Abstract
We designed a vector for the bacterial expression of recombinant antibodies fused to a double tag composed of 6xHis and the EPEA amino acid sequence. EPEA sequence (C tag) is tightly bound by a commercial antibody when expressed at the C-term end of a polypeptide. The antigen is released in the presence of 2 M MgCl2. Consequently, constructs fused to the 6xHis-C tags can be purified by two successive and orthogonal affinity steps. Single-domain antibodies were produced either in the periplasmic or in the cytoplasmic space of E. coli. Surprisingly, the first affinity purification step performed using the EPEA-binding resin already yielded homogeneous proteins. The presence of the C tag did not interfere with the binding activity of the antibodies, as assessed by FACS and SPR analyses, and the C tag was extremely effective for immunoprecipitating HER2 receptor. Finally, the Alexa488-coupled anti-C tag allowed for simplification of FACS and IF analyses. These results show that a tag of minimal dimensions can be effectively used to improve the applicability of recombinant antibodies as reagents. In our hands, C tag was superior to His-tag in affinity purification and pull-down experiments, and practical in any other standard immune technique.
1. Introduction
The possibility to express constructs in which the target polypeptide is fused to appropriate tags suitable for affinity purification is an evident advantage of recombinant protein technology. This chromatographic approach allows for the standardization and the automation of protein purification but usually is not sufficient to obtain homogeneous products. Tandem affinity purification systems have been proposed for both eukaryotic and prokaryotic systems to avoid setting time-consuming protein-specific protocols aimed at removing minor contaminants. These systems typically use two orthogonal separation principles such as the TAP tag (Protein A and calmodulin or streptavidin binding domains) or combinations of 6xHis and carriers such as GST, Strep, MBP, or Z domain able to reversibly bind to a specific molecule [1,2,3,4,5]. The approach is effective, but the introduction of two tags can result in the modification of the structural stability of the target protein or to determine steric hindrance. In both cases the protein native characteristics can be compromised.
Tags can critically modify the stability, the yields, and the binding features for the substrate of recombinant antibodies. On the other hand, the presence of fusion tags simplifies the purification step and enables the antibody (multi)labeling. Short tags that do not interfere with the antibody functional activity and that can be detected in standard biological assessments would transform the recombinant antibody fragments into user-friendly immune-reagents [6,7]. Consequently, we designed a vector for expressing polypeptides fused to a double tag based on 6xHis and the C-terminal four (EPEA) amino acid sequence and evaluated its reliability to produce single-domain (VHH, nanobodies) antibodies using two independent affinity purification methods.
2. Results and Discussion
2.1. Affinity Purification Using Anti-C Tag Activated Resin
Recombinant single-domain antibodies specific for the HER2 ectodomain were previously isolated by panning a synthetic phage library (manuscript in preparation). The 6xHis-3xmyc-tagged constructs expressed by the pHEN2 phagemid enabled affinity purification by IMAC and amplified detection using a monoclonal anti-myc antibody. However, the preliminary results showed that a consistent amount of the fusion construct was degraded (Figure 1a). Functional assays proved that the poly-myc tag was partially or completely lost (data not shown). The substitution of the 3xmyc with the C tag allowed for the production of a double-tagged antibody (6xHis-C tag) suitable for affinity purification. IMAC alone is not usually sufficient to recover pure His-tagged protein and, also in our case, a significant amount of non-specific proteins was visualized together with the recombinant antibody (Figure 1a).
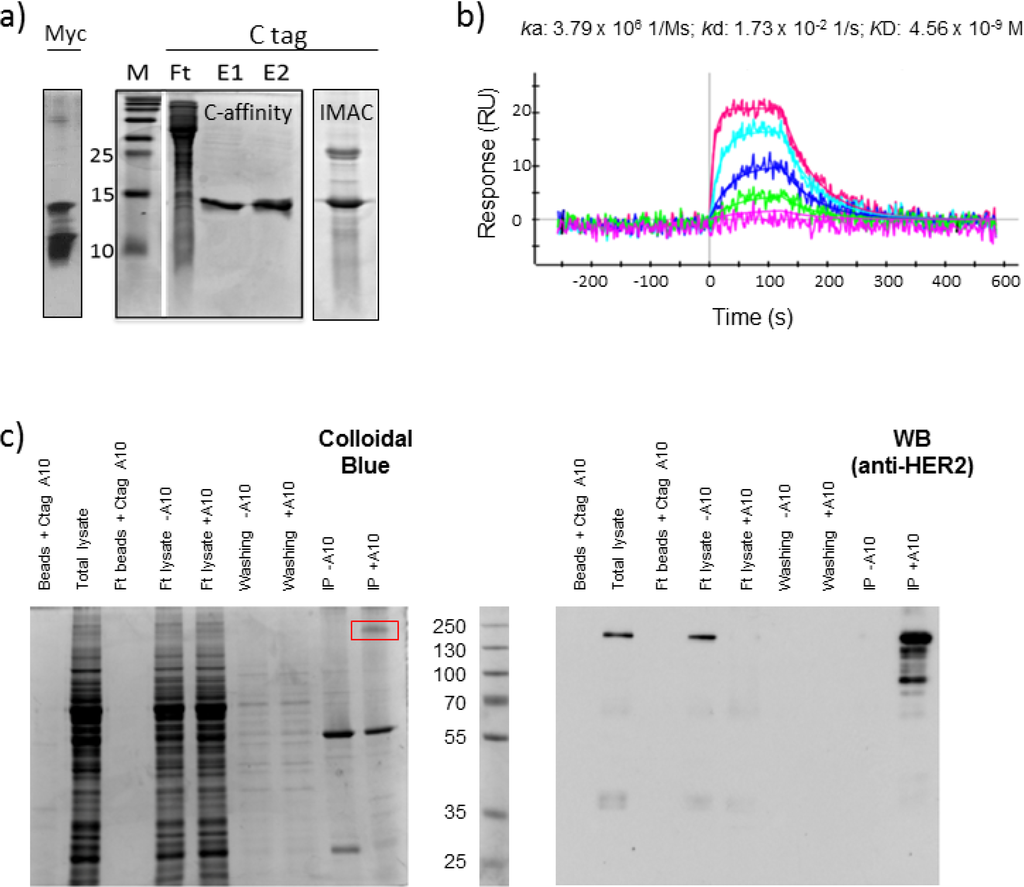
Figure 1.
Purification and functional validation of the single-domain antibody fused to C tag. (a) The single-domain antibody A10 expressed by the phagemid pHEN2 was purified by IMAC as a VHH-His-myc construct (Myc). The same antibody was also expressed as VHH-His-C tag and purified either by immune-affinity using an anti-C tag antibody or by IMAC (C tag). The immune- purified (E1 and E2) and IMAC elution fractions were separated by SDS-PAGE, compared with the flow-through (Ft) and the marker (M), and stained with colloidal blue. (b) The affinity of the VHH-His-C tag construct was assessed by surface plasmon resonance (SPR) in combination with the recombinant HER2 ectodomain (c) The same construct was used to precipitate HER2 from SKBR3 cell lysate. The control and experimental fractions used for immunoprecipitation were separated in parallel and either stained with colloidal blue or used for western blot in combination with a commercial anti-HER2.
Consequently, we consider exploiting the 6xHis and the C tag for two successive orthogonal affinity purification steps with the aim of removing any non-specific contaminant according to the scheme reported in Figure 2. Surprisingly, the sole C tag-dependent affinity purification using a resin coupled to anti C tag antibodies was sufficient to obtain a homogeneous VHH sample (Figure 1a). No protein contaminant or trace of degradation was detectable and, therefore, there was no reason to enchain a successive His-tag dependent IMAC.
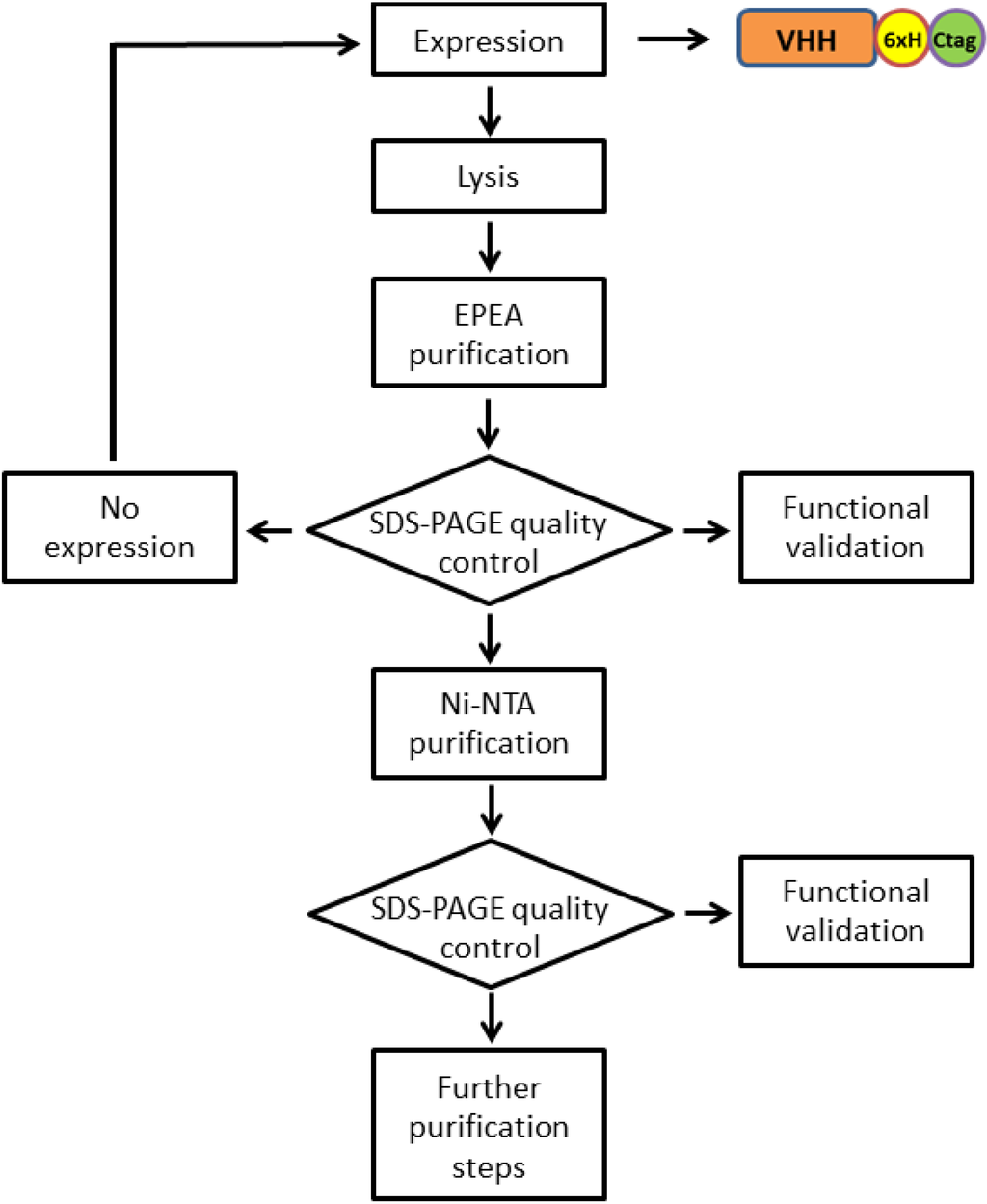
Figure 2.
Flow-chart of the purification protocol used for recovering homogeneous proteins fused to both 6xHis and C tags. The presence of both C and 6xHis tags enables two orthogonal steps of affinity purification. A protein quality control (functionality, contamination, degradation) can be performed after each purification step to evaluate the necessity to stop the procedure or to pass to the next purification modules.
Recently we showed that the production of single-domain antibodies in the cytoplasm of bacteria over-expressing a sulfhydryl oxidase could represent a convenient alternative to their periplasmic accumulation [8]. Apparently, the double His-C tag appended to VHHs did not interfere with the production of the three different nanobodies used for the test. The average yields were 4.7 mg/L culture when the VHHs accumulated in the cytoplasm and 0.9 mg/L culture for periplasmic production. Analytical gel filtration confirmed that the purified antibodies were monodisperse (Figure 3).
The A10 VHH purified using the C tag showed an affinity in the low nanomolar range (Figure 1b). This value is comparable to the control (manuscript in preparation) and confirms that the purification procedure does not modify the antibody binding capacity.
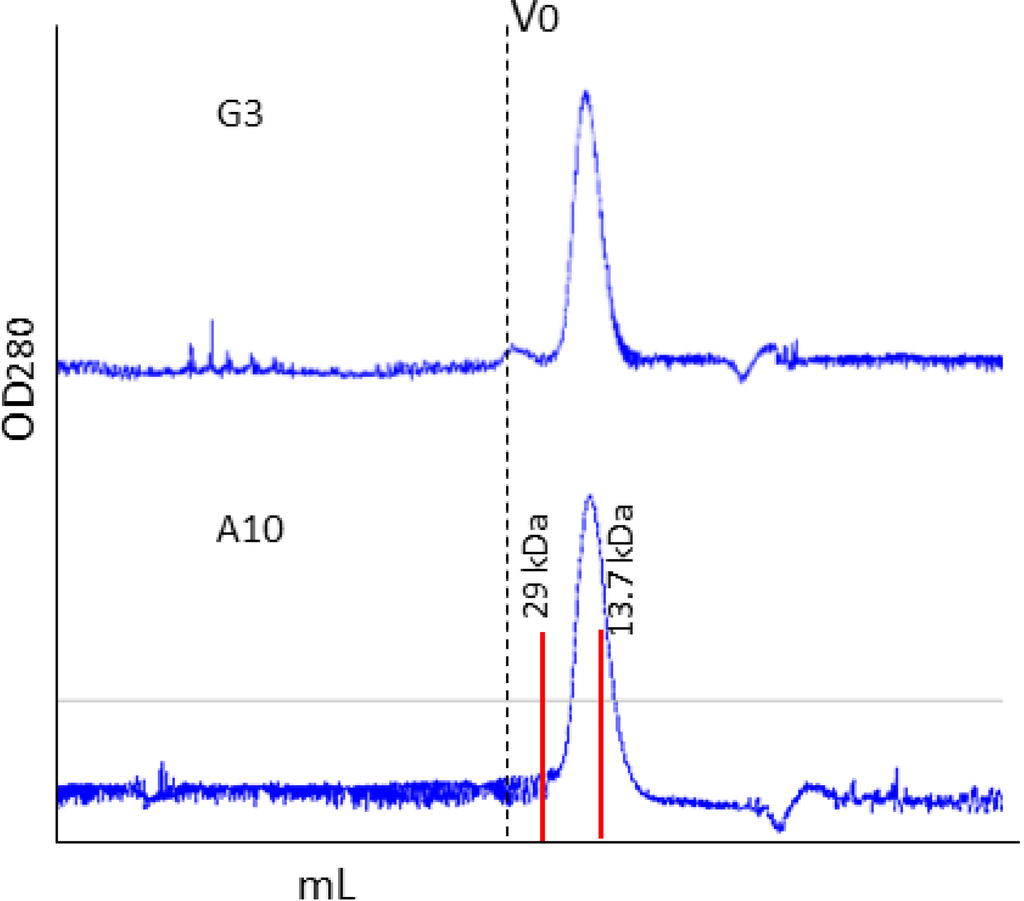
Figure 3.
Gel filtration of immunopurified VHH fractions The elution fractions corresponding to the VHH antibodies G3 and A10, immunopurified using the anti-C tag resin, have been purified by gel filtration to evaluate their monodispersity and aggregation/oligomerization state.
2.2. C Tag-Dependent Immunoprecipitation
The antibody A10 fused to C tag was used to bind the HER2 receptor present in SKBR3 cell lysate and the complex was successfully precipitated by using beads coupled to the anti-C tag antibody. Apart from a unique non-specific band at 55 kDa, only a major band corresponding to a large protein was detectable in the elution fraction of gel stained by colloidal blue (Figure 1c). When the same fractions were analyzed by western blot using a monoclonal anti-HER2 antibody, the band identified by SDS-PAGE was specifically stained together with a set of less intense bands corresponding to proteins of lower mass. This has been described as the typical pattern of HER2 present at the cell surface since both the full-length protein and its shedding products generated by metalloprotease activity are simultaneously present [9]. The result confirmed that the monovalent A10 was sufficient to specifically immunoprecipitate its antigen HER2 in the presence of 1% Triton X-100 and that C tag allowed for the recovery of a clean fraction after a single affinity purification step.
2.3. Functional Validation of His-C Double-Tagged VHHs
We used flow cytometry to compare alternative detection approaches and the functionality of VHHs produced in either the periplasm or the cytoplasm of E. coli. As reported in Figure 4, HER2 positive (SKBR3) and negative (MCF10A) cells were specifically identified by using any of the four strategies. The VHHs produced in the cytoplasm were at least as efficient as those produced in the periplasm when the intensity of their binding to the antigen was evaluated using an anti-His tag primary antibody followed by a labeled secondary antibody (Figure 4).
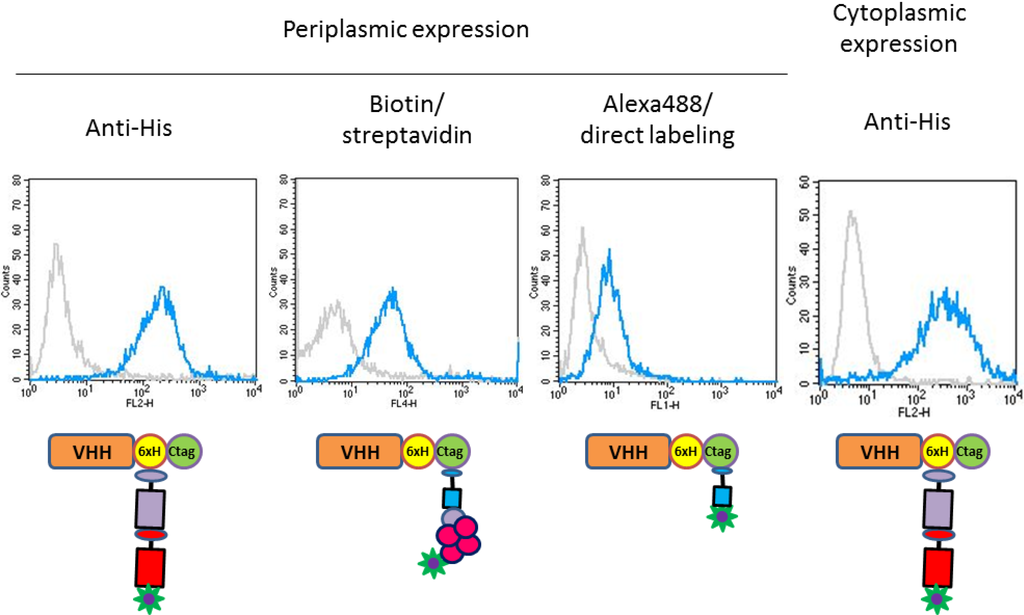
Figure 4.
FACS analyses performed with C tagged recombinant antibodies. HER2 detection on MCF10A (neg) and SKBR3 (pos) cells was obtained using the anti-HER2 His+C-tagged A10 VHHs produced either in bacterial periplasm or cytoplasm in combination with: (i) an anti-His primary antibody (TeBu Bio) plus a PE goat anti-mouse secondary antibody (BD Pharmingen); (ii) the anti-C tag nanobody coupled to biotin plus APC-labeled streptavidin; (iii) an Alexa488-labeled anti-C tag nanobody.
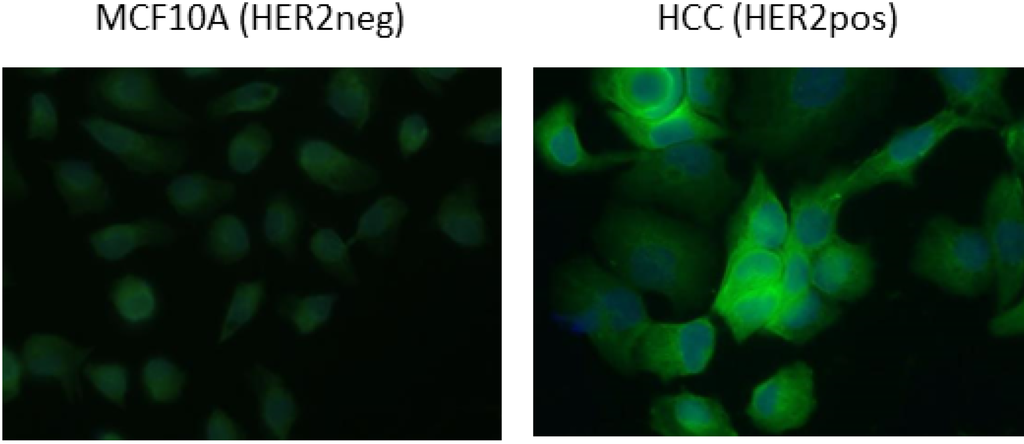
Figure 5.
Immunofluorescent staining of HCC (HER2 pos) cells. Direct staining of HER2 neg (MCF10A) and HER2 pos (HCC) cells performed using Alexa488-labeled A10 VHH.
The C tag was used for two alternative staining methods. In the first case, a biotinylated anti-C tag antibody was coupled to a streptavidin molecule linked to a chromophore, and in the second the anti-C tag antibody was directly linked to Alexa488 (Figure 4). The specific signal shift between negative and positive cells was evident, even though of lower intensity in comparison to the experiments performed using the anti-His primary antibody. The relatively higher signal background obtained using streptavidin could be due to its sticky nature whereas we suspect that the amine-labeling decreased the binding capacity of the Alexa488-labeled VHH. Although some technical improvements seem necessary to optimize the use of such reagents in flow cytometry, the Alexa488-labeled antibody succeeded in specifically staining the HER2 positive cell line HCC (Figure 5) and its use simplified the protocol since no secondary antibody was necessary.
3. Experimental
3.1. Vector Preparation for Recombinant Antibody Expression
A vector for the periplasmic expression of recombinant polypeptides fused to the C tag was prepared by replacing the sequence corresponding to the 6xHis-3xmyc tag in the pHEN2 vector with the adaptor constructed by pairing the two sequences 5'GGCCGCACATCATCATCACCATCACGGGGCCGCAGAACCGGAAGCGTAAGAATGCT3' and 5'CGTGTAGTAGTAGTGGTAGTGCCCCGGCGTCTTGGCCTTCGCATTCTTAC3'. The adaptor was inserted between the NotI and BsmI sites. The cytoplasmic C tag vector was subcloned from the periplasmic vector by cut-and-paste of the sequence excised with the restriction enzymes NcoI-BamHI into a pET14 vector.
Three anti-HER2 (A10, C8, G3) recombinant VHHs isolated from a pHEN2-based phage-display synthetic library were cloned NcoI-NotI into both expression vectors.
3.2. Antibody Production and Purification
Recombinant VHHs were expressed in E. coli BL21 (DE3) strain either overnight in the periplasm, as described previously [10], or in the cytoplasm after pre-expression of sulfhydryl oxidase [8]. Bacteria were grown at 37 °C in LB medium until OD600nm reached 0.4 and sulfhydryl oxidase was induced for 45 min at 30 °C by adding 0.5% (g/mL culture) of arabinose. Finally, the temperature was lowered to 20 °C, IPTG was added at 0.05 mM, and the bacteria were harvested the next morning. Frozen pellets corresponding to 500 mL culture were resuspended in 20 mL of 100 mM Tris pH8, 500 mM NaCl, 2.5 mM MgCl2. Lysozyme (0,5 mg/mL) and DNase (3U) were added for 30 min at room temperature. Samples were sonicated, centrifuged at 18,000xg for 20 min at 4 °C, and the lysates filtered before being affinity-purified. IMAC was performed using a HiTrap TALON® column and an ÄKTA-Pure FPLC system (GE Healthcare). C tag-dependent affinity purification was performed using CaptureSelect C tag affinity matrix (kindly provided by BAC BV-Life Technologies) both on batch and FPLC-assisted. C-tagged antibodies were eluted in the presence of 2 M MgCl2 and their concentration evaluated using the BCA colorimetric assay. CaptureSelect C tag affinity matrix can be regenerated using 1 M glycine, pH 2, and used for several cycles. Analytical size exclusion chromatographic was performed on Superdex™ 75 5/150 [11]. SDS-PAGE gels (15%) were Coomassie stained and recorded using a Gel Doc™ EZ System (Bio Rad).
3.3. Immunoprecipitation
Fifty Microliters of the affinity resin specific for the C-tag were centrifuged to remove the buffer excess. The resin was washed three times in PBS before being incubated 30 min at 4 °C in the presence of 2.5 nmol of the anti-HER2 (A10) VHH fused to the terminal C-tag. The coupled resin was washed three times in PBS before use. Confluent SKBR3 cells were scraped on ice, lysed in 500 mL of 20 mM HEPES-KOH, pH 7, 2, 50 mM beta-glycerophosphate, 100 mM KCl, 0.2 mM EDTA, 10% glycerol, 1% Triton X-100 and frozen. Thawed lysate was centrifuged 10 min at 11000 ×g and 4 °C. Two mg of the resulting protein supernatant were added to anti-HER2-activated C-tag beads and incubated for 2 h at 4 °C in a total volume of 500 μL. After a spin centrifugation, the beads were washed 3 times with 1 mL of lysis buffer and 50 μL of 2× loading buffer were finally added before evaluating the samples by SDS PAGE. Uncoupled resin was used as a negative control.
3.4. FACS Analysis and Cell Imaging
Purified antibodies were analyzed by flow cytometry using SKBR3 (HER2 positive sample) and MCF10A cells (HER2 negative sample). Cells were incubated in 96 well plates in the presence of 10% FCS-PBS and then 2 h at 4 °C with the antibodies. After 3 washing steps, cells were resuspended in 1% PFA-PBS and analyzed by using a FACSCalibur Flow Cytometer (BD Biosciences). Biotinylated C tag-binding antibody fragment and Alexa488 C tag-binding antibody fragment were kindly provided by BAC BV – Life Technologies (Leiden, NL).
Immunofluorescence was performed using MCF10A and SKBR3 cells seeded overnight on coverslips. The day after, cells were fixed 15 min using 4% PFA-PBS at room temperature, stained 2 h in the presence of the antibodies, and incubated 45 min in the dark in the presence of the Alexa488-conjugated antibodies. The samples were finally mounted on microscope slides by using Mowiol.
3.5. Surface Plasmon Resonance
Antibody affinity was measured at 25 °C using a ProteOn XPR36 (BioRad). Fc-HER2 ectodomain produced in mammalian cells (96 kDa) was diluted to 400 μg/mL in sodium acetate buffer (pH 5.0) and immobilized by amine-coupling on a GLC chip (BioRad) at 2000 RU. One hundred μL of antibodies were injected as the analyte at 100 μL/min. The complete kinetic data set was collected in a single run and data were fitted with a 1:1 Langmuir interaction model. Surface regeneration was performed using 10 mM glycine HCl, pH 2.5.
4. Conclusions
Affinity purification based on the recognition of an antibody for its specific epitope has already been widely exploited in the past but the general use of such approach can be impaired by several limitations such as the difficulty to produce cost-effective stable antibodies or the necessity to use harsh conditions to elute the target proteins. The nine terminal amino acid sequence of bovine rhodopsin (rhodopsin tag) have been used in combination with the corresponding 1D4 monoclonal antibody for immune purification of recombinant proteins in both eukaryotic and prokaryotic systems [12,13]. The target protein is then eluted by competition with free Rho peptides. We have now demonstrated that the C tag is a valid alternative. C tag is shorter than rhodopsin tag (the four EPEA amino acids) and this condition would reduce undesirable steric hindrance and instability effects. Furthermore, native elution is obtained by the addition of inexpensive MgCl2 and related reagents such as biotinylated and Alexa488-bound anti-C tag antibodies are already available. Finally, C tag does not interfere with polypeptide production in both the periplasmic and cytoplasmic space and the resulting proteins are monodisperse, functional, and resistant to the detergent concentrations used in pull-down experiments. The vectors we developed allow for multiple labeling and different derivatization strategies. For instance, the biotin/streptavidin system represents a flexible platform suitable to link the antigen-specific moiety (primary Ab plus biotinylated anti-C tag) to a reporter (the streptavidin) for which a large array of commercial fusion combinations exists. We demonstrated that C tag-coupled resin is very effective for IP and this application could be even improved in combination with magnetic beads. Finally, (strept)avidin biosensors could be used to capture and confer outward orientation to biotin-activated antibodies.
Acknowledgments
The authors wish to thank Pim Hermans (BAC BV- Life Technologies) for the support and the provided reagents and COPIO/ITMO-TS/Aviesan, Région île de France, and Labex DCBIOL (ANR-11-LABEX-0043–grant ANR-10-IDEX-0001-02 PSL) for financial support.
Author Contributions
S.D. developed the vectors, performed the cloning, and measured the antibody affinities, A.B. purified the antibodies and performed the IP and IF, A.S. performed the FACS. experiments, A.d.M. designed the experiments, analyzed the data, and wrote the manuscript. All authors reviewed and accepted the final text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rigaut, G.; Shevchenko, A.; Rutz, B.; Wilm, M.; Mann, M.; Séraphin, B. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 1999, 17, 1030–1032. [Google Scholar] [CrossRef]
- Dümmler, A.; Lawrence, A.M.; de Marco, A. Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb. Cell Factories 2005, 4, 34. [Google Scholar] [CrossRef]
- Yelissev, A.; Zoubak, L.; Gawrisch, K. Usual of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr. Purif. 2007, 53, 153–163. [Google Scholar] [CrossRef]
- Bürckstümmer, T.; Bennett, K.L.; Preradovic, A.; Schütze, G.; Hantschel, O.; Superti-Furga, G.; Bauch, A. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat. Methods 2013, 12, 1013–1019. [Google Scholar]
- Dammeyer, T.; Timmis, K.N.; Tinnefeld, P. Broad host range vectors for expression of proteins with (Twin-) Strep-tag, His-tag and engineered, export optimized yellow fluorescent protein. Microb. Cell Fact. 2013, 12, 49. [Google Scholar] [CrossRef]
- Aliprandi, M.; Sparacio, E.; Pivetta, F.; Ossolengo, G.; Maestro, R.; de Marco, A. The availability of a recombinant anti-SNAP antibody in VHH format amplifies the application flexibility of SNAP-tagged proteins. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef]
- de Marco, A. The choice of appropriate tags improves the application effectiveness of the selected binders: The generation of user-friendly expression plasmids. Methods Mol. Biol. 2012, 911, 507–522. [Google Scholar]
- Veggiani, G.; de Marco, A. Improved quantitative and qualitative production of single-domain intrabodies mediated by the co-expression of Erv1p sulfhydryl oxidase. Protein Expr. Purif. 2011, 79, 111–114. [Google Scholar] [CrossRef]
- Sperinde, J.; Jin, X.; Banerjee, J.; Penuel, E.; Saha, A.; Diedrich, G.; Huang, W.; Leitzel, K.; Weidler, J.; Ali, SM.; et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2010, 16, 4226–4235. [Google Scholar] [CrossRef]
- Monegal, A.; Ami, D.; Martinelli, C.; Huang, H.; Aliprandi, M.; Capasso, P.; Francavilla, C.; Ossolengo, G.; de Marco, A. Immunological applications of single domain llama recombinant antibodies isolated from a naïve library. Protein Eng. Des. Sel. 2009, 22, 273–280. [Google Scholar] [CrossRef]
- Sala, E.; de Marco, A. Screening optimized protein purification protocols by coupling small-scale expression and mini-size exclusion chromatography. Protein. Expr. Purif. 2010, 74, 231–235. [Google Scholar] [CrossRef]
- Shimada, M.; Chen, X.; Cvrk, T.; Hilfiker, H.; Parfenova, M.; Segre, GV. Purification and characterization of a receptor for human parathyroid hormone and parathyroid hormone-related peptide. J. Biol. Chem. 2002, 277, 31774–31780. [Google Scholar]
- Locatelli-Hoops, S.C.; Gorshkova, I.; Gawrisch, K.; Yeliseev, A.A. Expression, surface immobilization, and characterization of functional recombinant cannabinoid receptor CB2. Biochim. Biophys. Acta 2013, 1834, 2045–2056. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).