. When one hemisphere takes control: Metacontrol in pigeons (Columba livia). PLoS ONE 2009, 4, e5307. [Google Scholar] [CrossRef] [PubMed]
- Urgesi, C.; Bricolo, E.; Aglioti, S.M. Hemispheric metacontrol and cerebral dominance in healthy individuals investigated by means of chimeric faces. Cogn. Brain Res. 2005, 24, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Kavcic, V.; Fei, R.; Hu, S.; Doty, R.W. Hemispheric interaction, meta-control, and mnemonic processing in split-brain macaques. Behav. Brain Res. 2000, 111, 71–82. [Google Scholar] [CrossRef]
- Vallortigara, G. Comparative neuropsychology of the dual brain: A stroll through animals’ left and right perceptual worlds. Brain Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef]
- Freund, N.; Valencia-Alfonso, C.E.; Kirsch, J.; Brodmann, K.; Manns, M.; Güntürkün, O. Asymmetric top-down modulation of ascending visual pathways in pigeons. Neuropsychologia 2016, 83, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, C.; Maxfield, L. Varieties of interhemispheric inhibition, or how to keep a good hemisphere down. Brain Cogn. 1996, 30, 81–108. [Google Scholar] [CrossRef]
- Zeier, H.J.; Karten, H.J. Connections of the anterior commissure in the pigeon (Columba livia). J. Comp. Neurol. 1973, 150, 201–216. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Rogers, L.J. Asymmetry of brain and behavior in animals: Its development, function, and human relevance. Genesis 2014, 52, 555–571. [Google Scholar] [CrossRef]
- Valenti, A.; Sovrano, V.A.; Zucca, P.; Vallortigara, G. Visual lateralisation in quails (Coturnix coturnix japonica). Laterality 2003, 8, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O.; Kesch, S. Visual lateralization during feeding in pigeons. Behav. Neurosci. 1987, 101, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Aust, U.; Huber, L.; Hausmann, M.; Güntürkün, O. Lateralized cognition: Asymmetrical and complementary strategies of pigeons during discrimination of “human concept”. Cognition 2007, 104, 315–344. [Google Scholar] [CrossRef] [PubMed]
- Prior, H.; Wiltschko, R.; Stapput, K.; Güntürkün, O.; Wiltschko, W. Visual lateralization and homing in pigeons. Behav. Brain Res. 2004, 154, 301–310. [Google Scholar] [CrossRef]
- Rogers, L.J.; Munro, U.; Freire, R.; Wiltschko, R.; Wiltschko, W. Lateralized response of chicks to magnetic cues. Behav. Brain Res. 2008, 186, 66–71. [Google Scholar] [CrossRef]
- Rogers, L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008, 76, 235–244. [Google Scholar] [CrossRef]
- Diekamp, B.; Regolin, L.; Güntürkün, O.; Vallortigara, G. A left-sided visuospatial bias in birds. Curr. Biol. 2005, 15, R372–R373. [Google Scholar] [CrossRef]
- Vallortigara, G.; Andrew, R.J. Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behav. Proc. 1994, 33, 41–58. [Google Scholar] [CrossRef]
- Vallortigara, G.; Pagni, P.; Sovrano, V.A. Separate geometric and non-geometric modules for spatial reorientation: Evidence from a lopsided animal brain. J. Cogn. Neurosci. 2004, 16, 390–400. [Google Scholar] [CrossRef]
- Pollonara, E.; Guilford, T.; Rossi, M.; Bingman, V.P.; Gagliardo, A. Right hemisphere advantage in the development of route fidelity in homing pigeons. Anim. Behav. 2017, 123, 395–409. [Google Scholar] [CrossRef]
- Xiao, Q.; Güntürkün, O. Asymmetrical commissural control of the subdominant hemisphere in pigeons. Cell Rep. 2018, 25, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Letzner, S.; Simon, A.; Güntürkün, O. Connectivity and neurochemistry of the commissura anterior of the pigeon (Columba livia). J. Comp. Neurol. 2016, 524, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Otto, T.; Dittrich, L. The Biopsychology-Toolbox: A free, open-source Matlab-toolbox for the control of behavioral experiments. J. Neurosci. Meth. 2008, 175, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Karten, H.J.; Hodos, W. A Stereotaxic Atlas of the Brain of the Pigeon: Columba Livia; Johns Hopkins Press: Baltimore, MD, USA, 1967. [Google Scholar]
- Stüttgen, M.; Yildiz, A.; Güntürkün, O. Adaptive criterion setting in perceptual decision making. J. Exp. Anal. Behav. 2011, 96, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Holmes, P.; Rorie, A.; Newsome, W.T. Can monkeys choose optimally when faced with noisy stimuli and unequal rewards? PLoS Comput. Biol. 2009, 5, e1000284. [Google Scholar] [CrossRef] [PubMed]
- Teichert, T.; Ferrara, V.P. Suboptimal integration of reward magnitude and prior reward likelihood in categorical decisions by monkeys. Front. Neurosci. 2010, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O. Lateralization of visually controlled behavior in pigeons. Physiol. Behav. 1985, 34, 575–577. [Google Scholar] [CrossRef]
- Güntürkün, O. Avian visual lateralization: A review. Neuroreport 1997, 8, 3–11. [Google Scholar]
- Verhaal, J.; Kirsch, J.A.; Vlachos, I.; Manns, M.; Güntürkün, O. Lateralized reward-associated visual discrimination in the avian entopallium. Eur. J. Neurosci. 2012, 35, 1337–1343. [Google Scholar] [CrossRef]
- Folta, K.; Troje, N.; Güntürkün, O. Timing of ascending and descending visual signals predicts the response mode of single cells in the thalamic nucleus rotundus of the pigeon (Columba livia). Brain Res. 2007, 1132, 100–109. [Google Scholar] [CrossRef]
- Folta, K.; Diekamp, B.; Güntürkün, O. Asymmetrical modes of visual bottom-up and top-down integration in the thalamic nucleus rotundus of pigeons. J. Neurosci. 2004, 24, 9475–9485. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.H.; Rogers, L.J. Role of the tectal and posterior commissures in lateralization of the avian brain. Behav. Brain Res. 1993, 54, 153–164. [Google Scholar] [CrossRef]
- Güntürkün, O.; Böhringer, P.G. Lateralization reversal after intertectal commissurotomy in the pigeon. Brain Res. 1987, 408, 1–5. [Google Scholar] [CrossRef]
- Skiba, M.; Diekamp, B.; Prior, H.; Güntürkün, O. Lateralized interhemispheric transfer of color cues: Evidence for dynamic coding principles of visual lateralization in pigeons. Brain Lang. 2000, 73, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Keysers, C.; Diekamp, B.; Güntürkün, O. Evidence for physiological asymmetres in the phasic intertectal interactions in the pigeon (Columba livia) and their potential role in brain lateralisation. Brain. Res. 2000, 852, 406–413. [Google Scholar] [CrossRef]
- Watanabe, S. Interhemispheric transfer of visual discrimination in pigeons with supraoptic decussation (DSO) lesions before and after monocular learning. Behav. Brain Res. 1985, 17, 163–170. [Google Scholar] [CrossRef]
- Stacho, M.; Letzner, S.; Theiss, C.; Manns, M.; Güntürkün, O. A GABAergic tecto-tegmento-tectal pathway in pigeons. J. Comp. Neurol. 2016, 524, 2886–2913. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).